Abstract
Protein tyrosine kinase 6 (PTK6) (also called Brk or Sik) is an intracellular tyrosine kinase that is expressed in breast cancer and normal epithelial linings. In adult mice, PTK6 expression is high in villus epithelial cells of the small intestine. To explore functions of PTK6, we disrupted the mouse Ptk6 gene. We detected longer villi, an expanded zone of PCNA expression, and increased bromodeoxyuridine incorporation in the PTK6-deficient small intestine. Although differentiation of major epithelial cell types occurred, there was a marked delay in expression of intestinal fatty acid binding protein, suggesting a role for PTK6 in enterocyte differentiation. However, fat absorption was comparable in wild-type and Ptk6−/− mice. It was previously shown that the serine threonine kinase Akt is a substrate of PTK6 and that PTK6-mediated phosphorylation of Akt on tyrosine resulted in inhibition of Akt activity. Consistent with these findings, we detected increased Akt activity and nuclear β-catenin in intestines of PTK6-deficient mice and decreased nuclear localization of the Akt substrate FoxO1 in villus epithelial cells. PTK6 contributes to maintenance of tissue homeostasis through negative regulation of Akt in the small intestine and is associated with cell cycle exit and differentiation in normal intestinal epithelial cells.
The intracellular epithelial protein tyrosine kinase 6 (PTK6) was cloned from cultured human melanocytes (24) and human breast cancer cells (it is also called Brk for breast tumor kinase) (31). PTK6 was also identified in a screen for tyrosine kinases expressed in the mouse small intestine (it is also called Sik for Src-related intestinal kinase) (40, 45). While PTK6 is expressed in many breast carcinoma cell lines and in a high percentage of primary breast tumors that have been examined, it has not been detected in normal human breast tissue (3, 31) or in the mouse mammary gland (26). In normal tissues PTK6 is expressed in differentiated nondividing cells, with high levels in linings of the gastrointestinal tract (26, 45). PTK6 is expressed in skin (45), and overexpression of PTK6 in mouse keratinocytes resulted in increased expression of the differentiation marker filaggrin during calcium-induced differentiation, suggesting a positive role in differentiation (46). PTK6 was also recently implicated in the regulation of human keratinocyte differentiation (48). Higher levels of PTK6 were detected in normal human oral epithelial cells than in oral epithelial cancer cells (34). In the normal prostate, PTK6 is present in the nuclei of epithelial cells, but it is relocalized to the cytoplasm in prostate tumors (10).
PTK6 belongs to a novel subfamily of intracellular tyrosine kinases that is distinct from the Src family and includes Srms, FRK (Rak/Gtk/Iyk/Bsk), and Src42A/Dsrc41 (reviewed in reference 39). These kinases share a low degree of sequence homology with known kinases, including one another, but the exon structure of the genes encoding these kinases is highly conserved and distinct. Lack of myristoylation distinguishes most PTK6 family kinases from the Src family, and these proteins are not specifically targeted to the membrane. In fact, both PTK6 (10, 11, 15) and FRK (9) appear to have nuclear functions.
The first substrates identified for PTK6 were the novel adaptor protein BKS/STAP-2 (32), which has been implicated in the regulation of STAT3 (30), and the KH domain-containing RNA binding protein Sam68 (11). We also identified the Sam68-like mammalian proteins SLM-1 and SLM-2 as substrates of PTK6 (15). PTK6 phosphorylates Sam68, SLM-1, and SLM-2 in the nucleus and inhibits their RNA binding activities (11, 15). PTK6 was also found to phosphorylate the cytoskeletal protein paxillin following epidermal growth factor stimulation, which promoted activation of Rac1, cell migration, and invasion in vitro (8).
Recently the serine threonine kinase Akt/PKB was identified as a substrate of PTK6, and PTK6-mediated tyrosine phosphorylation led to inhibition of Akt activity in unstimulated cells (50). Overexpression of active PTK6 blocked the phosphorylation of the Forkhead transcription factor FoxO1 (FKHR), a downstream Akt target. These results suggested that PTK6 may function as a signaling molecule whose kinase activity normally limits the activity of Akt in unstimulated cells (50).
The dramatic induction of the PTK6 tyrosine kinase in a significant percentage of human breast tumors suggests a role for this tyrosine kinase in the etiology of breast cancer (17). However, expression patterns of PTK6 in normal epithelia are not consistent with a role in promoting growth. Ectopic expression of mouse PTK6 in Rat1a cells did not enhance growth but led to increased apoptosis following serum deprivation and UV exposure (16). To explore functions of PTK6, we developed and characterized a PTK6-deficient mouse model, focusing on the small intestine, where PTK6 is expressed at highest levels in adult animals.
MATERIALS AND METHODS
Targeting of the murine Ptk6 gene.
A targeting vector was developed that replaced the first exon and start site of transcription of the Ptk6 gene with a phosphoglycerate kinase (PGK) promoter driving expression of the selectable marker neomycin (PGK-neo) for positive selection. Transcription of neomycin gene occurred in the opposite direction of the Ptk6 gene, and a PGK-driven diphtheria toxin-A gene was added to the 3′ end of the construct for negative selection (41). Southern blot analyses and RNase protection assays were performed as previously described (16, 26). Offspring from matings of heterozygous Ptk6+/− mice derived from four independent embyonic stem cell clones were analyzed. Both C57BL/6J;129/Sv hybrid mice from early generations and mice backcrossed for 12 generations to C57BL/6J mice were used for the studies.
Histological and fecal fat measurements.
Corresponding regions of the jejunum and ileum were stained with hematoxylin and eosin and examined by light microscopy. Villus height and crypt depth were measured in age- and sex-matched mice (≥3 per genotype). For each animal, six well-oriented villi or crypts from at least five quadrants (for a total of 30) were measured. The data were subjected to the Student two-tailed t test to determine the P values.
