Abstract
Cyclic AMP response element binding protein (CREB) content is diminished in smooth muscle cells (SMCs) in remodeled pulmonary arteries from animals with pulmonary hypertension and in the SMC layers of atherogenic systemic arteries and cardiomyocytes from hypertensive individuals. Loss of CREB can be induced in cultured SMCs by chronic exposure to hypoxia or platelet-derived growth factor BB (PDGF-BB). Here we investigated the signaling pathways and mechanisms by which PDGF elicits depletion of SMC CREB. Chronic PDGF treatment increased CREB ubiquitination in SMCs, while treatment of SMCs with the proteasome inhibitor lactacystin prevented decreases in CREB content. The nuclear export inhibitor leptomycin B also prevented depletion of SMC CREB alone or in combination with lactacystin. Subsequent studies showed that PDGF activated extracellular signal-regulated kinase, Jun N-terminal protein kinase, and phosphatidylinositol 3 (PI3)-kinase pathways in SMCs. Inhibition of these pathways blocked SMC proliferation in response to PDGF, but only inhibition of PI3-kinase or its effector, Akt, blocked PDGF-induced CREB loss. Finally, chimeric proteins containing enhanced cyan fluorescent protein linked to wild-type CREB or CREB molecules with mutations in several recognized phosphorylation sites were introduced into SMCs. PDGF treatment reduced the levels of each of these chimeric proteins except for one containing mutations in adjacent serine residues (serines 103 and 107), suggesting that CREB loss was dependent on CREB phosphorylation at these sites. We conclude that PDGF stimulates nuclear export and proteasomal degradation of CREB in SMCs via PI3-kinase/Akt signaling. These results indicate that in addition to direct phosphorylation, proteolysis and intracellular localization are key mechanisms regulating CREB content and activity in SMCs.
Pulmonary hypertension (PH) and related vascular pathologies are characterized by changes in the structure of the arterial wall. These changes are largely due to the proliferation and hypertrophy of smooth muscle cells (SMCs) and increased SMC deposition of extracellular matrix in the vessel wall. The proliferation and hypertrophy of SMCs are stimulated by growth factors and proinflammatory agents such as platelet-derived growth factor BB (PDGF-BB), insulin-like growth factors I and II, epidermal growth factor, basic fibroblast growth factor, vascular endothelial growth factor, endothelin-1, and thrombospondin-1, which are produced by endothelial cells, SMCs, fibroblasts, and platelets in response to vascular injury (6, 11, 14, 15, 46, 59). Binding of these growth factors to their respective receptors activates associated tyrosine kinases, G proteins, and C-type phospholipases. Activation of receptor tyrosine kinases stimulates mitogen-activated protein kinase (MAPK) signaling cascades, with PDGF-BB stimulation of extracellular signal-regulated kinase 1 (ERK1)/ERK2 being a widely studied example (23, 44). G protein-coupled receptors may regulate numerous signaling pathways, with recent studies implicating RhoA/Rho kinase signaling in SMC growth and migration (52). These signaling pathways modulate the activity of downstream effectors of growth such as cyclin-dependent kinases (42) and immediate-early genes (49).
These growth-promoting pathways are normally restrained in healthy arteries by endogenous mediators such as prostacyclin and NO. These agents exert antiproliferative effects on SMCs largely by increasing intracellular levels of cyclic nucleotides (53, 54), which stimulate the activity of protein kinase A (PKA) and GMP-stimulated protein kinase. Many compounds that activate adenyl cyclase (39), inhibit phosphodiesterases (50), or mimic cyclic AMP (cAMP)/cGMP (34) exert antiproliferative effects on SMC growth. Interestingly, many drugs and therapeutic agents that reduce SMC proliferation act by increasing intracellular cAMP levels (22, 27, 44, 64). There is now substantial evidence that cAMP/PKA signaling acts as a molecular gate to block MAPK-induced proliferation in response to mitogens such as PDGF (5, 23, 30, 44). Activation of cAMP signaling in SMCs decreases the expression of cyclin D1 and Cdk2 (60), increases the expression of antiproliferative molecules such as p53 and p21 (25), and increases overall sensitivity to antiproliferative stimuli.
Given the potent proliferation-suppressing action of cAMP on SMCs, we hypothesized that the transcription factor CREB, a primary target of cAMP/PKA signaling, might participate in controlling SMC proliferation. In previous studies (33), we measured levels of phosphorylated CREB and total CREB in pulmonary artery (PA) and aortic SMCs grown in culture in response to various proliferative stimuli. We found that phosphorylated CREB and total CREB levels were reduced in SMCs under conditions that stimulate proliferation (PDGF-BB treatment or serum exposure) but were elevated in quiescent cells (serum-free medium). These data were confirmed in studies of lung and PA tissue samples from neonatal calves raised under hypoxic conditions to produce PH. We found that total CREB content was reduced in SMCs surrounding remodeled, hypertensive PAs compared to levels observed in normotensive PAs from control animals. Subsequent studies demonstrated that ectopic expression of wild-type or constitutively active forms of CREB in cultured cells inhibited basal and PDGF-induced SMC proliferation. These CREB isoforms also attenuated the ability of PDGF to stimulate migration, whereas dominant negative forms of CREB enhanced PDGF-stimulated migration. These data suggest that CREB content is not simply a marker of SMC proliferative potential but rather that it directly attenuates proliferation and migration, processes that contribute to vascular remodeling.
In this report, we investigate the regulatory mechanisms and intracellular signaling pathways by which PDGF-BB decreases CREB content in PA SMCs in vitro. Exposure of SMCs to PDGF-BB for periods from 24 to 72 h resulted in an increase in ubiquitinated CREB and a complete loss of CREB localized to the nucleus. These responses could be blocked with inhibitors of either nuclear export or proteasomal degradation. PDGF-BB activated ERK, Jun N-terminal protein kinase (JNK), and phosphatidylinositol 3 (PI3)-kinase/Akt signaling pathways in SMCs, and blockade of any one of these pathways inhibited PDGF-induced SMC proliferation. However, only inhibition of PI3-kinase/Akt signaling restored nuclear CREB to nearly normal levels. These data identify a novel regulatory process in the control of the SMC phenotype in which PDGF treatment leads to CREB nuclear export and degradation via activation of PI3-kinase/Akt signaling.
MATERIALS AND METHODS
Materials.
Recombinant human PDGF-BB was purchased from Bachem (King of Prussia, PA). Rapamycin, Akt inhibitor, SB203580, SP600125, and U0126 were purchased from Calbiochem/EMD Biosciences (San Diego, CA). LY294002 was obtained from Cayman Chemicals (Ann Arbor, MI), and SB216763 was from Biomol International, Inc. (Plymouth Meeting, PA). PepTag nonradioactive PKA and PKC assay kits, CellTiter 96 AQ nonradioactive cell proliferation assay kits, a dual luciferase reporter assay system, and plasmid pRL-SV40 were from Promega (Madison, WI). Oligofectamine, Lipofectamine Plus reagent, and a SuperScript III first-strand synthesis system were obtained from Invitrogen (Carlsbad, CA). SuperSignal West Pico chemiluminescent substrate and NE-PER nuclear and cytoplasmic extract reagents were from Pierce (Rockford, IL). Vector NovaRed peroxidase staining kits, VectaShield mounting medium with DAPI (4′,6′-diamidino-2-phenylindole), and peroxidase-conjugated goat anti-rabbit secondary antibody were from Vector Laboratories (Burlingame, CA). The plasmid pECFP-C1 was purchased from Clontech Laboratories, Inc. (Palo Alto, CA). Dulbecco's modified Eagle's medium (DMEM) was from HyClone (Logan, UT), and Optimem was from GIBCO/Invitrogen. RhoA activation assay kits were obtained from Upstate (Lake Placid, NY). Protein A-Sepharose CL4B, leptomycin B, lactacystin, and monoclonal anti-smooth muscle (SM) α-actin, anti-SM myosin, anti-β-actin, and anti-calponin antibodies were from Sigma (St. Louis, MO). AlexaFluor 594-conjugated goat anti-rabbit secondary antibody was from Molecular Probes (Eugene, OR). Mouse monoclonal anti-ubiquitin and anti-cyclin D1 antibodies and rabbit polyclonal antibodies to CREB, phospho-CREB, ERK, phospho-ERK, p38 MAPK, phospho-p38 MAPK, JNK, phospho-JNK, glycogen synthase kinase 3β (GSK-3β), phospho-GSK-3α/β, phospho-p70 S6 kinase, and Akt-2 were from Cell Signaling Technology, Inc. (Beverly, MA). Goat polyclonal ant-Akt-1, anti-Akt-3, and anti-phospho-Akt-1/2/3 antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Rat monoclonal anti-bromodeoxyuridine (BrdU) antibody was purchased from Abcam (Cambridge, MA). Proteasome/ubiquitin kits were from Chemicon (Temecula, CA). Three double-stranded small interfering RNA (siRNA) oligonucleotides to CREB (284-AAGATTCAACAGGAGTCTGTGG-305, 528-AATACAGCTGGCTAACAATGG-549, and 670-AACCAAGTTGTTGTTCAAGCT-691) and a control siRNA oligonucleotide to firefly luciferase (CGTACGCGGAATACTTCGA) were from Dharmacon (Lafayette, CO). Single-stranded DNA primers for PCR amplification of tissue inhibitor of metalloproteinases-3 (TIMP-3) (forward, AACTCCGACATCGTGATCCGG; reverse, ATCCTCGGTACCAGCTGCAGT), fibronectin (forward, ACCTTGACTGCCCAGTGGACAGCG; reverse, GCTCACTCTTCTGATTGTTCTTCA), β-actin (forward, CCAACCGTGAAAAGATGACC; reverse, TCTAGGGCAACATAGCACAGC), CREB (forward, AATGACCATGGAATC; reverse, CAAAATTTTCCTGTAGGAAGG), and SM myosin (forward, GAAGGCCAAGAAGGCCAT; reverse, GGTCTCAGGGCTTCACAGGC) were obtained from Integrated DNA Technologies, Inc. (Coralville, CA). Wild-type CREB-327 and CREB mutants S115A and S138A were generously provided by Joel Habener (Massachusetts General Hospital, Boston, MA). Adenoviruses expressing constitutively active myr-Akt and dominant negative Akt-AAA were from Kenneth Walsh (Boston University, MA).
