Abstract
A novel selection scheme has been developed to isolate bloodstream forms of Trypanosoma brucei, which are defective in their ability to differentiate to the procyclic stage. Detailed characterization of one selected cell line (defective in differentiation clone 1 [DiD-1]) has demonstrated that these cells are indistinguishable from the wild-type population in terms of their morphology, cell cycle progression, and biochemical characteristics but are defective in their ability to initiate differentiation to the procyclic form. Although a small proportion of DiD-1 cells remain able to transform, deletion of the genes for glycophosphatidyl inositol-phospholipase C demonstrated that this enzyme was not responsible for this inefficient differentiation. However, the attenuated growth of the Δ-glycophosphatidyl inositol-phospholipase C DiD-1 cells in mice permitted the expression of stumpy characteristics in this previously monomorphic cell line, and concomitantly their ability to differentiate efficiently was restored. Our results indicate that monomorphic cells retain expression of a characteristic of the stumpy form essential for differentiation, and that this is reduced in the defective cells. This approach provides a new route to dissection of the cytological and molecular basis of life cycle progression in the African trypanosome.
INTRODUCTION
Analysis of the life cycle control of microbial eukaryotes is providing an essential component to the understanding of the basic mechanisms of cell fate, determination, and differentiation (Rasmussen et al., 1996). In multicellular organisms these cellular responses are governed by the presence of particular growth factors or by hormonal stimulation. In contrast, differentiation events in unicellular organisms frequently represent a response to environmental cues such as changes in pH, temperature, the depletion of available nutrients, the accumulation of toxic factors, or the presence of specific signaling molecules (Zilberstein and Shapira, 1994; Parent and Devreotes, 1996; Lujan et al., 1997). Once initiated, however, both multicellular and unicellular organisms undergo a program of gene expression and protein synthesis, which culminates in irreversible cell specialization or development to a new life cycle stage. The initiation of such events is frequently coordinated with cell cycle control, often involving cell cycle arrest in metazoan cells, or cell cycle modulation in unicellular microbes as they adapt to new environments (Weeks and Weijer, 1994).
African trypanosomes are protozoan parasites that coordinate life cycle differentiation with cell cycle progression (Matthews and Gull, 1994; Mottram, 1994). Trypanosomes are the causative agents of sleeping sickness in humans and nagana in cattle and are transmitted between mammalian hosts by the bite of the blood-feeding tsetse fly (Vickerman, 1985). In the mammal bloodstream, trypanosomes survive through their expression of a variable surface glycoprotein (VSG) coat. This coat can be periodically changed, and the resultant antigenic variation allows the parasite to evade specific antibody responses (Cross et al., 1998). The VSG coat also protects against nonspecific lysis by complement activated by the alternative pathway (Ferrante and Allison, 1983).
The trypanosome life cycle is complex, with several distinct stages being present in both the insect vector and mammalian host. During the course of a bloodstream parasitemia the trypanosomes proliferate initially as morphologically slender forms. As cell numbers increase in the blood, however, slender forms differentiate to morphologically stumpy forms (Vickerman, 1965). Among the first events of this transformation is the cessation of cell division, with the resulting stumpy cell population being uniformly arrested in G1 or G0 (Shapiro et al., 1984). Based on the observation that stumpy cells are uniformly arrested in their cell cycle and transform synchronously, a model that describes the initiation of differentiation as being cell cycle position dependent has been developed (Ziegelbauer et al., 1990; Matthews and Gull 1994). Specifically, cells in the G1–G0 phase of the cell cycle would be able to receive the differentiation signal, whereas those outside this window would not be competent.
Stumpy forms can only reenter into a proliferative cell cycle as they differentiate to the procyclic form of the parasite, which multiplies in the tsetse fly midgut. Although the specific trigger inducing this differentiation in the tsetse is unidentified, transformation to the procyclic form can be mimicked efficiently in vitro by the addition of cis-aconitate and temperature reduction from 37 to 27°C (Czichos et al., 1986; Overath et al., 1986; Matthews, 1999). There are good cytological markers that define distinct phases of this differentiation, which if initiated with populations highly enriched for the stumpy form, occur very synchronously in the population (Pays et al., 1993; Matthews and Gull 1994). In the first 2 h, cells begin expression of the procyclic stage-specific surface coat procyclin (Mowatt and Clayton, 1987; Roditi et al., 1987), and this is followed after a further 2–4 h by loss of the bloodstream stage-specific VSG coat (Roditi et al., 1989; Pays et al., 1993; Matthews and Gull, 1994). Although there is a specific enzyme, glycophosphatidyl inositol-phospholipase C (GPI-PLC), capable of shedding the VSG by cleavage of its GPI anchor moiety (Fox et al., 1986; Hereld et al., 1986, 1988), biochemical and genetic experiments have shown that this is not responsible for active VSG loss during differentiation to the procyclic form (Bulow et al., 1989; Ziegelbauer et al., 1993; Bangs et al., 1997; Webb et al., 1997). Nevertheless, activation of GPI-PLC in response to cell stress has been proposed as a potential route for the stimulation of differentiation under particular experimental regimes (Rolin et al., 1996, 1998).
The trypanosome population in the mammalian bloodstream is described as being pleomorphic because of the morphological heterogeneity of slender and stumpy cells (Vickerman, 1965). However, by rapid passage through laboratory animals, it is possible to derive virulent trypanosome lines that no longer generate morphologically stumpy forms (Fairbairn and Culwick, 1947; Ashcroft, 1960). These monomorphic lines retain the capacity to differentiate to procyclic forms in vitro, although this differentiation is asynchronous in the population (Overath et al., 1986; Roditi et al., 1989; Ziegelbauer et al., 1990; Matthews and Gull 1994). The slender morphology and virulence of these lines, coupled with their ability to transform to procyclic forms, have led to the concept that stumpy forms are a nonessential step in the trypanosome life cycle (Bass and Wang, 1991).
A powerful means to understand the control events and dependency relationships in microbial differentiation is to study cells that are defective in these processes. In the present work we have developed a simple in vivo selection scheme to derive trypanosome lines that are defective in the events that occur very early in the differentiation process between bloodstream and procyclic cells. Detailed analysis of one line has established the criteria for the dissection of differentiation defects and has demonstrated that, although monomorphic, this line fails to differentiate through reduced expression of a stumpy form characteristic. This approach has demonstrated the central role of stumpy cells in the trypanosome life cycle and provided a new route for the analysis of the control and coordination of differentiation events in Trypanosoma brucei.
MATERIALS AND METHODS
Trypanosomes
The starting population for the selection of differentiation defective cells was T. b. rhodesiense East African Trypanosomiasis Research Organization (EATRO) 2340 GUP2965, which is monomorphic in rodents. This population was derived by serial syringe passage through laboratory animals from a pleomorphic line of T. b. rhodesiense EATRO 2340. Infections were initiated in a mouse host with an inoculum of 1 × 106 trypanosomes and reached a parasitemia of 5 × 108 parasites/ml 3 d after inoculation. Where protein and RNA were to be prepared, trypanosomes were isolated from a rat host at a parasitemia of between 5 × 108 and 1 × 109 trypanosomes/ml. In this case, trypanosomes were purified by DEAE cellulose chromatography (Lanham and Godfrey, 1970).
In Vitro Differentiation and Selection of Differentiation-defective Trypanosomes
Differentiation to the procyclic form was initiated by incubating trypanosomes directly isolated from a rodents in SDM-79 (Brun and Schonenberger, 1979) at 27°C containing 6 mM cis-aconitate. Differentiation was initiated at a cell density of 2 × 106/ml and was monitored by following loss of the VSG coat and gain of the procyclin coat by immunofluorescence. Thus, 1 ml of cell culture was concentrated at 1000 × g, and then air-dried smears were prepared from the parasite pellet. These were fixed in 100% methanol at −20°C for at least 30 min. Immunofluorescence was carried out as previously described (Matthews and Gull, 1994), using polyclonal rabbit anti-Glasgow University Trypanozoon antigen type (GUTat) 7.2 VSG antibody (1:250; a gift from Dr. C.M.R. Turner, University of Glasgow, Glasgow, United Kingdom) and a monoclonal anti-procyclin antibody (1:500; Cedar Lane Laboratories, Hornby, Ontario, Canada; Richardson et al., 1988) as primary antibodies. These were visualized by using FITC-conjugated anti-rabbit and TRITC-anti-mouse immunoglobulin (Sigma, St. Louis, MO). Slides were counterstained with DAPI to reveal the parasite nucleus and kinetoplast and were mounted in MOWIOL (Harlow Chemical, Harlow, United Kingdom) containing 1 mg/ml phenylene diamine as an antifading agent. Differentiation status was assessed by examining parasites on a Zeiss (Thornwood, NY) Axioscop microscope. Images were captured using NIH Image 1.58 and processed using Adobe (Mountain View, CA) Photoshop 5.0. The kinetoplast-posterior dimension on differentiating parasites was also used as an indicator of differentiation efficiency and was measured using NIH Image 1.58 (Matthews et al., 1995).