Wild-type and Ptk6−/− mice were fed a standard diet (5.2% fat; 22/5 Rodent Diet) or a high-fat diet (21.2% fat; Adjusted Calories Diet TD88137) (Harlan Teklad, Madison, WI). Body weight and chow intake were measured daily. Stool samples were collected, and fecal fat content was analyzed by organic extraction as described previously (38, 49).
BrdU labeling and immunohistochemistry.
Mice were injected with 5-bromo-2-deoxyuridine (BrdU) (Sigma) in phosphate-buffered saline at 50 μg/g of body weight 1 hour prior to being sacrificed, and tissues were fixed in Carnoy's fixative (10% glacial acetic acid, 60% ethanol, 30% chloroform) for 3 h at 4°C. BrdU incorporation was detected using immunohistochemistry and the M.O.M. immunodetection kit (Vector Laboratories Inc.). For other immunohistochemical analyses, tissues were fixed in 4% paraformaldehyde and the Vectastain ABC kit (Vector Laboratories Inc.) was used according to manufacturer's instructions. Antigen retrieval was performed by microwaving samples in 10 mM sodium-citrate buffer, pH 6.0. Antibody binding was visualized by incubation of the slides with the chromogenic substrate 3,3′-diaminobenzidine tetrahydrochloride (DAB) (Sigma). Controls were performed with equal dilutions of rabbit or mouse immunoglobulin G (Santa Cruz Biotechnology).
Antibodies and immunoblotting.
Antibodies against mouse PTK6 (Sik C-17), PCNA, and c-Myc were obtained from Santa Cruz Biotechnology, and anti-β-catenin and BrdU monoclonal antibodies were obtained from BD Biosciences. Anti-Akt, phospho-Akt(Ser473), phospho-glycogen synthase kinase 3 (GSK-3)α/β(Ser21/9), GSK-3, and FKHR (FoxO1) antibodies were obtained from Cell Signaling Technology. To examine differentiation, rabbit antilysozyme (DAKO), rabbit anti-intestinal fatty acid binding protein (anti-I-FABP) (36) (a gift from J. I. Gordon, Washington University, St. Louis, Mo.), and rabbit antisynaptophysin (DAKO) were used. Immunoblotting was performed as previously described (16).
In vitro Akt kinase assays.
In vitro Akt kinase assays were performed using a nonradioactive Akt kinase assay kit (Cell Signaling Technology) according to the manufacturer's instructions. Tissue lysates were incubated with immobilized Akt (1G1) monoclonal antibody overnight at 4°C. Resulting immune complexes were washed twice in cell lysis buffer and kinase buffer. In vitro kinase assays were performed using recombinant GSK-3 fusion protein as a substrate. Immunoprecipitated Akt was incubated with 0.2 mM ATP and 1 μg GSK-3 fusion protein in kinase buffer for 30 min at 30°C. Reactions were terminated with 25 μl of 2× sample buffer, the mixture was boiled for 5 min, and the supernatant was separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Phosphorylation of GSK-3 was measured by immunoblotting using phospho-GSK-3α/β(Ser21/9) polyclonal antibody.
RESULTS
Disruption of the mouse Ptk6 gene.
To determine in vivo functions of the non-receptor tyrosine kinase PTK6, we generated PTK6-deficient mice. The strategy is summarized in Fig. 1A, and successful targeting was confirmed by Southern blotting (Fig. 1B). Loss of PTK6 mRNA expression was confirmed by RNase protection assays (Fig. 1C). No PTK6 protein expression was observed in the intestines of knockout mice, confirming the loss-of-function mutation in the Ptk6 gene (Fig. 2A). PTK6-deficient mice were viable and fertile and did not develop spontaneous tumors over an 18-month period.
FIG. 1.
Disruption of the Ptk6 gene in the mouse. A. The general strategy is outlined, as described in Materials and Methods. B. Southern blotting was performed with digested tail DNA and probes derived from the 5′ and 3′ regions of the targeting region. Results of a typical hybridization with the 5′ probe highlighted in panel A and NdeI- and EcoRI-digested DNA confirm the appropriate targeting event. C. RNase protection assays performed with ileum RNAs isolated from three wild-type and four Ptk6−/− mice demonstrate absence of Ptk6 gene expression in the Ptk6−/− small intestine. Expression of cyclophilin was examined as a control.
FIG. 2.
PTK6 expression in the small and large intestines. A. PTK6 protein expression is highest in the neonatal colon and adult ileum. Immunoblotting was performed to examine PTK6 protein expression in wild-type and Ptk6−/− small and large intestines. Lysates from three wild-type and three Ptk6−/− neonatal (14-day-old) and adult (8-week-old) animals were probed with anti-mouse PTK6 (Sik) antibodies. No PTK6 was detected in the Ptk6−/− lysates. As a control, blots were also probed with antibodies specific for β-actin. B. PTK6 is expressed in nondividing differentiated cells of the small intestine and colon. The boxed region is magnified, and the arrows denote cells with reduced immunoreactivity in the villus epithelium of the jejunum. The colon lacks villi (V), and differentiation and cell cycle exit occur in the upper regions of the crypt (C). Immunohistochemistry was performed with adult wild-type jejunum and colon, using anti-PTK6 antibodies or anti-rabbit immunoglobulin G (IgG) antibodies as controls and DAB as a substrate (brown). Bar, 50 μm.