PA SMC isolation and cell culture.
PAs were recovered from adult rat lungs. Segments of the PAs were cut open and mechanically stripped of adventitia and endothelium. The segments were then placed lumen side down into individual wells of a six-well plate. Tissue explants were maintained in complete DMEM supplemented with 200 U/ml penicillin, 0.2 mg/ml streptomycin, and 10% fetal calf serum (FCS).
Since our goal was to obtain pure subpopulations of SMCs, we selectively isolated individual cell colonies with a distinct, although uniform, morphological appearance from primary culture by use of cloning cylinders. Expression of SMC-specific markers (α-SM actin and α-SM myosin heavy chains) in each isolated cell subpopulation was examined. Only cell subpopulations of uniform morphological appearance and with uniform patterns of expression of SMC markers were selected for further experimentation. Individual cell colonies growing from tissue explants in primary culture were isolated by placing cloning cylinders (5 to 10 mm in diameter; greased on the bottom) over each cell colony of interest. Cells within the ring were trypsinized and transferred to a 24-multiwell plate for expansion. All studies were carried out using cells at passages 1 to 8. Cell cultures were tested for mycoplasma contamination using a Gen-Probe mycoplasma tissue culture rapid detection system (Gen-Probe Inc., San Diego, CA).
siRNA treatments.
SMCs were plated at 30 to 50% confluence on six-well plates in complete medium. Twenty-four hours later, the cells were transferred to serum-free DMEM for transfection. Double-stranded CREB siRNA oligonucleotides (equal amounts of the three CREB siRNAs were mixed together) or the control luciferase-specific siRNA was complexed with Oligofectamine reagent and applied to the cells according to the manufacturer's recommendations. After 3 h, an equal volume of DMEM containing 30% FCS was added to the wells. Cells were allowed to recover for at least 48 h before subsequent manipulations.
Proliferation assays.
SMC proliferation was measured with a CellTiter 96 AQ assay kit according to the manufacturer's directions.
Subcellular fractionation and Western blots.
Cytosolic and nuclear fractions were prepared from SMCs using NE-PER reagents following the supplier's instructions. After a step correcting for protein concentrations, cell lysates were mixed with equal volumes of Laemmli sodium dodecyl sulfate (SDS) loading buffer, resolved on 10% polyacrylamide-SDS gels, and transferred to polyvinylidene difluoride (PVDF) membranes. The blots were blocked with phosphate-buffered saline (PBS) containing 5% dry milk and 0.1% Tween 20 and then treated overnight at 4°C with antibodies that recognize the target proteins indicated in each figure. The blots were washed and subsequently treated with appropriate secondary antibodies conjugated to horseradish peroxidase. After the blots are washed, specific immune complexes were visualized with SuperSignal West Pico chemiluminescent substrate.
pECFP-CREB vectors.
The coding regions for the wild-type, S115A, and S138A CREB molecules were inserted in frame into the plasmid pECFP-C1. CREB mutants containing serine-to-alanine transitions at serine 119 alone or dual transitions at serines 103 and 107 were generated by site-directed mutagenesis of the wild-type CREB-containing pECFP plasmid by the method of Huang and Sorkin (28).
Transfections, infections, and luciferase assays.
For plasmid transfections, SMCs were plated in complete medium at 50 to 80% confluence and transfected the following day. Plasmid complexes were formed with Lipofectamine Plus reagent as specified by the supplier. Cells were transferred to OptiMEM medium and incubated with the complexes for 3 h. The transfection reaction products were removed, and cells were maintained in complete medium for subsequent studies. For adenoviral infection, SMCs were plated in complete medium at 80% confluence. The purified adenoviruses were then added directly to the monolayers at a multiplicity of infection of 100.
Luciferase assays were conducted on cells transfected with a plasmid containing four copies of a consensus cAMP response element upstream of an enhancerless simian virus 40 (SV40) promoter linked to the firefly luciferase gene (pCRESV-Luc). Cells were cotransfected with the plasmid pRL-SV40, which contains the viral SV40 promoter linked to Renilla luciferase as an internal control. Cell lysates and luciferase reactions were performed with a dual luciferase reporter assay system on a Turner Designs 20/20 luminometer (Turner Designs, Sunnyvale, CA).
Reverse transcriptase PCR.
cDNA was prepared from SMCs using Cells-to-cDNA II (Ambion, Austin, TX) reagents according to the manufacturer's instructions. PCR amplification was performed with 3 μl transcribed cDNA and 1 pmol of each primer for 30 cycles of hot start at 94°C for 1 min, denaturation at 94°C for 1 min, annealing at the appropriate temperature for 45 seconds, and elongation at 72°C for 2 min followed by a final elongation for 8 min at 72°C. Negative PCR controls included omission of reverse transcriptase and omission of cDNA. β-Actin primers were used to validate each batch of the template before use. PCR products were resolved on 2% agarose gels containing ethidium bromide and photographed under UV illumination.
Immunostaining and microscopy.
Immunocytochemistry for CREB was performed on SMCs treated as indicated in the figure legends. Cells were fixed for 20 min at room temperature in PBS containing 4% paraformaldehyde. The slides were washed three times in PBS and blocked with PBS containing 5% horse serum and 0.2% Triton X-100 for 30 min at room temperature. The cells were then incubated overnight at 4°C in PBS-horse serum-Triton X-100 containing CREB antibody at the dilution recommended by the manufacturer. The slides were washed three times and incubated with a goat anti-rabbit secondary antibody conjugated to AlexaFluor 594 for 1 h at room temperature. The slides were washed three times with PBS, and coverslips were mounted with VectaShield with DAPI.
Staining for BrdU incorporation was performed on cells fixed in PBS-4% paraformaldehyde. Slides were incubated in 0.5% hydrogen peroxide for 10 min to quench endogenous catalase and briefly rinsed with tap water. The slides were then incubated in 2 N HCl at 37 C for 30 min and transferred to 0.1 N disodium tetraborate for 10 min. The slides were rinsed, and immunostaining was conducted as described above by use of a rat monoclonal anti-BrdU primary antibody and an anti-rat secondary antibody conjugated to horseradish peroxidase. Slides were then developed with Vector NovaRed reagents.
Microscopy was performed on a Nikon TE2000-U inverted epifluorescent microscope. Bright-field, phase-contrast, and fluorescent digital deconvolution images were captured to a personal computer with either a Spot RT/KE monochrome camera or a Spot Insight color camera (Diagnostic Imaging, Sterling Heights, MI). Images were analyzed and processed with MetaMorph 6.1 software (Molecular Devices, Sunnyvale, CA).
RESULTS
CREB loss produces an immature, proliferative, and synthetic SMC phenotype.
We previously demonstrated that upregulation of CREB activity in SMCs via ectopic expression of constitutively active forms of CREB inhibits proliferation and migration in response to PDGF and other stimuli (33). Downregulation of CREB activity in SMCs with dominant negative forms of CREB increased migration. To better mimic the in vivo phenomenon of decreased CREB protein expression, we blocked CREB expression in PA SMCs with specific siRNA and measured several parameters related to SMC maturity and behavior. We found that loss of CREB was associated with a decrease in several SMC markers, including SM myosin, calponin, fibronectin, and TIMP-3, and with a modest decrease in β-actin (Fig. 1A). SM α-actin levels were not changed with loss of CREB. We next assessed the impact of CREB depletion on SMC proliferation. The cyclin D1 gene, which is expressed early in the cell cycle and a putative CREB-regulated gene (10, 39, 40), and DNA synthesis, as assessed by BrdU incorporation, were upregulated by CREB depletion (Fig. 1A, B, and C). Pentachrome staining of SMCs treated with either PDGF or CREB siRNA revealed increased intracellular elastin production and the deposition of extracellular elastin fibers, a hallmark of dedifferentiated or immature SMCs, but these were not observed in untreated control cells or in cells treated with a nonspecific siRNA (Fig. 1D). The changes induced by loss of CREB are consistent with the changes in the SMC phenotype noted in cases of PH and other vascular pathologies, such as the decreased expression of SMC markers, elevated SMC cell cycle entry and DNA synthesis, and enhanced extracellular matrix deposition.
FIG. 1.
CREB depletion with siRNA induces a dedifferentiated, proliferative, and synthetic phenotype in PA SMCs. Rat PA SMCs were transfected with nonspecific (Cntrl) or CREB-specific (CREB) double-stranded siRNAs as indicated and incubated overnight in DMEM containing 15% FCS. Cells were then transferred to DMEM containing 0.2% FCS for 72 h. (A) Whole-cell lysates and RNA were prepared from the SMCs and subjected to Western blotting (left panels) or semiquantitative reverse transcriptase PCR (right panels) for the factors indicated to the left of each pair of panels. (B) BrdU (10 μM) was added to the siRNA-transfected cells for 12 h. Cells were then fixed in PBS containing 4% paraformaldehyde and subjected to immunostaining for BrdU incorporation as an index of cell proliferation. Representative bright-field images are shown. Magnification, ×200. (C) Eight randomly selected microscope fields were counted for BrdU-positive and total cell numbers by a blinded observer. Data shown are average percentages of BrdU-positive cells. (D) siRNA-treated cells and untreated or PDGF-treated (25 ng/ml for 72 h) cells were subjected to pentachrome staining. Representative bright-field images are shown. Magnification, ×400.
PDGF-BB induces loss of CREB in SMCs via nuclear export and proteasomal degradation.