To select for differentiation-defective cells, differentiation was monitored over 48 h. When ∼99% of cells had lost their VSG coat as assessed by immunofluorescence, 10 ml of the culture was concentrated by centrifugation at 1000 × g at 20°C. The parasite pellet was resuspended in 250 μl of HMI-9 medium (Hirumi and Hirumi, 1989) and inoculated into a mouse. Subsequent parasitemias were monitored over the course of 5 d or until the parasitemia reached 5 × 108 trypanosomes/ml. The parasites were then subjected to a subsequent round of in vitro differentiation, with a small proportion of the parasites being cryopreserved as a reference stock. This regimen was repeated until the differentiation kinetics in the population decreased significantly.
Northern and Western Blotting
RNA was prepared from DEAE-purified parasites by the guanidinium hydrochloride-acid phenol method (Chomczynski and Sacchi, 1987). Northern hybridization was carried out as described by Matthews and Gull (1998) using digoxygenin-labeled riboprobes for the VSG GUTat 7.2 transcript, GPI-PLC transcript (Hereld et al., 1988), or cytochrome oxidase c subunit II transcript (coxII). For the GPI-PLC and coxII transcripts, probes were prepared from amplified inserts of each gene cloned by PCR, using as primers oligonucleotides directed against either the first 20 nucleotides (nt) or the last 20 nt of the gene-coding region (for the GPI-PLC gene) or a 445-bp fragment of coxII (Stojdl and Clarke, 1996) (GPI-PLC A, GCGAATTCCTTTGAGACGGTTAAGAATC; GPI-PLC B, TACGAAGCTTGTCACAAACGCAGCAGAGAT; COX II A, TATGAATATGTGCATTG; COX II B, ACCTACCAGGTATAC). For the VSG GUTat 7.2 gene, an oligonucleotide directed to the first 20 nt of the spliced mini-exon sequence was used in combination with a primer directed to the sequence conserved in the 3′ end of VSG genes (5′-GGTGTTAAAATATATCAG-3′). Hybridization was in 50% formamide, 5× SSC, and 2% block (Boehringer Mannheim, Indianapolis, IN) in 0.1% SDS and 0.05% sodium lauryl sarcosine at 68°C (for the VSG and GPI-PLC transcripts) or at 50°C (for the coxII transcript). Posthybridization detection was by chemiluminescence, using CDP-star as a reaction substrate (Boehringer Mannheim).
Trypanosome protein was resolved on SDS-PAGE gels and blotted onto nitrocellulose membrane by electrotransfer. Antibody detection of proteins was by chemiluminescence, using alkaline phosphatase-conjugated secondary antibody and CDP-star as a reaction substrate (Amersham Pharmacia Biotech, Uppsala, Sweden).
Trypanosome Growth In Vitro
Bloodstream-form trypanosomes were grown in vitro on agarose-supported HMI-9 plates according to the method of Carruthers and Cross (1992). Clonal trypanosome populations were isolated by preparing a titration series of trypanosomes on HMI-9 plates and identifying well-isolated trypanosome colonies. Defective in differentiation clone 1 (DiD-1) was recloned to ensure its clonal origin. Long-term growth of the respective trypanosome lines was achieved in liquid HMI-9 medium (Hirumi and Hirumi, 1989).
GPI-PLC Gene Deletion
The two alleles of the GPI-PLC gene were deleted using the vectors pLN and pLH (kind gifts from Drs. H. Webb and M. Carrington, University of Cambridge, Cambridge, United Kingdom; Webb et al., 1997). Initially, DiD-1 cells adapted to in vitro culture were transfected at a density of 3 × 106 parasites/ml with 5 μg of pLN at 1.7 kV for three pulses on a BTX ECM830 electroporator (Kramel Biotech, Cramlington, Northumberland, United Kingdom). Transfectants were selected in 0.3 μg/ml G418, a concentration we had found suitable for the selecting for neomycin resistance with the DiD-1 cell line. Once established, a second round of transfection was performed with pLH, and these cells were selected in the presence of 1 μg/ml hygromycin plus 0.3 μg/ml G418. Southern blotting was then used to confirm deletion of the GPI-PLC gene in these cells. For mouse infections with GPI-PLC null mutants, BALB/c mice were immunosuppressed with cyclophosphamide (200 mg/kg) 24 h before infection with 2 × 106 parasites.
RESULTS
Selection for Differentiation-defective Cells
One of the major events during differentiation between bloodstream- and procyclic-form trypanosomes is the loss of the VSG coat. This represents a crucial event because cells that do not possess this coat are quickly lysed in the bloodstream by complement activated by the alternative pathway (Ferrante and Allison, 1983). This provided a powerful and straightforward approach for the selection of trypanosomes that are defective in their ability to differentiate to the procyclic form. Thus, bloodstream cells were stimulated to differentiate to procyclic forms in vitro, and once >90% had lost the VSG coat as part of this process, the population was concentrated and inoculated into a new mouse host. Even a single trypanosome is infective for a mouse, such that those few trypanosomes that survive and multiply must be those that have not successfully shed the VSG in response to differentiation conditions. Successive rounds of differentiation in vitro and elimination of uncoated forms in vivo result in strong selection for cells unable to lose the VSG coat.
An important component of such a selection scheme is the biological characteristics of the starting population. In a pleomorphic population, the stumpy form is irreversibly committed to division arrest unless it undergoes differentiation to the procyclic form (Vassella and Boshart, 1996, Vassella et al., 1997; Tyler et al., 1997). Thus, such cells cannot be used to regenerate a bloodstream population from cells that have failed to differentiate in vitro. Therefore, it was important to use a monomorphic line that could both maintain division in the bloodstream and differentiate efficiently to the procyclic form in vitro.
We used the GUP2965 line of T. b. rhodesiense EATRO 2340, which is monomorphic and which transforms to the procyclic form efficiently. It also has a very well-defined biological history, having been derived from a pleomorphic line by serial passage through mice (Turner et al., 1986; Graham et al., 1990). This pleomorphic line is of the same genetic origin as the reference line that we have previously used to map in detail the cytological events of differentiation and to define the requirements for its initiation and commitment (Matthews and Gull, 1994, 1997, 1998; Matthews et al., 1995). Therefore, the selection was carried out on a biologically well-characterized strain with predictable characteristics both in the bloodstream and during in vitro transformation to the procyclic form.
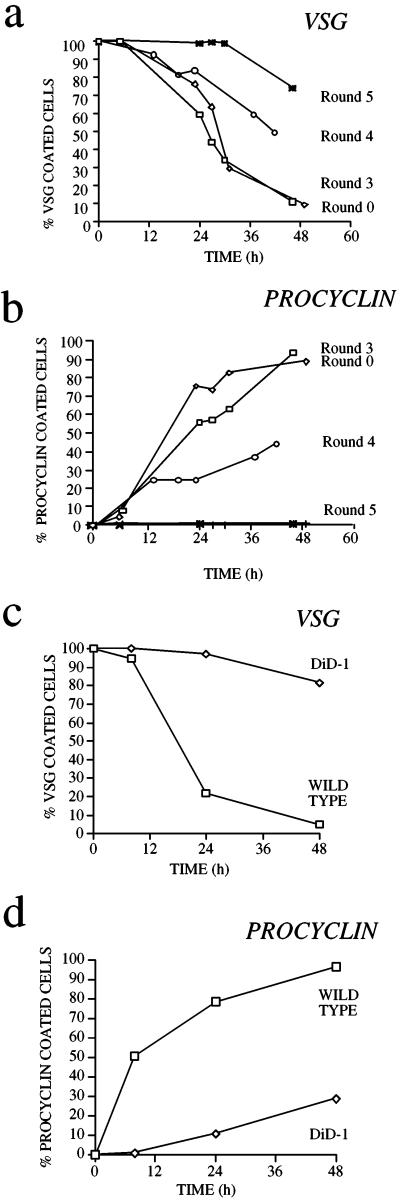
Figure 1a shows the kinetics of VSG coat loss during the selection regimen for differentiation-defective bloodstream trypanosomes. To assess differentiation efficiency an immunofluorescence assay detecting the GUTat 7.2 VSG coat was used, this being very effective at detecting the very small proportion of coated forms against a large background of uncoated differentiated forms. As expected for this monomorphic line, the starting population T. b. rhodesiense GUP2965 (henceforth called “wild type”) differentiated asynchronously, and >90% of the population had shed the VSG coat within 48 h (Figure 1a, Round 0). These differentiated cells had the morphology of the procyclic form and expressed the procyclin surface coat (Figure 1b). The residual ∼5% of cells remained brightly stained by immunofluorescence with VSG-specific antibody and were morphologically bloodstream form (our unpublished observations).
Figure 1.
Selection for differentiation-defective trypanosome lines. (a) VSG coat loss for trypanosome lines subjected to zero (Round 0; T. b. rhodesiense EATRO 2340 GUP2965.3; the starting wild-type population), three, four, and five rounds of in vitro differentiation and in vivo selection. (b) Expression of the procyclic stage-specific coat procyclin during the selection regimen. (c) VSG coat loss for DiD-1, a clonal line derived from Round 4 of the selection regimen, shown in comparison with the wild-type population. (d) Differentiation of the DiD-1 population assessed by gain of procyclin.
As the cell population progressed through successive rounds of selection, the kinetics of differentiation slowed with respect to the wild-type cell population (Figure 1, a and b). Thus, although the cells still went on to lose the VSG coat with time, an increasing proportion retained the coat for 24–48 h after induction, and the kinetics of detectable procyclin gain were reduced. After four and five rounds of selection a difference in the kinetics of differentiation from the wild-type cells became significant and reproducible (Figure 1a, Rounds 4 and 5), indicating that the experimental regimen had selected for cells with reduced ability to shed the VSG coat under in vitro differentiation conditions.