PTK6 expression is developmentally regulated and is first evident as epithelial linings mature (45). Initially, higher PTK6 levels are present in neonatal colon (14 day old), but in the adult 8-week old mouse, PTK6 is expressed at highest levels in the ileum of the small intestine (Fig. 2A) (15). The intestinal epithelium provides a useful developmental model in which continuous self-renewal is occurring and proliferation and differentiation are compartmentalized (reviewed in reference 35). In the small intestine, a rapidly proliferating progenitor population in each crypt compartment gives rise to cells that migrate bidirectionally and give rise to Paneth cells at the base of the crypts and to enterocytes, goblet cells, and enteroendocrine cells that reside on the villus. In both the small intestine and colon, PTK6 is localized to nondividing differentiated epithelial cells (Fig. 2B). In the small intestine, a few of the epithelial cells (Fig. 2B) have decreased immunoreactivity, and it appears that PTK6 may be most abundant in the enterocyte lineage.
Increased growth and proliferation in the Ptk6−/− small intestine.
We found no significant difference in body weight or length of the intestines in the mice with wild-type or PTK6-deficient intestine. However, comparison of corresponding regions of jejunum (Fig. 3A and B) and distal ileum (Fig. 3D and E) of age-matched male mice revealed a significant increase in villus length in the PTK6-deficient animals. A trend toward deeper crypts was also observed in the jejunum (Fig. 3C). No alterations in basal levels of apoptosis were detected (data not shown).
FIG. 3.
Defects in intestinal epithelial homeostasis in the absence of PTK6 signaling. Loss of PTK6 expression affects villus length and crypt depth in the jejunum and ileum. A and D. Representative corresponding sections of jejunum (A) and ileum (D) from wild-type and Ptk6−/− mice were stained with hematoxylin-eosin and measured. Bars, 100 μm. B and E. Average villus length ± standard deviation. P = 0.038 and P = 0.003 for jejunum and ileum, respectively. C. A trend toward deeper crypts was also observed in Ptk6−/− jejunum (P = 0.07).
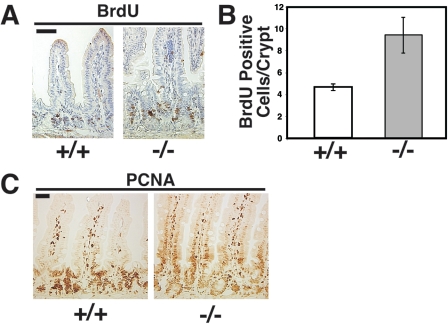
To examine proliferation, age-matched male wild-type and PTK6-deficient mice were injected with BrdU 1 hour prior to sacrifice, and paraffin sections of the small intestine were subjected to immunohistochemistry with antibodies directed against BrdU and PCNA. Analysis of BrdU-positive intestinal epithelial cells revealed an increased number of proliferating cells in PTK6 knockout mice compared with wild-type controls (Fig. 4A and B). PCNA-positive cells were detected in nondividing differentiating villus epithelial cells of the PTK6-deficient mice, while PCNA was more restricted to the crypt compartment in wild-type mice (Fig. 4C). The expanded proliferative zone and increased number of proliferating cells in PTK6-deficient mice suggested a deregulated balance between proliferation and differentiation in the intestinal epithelium in the absence of PTK6 signaling.
FIG. 4.
Increased proliferation in Ptk6−/− intestine. A. BrdU incorporation is increased in crypts of Ptk6−/− mice. Antibody binding was detected with DAB (brown), and sections were counterstained with hematoxylin (blue). B. The average number of BrdU-positive cells per crypt is significantly higher in Ptk6−/− small intestine (P = 0.037). Error bars indicate standard deviations. C. PCNA immunoreactivity is not restricted to the crypt compartment in PTK6-deficient mice. PCNA-positive epithelial cells are detected in villi of Ptk6−/− mice. Bars, 50 μm.
Increased proliferation and enhanced villus length are characteristics of intestinal adaptation following loss of functional small intestine (37, 42). To explore the possibility that the increased growth and proliferation in Ptk6−/− mice was an adaptive response due to poor absorption, mice were fed standard and high-fat diets, and fecal fat levels were measured to monitor intestinal absorption. No significant differences in excreted fecal lipid levels were detected (data not shown). In addition, no significant differences in chow intake or body weight were detected in mice fed a high-fat diet for 3 weeks. These data indicate that intestinal absorption is similar in wild-type and Ptk6−/− animals.
PTK6 regulates maturation of the enterocyte lineage in the small intestine.
Previous studies with cultured keratinocytes suggested that PTK6 might promote epithelial cell differentiation (46). To explore the consequences of disruption of the Ptk6 gene for differentiation of the various intestinal epithelial cell lineages, immunohistochemistry was performed using antibodies against common markers for the different epithelial cell types found in the small intestine (Fig. 5). Approximately equal numbers of cells were positive for lysozyme (Paneth cells) and synaptophysin (enteroendocrine cells). The periodic acid-Schiff reaction was used to identify mucin-containing goblet cells in the PTK6-deficient small intestine (Fig. 5), and the numbers of positive cells were similar in wild-type and knockout mice. The expression of mRNAs encoding cryptdin-1 (Paneth cells) and the intestinal trefoil factor TFF-3 (goblet cells) was also examined using RNase protection assays, and levels were equivalent (data not shown). No significant differences in expression of Paneth, goblet, or enteroendocrine cell markers were detected in the small intestine.
FIG. 5.
Differentiation of Paneth, enteroendocrine, and goblet cells in the Ptk6−/− intestine. The presence of Paneth cells and enteroendocrine cells in Ptk6−/− small intestine was confirmed by immunohistochemistry with antibodies against the Paneth cell marker lysozyme and the enteroendocrine marker synaptophysin. Goblet cells were detected using the periodic acid-Schiff reaction, which stains mucin-containing cells, such as goblet cells, bright pink. Arrowheads indicate examples of positive cells. Bar, 100 μm.