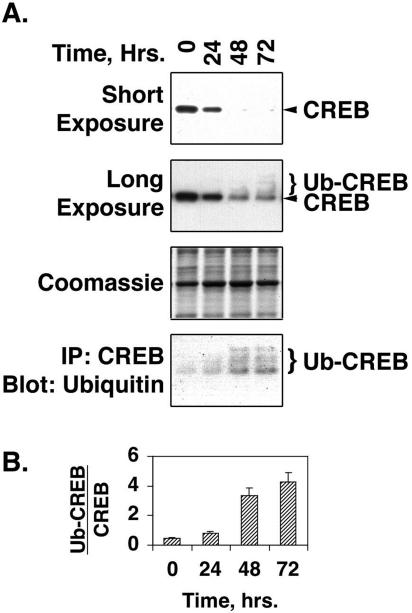
Our previous studies indicated that loss of CREB in response to proliferative stimuli might occur at the level of CREB gene expression (33). However, repeated attempts to demonstrate changes in transcription from the CREB gene promoter failed to produce reproducible results (data not shown). Taylor et al. (57) have reported hypoxia-induced decreases in endothelial cell CREB related to ubiquitination and proteasomal degradation, while Stevenson et al. (55) have demonstrated nuclear translocation of CREB in response to membrane depolarization or PDGF treatment. To examine the impact of these mechanisms on CREB loss in PA SMCs, we tracked the changes in total CREB levels in SMCs treated with PDGF-BB for times ranging from 24 to 72 h. As expected, total CREB levels declined markedly with PDGF treatment, reaching minimal levels by 48 h and remaining low out to 72 h (Fig. 2A). A long exposure of the Western blot revealed the presence of multiple bands of higher molecular weight than the single CREB band detected in untreated cells. To determine whether these bands represented polyubiquitinated forms of CREB, CREB was immunoprecipitated from cell lysates and then subjected to Western blotting for the detection of ubiquitin. The blots show a steady increase in polyubiquitinated CREB over the 72-hour time course (Fig. 2A). Band intensities were plotted as the ratio of ubiquitinated CREB to total CREB to provide an index of the stoichiometry of ubiquitination. This shows an significant increase in CREB ubiquitination in response to PDGF treatment out to 72 h (Fig. 2B).
FIG. 2.
PDGF stimulates ubiquitination of CREB. (A) Rat PA SMCs maintained in complete medium were transferred to DMEM containing 0.2% FCS for 24 h. The cells were then treated with 25 ng/ml PDGF, and medium and PDGF were replaced every 24 h for 72 h. Nuclear extracts were prepared from the cells at the times indicated above the lanes. Portions (25 μg protein) of the extracts were resolved on 10% polyacrylamide-SDS gels and transferred to PVDF membranes. Another portion was incubated with CREB-specific antibodies linked to Sepharose beads conjugated with protein A. After a 1-h incubation, the beads were washed three times with PBS containing 0.1% Tween 20 and eluted with Laemmli loading buffer. The precipitated (IP) material was also resolved on SDS-polyacrylamide gels and transferred to PVDF membranes. The blots were subjected to Western blotting with antibodies to CREB or ubiquitin, as indicated to the right of each blot. Short exposure of the CREB Western blot shows loss of CREB with PDGF treatment. Long exposure of the CREB Western blot shows both CREB and ubiquitinated CREB (Ub-CREB) bands. A representative Coomassie blue-stained blot is shown as a loading control. (B) The bar graph shows the levels of ubiquitinated CREB relative to total CREB levels at each time point.
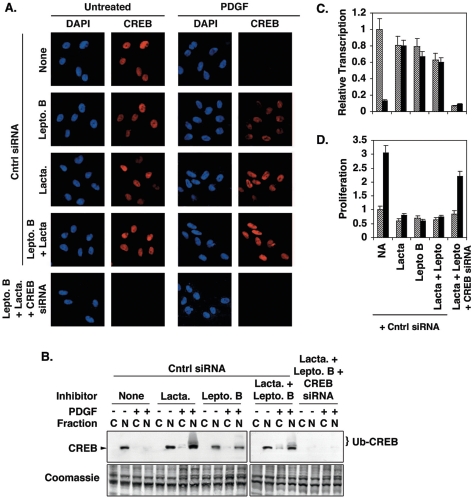
In subsequent experiments, SMCs were treated with leptomycin B, an inhibitor of crm-1-mediated nuclear export, and/or lactacystin, an inhibitor of 26S proteasomal degradation. Leptomycin B restored nuclear CREB levels in PDGF-treated cells to levels comparable to but slightly lower than those observed for untreated control cells (Fig. 3A and B). Lactacystin alone also restored nuclear levels. When both agents were used together, nuclear CREB levels were completely restored in essentially all of the cells. A long-exposure Western blot of nuclear and cytosolic extracts prepared from cells treated with PDGF and lactacytsin and/or leptomycin shows multiple high-molecular-weight CREB bands indicative of polyubiquitination, particularly in lactacystin-treated cells (Fig. 3B). These agents also restored CREB transcriptional activity in SMCs treated with PDGF, as determined by measuring luciferase expression from a CREB-responsive promoter (Fig. 3C), and blocked PDGF-induced SMC proliferation (Fig. 3D). However, CREB siRNA was able to inhibit CREB expression even in cells treated with lactacystin and/or leptomycin B, confirming that siRNA blocks CREB expression upstream of PDGF and PI3-kinase/Akt signaling. Likewise, CREB siRNA repressed CREB-dependent transcription and stimulated SMC proliferation even in cells treated with both lactacystin and leptomycin B. Lactacystin or leptomycin B alone or in combination produced small but statistically insignificant decreases in CREB transcriptional activity, likely due to mild toxicity. Changes in proteasome activity in PDGF-treated cells were not observed at any of the time points (data not shown).
FIG. 3.
Leptomycin B and lactacystin inhibit PDGF-induced CREB depletion, loss of CREB transcriptional activity, and SMC proliferation. Rat PA SMCs were transfected with nonspecific (Cntrl) or CREB-specific double-stranded siRNAs as indicated and incubated overnight in DMEM containing 15% FCS. The cells were transferred to DMEM containing 0.2% FCS for 24 h. The cells were then treated with 100 nM leptomycin B (Lepto. B) and/or 500 nM lactacystin (Lacta.) for 30 min prior to the addition of 25 ng/ml PDGF. Fresh medium containing PDGF and inhibitors was added every 24 h for 72 h. (A) Cells were fixed, subjected to immunostaining with a CREB-specific antibody, and counterstained with DAPI to indicate nuclei. Magnification, ×400. (B) Cytosolic (“C”) and nuclear (“N”) extracts were prepared and separated on 10% polyacrylamide-SDS gels and transferred to PVDF membranes. Western blotting was performed with a CREB-specific antibody. The positions of CREB and ubiquitinated CREB (Ub-CREB) are indicated. A representative Coomassie blue-stained blot is shown as a loading control. (C) Prior to being treated with PDGF and inhibitors, cells were transfected with a plasmid containing a CREB-responsive promoter linked to the firefly luciferase gene (pCRESV-Luc). Cells were cotransfected with the internal control vector pRL-SV40 to correct for transfection efficiency. Following treatment with PDGF and inhibitors, firefly luciferase levels were determined as an index of CREB transcriptional activity. The figure shows transcription relative to levels measured in cells not treated with PDGF and inhibitors. Untreated, cross-hatched bars; PDGF treated, solid bars. (D) SMC proliferation was measured with CellTiter 96 AQ reagents as described in Materials and Methods. Proliferation is expressed relative to absorbances measured with cells not treated with PDGF or inhibitors. Untreated, cross-hatched bars; PDGF treated, solid bars.
PDGF-BB stimulates ERK, JNK, and PI3-kinase pathways.
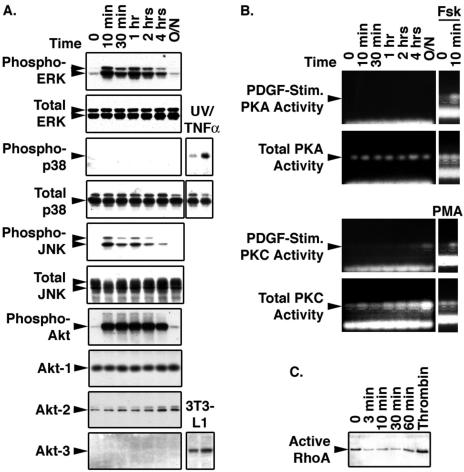
As a first step in identifying the signaling pathways that govern SMC proliferation and downregulation of CREB content, we examined which of the major pathways were activated by PDGF-BB (Fig. 4A). Western blotting was used to detect the phosphorylated and activated forms of ERK1/2, JNK, p38 MAPK, and the PI3-kinase substrate, Akt. PDGF treatment induced activation of ERK and JNK pathways, but not that of p38 MAPK, which was present in SMCs and could be activated by combined treatment with tumor necrosis factor alpha and exposure to UV light. PDGF also activated PI3-kinase signaling, as determined by the appearance of phosphorylated Akt. Akt1 and -2 were detected in SMC lysates, while Akt3 was absent.
FIG. 4.
PDGF stimulates ERK, JNK, and PI3-kinase intracellular signaling pathways. Rat PA SMCs maintained in complete medium were transferred to DMEM containing 0.2% FCS for 24 h. Cells were then treated with 25 ng/ml PDGF in the same medium for the times shown above each lane. (A) At each time point, whole-cell lysates were prepared and 25 μg of extract protein was resolved on 10% polyacrylamide-SDS gels and transferred to PVDF membranes. The blots were subjected to Western blot analysis with the antibodies to the proteins indicated on the left side of each panel. Where indicated, lysates from SMCs subjected to 20-min exposures to UV light and tumor necrosis factor alpha (TNFα) or lysates from 3T3-L1 cells were included as positive controls. (B) Total and PDGF-stimulated protein kinase A and C activities were measured with PepTag nonradioactive assay reagents as described in Materials and Methods. Cells treated with forskolin (Fsk) or phorbol myristate acetate (PMA) were included as positive controls as indicated. (C) Active RhoA was determined with RhoA activation assay reagents. Lysates from SMCs treated with thrombin for 30 min were included as a positive control.
Direct kinase activity assays were employed to assess the effect of PDGF on PKA and PKC activities (Fig. 4B). PDGF had no effect on PKA activity, but PKA was rapidly activated by forskolin. Total PKC levels rose during the 24-hour time course of the experiment, but minimal levels of PDGF-stimulated PKC activity were detected only after prolonged exposure to PDGF. However, PKC was strongly activated in cells treated with phorbol myristate acetate for 10 min. Measurements of active RhoA levels in membrane fractions from SMCs showed no effect of PDGF, while thrombin, a known activator of RhoA/Rho kinase signaling, elevated active RhoA levels (Fig. 4C). The data indicated that PDGF-BB treatment induced signaling via ERK, JNK, and PI3-kinase pathways in PA SMCs, with perhaps a small contribution from PKC.