We derived clonal cell lines from the selected populations to allow the cytological basis of the differentiation defect to be analyzed. To maximize the heterogeneity of the potential phenotypes of the clonal lines, we chose to clone from the Round 4 population. These cells were plated onto agarose plates, and well-isolated colonies picked, injected into a mouse host, and stabilated from the resulting parasitemia. The differentiation kinetics of the resulting clonal lines were then examined, and one of these, termed DiD-1, was selected as being representative of several that showed poor differentiation with respect to the wild-type population. The differentiation kinetics of this line are shown in relation to wild-type cells in Figure 1, c and d. When cultured in the presence of 6 mM cis-aconitate at 27°C, the wild-type cells differentiated very efficiently, with <25% remaining VSG-positive and >75% becoming procyclin-positive after 24 h (Figure 1, c and d). In contrast, the great majority of the DiD-1 population failed to undergo differentiation; in a large number of replicated experiments (>10; our unpublished data; but see later sections) 85–99% of the DiD-1 cells remain VSG-positive and procyclin-negative at 24 h after incubation in differentiation conditions. Significantly however, there remains a population of cells (comprising 1–15% of the total) that can successfully differentiate and eventually establish a proliferative procyclic population after 3–4 d in culture. Thus DiD-1 represents a cell line that shows a significantly reduced capacity for differentiation but that retains the ability to differentiate at low frequency. Initially we focused on the majority of cells that do not differentiate.
DiD-1 Cells Fail at the Initiation of Differentiation
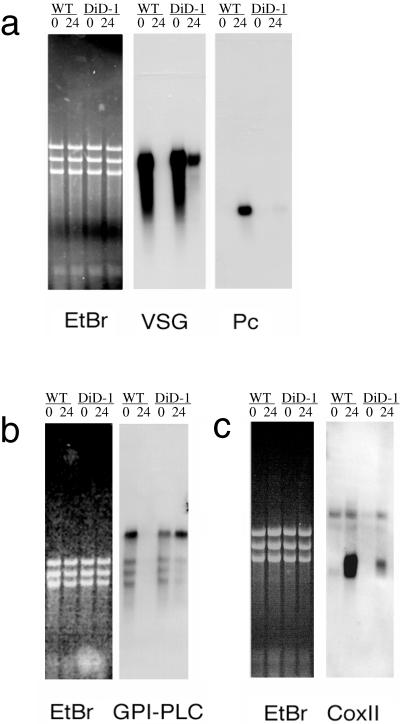
Transformation from the bloodstream to the procyclic form represents a highly regulated cascade of events, which provide the means to identify where DiD-1 cells fail in the differentiation process. First, we assessed stage-specific surface antigen mRNA expression in the wild-type and DiD-1 cell line, this being normally subjected to very rapid regulation in response to differentiation triggers. In particular, procyclin mRNAs are detectable 15–30 min after initiation of differentiation, and there is a concomitant rapid down-regulation of VSG mRNA (Vanhamme et al., 1995). Figure 2a shows a Northern blot of wild-type and DiD-1 cells immediately before and 24 h after exposure to cis-aconitate and temperature drop. This early time point was chosen to prevent the proliferation of differentiated procyclic cells complicating the mRNA expression patterns in each population. In both DiD-1 and wild-type cells, there is abundant VSG mRNA and no detectable procyclin expression (Figure 2a; 0-h time point). However, after 24-h exposure to the differentiation triggers, the wild-type population had no detectable VSG mRNA and expressed abundant procyclin mRNA. In contrast, the DiD-1 population showed almost no induction of procyclin mRNA, whereas the VSG mRNA was still abundant. Although this was reduced with respect to bloodstream cells at 37°C, this probably reflects the poor transcription of VSG genes observed at reduced temperature (Pays et al., 1989). Apparently, therefore, DiD-1 cells show greatly reduced surface antigen mRNA regulation in response to differentiation triggers.
Figure 2.
Stage-regulated RNA expression in the DiD-1 and wild-type populations 24 h after incubation in culture at 27°C containing 6 mM cis-aconitate. To demonstrate relative loading, an ethidium-stained image of each gel is shown, together with its corresponding Northern blot. (a) VSG and procyclin gene expression; (b) GPI-PLC gene expression; (c) cytochrome oxidase subunit II gene expression. The respective sizes for each transcript are VSG, ∼1.6 kb; procyclin, ∼0.8 kb; GPI-PLC, ∼4 kb, and coxII, 0.7 kb.
We were interested in whether other pathways in the differentiation process were also compromised. First, we assayed the expression of the transcript for GPI-PLC, an enzyme able to cleave the VSG anchor in the bloodstream. This transcript is bloodstream stage specific (Hereld et al., 1988), and its mRNA is quickly down-regulated during differentiation to the procyclic form (within 1 h; our unpublished observations). Figure 2b shows that the level of this transcript is similar in the wild-type and DiD-1 cell populations when in the bloodstream. However, after 24 h in differentiation conditions, GPI-PLC mRNA is significantly reduced in wild-type cells, whereas in the DiD-1 cells, it is maintained at the level in the bloodstream. Thus, this bloodstream-specific mRNA is not regulated in the DiD-1 population. We also examined the degree of procyclic stage-specific mitochondrial gene activation retained in DiD-1 by assaying regulation of the kinetoplast-encoded transcript for cytochrome oxidase subunit II (Figure 2c). This transcript is normally induced during differentiation to the procyclic form. In this case, we found that the wild-type cells show significant up-regulation in the levels of coxII after 24-h exposure to differentiation conditions. In contrast, although a low-level enhancement is detected because of the small proportion of cells that successfully initiate differentiation, DiD-1 does not induce significant levels of this transcript. Clearly, neither nuclear nor kinetoplast stage-specific genes are significantly regulated in DiD-1.
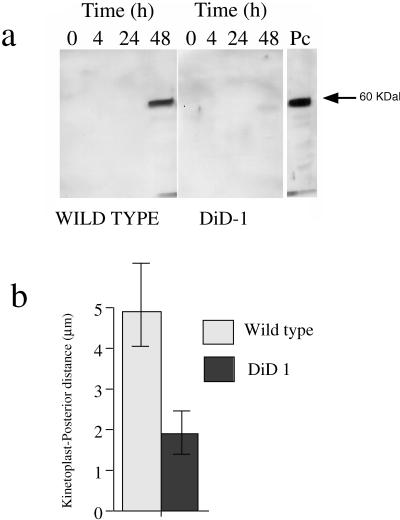
In addition to stage-regulated mRNAs, the metabolic development of DiD-1 was investigated by assessing elaboration of the glycosome. This organelle is unique to kinetoplastids and undergoes cyclical development during the trypanosome life cycle. A characteristic marker for this is the expression of phosphoenolpyruvate carboxykinase (PEPCK), an enzyme that is induced quite late during differentiation to the procyclic form (Bass and Wang, 1992). Using a PEPCK-specific antibody (Seebeck et al., 1988), we found that the wild-type cells strongly induced PEPCK expression, this being evident 48 h into the differentiation process (Figure 3a). In contrast, DiD-1 cells showed very little PEPCK induction even after 48 h (compare the respective levels of wild-type and DiD-1 cells in Figure 3a).
Figure 3.
Glycosomal development and morphological restructuring of DiD-1. (a) Glycosomal development of DiD-1 cells 4, 24, and 48 h after culture at 27°C in 6 mM cis-aconitate. The left panel shows equal protein loadings of the wild-type population undergoing differentiation, and the right panel shows DiD-1 samples under the same conditions. Each blot was probed with a rat anti-PEPCK antibody (a kind gift from Prof. T. Seebeck, University of Bern, Bern, Switzerland). A procyclic protein sample is shown at the extreme right. (b). Quantitative analysis of the relative distance between the kinetoplast and cell posterior in wild-type or DiD-1 populations 24 h after incubation in differentiation conditions. n = 100 cells for each cell type.
Finally, the extent of the morphological changes associated with development to the procyclic form was assessed in the defective cells. In particular, the repositioning of the kinetoplast, from the posterior end of the cell to midway between the nucleus and cell posterior, is a characteristic marker of this transition (Brown et al., 1973; Matthews et al., 1995). We, therefore, compared the linear distance between the cell posterior and kinetoplast in wild-type and DiD-1 cells after 24 h in differentiation conditions (Figure 3b). Although monomorphic cell differentiation is asynchronous, kinetoplast repositioning clearly occurred in the wild-type population with the mean posterior-kinetoplast distance being 4.9 μm. In contrast, this dimension for the DiD-1 population was far smaller, being uniformly in the region of 1.8 μm (Figure 3b). This closely corresponds to the distance seen in monomorphic bloodstream cells and indicates that DiD-1 cells retain the cytoskeletal architecture of the bloodstream form.
Basis of the Differentiation Defect
Our molecular and cytological analyses revealed that when exposed to cis-aconitate and temperature drop, the majority of the DiD-1 cells underwent no differentiation event for which we have markers. These markers define events that occur as soon as 15 min from the initiation of differentiation, demonstrating either that DiD-1 cannot receive the signal to differentiate or that they fail in differentiation very soon after the reception of this signal. To determine the basis of this failure, we evaluated a number of possible explanations.