I-FABP, a small intestine-specific fatty acid binding protein that represents approximately 3% of the cytoplasmic protein of enterocytes (2, 47), is commonly used as a marker of enterocyte differentiation. Differentiation of enterocytes, the major epithelial cell type, was examined using antibodies against I-FABP. While I-FABP protein was strongly induced in the lower villi of wild-type animals, strong induction of I-FABP was detected only in the upper half of villi in PTK6-deficient animals (Fig. 6A). Immunoblotting revealed that reduced levels of I-FABP protein were expressed in the PTK6-deficient intestine (Fig. 6B). These data suggest that PTK6 may play a specific role in promoting the differentiation or maturation of enterocyte lineage in the small intestine.
FIG. 6.
Expression of the enterocyte marker I-FABP is delayed and reduced in the Ptk6−/− intestine. A. Immunohistochemistry was performed with I-FABP antibodies and sections of jejunum from wild-type and Ptk6−/− mice. Antibody binding was detected with DAB (brown), and sections were counterstained with hematoxylin (blue). Induction of I-FABP protein expression appears strong only in the upper regions of the villi in the Ptk6−/− small intestine. Bar, 100 μm. B. Immunoblotting was performed with protein lysates prepared from distal ileum isolated from three wild-type and three Ptk6−/− animals. I-FABP protein levels are reduced in Ptk6−/− intestine.
Increased Akt activity and signaling in the PTK6-deficient small intestine.
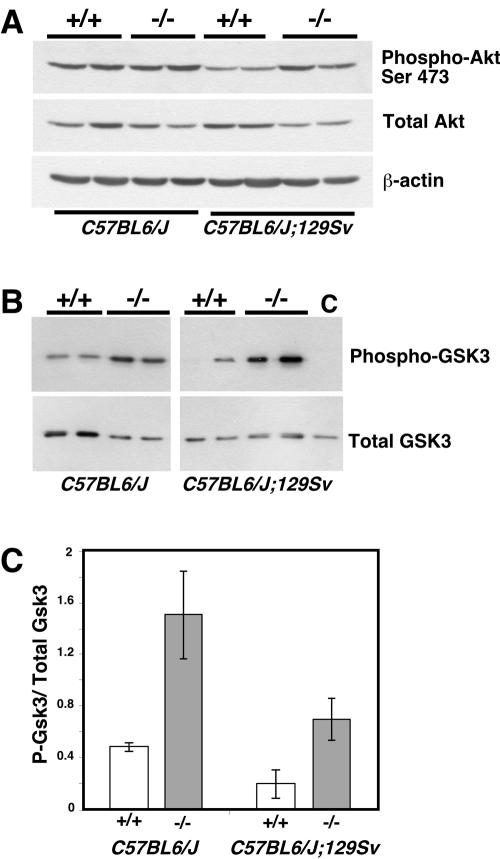
Akt is an important regulator of cell proliferation, growth, and survival (19). A recent study showed that PTK6 is able to phosphorylate Akt on tyrosine and inhibit its activity (50). Activated Akt is phosphorylated at Thr308 and Ser473 (reviewed in reference 23). Immunoblotting with total and phospho-Akt(Ser473) antibodies suggested possible differences in Akt activity in intestines of wild-type and PTK6-deficient mice (Fig. 7A). Phospho-Akt(Ser473) levels were higher relative to total Akt in cell lysates prepared from PTK6-deficient small intestines. To examine Akt activity, endogenous Akt was immunoprecipitated using immobilized Akt antibodies, and in vitro kinase assays were performed by incubation with ATP and purified recombinant GSK-3 protein as the substrate. Samples were subjected to immunoblotting using phospho-specific GSK-3 antibodies. Basal Akt kinase activity was increased in both hybrid C57BL/6J;129/Sv and congenic C57BL/6J PTK6-deficient mice compared with wild-type controls (Fig. 7B and C).
FIG. 7.
Increased Akt activity in the intestines of Ptk6−/− mice. A. Immunoblotting with lysates from two wild-type and two Ptk6−/− mice from congenic C57BL/6J and hybrid C57BL/6J;129/Sv backgrounds indicates increased levels of activated phospho-Akt(Ser473) relative to total Akt levels. B. Endogenous Akt was immunoprecipitated from total cell lysates of the ileums from congenic C57BL/6J and hybrid C57BL/6J;129/Sv mice. Data from two mice per genotype per background are shown. In vitro kinase assays were performed with purified recombinant GSK-3 and ATP. Reactions were stopped at 0 (control [C]) and 30 min, and products were subjected to Western blotting with phospho-GSK-3 and total GSK-3 antibodies. Equal amounts of total GSK-3 protein were present in the reactions. Loss of PTK6 results in increased Akt kinase activity in the intestinal epithelium. C. Quantitation of Akt kinase activity. The increase in Akt activity was significant in both congenic C57BL/6J (P = 0.014) and hybrid C57BL/6J;129Sv (P = 0.014) mice. Error bars indicate standard deviations.