ERK, JNK, and PI3-kinase pathways are required for PDGF-induced SMC proliferation.
To determine which of the signaling pathways regulated by PDGF was required for SMC proliferation, we initially tested siRNA directed against ERK1/2, JNK, Akt1 and -2, and other signaling molecules. These treatments were effective at downregulating the expression of the targeted molecules but repeatedly resulted in significant cell death when the cells were transferred to low-serum medium. Adenoviruses expressing dominant negative forms of MEK1 (upstream activator of ERK1/2) and JNK1/2 were also tested, but cells transduced with these viruses also died when transferred to serum-free medium. To circumvent these problems, we assembled a panel of pharmacological inhibitors to block signaling by the relevant pathways.
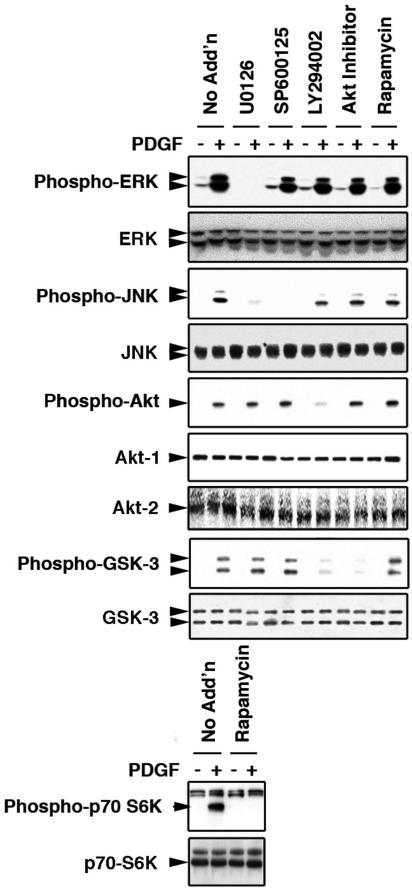
Figure 5 shows Western blot results in which the specificities of the inhibitors were assessed. U0126, a MEK1/2 inhibitor, blocked PDGF activation of ERK. This compound also inhibited activation of JNK, consistent with reports showing cross talk between ERK and JNK pathways in SMCs (31, 41). The JNK inhibitor SP600125 prevented PDGF activation of JNK but did not affect other signaling pathways. LY294002 was employed as an inhibitor of PI3-kinase, and this compound blocked the phosphorylation of the PI3-kinase target, Akt. This compound also prevented phosphorylation of GSK-3, a downstream target of Akt. The Akt inhibitor did not affect Akt phosphorylation but did block activation of GSK-3. Finally, rapamycin, an inhibitor of the protein kinase mTOR, attenuated PDGF-induced phosphorylation of p70 S6 kinase, a primary mTOR substrate, but had no impact on other pathways. These data indicate that the inhibitors on the panel act predictably and with considerable specificity.
FIG. 5.
Efficacies and specificities of intracellular signaling inhibitors. Rat PA SMCs were maintained in complete medium and transferred to DMEM containing 0.2% FCS 24 h before treatments. Cells were treated with 20 μM U0126, 40 μM SP600125, 40 μM LY294002, 10 μM Akt inhibitor, or 40 nM rapamycin for 30 min. “No Add'n” indicates cells not treated with an inhibitor. Duplicate wells either were left untreated or were subjected to the addition of PDGF to a final concentration of 25 ng/ml. After 30 min of incubation, the cells were lysed, and 25 μg of lysate protein was resolved on 10% polyacrylamide-SDS gels and transferred to PVDF membranes. The blots were subjected to Western blot analysis with antibodies to the phosphorylated and total signaling intermediates indicated to the left of each panel.
The panel of inhibitors was first used to identify PDGF-induced signaling pathways required for SMC proliferation. Cells were grown in 96-well plates in serum-free medium supplemented with PDGF in the presence or absence of each inhibitor for a period of 72 h. During this time, the medium, PDGF, and inhibitor were replaced daily. Individual inhibition of ERK, JNK, or PI3-kinase signaling was sufficient to repress PDGF-induced SMC proliferation (Fig. 6A and B). Neither blockade of p38 MAPK with SB203580 nor treatment with rapamycin had any effect on cell growth (Fig. 6A). Consistent with the absence of RhoA activation, the Rho kinase inhibitor Y27632 also failed to reduce PDGF-induced proliferation (data not shown). These data indicate that the three primary signaling pathways activated by PDGF, namely, ERK, JNK, and PI3-kinase (Fig. 4), are all required for the induction of proliferation by PDGF (Fig. 6A and B).
FIG. 6.
Inhibition of ERK, JNK, and PI3-kinase pathways blocks SMC proliferation. (A) Rat PA SMCs grown in complete medium on 96-well plates were transferred to DMEM containing 0.2% FCS for 24 h. The cells were then treated with the indicated signaling inhibitors at the concentrations specified in the legend to Fig. 5 for 30 min. PDGF was then added to a concentration of 25 ng/ml, and medium, PDGF, and inhibitors were replaced every 24 h for 72 h. Untreated, cross-hatched bars; PDGF treated, solid black bars. (B) Rat PA SMCs were transfected with nonspecific (control), CREB-specific, or Akt-specific double-stranded siRNAs as indicated and incubated overnight in DMEM containing 15% FCS. The cells were transferred to DMEM containing 0.2% FCS for 24 h. The cells were then treated with the indicated signaling inhibitors, and medium, PDGF, and inhibitors were replaced every 24 h for 72 h. Untreated, open bars; PDGF-treated, cross-hatched bars; CREB siRNA, shaded bars; CREB siRNA plus PDGF, solid black bars. Proliferation was measured with CellTiter 96 AQ reagents and is expressed relative to absorbances measured with untreated cells in both panel A and panel B.
In subsequent experiments, we tested whether depletion of CREB with siRNA could overcome the inhibition of PDGF-stimulated proliferation observed with ERK, JNK, and PI3-kinase/Akt inhibitors. As shown in Fig. 6B, loss of CREB alone increased SMC proliferation, as we had seen previously (Fig. 1B and C). CREB depletion was also able to restore PDGF-induced proliferation in cells treated with the ERK pathway inhibitor U0126. Likewise, decreased CREB levels partially restored basal and PDGF-stimulated proliferation in cells treated with the PI3-kinase inhibitor LY294002 or depleted of Akt with siRNA. However, the inhibition of proliferation observed with blockade of JNK signaling with SP600125 could not be overcome by CREB depletion.
PI3-kinase and Akt mediate PDGF-induced CREB loss in SMCs.
Subsequent studies examined the impact of the signaling pathway inhibitors on PDGF-induced CREB loss in SMCs. Cells were grown on eight-well microscope slides in the presence or absence of PDGF and of each of the individual inhibitors for 48 to 72 h, after which they were subjected to immunostaining for CREB. Fluorescent deconvolution imaging showed that the ERK, JNK, and mTOR inhibitors alone had no effect on CREB localization or levels (Fig. 7A and B). However, inhibition of PI3-kinase or Akt prevented CREB depletion by PDGF. A long-exposure Western blot of CREB in whole-cell lysates again showed that PDGF not only decreased CREB levels but also produced multiple high-molecular-weight bands due to polyubiquitination of CREB (Fig. 7C). No polyubiquitinated CREB was evident in cells treated with Akt inhibitor.
FIG. 7.
Inhibition of PI3-kinase/Akt signaling blocks PDGF-induced CREB loss in SMCs. Rat PA SMCs were plated on eight-well slides at 20% confluence. The cells were transferred from complete medium to DMEM containing 0.2% FCS for 24 h and then treated with the signaling inhibitors indicated to the left of each row for 30 min at the concentrations indicated in the legend to Fig. 5. PDGF was then added to a concentration of 25 ng/ml. Duplicate wells were left untreated. Medium, PDGF, and inhibitors were replaced every 24 h for 72 h. (A) Cells were fixed and subjected to immunostaining for total CREB using an AlexaFluor 594-conjugated secondary antibody. The cells were also mounted with VectaShield with DAPI to visualize nuclei. Figure shows representative fluorescent deconvolution photomicrographs at a magnification of ×400. Rapa., rapamycin; Cntrl, control. (B) Cytosolic (“C”) and nuclear (“N”) fractions were resolved on 10% polyacrylamide-SDS gels and transferred to PVDF membranes. The figure shows a representative Western blot for total CREB and a Coomassie blue-stained gel as a loading control. (C) Whole-cell lysates (25 μg protein) from cells treated with and without PDGF and/or Akt inhibitor were resolved on polyacrylamide-SDS gels and transferred to PVDF membranes. The figure shows a representative long-exposure Western blot for total CREB and a Coomassie blue-stained gel as a loading control. Positions of CREB and ubiquitinated CREB (Ub-CREB) are indicated in the figure.
To ensure that the protective effects of leptomycin B and lactacystin on nuclear CREB levels were not due to changes in PI3-kinase/Akt signaling, the impact of these agents on PI3-kinase and Akt activity was measured in PDGF-treated and untreated cells. Leptomycin B and lactacystin, alone or in combination, had no effect on basal or PDGF-stimulated PI3-kinase or Akt activity (data not shown).
