Abstract
Maize (Zea mays) stem gravitropism involves differential elongation of cells within a highly specialized region, the stem internodal pulvinus. In the present study, we investigated factors that control gravitropic responses in this system. In the graviresponding pulvinus, hexose sugars (d-Glc and d-Fru) accumulated asymmetrically across the pulvinus. This correlated well with an asymmetric increase in acid invertase activity across the pulvinus. Northern analyses revealed asymmetric induction of one maize acid invertase gene, Ivr2, consistent with transcriptional regulation by gravistimulation. Several lines of evidence indicated that auxin redistribution, as a result of polar auxin transport, is necessary for gravity-stimulated Ivr2 transcript accumulation and differential cell elongation across the maize pulvinus. First, the auxin transport inhibitor, N-1-naphthylphthalamic acid, inhibited gravistimulated curvature and Ivr2 transcript accumulation. Second, a transient gradient of free indole-3-acetic acid (IAA) across the pulvinus was apparent shortly after initiation of gravistimulation. This temporarily free IAA gradient appears to be important for differential cell elongation and Ivr2 transcript accumulation. This is based on the observation that N-1-naphthylphthalamic acid will not inhibit gravitropic responses when applied to pulvinus tissue after the free IAA gradient peak has occurred. Third, IAA alone can stimulate Ivr2 transcript accumulation in non-gravistimulated pulvini. The gravity- and IAA-stimulated increase in Ivr2 transcripts was sensitive to the protein synthesis inhibitor, cycloheximide. Based on these results, a two-phase model describing possible relationships between gravitropic curvature, IAA redistribution, and Ivr2 expression is presented.
Maintaining plant organs with respect to the gravity vector is crucial for proper plant development. Plants use the gravity vector as a cue to orient shoots and roots, positioning leaves for maximum light and roots for maximum water and nutrient uptake (Chen et al., 1999). Plants are able to perceive a change in the direction of the gravity vector. Signal transduction pathway(s) transmit this information, resulting in a differential growth response that returns the plant back to its normal position relative to the gravity vector. Using a genetic approach, some of the early key components of gravitropism have been elucidated (Chen et al., 1999). However, the pathways and mechanisms involved in gravity perception and signal transduction are still not very well understood.
Grass shoots have recently emerged as an excellent model system for studying gravitropism signaling events and responses. In grass shoots, gravitropism occurs at a specific region called the pulvinus. In maize (Zea mays), the focus of this study, the pulvinus is located at the base of the internode (Collings et al., 1998). Surrounding the vascular bundles within the maize pulvinus are several layers of bundle sheath cells. These cells contain starch-filled, sedimentable amyloplasts and they remain immature and unexpanded. Upon reorientation of maize stems relative to the gravity vector, amyloplasts within the bundle sheath cells sediment to the new physical cell bottom, indicating the new position of the gravity vector. Amyloplast resedimentation has been proposed to be the primary gravity sensing mechanism in plants (Kiss, 2000). Statolith sedimentation triggers a cascade of signal transduction events that leads to differential growth across the pulvinus, thus facilitating the return of the plant to an upright orientation. In mature maize shoots, typically three or four pulvini respond, each pulvinus reaching a maximum of 30° upward curvature, returning the maize shoot to a vertical position within 6 d (Collings et al., 1998).
Maize and oat (Avena sativa) pulvini have been used to answer specific questions in gravitropism (Brock et al., 1992; Chang and Kaufman, 2000; Kim and Kaufman, 1995; Collings et al., 1998; Perera et al., 1999, 2001; Johannes et al., 2001). There are a number of advantages to using these systems for studying gravity signaling and responses. First, gravity perception and responses are confined to the pulvinus. Second, the pulvinus of grass shoots is a highly specialized tissue and is unresponsive to a number of other stimuli such as phototropism. Third, the pulvinus yields a greater amount of tissue compared with other systems such as Arabidopsis and maize coleoptiles. This is particularly beneficial for biochemical studies, with the additional benefit that the pulvinus can easily be divided into upper and lower halves (Winter et al., 1997; Perera et al., 1999). Finally, and perhaps most importantly, gravitropism occurs over a time scale of days (Kaufman et al., 1995; Collings et al., 1998) such that early signaling events occur slowly enough that they can be observed. The time scale of the response supports a detailed biochemical dissection of early signaling events.