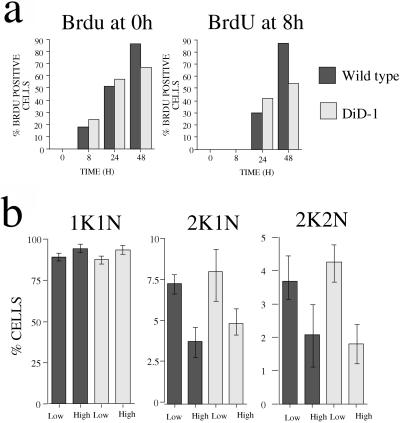
Initially we examined whether DiD-1 cells have a defective cell cycle and are not competent to receive the differentiation signal. The ability to initiate differentiation to the procyclic form is believed to be cell cycle position dependent, with G1–G0 being the window at which cells are competent to receive the differentiation signal (Matthews and Gull, 1994). To address the possibility that DiD-1 has a cell cycle defect, bloodstream populations of either wild-type or DiD-1 cells were harvested before peak parasitemia (when cells were in mid–late log growth, 3–5 × 108/ml), and their ability to progress through the cell cycle was assessed in the presence of cis-aconitate at 27°C. As the assay for cell cycle progression, we monitored incorporation of 5′-bromo-2′-deoxyuridine (BrdU) into the DNA of the parasites by immunofluorescence (Woodward and Gull, 1990). More specifically, BrdU was added to the cultures at the moment of cis-aconitate addition (t = 0 h) and, in a separate flask, 8 h after this (t = 8 h). This second treatment distinguished cells beginning a new cell cycle from those that are merely completing an ongoing cell cycle initiated in the bloodstream.
Figure 4a shows the incorporation of BrdU into the wild-type and DiD-1 populations after the addition of cis-aconitate. In both populations, there is ∼50–60% BrdU incorporation in the first 24 h in those samples in which BrdU was added at t = 0 h (Figure 4a, left graph). This was not entirely the result of cells completing an already ongoing cell cycle: where the cell populations were examined in which BrdU had been added at 8 h after incubation in differentiation conditions, 30–40% of cells had also successfully incorporated BrdU (Figure 4a, right graph). Thus, DiD-1 cells were not arresting in the cell cycle when under differentiation conditions: active and ongoing proliferation was possible, at least within a 24-h time frame. With further incubation, a greater proportion of cells in the wild-type population went on to incorporate BrdU, whereas the DiD-1 population increased only slowly (Figure 4a, 48-h time samples). This was expected because the wild-type cells at 48 h have successfully transformed to the procyclic form and are actively proliferating as that form. In contrast, only a small proportion of DiD-1 cells successfully complete this transformation, and the vast majority arrest in their cell cycle as the bloodstream form at 27°C and eventually die.
Figure 4.
DiD-1 does not show a cell cycle defect. (a) BrdU incorporation of DiD-1 and wild-type cells in culture at 27°C, with 6 mM cis-aconitate. In the left graph, cells were incubated in the presence of 50 mM BrdU and 50 mM 2′-deoxycytidine from the outset. In the right graph, BrdU and 2′-deoxycytidine were not added until 8 h after the cells had been placed into differentiation conditions. (b) Cell cycle progression of wild-type and DiD-1 cells during the course of a bloodstream parasitemia. The proportions of cells with a configuration of one kinetoplast and one nucleus (1K1N), 2K1N, and 2K2N were scored for each population when the parasite density was 5 × 107 cells/ml (low) or 5 × 108 cells/ml (high). The data represent four individual parasitemias for each cell type.
The cell cycle status of the wild-type and DiD-1 cells was also assayed in the bloodstream by monitoring the course of a parasitemia for each in a mouse host. The trypanosome cell cycle position can be rapidly defined by the nucleus and kinetoplast number in each cell, such that G1 and S phase cells have one kinetoplast and one nucleus (1K1N), G2 cells are 2K1N, whereas post mitotic cells are 2K2N (Sherwin and Gull, 1989). When these parameters were measured for the DiD-1 and wild-type population, no difference was found in their cell cycle progression in the bloodstream. Furthermore, Figure 4b shows that both populations accumulate in the 1K1N configuration as the parasitemia develops from 5 × 107 to 5 × 108/ml. Concomitant with this, there was a decrease in the proportion of cells in other cell cycle stages with the numbers of both 2K1N and 2K2N cells decreasing as the cells approached peak parasitemia. Evidently, although these populations are both monomorphic, each accumulated in the G1 phase of the cell cycle as the parasitemia increased, and there is no detectable difference in this capacity in the wild-type and DiD-1 populations. Thus, no cell cycle defect was detectable in the DiD-1 population.
We next investigated whether DiD-1 had lost a stumpy cell characteristic required for differentiation. A naturally occurring bloodstream trypanosome population is pleomorphic, being composed of slender cells at low parasitemia and stumpy cells at peak parasitemia. Although stumpy cells are believed to be preadapted for differentiation to the procyclic form, DiD-1 had been derived from a cell population that was monomorphic, could not generate morphologically stumpy forms in vivo, and yet was able to differentiate to the procyclic form efficiently. Thus, these monomorphic cells either can differentiate to the procyclic forms directly or retain some stumpy-form attributes that allow them to do so. If the latter were true then we reasoned that the DiD-1 population could potentially have lost such stumpy-like characteristics.
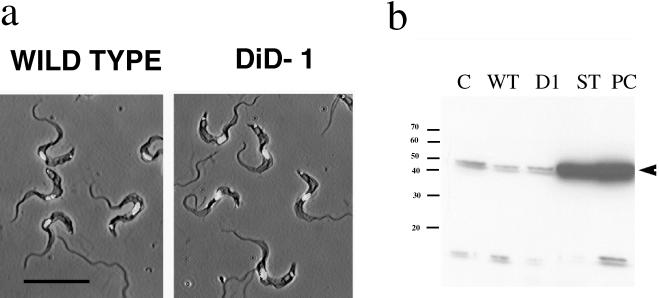
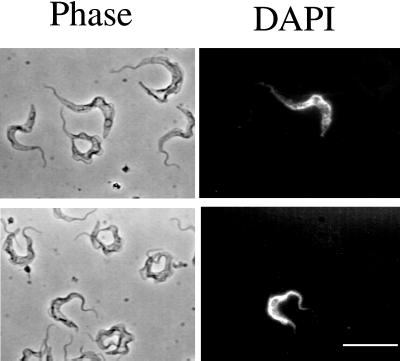
In addition to cell cycle arrest, the stumpy form is characterized by its morphology and through their expression of NADH diaphorase activity (Vickerman, 1965). Therefore, we first examined the morphology of the wild-type and DiD-1 cell population in terms of their cell shape, length of the free flagellum, and position of the nucleus and kinetoplast. However, careful examination of each cell population revealed them to be indistinguishable in the bloodstream, and we could not detect stumpy forms in either the wild-type or DiD-1 populations even at a very low level (Figure 5a). Apparently the defective cells had not detectably progressed toward a more slender morphology as a result of the selection regimen. Second, we examined DiD-1 cells for the biochemical expression of a stumpy cell marker. Although the NADH diaphorase assay represents a valuable cytochemical marker with which to confirm the identity of slender and stumpy cells (Vickerman, 1965), it cannot be used as a quantitative assay to categorize intermediates between these morphological extremes. Instead, we examined the expression of dihydrolipoamide dehydrogenase (DHLADH), because this enzyme is believed to be responsible for the cytochemical diaphorase activity and is significantly induced during the slender-stumpy transition (Tyler et al., 1997). Protein samples were prepared from each population harvested from a mouse host at very high cell density (>7 × 108 trypanosomes/ml), when stumpy characteristics are most likely to be manifest. These samples were then assayed with an antibody against DHLADH from the related kinetoplastid Trypanosoma cruzi (a kind gift from Prof. R.L. Krauth-Siegel, University of Heidelberg, Heidelberg, Germany; Lohrer and Krauth-Siegel, 1990). This antibody has been previously shown to faithfully reflect the level of stumpy cell expression of DHLADH in a pleomorphic trypanosome population (Tyler et al., 1997). Figure 5b demonstrates that there was no significant quantitative difference in the expression of DHLADH in the wild-type and DiD-1 cells, but that each showed significantly less expression of this marker than stumpy form cells or cells that have undergone differentiation to the procyclic form.
Figure 5.
Characteristics of DiD-1 in the bloodstream. (a) Phase-contrast images of either wild-type (left panel) or DiD-1(right panel) cells from a bloodstream parasitemia of 5 × 108 cells/ml. In each case cells have been counterstained with DAPI to reveal the nucleus and kinetoplast. Bar, 20 μm. (b) Expression of DHLADH in cultured monomorphic cells (C), wild-type cells isolated from a bloodstream parasitemia (WT), DiD-1 cells isolated from a bloodstream parasitemia (D1), bloodstream stumpy forms (ST), and cells induced to differentiate from the stumpy form to the procyclic form and isolated after 24 h (Pc). Proteins from the wild-type and DiD-1 populations were prepared from a parasitemia at a cell density of 7 × 108 cells/ml. Note that stumpy and differentiated procyclic form cells express abundant DHLADH, whereas a uniformly low level is detected in each of the other populations. A small cross-reacting band is also detected with this antibody of ∼15 kDa.