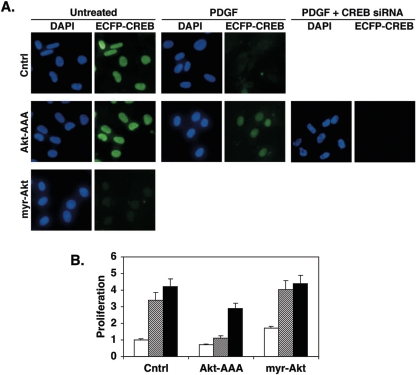
The ability of Akt to regulate CREB levels in SMCs was verified in experiments using stably transfected cells expressing chimeric enhanced cyan fluorescent protein (ECFP)-CREB. These cells were infected with adenoviruses expressing a dominant negative Akt (Akt-AAA), a constitutively active Akt (myr-Akt), or LacZ (control). Expression of myr-Akt resulted in loss of SMC CREB even in cells not treated with PDGF (Fig. 8A). Alternately, Akt-AAA expression prevented the depletion of CREB normally observed with PDGF. However, CREB siRNA was able to reduce CREB levels in cells expressing Akt-AAA, indicating that siRNA and Akt-AAA act at different stages of CREB expression. The active and inhibitory forms of Akt were also tested for their effects on SMC proliferation. Dominant negative Akt slightly reduced basal SMC proliferation and effectively blocked PDGF-induced increases in cell number (Fig. 8B). Constitutively active Akt slightly increased both basal and PDGF-stimulated proliferation. CREB depletion with siRNA increased SMC proliferation under all conditions, although the effect was somewhat blunted in cells expressing Akt-AAA. These data are consistent with the ability of siRNA to diminish CREB levels even when Akt is inactivated. These results support the concept that Akt plays a central role in regulating CREB levels in SMCs and is required for SMC proliferation in cooperation with other signaling pathways.
FIG. 8.
Regulation of Akt activity modulates SMC CREB content. Rat PA SMCs were grown in complete medium and stably transfected with a vector expressing wild-type CREB linked to ECFP. After selection, rapidly growing colonies exhibiting high levels of ECFP fluorescence were pooled and expanded. These cells were infected with adenoviruses expressing dominant negative Akt-AAA, constitutively active myr-Akt, or LacZ (Cntrl) at a multiplicity of infection of 100. Some cells also received CREB-specific siRNA as indicated. Twenty-four hours later, the cells were transferred to DMEM containing 0.2% FCS and incubated overnight before the addition of PDGF to a concentration of 25 ng/ml. Duplicate plates were left untreated as a control (Cntrl). Medium and PDGF were replaced every 24 h for 72 h. (A) Cells were fixed in PBS with 4% paraformaldehyde, and coverslips were affixed with VectaShield with DAPI to visualize nuclei. The figure shows representative fluorescence deconvolution images of DAPI or ECFP fluorescence at a magnification of ×416. (B) Cell proliferation was measured with CellTiter 96 AQ reagents. Open bars, untreated; cross-hatched bars, PDGF; solid bars, CREB siRNA.
GSK-3 is a target for Akt phosphorylation (9, 61), can phosphorylate CREB at serine 115 (of CREB-327 [8, 17]), and, therefore, could potentially mediate the effects of Akt on CREB. However, inhibition of GSK-3 had no effect on basal or PDGF-regulated CREB levels (data not shown). These data indicate that the impact of Akt on CREB in SMCs is independent of either GSK-3 or mTOR, each of which is a downstream mediator of Akt function.
PDGF-induced CREB loss is dependent on phosphorylation of CREB at serines 103 and 107.
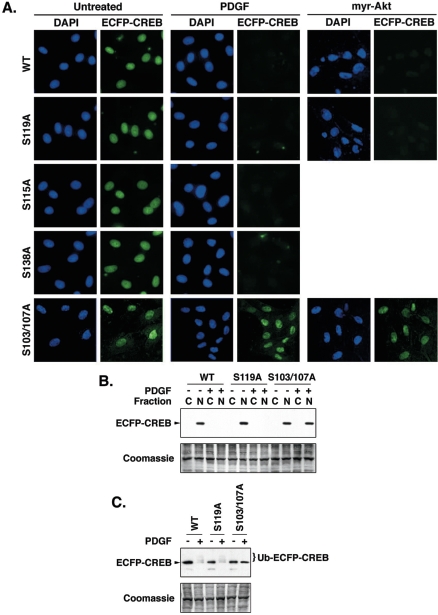
CREB transcriptional activity is regulated primarily by phosphorylation at various serine residues. Therefore, we determined whether phosphorylation of CREB at consensus PKA (serine 119 [67]), GSK-3 (serine 115 [8, 17]), CKII (serines 103 and 107), and Ca2+-calmodulin-stimulated protein kinase IV (serine 138 [13, 56]) target sites was required for PDGF-induced CREB loss. Chimeric proteins consisting of ECFP linked to full-length CREB containing individual serine-to-alanine mutations at position 115, 119, or 138 or dual transitions at serines 103 and 107 were expressed in SMCs that were then treated with PDGF. As anticipated, wild-type ECFP-CREB was depleted by PDGF treatment (Fig. 9A, B, and C). Each of the phosphorylation site mutants was also reduced by PDGF, with the exception of the dual serine 103/107 mutant. A long-exposure Western blot (Fig. 9C) shows the appearance of polyubiquitinated wild-type and S119A ECFP-CREB bands in cells treated with PDGF. However, in cells expressing the dual S103/107A mutant ECFP-CREB, a single ECFP-CREB was detected. These data indicate that serines 103 and 107 are required for ubiquitination of CREB. Thus, PDGF-induced CREB loss was dependent on the phosphorylation of CREB at serines 103 and 107 but independent of phosphorylation at other sites.
FIG. 9.
PDGF-induced CREB depletion occurs via phosphorylation of CREB serines 103 and 107. Wild-type (WT) CREB-327 and CREB mutants containing serine-to-alanine mutations at serines 115, 119, and 138 and a dual mutation of serines 103 and 107 were linked in frame to ECFP in the plasmid pECFP-C1. Plasmids were stably transfected into rat PA SMCs as described in Materials and Methods. After selection in G418, rapidly growing colonies exhibiting bright ECFP fluorescence were pooled and expanded. The cells were plated on an eight-well microscope slide at 20% confluence. Some cells were infected with an adenovirus expressing constitutively active myr-Akt at a multiplicity of infection of 100 as indicated. The cells were transferred to DMEM containing 0.2% FCS for 24 h, and PDGF was added to a concentration of 25 ng/ml. Medium and PDGF was replaced every 24 h for 72 h. Duplicate wells were left untreated as a control. (A) Cells were fixed and coverslips were attached with VectaShield plus DAPI to visualize nuclei. The figure shows representative fluorescence deconvolution images of DAPI and ECFP fluorescence at a magnification of ×400. (B) Duplicate plates of cells harvested and separated into cytosolic (“C”) and nuclear (“N”) fractions, which were resolved on 10% polyacrylamide-SDS gels and transferred to PVDF membranes. The figure shows a representative Western blot for ECFP-CREB and a Coomassie blue-stained gel as a loading control. (C) Whole-cell lysates (25 μg protein) from cells expressing the indicated ECFP-CREB isoforms were resolved on polyacrylamide-SDS gels and transferred to PVDF membranes. The figure shows a representative long-exposure Western blot for ECFP-CREB and a Coomassie blue-stained gel as a loading control. The positions of ECFP-CREB and ubiquitinated ECFP-CREB (Ub-ECFP-CREB) are indicated in the figure.
DISCUSSION
Initial studies described in this paper confirmed that CREB depletion in vascular SMCs elicits changes consistent with those observed in SMCs from pathologically remodeled arteries in vivo. Such changes include changes in the expression of SMC markers and contractile factors such as SM myosin, calponin, and fibronectin; increases in proliferation and proliferation-related factors such as cyclin D1; and increases in extracellular matrix production. The data support previous experiments showing that inhibition of CREB activity with dominant negative forms of CREB enhances SMC proliferation and migratory behavior (33). These studies further establish CREB as a key regulator of the SMC phenotype.
Several reports have suggested that CREB is required for mitogen-induced SMC proliferation rather than acting as an inhibitor of growth (18, 19, 43, 48, 58, 69). These results differ from ours likely because they were obtained by studies in which cells were exposed to mitogenic stimulus for brief periods of time (minutes to hours), rather than the 48- to 72-h time periods we have used. We too observe rapid CREB phosphorylation when cells are treated with PDGF-BB for periods from 10 min to 24 h. However, the rapid and transient activation of CREB is likely a braking mechanism by which cells attempt to prevent uncontrolled proliferation. We would also argue that vascular remodeling is a protracted process, one in which SMCs are subjected to various mitogens and cytokines over a period of days to weeks or months. Thus, our experiments, which assess changes in CREB content and activity over several days, may more accurately reflect what happens to CREB and its impact on SMCs in the clinically relevant in vivo situation.
Differences between acute and chronic receptor-mediated intracellular signaling are common in many biological systems. Differences in gene expression following acute versus chronic exposure to ethanol (63), antipsychotic drugs (2), and various analgesics (45, 47) are well-known examples. Likewise, differential activation of type 1 and 2 tumor necrosis factor alpha receptors has been reported in acute versus chronic inflammatory conditions (26). The PI3-kinase/Akt pathway in particular exhibits differential regulation in response to acute or chronic activation by a number of extracellular stimuli, including insulin, insulin-like growth factor-1, angiotensin II, and PDGF (1, 29, 35, 37, 62). Kaplan-Albuquerque and colleagues (32) have reported that agents which promote SMC differentiation, such as thrombin, produce transient activation of PI3-kinase/Akt and sustained activation of MAPK cascades, while agents that promote SMC proliferation, such as PDGF, elicit sustained activation of PI3-kinase/Akt and transient MAPK activation. Interestingly, chronic activation of Akt has been shown to diminish CREB levels in PC12 cells, although the mechanism was not determined (68). Thus, there is substantial precedent in the literature to support a model in which sustained activation of PI3-kinase/Akt signaling by PDGF leads to decreased CREB levels in SMCs.
The mechanisms that link chronic Akt activation to CREB depletion are the focus of ongoing studies. Although Du and Montminy (12) have reported that Akt can phosphorylate CREB at serine 119, we have been unable to replicate these results, and there is no evidence that Akt can phosphorylate CREB serines 103 and 107. These sites are substrates for CKII in vitro, but their phosphorylation by CKII in vivo has not been firmly established. Moreover, CKII is generally unregulated by extracellular stimuli and thus has constitutive kinase activity, making its participation in the depletion of CREB in response to PDGF unlikely. We have attempted to block PDGF-induced CREB loss in SMCs with pharmacological inhibitors of CKII with no success (unpublished data). Another possibility is that phosphorylation of CREB serines 103 and 107 is regulated by protein phosphatases. Taylor et al. (57) have reported that these sites are recognized by protein phosphatase 1. However, this phosphatase is not known to be regulated by PI3-kinase or Akt. Thus, the mechanism(s) that couples Akt signaling to CREB nuclear export, ubiquitination, and proteasomal degradation remains unresolved but is the focus of ongoing studies in our laboratory.