In maize, some early signaling events that occur in response to gravistimulation have been characterized. Within minutes of gravistimulation, a transient increase in inositol 1,4,5-trisphosphate is observed (Perera et al., 1999) and cytosolic pH changes are evident only in the bundle sheath cells (Johannes et al., 2001). We are also beginning to understand some of the downstream biochemical responses. Winter et al. (1997) reported that Suc synthase is targeted to the plasma membrane in response to gravistimulation in maize pulvini. Suc synthase is a Suc-hydrolyzing enzyme (Winter and Huber, 2000), cleaving Suc in the presence of UDP into d-Fru and UDP-Glc. In this way, the UDP-Glc generated by Suc synthase activity can be funneled directly into the cellulose synthase complex, and can participate in cell wall expansion.
Cell elongation also requires increased solute accumulation to drive water uptake and cell expansion. In the oat leaf sheath pulvinus, differential changes in soluble acid invertase activity (Gibeaut et al., 1990) and changes in vacuolar invertase gene expression (Wu et al., 1993a, 1993b) have been documented. Soluble acid invertase is a Suc-hydrolyzing enzyme in plants localized to the vacuole (Tymowska-Lalanne and Kreis, 1998a). Invertase activity generates the soluble hexose products d-Glc and d-Fru. Differential accumulation of these solutes within the vacuole could be expected to drive water uptake and differential cell elongation in this system. Asymmetric accumulation of hexose sugars has also been implicated in grass shoot gravitropism in wheat (Triticum aestivum; Bridges and Wilkins, 1974) and maize seedlings (Momonoki, 1988). Recently, a K+ channel has been implicated in etiolated maize coleoptile gravitropism (Philippar et al., 1999). Determining the mechanism for increasing solute accumulation across the maize pulvinus was one of the aims of this study.
Perhaps the best-studied response to a gravity signal in plants is the effect on indole-3-acetic acid (IAA). The Cholodny-Went hypothesis proposes that a gradient of auxin is generated as a result of a gravity signal, stimulating a gradient of growth rate changes in cells across the tissue (Lomax, 1997). This hypothesis has been supported by studies on auxin redistribution (Lomax, 1997) and by the observation that gravistimulation results in asymmetrically induced auxin-responsive genes (McClure and Guilfoyle, 1989; Li et al., 1991; Kamada et al., 2000; Rashotte et al., 2001). In the oat pulvinus, 24 h of gravistimulation results in asymmetrical changes in free IAA levels with a 2.5-fold increase in free IAA in the lower one-half compared with the upper one-half of the oat pulvinus (Brock et al., 1991; Kaufman et al., 1995). In addition, gravistimulation resulted in asymmetry of auxin in maize etiolated coleoptiles (Philippar et al., 1999). However, there is no information on the effect of a gravity signal on maize pulvinus auxin dynamics. Important questions we need to answer are: What are the dynamics of free IAA across the maize pulvinus in response to a gravity signal? How do these auxin dynamics relate to some of the early signaling events previously described? What effect, if any, does auxin have on Suc metabolism in the maize pulvinus?
To begin to address these questions, a number of related gravity-stimulated events in the maize internodal pulvinus were examined. Gravistimulation-induced changes in the accumulation of soluble hexose sugars and K+, acid invertase activity, and invertase gene expression in upper and lower pulvinus halves throughout the growth response were characterized. In an effort to dissect some of the underlying molecular events regulating these gravity responses, we focused our further studies on gravistimulation-induced auxin responses. Several different approaches were used. First, using a pharmacological approach, the effect of the polar auxin transport inhibitor N-1-naphthylphthalamic acid (NPA) on gravity responses was investigated. Second, changes in free IAA concentration across the pulvinus in response to a gravity signal were monitored by gas chromatography-mass spectroscopy (GC-MS) over the first 24 h of gravistimulation. Third, to begin to determine whether IAA is sufficient alone to stimulate changes in Suc metabolism in the maize pulvinus, the effect of IAA on invertase gene expression in ungravistimulated pulvinus was monitored. The results are discussed and presented in a two-phase gravity response model. This model highlights the value of the maize stem pulvinus as a model system for dissecting gravitropism events in cereal grass shoots.