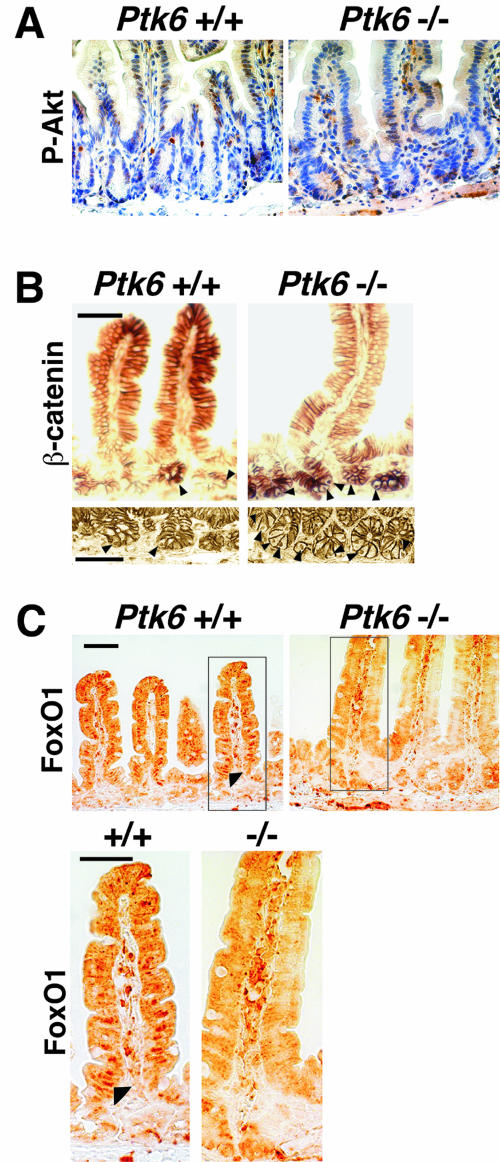
To further explore the increase in Akt activation, anti-phospho-Akt(Ser473) antibodies were used in immunohistochemistry experiments. In accordance with the immunoblotting data (Fig. 7), immunohistochemistry also revealed increased levels of phospho-Akt in the epithelia of PTK6-deficient mice. As reported by others (20), immunoreactive phospho-Akt is most striking in a few cells in the intestinal crypts of wild-type mice (Fig. 8A). However, in PTK6-deficient mice, phospho-Akt immunoreactivity appears to be more widespread and includes many cells in both the crypts and villi.
FIG. 8.
Increased Akt activation and nuclear β-catenin, and altered FoxO1 localization, the in Ptk6−/− intestine. A. Increased phospho-Akt(Ser473) immunoreactivity is evident in crypts and villi of the Ptk6−/− small intestine. Antibody binding was detected with DAB (brown), and sections were counterstained with hematoxylin. B. Increased numbers of β-catenin-positive nuclei were detected in crypts of PTK6-deficient mice. β-Catenin is localized at the membrane in the crypt and villus epithelial cells and in nuclei of crypt epithelial cells (arrowheads). C. Decreased nuclear localization of the Akt substrate FoxO1 is detected in Ptk6−/− intestine. The boxed region is magnified, and altered and the arrowhead shows nuclear FoxO1 in the wild-type epithelium. This supports our observation that Akt activity is increased in Ptk6−/− intestine because Akt negatively regulates its FoxO protein substrates by promoting nuclear exclusion of these transcription factors. FoxO1 antibody binding was detected with DAB (brown). No counterstain was used. Bars, 50 μm.
Akt phosphorylates and negatively regulates GSK-3, which plays a central role in the regulation of β-catenin. Nuclear β-catenin in complex with the T-cell factor DNA binding proteins controls proliferation versus differentiation in intestinal epithelial cells (reviewed in reference 14). We detected equivalent levels of β-catenin protein by immunoblotting using intestinal lysates from wild-type and Ptk6−/− mice (data not shown). Immunostaining with anti-β-catenin antibodies revealed membrane-localized β-catenin along the crypt-villus axis as well as nuclear β-catenin in crypt cells of the small intestine (Fig. 8B). No consistent differences in the membrane localization of β-catenin were observed in the sections examined. However, in comparable regions where we detected nuclear β-catenin, PTK6-deficient crypts often had two or three positive nuclei, while wild-type crypts usually had only one detectable positive nucleus. Views from regions of two different wild-type and Ptk6−/− mice are shown in Fig. 8B. An increased number of cells positive for nuclear β-catenin correlated with the increase in Akt activity and enhanced proliferation in the PTK6-deficient intestine.
The Forkhead box O (FoxO) transcription factors regulate cell cycle progression and differentiation, and they target a number of genes that promote growth arrest (reviewed in references 1 and 44). These transcription factors are direct substrates of Akt. Akt phosphorylates FoxO transcription factors and promotes their export from the nucleus, thereby inhibiting their transcription factor functions (4, 6). We examined the intracellular localization of FoxO1 as a marker for Akt activity (28). Increased activation of Akt would lead to enhanced phosphorylation and nuclear exclusion of FoxO1. FoxO1 nuclear localization was evident in villus epithelial cells of wild-type mice. However, nuclear FoxO1 was not detected in the PTK6-deficient small intestine (Fig. 8C), providing further evidence of enhanced Akt activity.
DISCUSSION
Although PTK6 is expressed at high levels in breast tumor cells, its expression in normal mouse and human tissues is associated with cell cycle exit and differentiation. In the small intestine it is expressed in a pattern similar to that for the Cdk inhibitor p21 (13), and it may be poised to serve as part of a braking mechanism for rapidly proliferating epithelial cells that are exiting the crypt compartments. We found that disruption of the mouse Ptk6 gene led to enhanced proliferation and growth and to delayed expression of the differentiation marker I-FABP in enterocytes of the small intestine.
While I-FABP gene expression has been found to increase during adaptation following intestinal resection (37), we detected delayed and decreased I-FABP expression in the Ptk6−/− small intestine. I-FABP does not play an essential role in fat absorption (47). Wild-type and Ptk6−/− mice fed standard and high-fat diets had no significant differences in weight gain or excreted fecal fat levels, suggesting that absorption was comparable for the two genotypes. The delayed expression of I-FABP is most likely due to altered differentiation as a consequence of disruption of PTK6 signaling. In earlier studies, ectopic expression of PTK6 in mouse keratinocytes enhanced expression of the epidermal differentiation marker filaggrin in differentiating cells (46), also suggesting a role for PTK6 in promoting epithelial cell differentiation.
Akt is a recently identified substrate of PTK6, and phosphorylation of Akt on tyrosine by PTK6 was previously shown to inhibit Akt activity (50). Inhibition of Akt may be important for efficient cell cycle exit and differentiation in the rapidly renewing epithelium of the small intestine, because hyperactive Akt may lead to increased cell growth and proliferation (reviewed in references 19, 23, and 25). We found that increased growth in the small intestine in PTK6-deficient mice correlated with an increase in Akt activation. Further evidence of enhanced Akt activation in Ptk6−/− intestine was the nuclear exclusion and inactivation of the Akt substrate FoxO1. FoxO transcription factors may promote growth arrest and differentiation (reviewed in reference 1), and Akt-mediated inactivation of FoxO proteins could contribute the enhanced proliferation and delayed enterocyte differentiation observed in the PTK6-deficient small intestine.