Thus, the wild-type and DiD-1 population were indistinguishable with respect to their cell cycle progression, morphology, and DHLADH expression. We conclude that no difference in the ability of the wild-type and DiD-1 cells to express stumpy cell characteristics is detectable.
Expression of Stumpy Characteristics Rescues the DiD-1 Defect
Although DiD-1 cells showed a compromised ability to differentiate, a small proportion were always found to transform to procyclic forms. Indeed, ∼1–15% of cells consistently differentiated in the first 24 h, and this proportion increased to 15–40% after 48 h and thereafter proceeded to establish a successful proliferative procyclic cell population. These cells do not represent a contaminant of DiD-1 cells, because this small differentiation-competent population is retained after further recloning (our unpublished observations). Figure 6 shows a representation of DiD-1 cells fixed 4 h after exposure to 6 mM cis-aconitate and identified as procyclin-positive among a background population of >10,000 undifferentiated cells. This early time point allows the identification of cells able to differentiate at a time when they retain their bloodstream-form morphology. Careful examination of this cell population did not demonstrate any obvious characteristic that distinguished them from the remainder of the population. Indeed, we closely examined the cells to determine whether they represented a small stumpy cell population inherent among the monomorphic cell background but found no cells that were unambiguously stumpy in morphology.
Figure 6.
A small proportion of DiD-1 cells are able to differentiate. The left panels shows the morphology of DiD-1 cells 4 h after incubation in culture at 27°C with 6 mM cis-aconitate. In each case cells were sought that demonstrated the expression of procyclin (right panel), and their morphology was examined for the expression of stumpy cell characteristics. Bar, 30 μm.
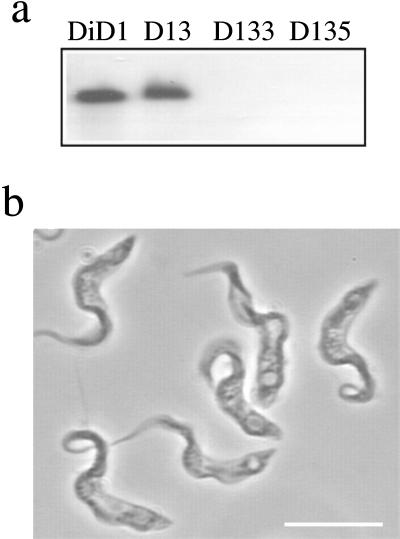
It has been proposed that bloodstream-form trypanosomes are able to differentiate inefficiently via a GPI-PLC-dependent pathway (Rolin et al., 1998). This pathway has been proposed to be stimulated in response to treatments that result in cell stress and to operate independently of the action of cis-aconitate. To establish whether the limited DiD-1 differentiation was dependent on this proposed alternative differentiation pathway, both alleles of the gene encoding GPI-PLC were deleted from the DiD-1 cells by homologous recombination using those transfection constructs previously used to delete this gene from a pleomorphic line of T. b. brucei (pLN and pLH; Webb et al., 1997). Three recombinant cell lines of DiD-1 were generated: DiD-1–3 (where one copy of the GPI-PLC gene has been replaced with a neomycin resistance gene, Δgpi-plc::NEO) and DiD-1–3.3 and DiD-1–3.5 (two independent lines in which DiD-1–3 was transfected with pLH to ablate the remaining GPI-PLC gene from the genome by selecting for both hygromycin and neomycin resistance, Δgpi-plc::NEO/Δgpi-plc::HYG). The genotype of the null mutants was then verified by Southern analysis (Figure 7a) and PCR (our unpublished results).
Figure 7.
GPI-PLC gene deletion in DiD-1. (a) Genomic Southern blots of DiD-1 cells (DI), DiD-1 cells deleted for one allele of GPI-PLC (D13), or DiD-1 cells deleted for both alleles of GPI-PLC (D133 and D135). Each was digested with BamHI and probed with the gene for GPI-PLC. (b) Development of stumpy cell characteristics after prolonged growth of the DiD-1–3.5 GPI-PLC null mutant in the bloodstream. Bar, 10 μm.
GPI-PLC gene ablation has been previously reported to reduce the virulence of a pleomorphic line of trypanosomes in mice (Webb et al., 1997). The DiD-1 GPI-PLC null mutants also showed reduced growth in mice when compared with the progenitor DiD-1 population. Thus, although the cells grew well in culture and with no obvious cellular phenotype, high doses of the null mutants were unable to establish significant infection in immunocompetent mice (<5 × 106 cells/ml 7 d after inoculation with 2 × 106 parasites). In contrast, culture-adapted DiD-1 cells and the single allele deletant DiD-1–3 were both able to establish high-level infections (>108cells/ml after 5d). Although they grew poorly in immunocompetent mice, the null mutant lines were able to generate parasitemias of >108cells/ml after 6–8 d of infection with 2 × 106 parasites when grown in mice immunosuppressed with cyclophosphamide. Strikingly, however, under this regime the GPI-PLC null mutant lines were no longer exclusively monomorphic; instead they generated variable levels (10–70%) of parasites, which noticeably resembled stumpy forms after >6 d of growth in mice (Figure 7b). Apparently the reduced virulence, and thereby extended parasitemia, associated with the deletion of the GPI-PLC genes had allowed the monomorphic DiD-1 cells to develop the expression of stumpy form characteristics.
The expression of stumpy characteristics in the DiD-1 Δgpi-plc cells provided the opportunity to directly discriminate whether the inefficient transformation of the DiD-1 cells was dependent on GPI-PLC activity, reduced ability to respond to cis-aconitate, or the retention in a small proportion of cells of some cryptic characteristic of the stumpy form essential for differentiation. In the first case we expected that DiD-1 differentiation would be retained in the absence of cis-aconitate but lost in the GPI-PLC null mutant. In the second case, we expected the inefficient differentiation to be retained in the null mutant but only in the presence of cis-aconitate. In the final case, however, we predicted that DiD-1 differentiation would be dependent on cis-aconitate and significantly enhanced when the proportion of stumpy cells was elevated in the Δgpi-plc mutant grown in vivo.
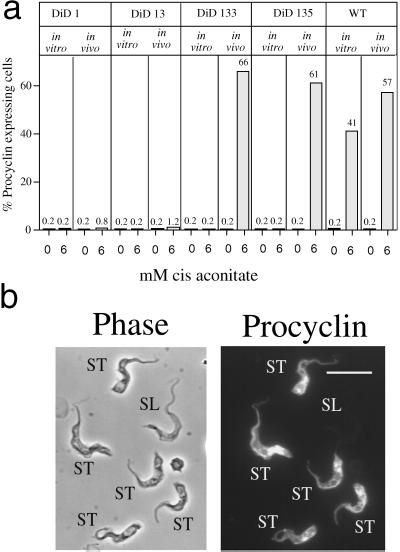
Figure 8a shows the differentiation of DiD-1 and DiD-1 Δgpi-plc cells in response to either 0 or 6 mM cis-aconitate when cells were grown in vitro (when no stumpy characteristics were expressed in the null mutant) or in vivo (when stumpy characteristics were clearly expressed). It is important to note in these experiments that all parasite lines were grown in mice immunocompromised with cyclophosphamide and that they were harvested using heparin rather than citrate as an anticoagulant to eliminate the possibility that transient exposure to this Krebs cycle intermediate was responsible for any observed differentiation.
Figure 8.
Differentiation of wild-type, DiD-1, and DiD-1 ΔGPI-PLC mutants grown in vitro and in vivo. (a) Percentage of procyclin expressers in the respective cell lines either 72 h (in vitro) or 24 h (in vivo) after incubation in differentiation conditions; the concentration of cis-aconitate used (0 or 6 mM) is shown in each case. Note that all parasites isolated in vivo were grown in mice immunocompromised with cyclophosphamide. (b) Representative image of the GPI-PLC null mutant DiD-1–3.5 cells 4 h after incubation in differentiation conditions, when bloodstream morphology is preserved. Note that those cells with a stumpy or stumpy-like morphology (ST) have initiated the expression of procyclin, whereas the morphologically slender cell (SL) does not express this marker.
When grown in vitro, the DiD-1 and GPI-PLC gene knockout lines differentiated very poorly when compared with the wild-type cells. For each cell line, <0.2% of cells expressed procyclin, regardless of the presence or absence of cis-aconitate. This confirmed that in vitro, when stumpy characteristics are not expressed, the GPI-PLC gene-deleted lines retained the DiD-1 differentiation defect. In vivo, ∼1% of DiD-1 and DiD-1–3 cells consistently expressed procyclin after 24 h in the presence of cis-aconitate, whereas in the absence of this trigger only ∼1 of 500 cells was detected expressing this marker. This very minor residual differentiation detected in the absence of cis-aconitate was not only observed in DiD-1 and DiD-1–3, however, but was also seen in the Δgpi-plc null mutants DiD-1–33 and DiD-1–35. This demonstrated that the limited differentiation of DiD-1 was almost exclusively cis-aconitate dependent, and that the residual differentiation in the absence of this trigger was not GPI-PLC dependent.