RESULTS
Gravistimulation Increases K+ Content, Acid Invertase Activity, and Ivr2 Transcript Accumulation
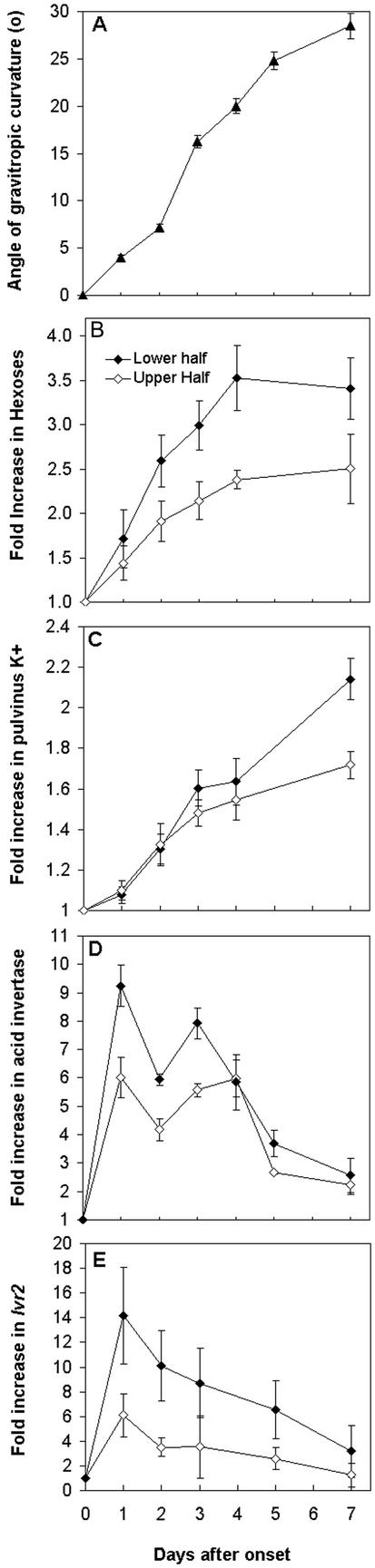
Maize plants were reoriented 90° relative to the gravity vector simply by laying plants horizontally. In maize plants of the age used in this study, the first three pulvini above soil level were competent to respond to gravity. Although the first three pulvini are gravitropic, the mean angle of curvature at the first pulvini, which was used for biochemical analyses, is shown in Figure 1A. In this study, curvature of each pulvinus reached a maximum at 6 to 7 d, with a final angle of around 30°. Therefore, total stem bending (as measured by the sum of the bending angle at each pulvinus) at the end of the time course was 90° to 100°, which resulted in a return of the stem to a vertical position (data not shown).
Figure 1.
Kinetic studies of gravitropic curvature, hexose and K+ accumulation, acid invertase activity, and Ivr2 transcript abundance in maize stem pulvini. Vertical maize plants were gravistimulated for the time indicated by displacing to a horizontal position. At each time point, angle of gravitropic curvature was measured (A) and pulvini was harvested into upper (⋄) and lower (♦) halves. Hexose sugars (B), K+ content (C), and acid invertase activity (D) were determined and values are expressed as a fold increase over the vertical control value (set to 1). Mean values (±se) from three independent experiments are shown. E, Total RNA was isolated and hybridized with an Ivr2 cDNA probe, radioactivity stripped, and the membrane was rehybridized with an 18S rRNA probe. Ivr2 and 18S hybridization signals were quantified using a densitometer, and Ivr2 expression levels in each treatment were normalized to the 18S loading control. Transcript accumulation in gravistimulated samples is expressed relative to the vertical control (set to 1). Mean values (±se) from three independent experiments are shown.