Increased Akt activity may enhance nuclear activity of β-catenin in the intestine (20), and we observed enhanced nuclear localization of β-catenin in crypts of the Ptk6−/− small intestine. Our detection of increased numbers of cells positive for nuclear β-catenin in the crypts may reflect expansion of the proliferative zone in the absence of functional PTK6. Increased nuclear β-catenin could contribute to the increased proliferation that we observed in the small intestine. Akt may facilitate the stabilization and nuclear accumulation of β-catenin, acting not only through Dishevelled and GSK-3 but also through direct phosphorylation of β-catenin (12, 33, 43). Negative regulation of Akt activity by PTK6 could keep proliferation and enhanced activation of β-catenin in check.
Similar to our findings with PTK6, growth-inhibitory functions have been reported for other members of the PTK6 kinase family (39). Human FRK, which was also initially isolated from breast tumor cells (7), was shown to associate with the retinoblastoma protein pRb, and its overexpression in NIH 3T3 cells inhibited colony formation (9). Overexpression of FRK in breast tumor cell lines also resulted in a block in proliferation (29). The Drosophila PTK6 family kinase Src42A was found to negatively regulate epidermal growth factor and Torso receptor tyrosine kinase signaling (27, 51).
Paradoxically, several studies suggest that PTK6 enhances ErbB family receptor signaling and positively regulates breast cancer cell growth and migration (8, 18, 21, 22). It is possible that aberrant overexpression of PTK6, along with other signaling proteins such as members of the ErbB family of receptors, leads to the recruitment of PTK6 to the membrane in breast cancer cells, where it may gain transforming potential. A correlation between PTK6 and ErbB2 (Her2/neu) overexpression in breast cancer cells was recently reported (5). Our data suggest that functions of PTK6 may be distinct in normal epithelia and cancer cells and may be dependent on cell type, intracellular localization, and access to specific substrates and other signaling molecules.
Acknowledgments
This work was supported by National Institutes of Health grant DK44525 (to A.L.T.). A.H. received support from the German Academic Exchange Service (DAAD) and the Schering Foundation.
We thank Linda Degenstein for help with generating the mouse model and Deborah Rubin, Andrei Gartel, Helena Palka Hamblin, and Patrick Brauer for helpful comments and suggestions. We thank Jeffrey Gordon for supplying the I-FABP antibody and Hans Clevers and Harry Begthel for their generous support.
REFERENCES
- 1.Accili, D., and K. C. Arden. 2004. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell 117:421-426. [DOI] [PubMed] [Google Scholar]
- 2.Agellon, L. B., M. J. Toth, and A. B. Thomson. 2002. Intracellular lipid binding proteins of the small intestine. Mol. Cell Biochem. 239:79-82. [PubMed] [Google Scholar]
- 3.Barker, K. T., L. E. Jackson, and M. R. Crompton. 1997. BRK tyrosine kinase expression in a high proportion of human breast carcinomas. Oncogene 15:799-805. [DOI] [PubMed] [Google Scholar]
- 4.Biggs, W. H., III, J. Meisenhelder, T. Hunter, W. K. Cavenee, and K. C. Arden. 1999. Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc. Natl. Acad. Sci. USA 96:7421-7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Born, M., L. Quintanilla-Fend, H. Braselmann, U. Reich, M. Richter, P. Hutzler, and M. Aubele. 2005. Simultaneous over-expression of the Her2/neu and PTK6 tyrosine kinases in archival invasive ductal breast carcinomas. J. Pathol. 205:592-596. [DOI] [PubMed] [Google Scholar]
- 6.Brunet, A., A. Bonni, M. J. Zigmond, M. Z. Lin, P. Juo, L. S. Hu, M. J. Anderson, K. C. Arden, J. Blenis, and M. E. Greenberg. 1999. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96:857-868. [DOI] [PubMed] [Google Scholar]
- 7.Cance, W. G., R. J. Craven, M. Bergman, L. Xu, K. Aitalo, and E. T. Liu. 1994. Rak, a novel nuclear tyrosine kinase expressed in epithelial cells. Cell Growth Diff. 5:1347-1355. [PubMed] [Google Scholar]
- 8.Chen, H. Y., C. H. Shen, Y. T. Tsai, F. C. Lin, Y. P. Huang, and R. H. Chen. 2004. Brk activates rac1 and promotes cell migration and invasion by phosphorylating paxillin. Mol. Cell. Biol. 24:10558-10572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Craven, R. J., W. G. Cance, and E. T. Liu. 1995. The nuclear tyrosine kinase Rak associates with the retinoblastoma protein pRb. Cancer Res. 55:3969-3972. [PubMed] [Google Scholar]
- 10.Derry, J. J., G. S. Prins, V. Ray, and A. L. Tyner. 2003. Altered localization and activity of the intracellular tyrosine kinase BRK/Sik in prostate tumor cells. Oncogene 22:4212-4220. [DOI] [PubMed] [Google Scholar]