When the Δgpi-plc null mutant cells were grown in vivo and expressed stumpy characteristics, a striking result was obtained. In this case, the Δgpi-plc cells differentiated to high level in the presence of cis-aconitate, even exceeding that of the wild-type control (>60% procyclin-positive cells at 24 h in DiD-1–33 and DiD-1–35 compared with 57% positive cells in the control). Furthermore, the kinetics of this differentiation were accelerated, with procyclin expression being clearly detectable within 4 h. Figure 8b shows a representation of these cells at 4 h, when the bloodstream morphology of the differentiating population was retained. Significantly, examination of these cells demonstrated that the earliest cells to initiate differentiation in this population were those expressing the stumpy or stumpy-like morphology, whereas those cells retaining a slender, monomorphic morphology remained undifferentiated. Apparently, therefore, the expression of stumpy characteristics permitted by attenuated growth in the bloodstream can restore the capacity for efficient differentiation to DiD-1 cells. We conclude that the DiD-1 cells are disabled in their ability to differentiate through reduced ability to express a stumpy characteristic present in the initial monomorphic population.
DISCUSSION
In the bloodstream of mammalian hosts, African trypanosomes exist either as proliferative slender forms or as nonproliferative stumpy forms. Since the earliest studies of the trypanosome life cycle nearly 100 years ago, there has been close examination of the factors that influence the respective ability of these forms to infect their tsetse fly vector. Robertson (1912) reported the morphological heterogeneity of the bloodstream trypanosome population and suggested that the stumpy-form cells might be preadapted for fly transmission. Subsequently, Wijers and Willett (1960) and Ashcroft (1960) performed a direct comparison of the infectivity for the tsetse of either slender or stumpy cells and concluded that the stumpy cell was most readily transmitted, whereas slender cells failed to establish tsetse infections. With the development of suitable in vitro differentiation conditions, however, the situation has become less straightforward. In particular it has been found that monomorphic lines, which cannot generate stumpy cells at a detectable level, can differentiate efficiently, although asynchronously. In consequence, it has been suggested that stumpy cells are not obligatory for differentiation to the procyclic form (Bass and Wang, 1991).
Here we have exploited the ability of monomorphic cells either to differentiate to the procyclic form or to continue division in the bloodstream to isolate trypanosome lines that are defective in life cycle progression. Our approach was to select for trypanosomes unable to shed their VSG coat during differentiation and then to exploit the existing detailed knowledge of the temporal order of events during this transition to identify where and why these cells failed in the normal developmental program. This regime successfully isolated a number of differentiation-defective trypanosome clones of which one was selected for study in detail. By the application of a large array of well-defined molecular and cytological markers for differentiation processes, we have been able to establish that this line 1) fails very early in the differentiation program, 2) is indistinguishable from wild-type cells in the bloodstream in terms of cell cycle progression and morphology, and 3) is specifically disabled in its ability to express a stumpy characteristic essential for differentiation.
The ability to initiate the differentiation to the procyclic form is believed to be dependent on both cell cycle position and responsiveness of cells to the presence of a differentiation signal. However, analysis of several characteristics of the DiD-1 population failed to detect any cell cycle defects. Although these cells are monomorphic and do not generate morphologically stumpy forms, we found that both the wild-type population and the differentiation-defective DiD-1 population were able to modulate cell cycle progression in the bloodstream. Thus, although both populations are capable of killing a rodent host within 3–5 d, these cells accumulated in the G1 phase of their cell cycle at high parasitemia and did not generate a large number of cells with aberrant karyotypes, a characteristic of nonphysiological cell cycle inhibition (Vassella et al., 1997). This ability to modulate cell cycle progression has also been observed by Ashcroft (1960), who found that a monomorphic derivative of T. b. rhodesiense accumulated in a nondividing stage at high parasitemia even after 20 y of passage through laboratory animals. Monomorphism is therefore not necessarily associated with loss of growth control in the bloodstream, and the differentiation defect was not due to a failure to accumulate in the G1 phase of the cell cycle.
The DiD-1 defect was found not to be absolute; instead a small proportion of cells were consistently seen as becoming procyclin-positive within 24 h of exposure to cis-aconitate. We investigated whether this limited differentiation operated via a proposed GPI-PLC-dependent pathway by generating null mutants for the GPI-PLC gene in the DiD-1 cell background. Although a null mutant for this gene has been previously isolated by transfection of procyclic-form trypanosomes followed by cyclical transmission through tsetse flies, an inability to directly generate null mutants for this gene in S427 monomorphic bloodstream trypanosomes has been reported (Ochatt et al., 1999). However, using those same transfection constructs as Webb et al. (1997), we were successful in isolating GPI-PLC null mutants in the DiD-1 population, demonstrating that this gene is not essential in monomorphic bloodstream form trypanosomes. Cell line- or construct-specific differences might account for this apparent discrepancy.
Evaluation of the ability of DiD-1 and DiD-1 Δgpi-plc cells to express procyclin demonstrated that the activity of GPI-PLC was not a significant contributor to their differentiation under our experimental conditions. Indeed, we have attempted several procedures that might stimulate a GPI-PLC-mediated differentiation response in cells grown in vitro (mild acid treatment, extended parasite manipulation, calcium ionophores, and calphostin C; Voorheis et al., 1982; Rolin et al., 1996, 1998) but could not elicit detectable differentiation by these means: a low level of differentiation was always observed in the absence of cis-aconitate, but this was irrespective of presence of the GPI-PLC gene (our unpublished observations).
Although GPI-PLC activity did not account for the limited ability of DiD-1 cells to differentiate, the mutation of this gene was highly informative in determining the basis of the DiD-1 defect. Specifically, the null mutants exhibited reduced virulence in mice, a consequence of which was the development of cells that morphologically resembled stumpy cells in the bloodstream. Significantly, these cells were now fully competent to transform to procyclic cells, indicating that the DiD-1 cells are not defective in differentiation per se. Instead, loss of a characteristic specific to the stumpy form was implicated as the basis of their differentiation defect.
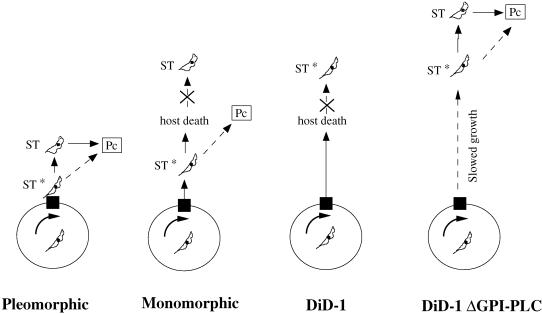
Figure 9 shows a model relating the ability to differentiate with the phenotypes of pleomorphic, monomorphic, and differentiation-defective DiD-1 cells. In this model, cells accumulate in the G1 phase of the cell cycle in response to cell density in the bloodstream, presumably this being induced by a proposed stumpy induction factor (Vassella et al., 1997). However, accumulation in G1 of the cell cycle is not sufficient to elicit competence to differentiate; instead cells must progress to a form that does not have a stumpy morphology but that has acquired some characteristic of that form essential for differentiation (ST*). This may represent progression from G1 into G0 or acquisition of the ability to detect the differentiation signal. For pleomorphic cells, passage to this differentiation-competent state would be followed by development to the stumpy form, whereas in monomorphic cells, the trypanosomes become competent to differentiate, but their ability to undergo morphological transformation is reduced and superseded by host death. In the DiD-1 population, the ability to achieve the differentiation-competent state has been reduced in vivo (although they retain the ability to generate morphologically stumpy-like forms when cultured in vitro in the presence of difluoromethylornithine; our unpublished observations). Nevertheless, when the parasitemia in the bloodstream is extended by Δgpi-plc-mediated growth attenuation, these cells retain the ability to reach the ST* form and can develop toward the stumpy cell morphology. The important consequences of this model are that slender cells are not competent to differentiate unless they at least initiate transformation to the stumpy form and that the achievement of the differentiation-competent state precedes detectable morphological transformation to the stumpy form. The isolation of the DiD-1, which is morphologically and biochemically indistinguishable from monomorphic wild-type cells and differs only in its capacity for differentiation, provides an excellent tool to identify the key molecules responsible for the initiation of transformation to the procyclic form.
Figure 9.
Model for the ability of bloodstream cells to differentiate based on their progression toward a differentiation-competent ST* state. For each cell type (pleomorphic, monomorphic, and differentiation-defective DiD-1; Did-1ΔGPI-PLC) the circle represents the cell cycle of the trypanosome, with the black box indicating the receptive window of the cell cycle at which parasites must accumulate to be able to differentiate. Cells must then progress toward an ST* state before transformation to procyclic cells either directly or via the morphologically stumpy form.
The evaluation of the basis of the failure to differentiate in vitro is complicated by our absence of any clear understanding of what triggers operate in the tsetse fly. Analyses of the ability of DiD-1 cells to infect tsetse flies have found that these cells are able to establish midgut infections at frequencies similar to those of the wild-type monomorphic population, indicating that an efficient differentiation in vitro does not necessarily reflect the efficiency of infectivity for the fly (M. Tasker, C.M.R. Turner, and K. Matthews, unpublished results). It may be, therefore, that trypanosomes possess multiple redundant pathways by which differentiation can be successfully initiated, or that a very few transforming cells may be sufficient for successful colonization of the fly. Nevertheless, the ability to compare in vitro the efficient response of bloodstream trypanosomes to cis-aconitate with the severe disability of this response in the DiD-1 line provides a valuable approach to dissecting the molecular and biochemical processes that underlie this transition.