Our data also show that SMC proliferation in response to PDGF-BB is mediated by a combination of ERK, JNK, and PI3-kinase/Akt signaling, while CREB loss is regulated by PI3-kinase/Akt alone. We envision a model in which concomitant activation of ERK, JNK (either via ERK or via MEK3/6), and PI3-kinase/Akt signaling pathways is required for SMC proliferation (Fig. 10). Loss of any one pathway by pharmacological blockade is sufficient for the attenuation of SMC growth. PI3-kinase/Akt signaling contributes to proliferation via numerous mechanisms, at least one of which is the downregulation of CREB content. How then does depletion of CREB alone (via siRNA) induce SMC proliferation, which is governed by multiple signaling pathways? The answer may lie in the ability of other signaling pathways, such as ERK, to regulate CREB activity without causing CREB loss. These pathways have been implicated in regulating CREB activity in other cell types (65, 66). The coordinate control of CREB phosphorylation/activity and CREB content underscores the importance of CREB as a regulatory target and a central control element in the regulation of the SMC phenotype.
FIG. 10.
Model of PDGF-induced SMC proliferation and CREB depletion. PDGF stimulates SMC proliferation via activation of ERK, JNK, and PI3-kinase signaling pathways. Inhibition of any one of these pathways blocks PDGF's mitogenic impact. PDGF-induced CREB loss is mediated by PI3-kinase and Akt signaling. Loss of CREB and other PI3-kinase/Akt-regulated events participate in SMC proliferation.
The importance of PDGF, PI3-kinase/Akt, and CREB in pulmonary vascular biology is underscored by recent reports showing that blockade of PDGF receptor signaling with imatinib mesylate (Gleevec) blocks the development of pulmonary hypertension and attenuates pulmonary arterial remodeling in rodents and humans (20, 51). In recent studies, we tested the abilities of the PI3-kinase inhibitor LY294002 and the Akt inhibitor triciribine to prevent pulmonary artery remodeling in a rat model of chronic hypoxic pulmonary hypertension. We found that these agents effectively block pulmonary artery remodeling, inhibit SMC proliferation, and prevent loss of CREB in arterial SMCs (unpublished data). Studies to measure pulmonary arterial remodeling and the development of pulmonary hypertension in mice overexpressing CREB or in which CREB is diminished in vascular SMCs are under way. These studies should confirm the role of CREB in maintaining normal vascular structure.
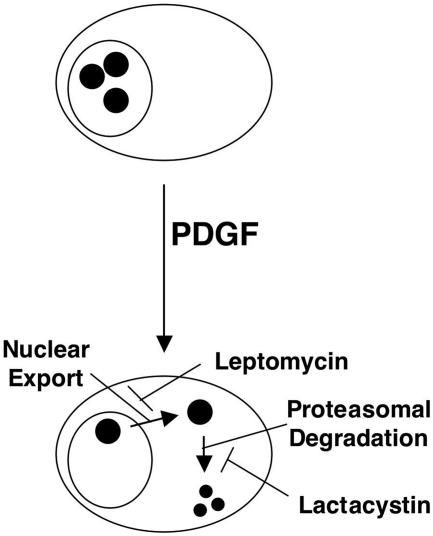
Our results are also the first to demonstrate that both ubiquitination/degradation and nuclear export are involved in the regulation of CREB content in SMCs (Fig. 11). Ubiquitination and degradation of CREB in epithelial cells have been previously reported to occur in response to hypoxia (57). The loss of CREB in these studies required phosphorylation of CREB at putative CKII sites, since phosphorylated but not unphosphorylated decoy peptides corresponding to these sites could block hypoxia-induced CREB depletion. In our studies, mutation of these same sites also blocked PDGF-induced CREB depletion. Stevenson and colleagues (55) reported nuclear translocation of CREB in SMCs in response to membrane depolarization or PDGF-BB. These studies were conducted over a period of only a few hours, and the ability of PDGF to stimulate nuclear export over longer time periods was not examined. The rapid nuclear import of CREB in response to PDGF or membrane depolarization was contemporaneous with CREB phosphorylation, although it remains unclear whether phosphorylation of CREB was required for nuclear translocation. Our observations support these previous studies and underscore the importance of regulatory mechanisms other than direct phosphorylation/dephosphorylation in the regulation of CREB activity. They also highlight the numerous mechanisms that can target a single factor to bring about a particular response.
FIG. 11.
PDGF induces leptomycin-sensitive nuclear export and lactacystin-sensitive degradation of CREB in PA SMCs. Large oval, cell membrane; smaller circle, nuclear membrane; small, solid circles, CREB.
The PI3-kinase/Akt signaling pathway and its downstream effector, GSK-3, have been shown to regulate the intracellular location and degradation of a number of other factors. For example, PI3-kinase/Akt signaling leads to the self-ubiquitination of the ubiquitin ligase Mdm2 (16). PI3-kinase/Akt inhibition of GSK-3 results in the stabilization of hypoxia-inducible factor 1α (38) but increases proteasomal degradation of the basic helix-loop-helix protein p8 (21). In other studies, Akt has been shown to promote nuclear exclusion of the forkhead transcription factor FKHR1 (4) and block nuclear import of another forkhead protein, AFX (7). Beals and colleagues (3) have shown that GSK-3 stimulates nuclear export of the transcription factor NFATc. The concomitant ubiquitination and nuclear export of CREB more closely resemble the effect of the ubiquitin ligase Mdm2 on p53. Here, activation of Mdm2 leads to C-terminal ubiquitination of p53 (36), which in turn reveals a nuclear export sequence in p53 (24). As noted earlier, Mdm2 is a target for the PI3-kinase/Akt pathway and perhaps could function as a CREB ubiquitin ligase. Whether Mdm2 ubiquitinates CREB and whether ubiquitination of CREB alters its conformation to reveal a nuclear export signal will be the focus of future studies.
One issue left unresolved by these studies is whether proteasomal degradation of CREB occurs in the nucleus, in the cytosol, or in both locations and whether nuclear export occurs prior to or simultaneously with degradation. Both leptomycin B (nuclear export blockade) and lactacystin (proteasome inhibition) individually restore nuclear CREB content in PDGF-treated cells. If degradation occurs in the nucleus, then leptomycin B should have no effect on CREB loss, while lactacystin should block nuclear degradation. Since leptomycin B alone restores nuclear CREB levels, proteasomal degradation in the nucleus is unlikely. A second possibility is that CREB is exported from the nucleus and degraded in the cytosol. Here, leptomycin B would maintain nuclear CREB levels by preventing export and degradation. However, lactacystin would be expected to protect CREB from degradation, but CREB would accumulate in the cytosol, since nuclear export would be unaffected. Since significant levels of CREB were never detected in the cytosol by immunocytochemistry or by Western blotting in our studies, CREB must be reimported into the nucleus when it cannot be degraded. Stevenson and colleagues (55) have reported the Ran-dependent nuclear import of CREB in vascular SMCs, making this the most parsimonious explanation of our results.
In conclusion, depletion of CREB in PA SMCs produces phenotypic changes consistent with changes observed in pathologically remodeled vessel walls in vivo. PDGF-BB, a mitogen released in response to vascular damage and during vascular remodeling, leads to a depletion of CREB in SMCs. This depletion is mediated by PI3-kinase/Akt signaling but does not involve GSK-3 or mTOR. CREB loss in response to PDGF is not due to changes in CREB gene transcription but rather to nuclear export and proteasomal degradation. The loss of CREB with PDGF treatment appears to be dependent on CREB phosphorylation at putative CKII sites.
Acknowledgments
This work was funded by a Veteran Affairs MERIT Award and NIH grant HL14985 (to D.J.K.).