- 11.Derry, J. J., S. Richard, H. Valderrama Carvajal, X. Ye, V. Vasioukhin, A. W. Cochrane, T. Chen, and A. L. Tyner. 2000. Sik (BRK) phosphorylates Sam68 in the nucleus and negatively regulates its RNA binding ability. Mol. Cell. Biol. 20:6114-6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fukumoto, S., C. M. Hsieh, K. Maemura, M. D. Layne, S. F. Yet, K. H. Lee, T. Matsui, A. Rosenzweig, W. G. Taylor, J. S. Rubin, M. A. Perrella, and M. E. Lee. 2001. Akt participation in the Wnt signaling pathway through Dishevelled. J. Biol. Chem. 276:17479-17483. [DOI] [PubMed] [Google Scholar]
- 13.Gartel, A. L., M. S. Serfas, M. Gartel, E. Goufman, G. S. Wu, W. S. El-Deiry, and A. L. Tyner. 1996. p21 (WAF1/CIP1) expression is induced in newly nondividing cells in diverse epithelial and during differentiation of the Caco-2 intestinal cell line. Exp. Cell Res. 227:171-181. [DOI] [PubMed] [Google Scholar]
- 14.Gregorieff, A., and H. Clevers. 2005. Wnt signaling in the intestinal epithelium: from endoderm to cancer. Genes Dev. 19:877-890. [DOI] [PubMed] [Google Scholar]
- 15.Haegebarth, A., D. Heap, W. Bie, J. J. Derry, S. Richard, and A. L. Tyner. 2004. The nuclear tyrosine kinase BRK/Sik phosphorylates and inhibits the RNA-binding activities of the Sam68-like mammalian proteins SLM-1 and SLM-2. J. Biol. Chem. 279:54398-54404. [DOI] [PubMed] [Google Scholar]
- 16.Haegebarth, A., R. Nunez, and A. L. Tyner. 2005. The intracellular tyrosine kinase Brk sensitizes non-transformed cells to inducers of apoptosis. Cell Cycle 4:1239-1246. [DOI] [PubMed] [Google Scholar]
- 17.Harvey, A. J., and M. R. Crompton. 2004. The Brk protein tyrosine kinase as a therapeutic target in cancer: opportunities and challenges. Anticancer Drugs 15:107-111. [DOI] [PubMed] [Google Scholar]
- 18.Harvey, A. J., and M. R. Crompton. 2003. Use of RNA interference to validate Brk as a novel therapeutic target in breast cancer: Brk promotes breast carcinoma cell proliferation. Oncogene 22:5006-5010. [DOI] [PubMed] [Google Scholar]
- 19.Hay, N. 2005. The Akt-mTOR tango and its relevance to cancer. Cancer Cell 8:179-183. [DOI] [PubMed] [Google Scholar]
- 20.He, X. C., J. Zhang, W. G. Tong, O. Tawfik, J. Ross, D. H. Scoville, Q. Tian, X. Zeng, X. He, L. M. Wiedemann, Y. Mishina, and L. Li. 2004. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-beta-catenin signaling. Nat. Genet. 36:1117-1121. [DOI] [PubMed] [Google Scholar]
- 21.Kamalati, T., H. E. Jolin, M. J. Fry, and M. R. Crompton. 2000. Expression of the BRK tyrosine kinase in mammary epithelial cells enhances the coupling of EGF signalling to PI 3-kinase and Akt, via erbB3 phosphorylation. Oncogene 19:5471-5476. [DOI] [PubMed] [Google Scholar]
- 22.Kamalati, T., H. E. Jolin, P. J. Mitchell, K. T. Barker, L. E. Jackson, C. J. Dean, M. J. Page, B. A. Gusterson, and M. R. Crompton. 1996. Brk, a breast tumor-derived non-receptor protein-tyrosine kinase, sensitizes mammary epithelial cells to epidermal growth factor. J. Biol. Chem. 271:30956-30963. [DOI] [PubMed] [Google Scholar]
- 23.Kandel, E. S., and N. Hay. 1999. The regulation and activities of the multifunctional serine/threonine kinase Akt/PKB. Exp. Cell Res. 253:210-229. [DOI] [PubMed] [Google Scholar]
- 24.Lee, S. T., K. M. Strunk, and R. A. Spritz. 1993. A survey of protein tyrosine kinase mRNAs expressed in normal human melanocytes. Oncogene 8:3403-3410. [PubMed] [Google Scholar]
- 25.Liang, J., and J. M. Slingerland. 2003. Multiple roles of the PI3K/PKB (Akt) pathway in cell cycle progression. Cell Cycle 2:339-345. [PubMed] [Google Scholar]
- 26.Llor, X., M. S. Serfas, W. Bie, V. Vasioukhin, M. Polonskaia, J. Derry, C. M. Abbott, and A. L. Tyner. 1999. BRK/Sik expression in the gastrointestinal tract and in colon tumors. Clin. Cancer Res. 5:1767-1777. [PubMed] [Google Scholar]
- 27.Lu, X., and Y. Li. 1999. Drosophila Src42A is a negative regulator of RTK signaling. Dev. Biol. 208:233-243. [DOI] [PubMed] [Google Scholar]
- 28.Manning, B. D., M. N. Logsdon, A. I. Lipovsky, D. Abbott, D. J. Kwiatkowski, and L. C. Cantley. 2005. Feedback inhibition of Akt signaling limits the growth of tumors lacking Tsc2. Genes Dev. 19:1773-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meyer, T., L. Xu, J. Chang, E. T. Liu, R. J. Craven, and W. G. Cance. 2003. Breast cancer cell line proliferation blocked by the Src-related Rak tyrosine kinase. Int. J. Cancer 104:139-146. [DOI] [PubMed] [Google Scholar]
- 30.Minoguchi, M., S. Minoguchi, D. Aki, A. Joo, T. Yamamoto, T. Yumioka, T. Matsuda, and A. Yoshimura. 2003. STAP-2/BKS, an adaptor/docking protein, modulates STAT3 activation in acute-phase response through its YXXQ motif. J. Biol. Chem. 278:11182-11189. [DOI] [PubMed] [Google Scholar]