In summary, our selection regimen has resulted in the isolation of bloodstream-form trypanosomes that have lost the capacity to differentiate efficiently to the procyclic form. We have defined the characteristics of one of these cell lines and found that it cannot differentiate because it has reduced ability to respond to cis-aconitate. Null mutants for the GPI-PLC gene have been generated from this cell line, thereby effectively ablating a second proposed route for the initiation of differentiation and revealing the relative importance of each of these respective differentiation pathways. Comparative analysis of the phenotype of these respective lines has indicated that the expression of a stumpy characteristic is a key event in regulating the ability of bloodstream cells to differentiate to the procyclic form. Molecular analysis of these well-defined cell lines will now provide an excellent approach to identifying key regulators in the differentiation process, and, moreover, the defective lines provide valuable indicator strains in which genes that are candidates for a role in life cycle progression can be tested. The selection and analysis of differentiation-defective trypanosomes have, therefore, provided highly valuable new routes to understanding the mechanisms of the control and coordination of important events in the trypanosome life cycle.
ACKNOWLEDGMENTS
We thank Drs. Iain Hagan, Mark Carrington, Ricky van Deursen, and Mark Timms for critical reading of the manuscript. Also, we thank Drs. Mark Carrington and Helena Webb (University of Cambridge) for the gift of GPI-PLC gene deletion constructs, Dr. Mike Turner (University of Glasgow) for VSG GUTat 7.2 antisera, Prof. Thomas Seebeck (University of Bern) for PEPCK antisera, and Prof. R. Luise Krauth-Siegel (University of Heidelberg) for DHLADH antisera. This work was supported by a project grant from the Royal Society and by the Wellcome Trust. M.T. was funded by the Biotechnology and Biological Sciences Research Council. M.S. was funded by the Dr. Hadwen Trust for Humane Research. K.M. is a Dunkerly Fellow.
Abbreviations used:
- BrdU
5′-bromo-2′-deoxyuridine
- cox
cytochrome oxidase c
- DHLADH
dihydrolipoamide dehydrogenase
- DiD-1
defective in differentiation clone 1
- EATRO
East African Trypanosomiasis Research Organization
- GPI-PLC
glycophosphatidyl inositol-phospholipase C
- GUTat
Glasgow University Trypanozoon antigen type
- K
kinetoplast
- N
nucleus
- nt
nucleotides
- PEPCK
phosphoenolpyruvate carboxykinase
- VSG
variable surface glycoprotein
REFERENCES
- Ashcroft MT. A comparison between a syringe passaged and a tsetse fly transmitted line of a strain of Trypanosoma rhodesiense. Ann Trop Med Parasitol. 1960;54:44–70. doi: 10.1080/00034983.1960.11685955. [DOI] [PubMed] [Google Scholar]
- Bangs JD, Ransom DM, McDowell MA, Brouch EM. Expression of bloodstream variant surface glycoproteins in procyclic stage Trypanosoma brucei: role of GPI anchors in secretion. EMBO J. 1997;16:4285–4294. doi: 10.1093/emboj/16.14.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass KE, Wang CC. The in vitro differentiation of pleomorphic Trypanosoma brucei from bloodstream into procyclic form requires neither intermediary nor short-stumpy stage. Mol Biochem Parasitol. 1991;44:261–270. doi: 10.1016/0166-6851(91)90012-u. [DOI] [PubMed] [Google Scholar]
- Bass KE, Wang CC. Transient inhibition of protein synthesis accompanies differentiation of Trypanosoma brucei from bloodstream to procyclic forms. Mol Biochem Parasitol. 1992;56:129–140. doi: 10.1016/0166-6851(92)90160-l. [DOI] [PubMed] [Google Scholar]
- Brown RC, Evans DA, Vickerman K. Changes in oxidative metabolism and ultrastructure accompanying differentiation of the mitochondrion in Trypanosoma brucei. Int JParasitol. 1973;3:691–704. doi: 10.1016/0020-7519(73)90095-7. [DOI] [PubMed] [Google Scholar]
- Brun R, Schonenberger M. Cultivation and in vitro cloning of procyclic culture forms of Trypanosoma brucei in a semi-defined medium. Acta Trop. 1979;36:289–292. [PubMed] [Google Scholar]
- Bulow R, Nonnengasser C, Overath P. Release of the variant surface glycoprotein during differentiation of bloodstream to procyclic forms of Trypanosoma brucei. Mol Biochem Parasitol. 1989;32:85–92. doi: 10.1016/0166-6851(89)90132-1. [DOI] [PubMed] [Google Scholar]
- Carruthers VB, Cross GAM. High-efficiency clonal growth of bloodstream- and insect-form Trypanosoma brucei on agarose plates. Proc Natl Acad Sci USA. 1992;89:8818–8821. doi: 10.1073/pnas.89.18.8818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cross GAM, Wirtz LE, Navarro M. Regulation of vsg expression site transcription and switching in Trypanosoma brucei. Mol Biochem Parasitol. 1998;91:77–91. doi: 10.1016/s0166-6851(97)00186-2. [DOI] [PubMed] [Google Scholar]
- Czichos J, Nonnengasser C, Overath P. Trypanosoma brucei: cis-aconitate and temperature reduction as triggers of synchronous transformation of bloodstream to procyclic trypomastigotes in vitro. Exp Parasitol. 1986;62:283–291. doi: 10.1016/0014-4894(86)90033-0. [DOI] [PubMed] [Google Scholar]
- Fairbairn H, Culwick A. The modification of Trypanosoma rhodesiense on prolonged syringe passage. Ann Trop Med Parasitol. 1947;41:26. doi: 10.1080/00034983.1947.11685308. [DOI] [PubMed] [Google Scholar]
- Ferrante A, Allison AC. Alternative pathway activation of complement by African trypanosomes. Parasite Immunol. 1983;5:491–498. doi: 10.1111/j.1365-3024.1983.tb00763.x. [DOI] [PubMed] [Google Scholar]
- Fox JA, Duszenko M, Ferguson MA, Low MG, Cross GA. Purification and characterization of a novel glycan-phosphatidylinositol-specific phospholipase C from Trypanosoma brucei. J Biol Chem. 1986;261:15767–15771. [PubMed] [Google Scholar]
- Graham SV, Matthews KR, Shiels PG, Barry JD. Distinct, developmental stage-specific activation mechanisms of trypanosome VSG genes. Parasitology. 1990;101:361–367. doi: 10.1017/s0031182000060558. [DOI] [PubMed] [Google Scholar]
- Hereld D, Hart GW, Englund PT. cDNA encoding the glycosyl-phosphatidylinositol-specific phospholipase C of Trypanosoma brucei. Proc Natl Acad Sci USA. 1988;85:8914–8918. doi: 10.1073/pnas.85.23.8914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hereld D, Krakow JL, Bangs JD, Hart GW, Englund PT. A phospholipase C from Trypanosoma brucei which selectively cleaves the glycolipid on the variant surface glycoprotein. J Biol Chem. 1986;261:13813–13819. [PubMed] [Google Scholar]
- Hirumi H, Hirumi K. Continuous cultivation of Trypanosoma brucei blood stream forms in a medium containing a low concentration of serum protein without feeder cell layers. J Parasitol. 1989;75:985–989. [PubMed] [Google Scholar]
- Lanham SM, Godfrey DG. Isolation of salivarian trypanosomes from man and other animals on DEAE cellulose. Exp Parasitol. 1970;28:521–532. doi: 10.1016/0014-4894(70)90120-7. [DOI] [PubMed] [Google Scholar]
- Lohrer H, Krauth-Siegel RL. Purification and characterization of lipoamide dehydrogenase from Trypanosoma cruzi. Eur J Biochem. 1990;194:863–869. doi: 10.1111/j.1432-1033.1990.tb19480.x. [DOI] [PubMed] [Google Scholar]
- Lujan HD, Mowatt MR, Nash TE. Mechanisms of Giardia lamblia differentiation into cysts. Microbiol Mol Biol Rev. 1997;61:294–307. doi: 10.1128/mmbr.61.3.294-304.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews KR. Developments in the differentiation of T. brucei. Parasitol Today. 1999;15:76–80. doi: 10.1016/s0169-4758(98)01381-7. [DOI] [PubMed] [Google Scholar]
- Matthews KR, Gull K. Evidence for an interplay between cell cycle progression and the initiation of differentiation between life cycle forms of African trypanosomes. J Cell Biol. 1994;125:1147–1156. doi: 10.1083/jcb.125.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews KR, Gull K. Commitment to differentiation and cell cycle re-entry are coincident but separable events in the transformation of African trypanosomes from their bloodstream to their insect form. J Cell Sci. 1997;110:2609–2618. doi: 10.1242/jcs.110.20.2609. [DOI] [PubMed] [Google Scholar]
- Matthews K, Gull K. Identification of stage specific and differentiation enriched transcripts during transformation of the African trypanosomes from its bloodstream to procyclic form. Mol Biochem Parasitol. 1998;95:81–95. doi: 10.1016/s0166-6851(98)00100-5. [DOI] [PubMed] [Google Scholar]