REFERENCES
- 1.Allen, R. T., K. D. Krueger, A. Dhume, and D. K. Agrawal. 2005. Sustained Akt/PKB activation and transient attenuation of c-Jun N-terminal kinase in the inhibition of apoptosis by IGF-1 in vascular smooth muscle cells. Apoptosis 10:525-535. [DOI] [PubMed] [Google Scholar]
- 2.Atkins, J. B., J. Chlan-Fourney, H. E. Nye, N. Hiroi, W. A. Carlezon, and E. J. Nestler. 1999. Region-specific induction of dFosB by repeated administration of typical versus atypical antipsychotic drugs. Synapse 33:118-128. [DOI] [PubMed] [Google Scholar]
- 3.Beals, C. R., C. M. Sheridan, C. W. Turck, P. Gardner, and G. R. Crabtree. 1997. Nuclear export of NF-ATc enhanced by glycogen synthase kinase-3. Science 275:1930-1933. [DOI] [PubMed] [Google Scholar]
- 4.Biggs, W. H., III, J. Meisenhelder, T. Hunter, W. K. Cavenee, and K. C. Arden. 1999. Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc. Natl. Acad. Sci. USA 96:7421-7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bornfeldt, K. E., and E. G. Krebs. 1999. Crosstalk between protein kinase A and growth factor receptor signaling pathways in arterial smooth muscle. Cell. Signal. 11:465-477. [DOI] [PubMed] [Google Scholar]
- 6.Botney, M. D., L. Bahadori, and L. I. Gold. 1994. Vascular remodeling in primary pulmonary hypertension. Potential role for transforming growth factor-b. Am. J. Pathol. 140:286-295. [PMC free article] [PubMed] [Google Scholar]
- 7.Brownawell, A. M., G. J. Kops, I. G. Macara, and B. M. T. Burgering. 2001. Inhibition of nuclear import by protein kinase B (Akt) regulates the subcellular distribution and activity of the forkhead transcription factor AFX. Mol. Cell. Biol. 21:3534-3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bullock, B. P., and J. F. Habener. 1998. Phosphorylation of the cAMP response element binding protein CREB by cAMP-dependent protein kinase A and glycogen synthase kinase-3 alters DNA-binding affinity, conformation, and increases net charge. Biochemistry 37:3795-3809. [DOI] [PubMed] [Google Scholar]
- 9.Cross, D. A., D. R. Alessi, P. Cohen, M. Andjelkovich, and B. A. Hemmings. 1995. Inhibition of glycogen synthase kinase-3 by insulin mediated protein kinase B. Nature 378:785-789. [DOI] [PubMed] [Google Scholar]
- 10.D'Amico, M., J. Hulit, D. F. Amanatullah, B. T. Zafonte, C. Albanese, B. Bouzahzah, M. Fu, L. H. Augenlicht, L. A. Donehower, K.-I. Takemaru, R. T. Moon, R. Davis, M. P. Lisanti, M. Shtutman, J. Zhurinsky, A. Ben-Ze'ev, A. A. Troussard, S. Dedahr, and R. G. Pestell. 2000. The integrin-linked kinase regulates the cyclin D1 gene through glycogen synthase kinase 3β and cAMP-responsive element-binding protein-dependent pathways. J. Biol. Chem. 275:32469-32657. [DOI] [PubMed] [Google Scholar]
- 11.Dawes, K. E., A. J. Peacock, A. J. Gray, J. E. Bishop, and G. J. Laurent. 1994. Characterization of fibroblast mitogens and chemoattractants produced by endothelial cells exposed to hypoxia. Am. J. Respir. Cell Mol. Biol. 10:552-559. [DOI] [PubMed] [Google Scholar]
- 12.Du, K., and M. Montminy. 1998. CREB is a regulatory target for the protein kinase Akt/PKB. J. Biol. Chem. 273:32377-32379. [DOI] [PubMed] [Google Scholar]
- 13.Enslen, H., H. Tokumitsu, and T. R. Soderling. 1995. Phosphorylation of CREB by CaM-kinase IV activated by CaM-kinase IV kinase. Biochem. Biophys. Res. Commun. 207:1038-1043. [DOI] [PubMed] [Google Scholar]
- 14.Faller, D. V. 1999. Endothelial cell responses to hypoxic stress. Clin. Exp. Pharmacol. Physiol. 26:74-84. [DOI] [PubMed] [Google Scholar]
- 15.Fanburg, B., and S.-L. Lee. 2000. A role for the serotonin transporter in hypoxia-induced pulmonary hypertension. J. Clin. Investig. 105:1521-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng, J., R. Tamaskovic, Z. Yang, D. P. Brazil, A. Merlo, D. Hess, and B. A. Hemmings. 2004. Stabilization of Mdm2 via decreased ubiquitination is mediated by protein kinase B/Akt-dependent phosphorylation. J. Biol. Chem. 279:35510-35517. [DOI] [PubMed] [Google Scholar]
- 17.Fiol, C. J., J. S. Williams, C. H. Chou, Q. M. Wang, P. J. Roach, and O. M. Andrisani. 1994. A secondary phosphorylation of CREB341 at Ser129 is required for the cAMP-mediated control of gene expression. A role for glycogen synthase kinase-3 in the control of gene expression. J. Biol. Chem. 269:32187-32193. [PubMed] [Google Scholar]
- 18.Fukuyama, K., T. Ichiki, H. Ono, T. Tokunou, N. Iino, S. Masuda, H. Ohtsubo, and A. Takeshita. 2005. cAMP-response element-binding protein mediates prostaglandin F(2alpha)-induced hypertrophy of vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 338:910-918. [DOI] [PubMed] [Google Scholar]
- 19.Funakoshi, Y., T. Ichiki, K. Takeda, T. Tokuno, N. Iino, and A. Takeshita. 2002. Critical role of cAMP-response element-binding protein for angiotensin II-induced hypertrophy of vascular smooth muscle cells. J. Biol. Chem. 277:18710-18717. [DOI] [PubMed] [Google Scholar]
- 20.Ghofrani, H. A., W. Seeger, and F. Grimminger. 2005. Imatinib for the treatment of pulmonary arterial hypertension. N. Engl. J. Med. 353:1412-1413. [DOI] [PubMed] [Google Scholar]
- 21.Goruppii, S., and J. M. Kyriakis. 2004. The pro-hypertrophic basic helix-loop-helix protein p8 is degraded by the ubiquitin/proteosome system in a protein kinase B/Akt- and glucogen synthase kinase-3-dependent manner, whereas endothelin induction of p8 mRNA and renal mesangial cell hypertrophy require NFAT4. J. Biol. Chem. 279:20950-20958. [DOI] [PubMed] [Google Scholar]
- 22.Graves, L. M., K. E. Bornfeldt, E. W. Raines, B. C. Potts, S. G. Macdonald, R. Ross, and E. G. Krebs. 1993. Protein kinase A antagonizes platelet-derived growth factor-induced signaling by mitogen-activated protein kinase in human arterial smooth muscle cells. Proc. Natl. Acad. Sci. USA 90:10300-10304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graves, L. M., and J. C. Lawrence, Jr. 1996. Insulin, growth factors, and cAMP. Antagonism in the signal transduction pathways. Trends Endocrinol. Metab. 7:43-50. [DOI] [PubMed] [Google Scholar]
- 24.Gu, J., L. Nie, D. Weiderschain, and Z.-M. Yuan. 2001. Identification of p53 sequence elements that are required for Mdm2-mediated nuclear export. Mol. Cell. Biol. 21:8533-8546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayashi, S., R. Morishita, H. Matsushita, H. Nakagami, Y. Taniyama, T. Nakamura, M. Aoki, K. Yamamoto, J. Higaki, and T. Ogihara. 2000. Cyclic AMP inhibited proliferation of human aortic vascular smooth muscle cells, accompanied by induction of p53 and p21. Hypertension 35:237-243. [DOI] [PubMed] [Google Scholar]
- 26.Holtmann, M. H., and M. F. Neurath. 2004. Differential TNF-signaling in chronic inflammatory disorders. Curr. Mol. Med. 4:439-444. [DOI] [PubMed] [Google Scholar]
- 27.Hoshiya, M., and M. Awazu. 1998. Trapidil inhibits platelet-derived growth factor-stimulated mitogen-activated protein kinase cascade. Hypertension 31:665-671. [DOI] [PubMed] [Google Scholar]
- 28.Huang, F., and A. Sorkin. 2005. Growth factor receptor binding protein 2-mediated recruitment of the RING domain of Cbl to the epidermal growth factor receptor is essential and sufficient to support receptor endocytosis. Mol. Biol. Cell 16:1268-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Isenovic, E. R., Y. Meng, A. Divald, N. Milivojevic, and J. R. Sowers. 2002. Role of phosphatidylinositol 3-kinase/Akt pathway in angiotensin II and insulin-like growth factor-1 modulation of nitric oxide synthase in vascular smooth muscle cells. Endocrine 19:287-292. [DOI] [PubMed] [Google Scholar]
- 30.Iyengar, R. 1996. Gating by cyclic AMP: expanded role for an old signaling pathway. Science 271:461-463. [DOI] [PubMed] [Google Scholar]
- 31.Kang, Y. J., E. S. Jeon, H. Y. Song, J. S. Woo, J. S. Jung, Y. K. Kim, and J. H. Kim. 2005. Role of c-Jun N-terminal kinase in the PDGF-induced proliferation and migration of human adipose tissue-derived mesenchymal stem cells. J. Cell. Biochem. 95:1135-1145. [DOI] [PubMed] [Google Scholar]
- 32.Kaplan-Albuquerque, N., C. Garat, C. Desseva, P. L. Jones, and R. A. Nemenoff. 2003. Platelet-derived growth factor-BB-mediated activation of Akt suppresses smooth muscle-specific gene expression through inhibition of mitogen-activated protein kinase and redistribution of serum response factor. J. Biol. Chem. 278:39830-39838. [DOI] [PubMed] [Google Scholar]
- 33.Klemm, D. J., P. Watson, M. G. Frid, E. C. Dempsey, J. Schaack, L. A. Colton, A. Nesterova, K. R. Stenmark, and J. E. B. Reusch. 2001. cAMP response element-binding protein content is a molecular determinant of smooth muscle cell proliferation and migration. J. Biol. Chem. 276:46132-46141. [DOI] [PubMed] [Google Scholar]
- 34.Koyama, H., K. E. Bornfeldt, S. Fukumoto, and Y. Nishizawa. 2001. Molecular pathways of cyclic nucleotide-induced inhibition of arterial smooth muscle cell proliferation. J. Cell. Physiol. 186:1-10. [DOI] [PubMed] [Google Scholar]
- 35.Li, F., C. Zhang, S. Schaefer, A. Estes, and K. U. Malik. 2005. ANG II-induced neointimal growth is mediated via cPLA2- and PLD2-activated Akt in balloon-injured rat carotid artery. Am. J. Physiol. Heart Circ. Physiol. 289:H2592-H2601. [DOI] [PubMed] [Google Scholar]