- 31.Mitchell, P. J., K. T. Barker, J. E. Martindale, T. Kamalati, P. N. Lowe, M. J. Page, B. A. Gusterson, and M. R. Crompton. 1994. Cloning and characterisation of cDNAs encoding a novel non-receptor tyrosine kinase, brk, expressed in human breast tumours. Oncogene 9:2383-2390. [PubMed] [Google Scholar]
- 32.Mitchell, P. J., E. A. Sara, and M. R. Crompton. 2000. A novel adaptor-like protein which is a substrate for the non-receptor tyrosine kinase, BRK. Oncogene 19:4273-4282. [DOI] [PubMed] [Google Scholar]
- 33.Persad, S., A. A. Troussard, T. R. McPhee, D. J. Mulholland, and S. Dedhar. 2001. Tumor suppressor PTEN inhibits nuclear accumulation of beta-catenin and T cell/lymphoid enhancer factor 1-mediated transcriptional activation. J. Cell Biol. 153:1161-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petro, B. J., R. C. Tan, A. L. Tyner, M. W. Lingen, and K. Watanabe. 2004. Differential expression of the non-receptor tyrosine kinase BRK in oral squamous cell carcinoma and normal oral epithelium. Oral Oncol. 40:1040-1047. [DOI] [PubMed] [Google Scholar]
- 35.Radtke, F., and H. Clevers. 2005. Self-renewal and cancer of the gut: two sides of a coin. Science 307:1904-1909. [DOI] [PubMed] [Google Scholar]
- 36.Rubin, D. C., D. E. Ong, and J. I. Gordon. 1989. Cellular differentiation in the emerging fetal rat small intestinal epithelium: mosaic patterns of gene expression. Proc. Natl. Acad. Sci. USA 86:1278-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rubin, D. C., E. A. Swietlicki, J. L. Wang, B. D. Dodson, and M. S. Levin. 1996. Enterocytic gene expression in intestinal adaptation: evidence for a specific cellular response. Am. J. Physiol. 270:G143-G152. [DOI] [PubMed] [Google Scholar]
- 38.Schwarz, M., E. G. Lund, K. D. Setchell, H. J. Kayden, J. E. Zerwekh, I. Bjorkhem, J. Herz, and D. W. Russell. 1996. Disruption of cholesterol 7alpha-hydroxylase gene in mice. II. Bile acid deficiency is overcome by induction of oxysterol 7alpha-hydroxylase. J. Biol. Chem. 271:18024-18031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Serfas, M. S., and A. L. Tyner. 2003. Brk, Srm, Frk, and Src42A form a distinct family of intracellular Src-like tyrosine kinases. Oncol. Res. 13:409-419. [DOI] [PubMed] [Google Scholar]
- 40.Siyanova, E. Y., M. S. Serfas, I. A. Mazo, and A. L. Tyner. 1994. Tyrosine kinase gene expression in the mouse small intestine. Oncogene 9:2053-2057. [PubMed] [Google Scholar]
- 41.Soriano, P. 1999. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 21:70-71. [DOI] [PubMed] [Google Scholar]
- 42.Tappenden, K. A. 2006. Mechanisms of enteral nutrient-enhanced intestinal adaptation. Gastroenterology 130:S93-S99. [DOI] [PubMed] [Google Scholar]
- 43.Tian, Q., M. C. Feetham, W. A. Tao, X. C. He, L. Li, R. Aebersold, and L. Hood. 2004. Proteomic analysis identifies that 14-3-3zeta interacts with beta-catenin and facilitates its activation by Akt. Proc. Natl. Acad. Sci. USA 101:15370-15375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tran, H., A. Brunet, E. C. Griffith, and M. E. Greenberg. 2003. The many forks in FOXO's road. Sci. STKE 2003:RE5. [DOI] [PubMed] [Google Scholar]
- 45.Vasioukhin, V., M. S. Serfas, E. Y. Siyanova, M. Polonskaia, V. J. Costigan, B. Liu, A. Thomason, and A. L. Tyner. 1995. A novel intracellular epithelial cell tyrosine kinase is expressed in the skin and gastrointestinal tract. Oncogene 10:349-357. [PubMed] [Google Scholar]
- 46.Vasioukhin, V., and A. L. Tyner. 1997. A role for the epithelial-cell-specific tyrosine kinase Sik during keratinocyte differentiation. Proc. Natl. Acad. Sci. USA 94:14477-14482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vassileva, G., L. Huwyler, K. Poirier, L. B. Agellon, and M. J. Toth. 2000. The intestinal fatty acid binding protein is not essential for dietary fat absorption in mice. FASEB J. 14:2040-2046. [DOI] [PubMed] [Google Scholar]
- 48.Wang, T. C., S. H. Jee, T. F. Tsai, Y. L. Huang, W. L. Tsai, and R. H. Chen. 2005. Role of breast tumour kinase in the in vitro differentiation of HaCaT cells. Br. J. Dermatol. 153:282-289. [DOI] [PubMed] [Google Scholar]
- 49.Wang, Y., H. Iordanov, E. A. Swietlicki, L. Wang, C. Fritsch, T. Coleman, C. F. Semenkovich, M. S. Levin, and D. C. Rubin. 2005. Targeted intestinal overexpression of the immediate early gene tis7 in transgenic mice increases triglyceride absorption and adiposity. J. Biol. Chem. 280:34764-34775. [DOI] [PubMed] [Google Scholar]
- 50.Zhang, P., J. H. Ostrander, E. J. Faivre, A. Olsen, D. Fitzsimmons, and C. A. Lange. 2005. Regulated association of protein kinase B/Akt with breast tumor kinase. J. Biol. Chem. 280:1982-1991. [DOI] [PubMed] [Google Scholar]
- 51.Zhang, Q., Q. Zheng, and X. Lu. 1999. A genetic screen for modifiers of Drosophila Src42A identifies mutations in Egfr, rolled and a novel signaling gene. Genetics 151:697-711. [DOI] [PMC free article] [PubMed] [Google Scholar]