- Matthews KR, Sherwin T, Gull K. Mitochondrial genome repositioning during the differentiation of the African trypanosome between life cycle forms is microtubule mediated. J Cell Sci. 1995;108:2231–2239. doi: 10.1242/jcs.108.6.2231. [DOI] [PubMed] [Google Scholar]
- Mottram JC. cdc2-related protein kinases and cell cycle control in trypanosomatids. Parasitol Today. 1994;10:253–257. doi: 10.1016/0169-4758(94)90136-8. [DOI] [PubMed] [Google Scholar]
- Mowatt MR, Clayton CE. Developmental regulation of a novel repetitive protein of Trypanosoma brucei. Mol Cell Biol. 1987;7:2838–2844. doi: 10.1128/mcb.7.8.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochatt CM, Butikafer P, Navarro M, Wirtz E, Boschung M, Armah D, Cross GA. Conditional expression of glycosylphosphatidylinositol phospholipase C in Trypanosoma brucei. Mol Biochem Parasitol. 1999;103:35–48. doi: 10.1016/s0166-6851(99)00111-5. [DOI] [PubMed] [Google Scholar]
- Overath P, Czichos J, Haas C. The effect of citrate/cis-aconitate on oxidative metabolism during transformation of Trypanosoma brucei. Eur J Biochem. 1986;160:175–182. doi: 10.1111/j.1432-1033.1986.tb09955.x. [DOI] [PubMed] [Google Scholar]
- Parent CA, Devreotes PN. Molecular genetics of signal transduction in Dictyostelium. Annu Rev Biochem. 1996;65:411–440. doi: 10.1146/annurev.bi.65.070196.002211. [DOI] [PubMed] [Google Scholar]
- Pays E, Coquelet H, Pays A, Tebabi P, Steinert M. Trypanosoma brucei: posttranscriptional control of the variable surface glycoprotein gene expression site. Mol Cell Biol. 1989;9:4018–4021. doi: 10.1128/mcb.9.9.4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pays E, Hanocq-Quertier J, Hanocq F, Van Assel S, Nolan D, Rolin S. Abrupt RNA changes precede the first cell division during the differentiation of Trypanosoma brucei bloodstream forms into procyclic forms in vitro. Mol Biochem Parasitol. 1993;61:107–114. doi: 10.1016/0166-6851(93)90163-r. [DOI] [PubMed] [Google Scholar]
- Rasmussen L, Christensen ST, Schousboe P, Wheatley DN. Cell survival and multiplication—the overriding need for signals: from unicellular to multicellular systems. FEMS Microbiol Lett. 1996;137:123–128. doi: 10.1016/0378-1097(96)00053-5. [DOI] [PubMed] [Google Scholar]
- Richardson JP, Beecroft RP, Tolson DL, Liu MK, Pearson TW. Procyclin: an unusual immunodominant glycoprotein surface antigen from the procyclic stage of African trypanosomes. Mol Biochem Parasitol. 1988;31:203–216. doi: 10.1016/0166-6851(88)90150-8. [DOI] [PubMed] [Google Scholar]
- Robertson M. Notes on the polymorphism of Trypanosoma gambiense in the blood and its relation to the exogenous cycle in Glossina palpalis. Proc R Soc Lond B Biol Sci. 1912;85:241–539. [Google Scholar]
- Roditi I, Carrington M, Turner M. Expression of a polypeptide containing a dipeptide repeat is confined to the insect stage of Trypanosoma brucei. Nature. 1987;325:272–274. doi: 10.1038/325272a0. [DOI] [PubMed] [Google Scholar]
- Roditi I, et al. Procyclin gene expression and loss of the variant surface glycoprotein during differentiation of Trypanosoma brucei. J Cell Biol. 1989b;108:737–746. doi: 10.1083/jcb.108.2.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolin S, Hanocq-Quertier J, Paturiaux-Hanocq F, Nolan D, Salmon D, Webb H, Carrington M, Voorheis P, Pays E. Simultaneous but independent activation of adenylate cyclase and glycosylphosphatidylinositol-phospholipase C under stress conditions in Trypanosoma brucei. J Biol Chem. 1996;271:10844–10852. doi: 10.1074/jbc.271.18.10844. [DOI] [PubMed] [Google Scholar]
- Rolin S, Hanocq-Quertier J, Paturiaux-Hanocq F, Nolan DP, Pays E. Mild acid stress as a differentiation trigger in Trypanosoma brucei. Mol Biochem Parasitol. 1998;93:251–262. doi: 10.1016/s0166-6851(98)00046-2. [DOI] [PubMed] [Google Scholar]
- Seebeck T, Kung V, Wyler T, Muller M. A 60-kDa cytoskeletal protein from Trypanosoma brucei brucei can interact with membranes and with microtubules. Proc Natl Acad Sci USA. 1988;85:1101–1104. doi: 10.1073/pnas.85.4.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro SZ, Naessens J, Liesegang B, Moloo SK, Magondu J. Analysis by flow cytometry of DNA synthesis during the life cycle of African trypanosomes. Acta Trop. 1984;41:313–323. [PubMed] [Google Scholar]
- Sherwin T, Gull K. The cell division cycle of Trypanosoma brucei brucei: timing of event markers and cytoskeletal modulations. Philos Trans R Soc Lond B Biol Sci. 1989;323:573–88. doi: 10.1098/rstb.1989.0037. [DOI] [PubMed] [Google Scholar]
- Stojdl DF, Clarke MW. Trypanosoma brucei: analysis of cytoplasmic Ca2+ during differentiation of bloodstream stages in vitro. Exp Parasitol. 1996;83:134–146. doi: 10.1006/expr.1996.0057. [DOI] [PubMed] [Google Scholar]
- Turner CM, Barry JD, Vickerman K. Independent expression of the metacyclic and bloodstream variable antigen repertoires of Trypanosoma brucei rhodesiense. Parasitology. 1986;92:67–73. doi: 10.1017/s0031182000063459. [DOI] [PubMed] [Google Scholar]
- Tyler KM, Matthews KR, Gull K. The bloodstream differentiation-division of Trypanosoma brucei studied using mitochondrial markers. Proc R Soc Lond B Biol Sci. 1997;264:1481–1490. doi: 10.1098/rspb.1997.0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhamme L, Berberof M, Le R-D, Pays E. Stimuli of differentiation regulate RNA elongation in the transcription units for the major stage-specific antigens of Trypanosoma brucei. Nucleic Acids Res. 1995;23:1862–1869. doi: 10.1093/nar/23.11.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassella E, Boshart M. High-molecular-mass agarose matrix supports growth of blood-stream forms of pleomorphic Trypanosoma brucei strains in axenic culture. Mol Biochem Parasitol. 1996;82:91–105. doi: 10.1016/0166-6851(96)02727-2. [DOI] [PubMed] [Google Scholar]
- Vassella E, Reuner B, Yutzy B, Boshart M. Differentiation of African trypanosomes is controlled by a density sensing mechanism which signals cell cycle arrest via the cAMP pathway. J Cell Sci. 1997;110:2661–2671. doi: 10.1242/jcs.110.21.2661. [DOI] [PubMed] [Google Scholar]
- Vickerman K. Polymorphism and mitochondrial activity in sleeping sickness. Nature. 1965;208:762–766. doi: 10.1038/208762a0. [DOI] [PubMed] [Google Scholar]
- Vickerman K. Developmental cycles and biology of pathogenic trypanosomes. Br Med Bull. 1985;41:105–114. doi: 10.1093/oxfordjournals.bmb.a072036. [DOI] [PubMed] [Google Scholar]
- Voorheis HP, Bowles DJ, Smith GA. Characteristics of the release of the surface-coat protein from bloodstream forms of Trypanosoma brucei. J Biol Chem. 1982;257:2300–2304. [PubMed] [Google Scholar]
- Webb H, Carnall N, Vanhamme L, Rolin S, VandenAbbeele J, Welburn S, Pays E, Carrington M. The GPI-phospholipase C of Trypanosoma brucei is nonessential but influences parasitemia in mice. J Cell Biol. 1997;139:103–114. doi: 10.1083/jcb.139.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks G, Weijer CJ. The Dictyostelium cell-cycle and its relationship to differentiation. FEMS Microbiol Lett. 1994;124:123–130. doi: 10.1016/0378-1097(94)90239-9. [DOI] [PubMed] [Google Scholar]
- Wijers DJB, Willett KC. Factors that may influence the infection rate of Glossina palpalis with Trypanosoma gambiense: II. The number and morpholgy of the trypanosomes present in the blood of the host at the time of the infected feed. Ann Trop Med Parasitol. 1960;54:341–350. [PubMed] [Google Scholar]
- Woodward R, Gull K. Timing of nuclear and kinetoplast DNA replication and early morphological events in the cell cycle of Trypanosoma brucei. J Cell Sci. 1990;95:49–57. doi: 10.1242/jcs.95.1.49. [DOI] [PubMed] [Google Scholar]
- Ziegelbauer K, Quinten M, Schwarz H, Pearson TW, Overath P. Synchronous differentiation of Trypanosoma brucei from bloodstream to procyclic forms in vitro. Eur JBiochem. 1990;192:373–378. doi: 10.1111/j.1432-1033.1990.tb19237.x. [DOI] [PubMed] [Google Scholar]
- Ziegelbauer K, Stahl B, Karas M, Stierhof YD, Overath P. Proteolytic release of cell surface proteins during differentiation of Trypanosoma brucei. Biochemistry. 1993;32:3737–3742. doi: 10.1021/bi00065a028. [DOI] [PubMed] [Google Scholar]
- Zilberstein D, Shapira M. The role of pH and temperature in the development of Leishmania parasites. Annu Rev Microbiol. 1994;48:449–470. doi: 10.1146/annurev.mi.48.100194.002313. [DOI] [PubMed] [Google Scholar]