- 36.Lohrum, M. A., D. B. Woods, R. L. Ludwig, E. Balint, and K. H. Vousden. 2001. C-terminal ubiquitination of p53 contributes to nuclear export. Mol. Cell. Biol. 21:8521-8532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lymn, J. S., K. L. Gallagher, G. F. Clunn, S. E. Fexby, M. K. Patel, and A. D. Hughes. 2003. PDGF stimulates DNA synthesis in human vascular smooth muscle cells via a novel wortmannin-insensitive phosphatidylinositol 3-kinase. FEBS Lett. 555:591-596. [DOI] [PubMed] [Google Scholar]
- 38.Mottet, D., V. Dumont, Y. Deccache, C. Demazy, N. Ninane, M. Raes, and C. Michiels. 2003. Regulation of hypoxia-inducible factor-1a protein level during hypoxic conditions by the phosphatidylinositol 3-kinase/Akt/glycogen synthase kinase 3b pathway in HepG2 cells. J. Biol. Chem. 278:31277-31285. [DOI] [PubMed] [Google Scholar]
- 39.Musa, N. L., M. Ramakrishnan, J. Li, S. Kartha, P. Liu, R. G. Pestell, and M. B. Hershenson. 1999. Forskolin inhibits cyclin D1 expression in cultured airway smooth muscle cells. Am. J. Respir. Cell Mol. Biol. 20:352-358. [DOI] [PubMed] [Google Scholar]
- 40.Nagata, D., E. Suzuki, H. Nishimatsu, H. Satonaka, A. Goto, M. Omata, and Y. Hirata. 2001. Transcriptional activation of the cyclin D1 gene is mediated by multiple cis-elements, including SP1 sites and a cAMP-responsive element in vascular endothelial cells. J. Biol. Chem. 276:662-669. [DOI] [PubMed] [Google Scholar]
- 41.Nakata, S., M. Tsutsui, H. Shimokawa, M. Tamura, H. Tasaki, T. Morishita, O. Suda, S. Ueno, Y. Toyohira, Y. Nakashima, and N. Yanagihara. 2005. Vascular neuronal NO synthase is selectively upregulated by platelet-derived growth factor. Involvement of the mitogen-activated protein kinase kinase/extracellular signal-regulated kinase pathway. Arterioscler. Thromb. Vasc. Biol. 25:2502-2508. [DOI] [PubMed] [Google Scholar]
- 42.Newby, A. C., and A. B. Zaltsman. 2000. Molecular mechanisms in intimal hyperplasia. J. Pathol. 190:300-309. [DOI] [PubMed] [Google Scholar]
- 43.Ono, H., T. Ichiki, K. Fukuyama, N. Iino, S. Masuda, K. Egashira, and A. Takeshita. 2004. cAMP-response element-binding protein mediates tumor necrosis factor-alpha-induced vascular smooth muscle cell migration. Arterioscler. Thromb. Vasc. Biol. 24:1634-1639. [DOI] [PubMed] [Google Scholar]
- 44.Osinski, M. T., and K. Schror. 2000. Inhibition of platelet-derived growth factor-induced mitogenesis by phosphodiesterase 3 inhibitors: role of protein kinase A in vascular smooth muscle cell mitogenesis. Biochem. Pharmacol. 60:381-387. [DOI] [PubMed] [Google Scholar]
- 45.Parada, C. A., D. B. Reichling, and J. D. Levine. 2005. Chronic hyperalgesic priming in the rat involves a novel interaction between cAMP and PKCe second messenger pathways. Pain 113:185-190. [DOI] [PubMed] [Google Scholar]
- 46.Perkett, E. A., D. B. Badesch, M. K. Roessler, K. R. Stenmark, and B. Meyrick. 1992. Insulin-like growth factor-1 and pulmonary hypertension induced by continuous air embolism in sheep. Am. Rev. Respir. Cell Mol. Biol. 6:82-87. [DOI] [PubMed] [Google Scholar]
- 47.Radwanska, K., J. Caboche, and L. Kaczmarek. 2005. Extracellular signal-regulated kinases (ERKs) modulate cocaine-induced gene expression in the mouse amygdala. Eur. J. Neurosci. 22:939-948. [DOI] [PubMed] [Google Scholar]
- 48.Rius, J., J. Martinez-Gonzalez, J. Crespo, and L. Badimon. 2004. Involvement of neuron-derived orphan receptor-1 (NOR-1) in LDL-induced mitogenic stimulus in vascular smooth muscle cells: role of CREB. Arterioscler. Thromb. Vasc. Biol. 24:697-702. [DOI] [PubMed] [Google Scholar]
- 49.Rothman, A., B. Wolner, D. Button, and P. Taylor. 1994. Immediate-early gene expression in response to hypertrophic and proliferative stimuli in pulmonary arterial smooth muscle cells. J. Biol. Chem. 269:6399-6404. [PubMed] [Google Scholar]
- 50.Rybalkin, S. D., K. E. Bornfeldt, W. K. Sonnenburg, I. G. Rybalkina, K. S. Kwak, K. Hanson, E. G. Krebs, and J. A. Beavo. 1997. Calmodulin-stimulated cyclic nucleotide phosphodiesterase (PDE1C) is induced in the human arterial smooth muscle cells of the synthetic, proliferative phenotype. J. Clin. Investig. 100:2611-2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schermuly, R. T., E. Dony, H. A. Ghofrani, S. Pullamsetti, R. Savai, M. Roth, A. Sydykov, Y. J. Lai, N. Weissmann, W. Seeger, and F. Grimminger. 2005. Reversal of experimental pulmonary hypertension by PDGF inhibition. J. Clin. Investig. 115:2691-2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seasholtz, T. M., T. Zhang, M. R. Morissette, A. L. Howes, A. H. Yang, and J. H. Brown. 2001. Increased expression and activity of RhoA are associated with increased DNA synthesis and reduced p27(Kip1) expression in the vasculature of hypertensive rats. Circ. Res. 89:488-495. [DOI] [PubMed] [Google Scholar]
- 53.Shaul, P. W., B. Kinanae, M. A. Farrar, M. Buja, and R. R. Magness. 1991. Prostacyclin production and mediation of adenylate cyclase activity in the pulmonary artery: alteration after prolonged hypoxia in the rat. J. Clin. Investig. 88:447-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stenmark, K. R., and R. P. Mecham. 1997. Cellular and molecular mechanisms of pulmonary vascular remodeling. Ann. Rev. Physiol. 59:89-144. [DOI] [PubMed] [Google Scholar]
- 55.Stevenson, A. S., L. Cartin, T. L. Wellman, M. H. Dick, M. T. Nelson, and K. M. Lounsbury. 2001. Membrane depolarization mediates phosphorylation and nuclear translocation of CREB in vascular smooth muscle cells. Exp. Cell Res. 263:118-130. [DOI] [PubMed] [Google Scholar]
- 56.Sun, P., H. Enslen, P. S. Myung, and R. A. Maurer. 1994. Differential activation of CREB by Ca2+/calmodulin-dependent protein kinases type II and type IV involves phosphorylation of a site that negatively regulates activity. Genes Dev. 8:2527-2539. [DOI] [PubMed] [Google Scholar]
- 57.Taylor, C. T., G. T. Furuta, K. Synnestvedt, and S. P. Colgan. 2000. Phosphorylation-dependent targeting of cAMP response element binding protein to the ubiquitin/proteosome pathway in hypoxia. Proc. Natl. Acad. Sci. USA 97:12091-12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tokunou, T., T. Ichiki, K. Takeda, Y. Funakoshi, N. Iino, and A. Takeshita. 2001. cAMP response element-binding protein mediates thrombin-induced proliferation of vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 21:1764-1769. [DOI] [PubMed] [Google Scholar]
- 59.Townsend, S. F., V. K. M. Han, and K. R. Stenmark. 1994. Persistence of the fetal pattern of IGF-II expression in the adventitia of small pulmonary arteries in neonatal hypoxic pulmonary hypertension. Growth Regul. 4:S12. [Google Scholar]
- 60.Vadiveloo, P. K., E. L. Filonzi, H. R. Stanton, and J. A. Hamilton. 1997. G1 phase arrest of human smooth muscle cells by heparin, IL-4 and cAMP is linked to repression of cyclin D1 and cdk2. Atherosclerosis 133:61-69. [DOI] [PubMed] [Google Scholar]
- 61.van Weeren, P. C., K. M. de Bruyn, A. M. de Vries-Smits, J. van Lint, and B. M. Burgering. 1998. Essential role for protein kinase B (PKB) in insulin-induced glycogen synthase kinase 3 inactivation. Characterization of dominant-negative mutant of PKB. J. Biol. Chem. 273:13150-13156. [DOI] [PubMed] [Google Scholar]
- 62.Vecchione, C., E. Patrucco, G. Marino, L. Barberis, R. Poulet, A. Aretini, A. Maffei, M. T. Gentile, M. Storto, O. Azzolino, M. Brancaccio, G. L. Colussi, U. Bettarini, F. Altruda, L. Silengo, G. Tarone, M. P. Wymann, E. Hirsch, and G. Lembo. 2005. Protection from angiotensin II-mediated vasculotoxic and hypertensive response in mice lacking PI3Kgamma. J. Exp. Med. 18:1217-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weng, Y.-I., and S. D. Shukla. 2003. Effects of chronic ethanol treatment on the angiotensin II-mediated p42/p44 mitogen-activated protein kinase and phosphorylase a activation in rat hepatocytes. Alcohol 29:83-90. [DOI] [PubMed] [Google Scholar]
- 64.Woods, M., E. G. Wood, J. A. Mitchell, and T. D. Warner. 2000. Cyclic AMP regulates cytokine stimulation of endothelin-1 release in human vascular smooth muscle cells. J. Cardiovasc. Pharmacol. 36:S404-S406. [DOI] [PubMed] [Google Scholar]
- 65.Xing, J., D. D. Ginty, and M. E. Greenberg. 1996. Coupling of the RAS-MAPK pathway to gene activation by RSK2, a growth factor-regulated CREB kinase. Science 273:959-963. [DOI] [PubMed] [Google Scholar]
- 66.Xing, J., J. M. Kornhauser, Z. Xia, E. A. Thiele, and M. E. Greenberg. 1998. Nerve growth factor activates extracellular signal-regulated kinase and p38 mitogen-activated protein kinase pathways to stimulate CREB serine 133 phosphorylation. Mol. Cell. Biol. 18:1946-1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yamamoto, K. K., G. A. Gonzalez, W. H. Biggs III, and M. R. Montminy. 1988. Phosphorylation-induced binding and transcriptional efficacy of nuclear factor CREB. Nature 334:494-498. [DOI] [PubMed] [Google Scholar]
- 68.Zauli, G., D. Milani, P. Mirandola, M. Mazzoni, P. Secchiero, S. Miscia, and S. Capitani. 2001. HIV-1 Tat protein downregulates CREB transcription factor expression in PC12 neuronal cells through a phosphatidylinositol 3-kinase/Akt/cyclic nucleoside phosphodiesterase pathway. FASEB J. 15:483-491. [DOI] [PubMed] [Google Scholar]
- 69.Zhang, S., C. V. Remillard, I. Fantozzi, and J. X. Yuan. 2004. ATP-induced mitogenesis is mediated by cyclic AMP response element-binding protein-enhanced TRPC4 expression and activity in human pulmonary artery smooth muscle cells. Am. J. Physiol. Cell Physiol. 287:C1192-C1201. [DOI] [PubMed] [Google Scholar]