The first pulvinus above soil level was harvested and separated into upper and lower halves. The upper and lower halves were used to characterize changes in hexose and K+ content, invertase activity, and Ivr2 gene expression during maize pulvini gravitropism. As shown in Figure 1B, there was an increase in hexose content, with greater hexose accumulation in the lower one-half than the upper one-half. Hexose accumulation paralleled gravistimulated growth very closely, with hexoses accumulating up until the time when growth had slowed down. At the end of the growth response there was three to four times as much hexose sugar in the lower pulvinus one-half than in the vertical control, and roughly 2-fold more hexose sugar in the upper pulvinus one-half than in the vertical control.
K+ content was measured in upper and lower halves throughout the growth response, as shown in Figure 1C. There was no obvious asymmetry between upper and lower halves, although an increase in K+ content that closely followed growth was evident. At the end of the growth response, K+ content was 2-fold higher than in the vertical control.
Upper and lower pulvini halves were assayed for soluble acid invertase activity (Fig. 1D). After 1 d of gravistimulation there was a substantial increase in soluble acid invertase activity on both sides of the pulvinus. The increase in the lower one-half was 9-fold over that of vertical controls, and only a 6-fold effect was observed in the upper one-half of the pulvinus. Invertase activity remained high until around 5 d after reorientation, and declined to near vertical control levels at the end of the time course. In all samples taken, there was higher activity in the lower than in the upper halves. The activities of Suc synthase, cell wall, and alkaline invertases did not change during gravistimulated growth (data not shown).
To test whether there was an effect of gravistimulation on acid invertase gene expression, total RNA was isolated from upper and lower pulvini samples throughout the time course. There are currently two known maize vacuolar acid invertase genes, Ivr1 and Ivr2 (Xu et al., 1996). Northern blots were probed with an Ivr1 and Ivr2 cDNA. No signal was ever detected for Ivr1 (data not shown), suggesting that Ivr1 is expressed in this tissue at very low levels that are below detection using this northern hybridization approach. In contrast, the Ivr2 transcript was detected, and an asymmetry in expression levels was evident, with more Ivr2 accumulating in the lower one-half than in the upper one-half, as shown in Figure 1E. The increases in Ivr2 transcript were very similar to the invertase activity expression pattern.
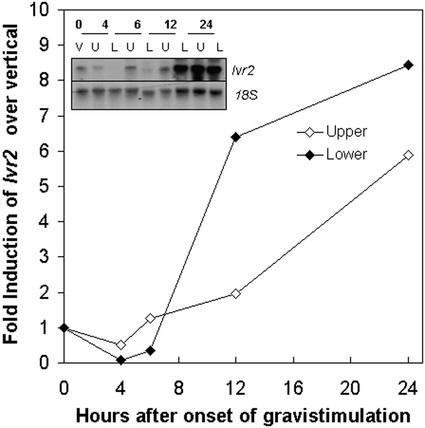
The above results suggest that asymmetrical transcriptional up-regulation of Ivr2 occurs during maize stem gravitropism. To characterize changes in Ivr2 transcript accumulation during the first 24 h, maize plants were gravistimulated and the pulvini was harvested into upper and lower halves at the times indicated in Figure 2. Total RNA was isolated, probed with Ivr2 cDNA, and the relative increase in Ivr2 over the vertical control was determined. A representative experiment is shown in Figure 2. Again, there was an asymmetric induction, with more Ivr2 accumulating in the lower than the upper one-half. Induction of Ivr2 transcript occurred at around 6 h after gravistimulation, and continued to increase in both upper and lower halves up until the last time point taken at 24 h.
Figure 2.
Time course of gravistimulated Ivr2 transcript accumulation in maize stem pulvini. Maize plants were gravistimulated for the time indicated and were harvested into upper and lower halves. Total RNA was isolated and hybridized with an Ivr2 cDNA probe, radioactivity stripped, and the membrane was rehybridized with an 18S rRNA probe. Ivr2 and 18S hybridization signals were quantified using a densitometer, and Ivr2 expression levels in each treatment were normalized to the 18S loading control. Transcript accumulation in gravistimulated samples is expressed relative to the vertical control (set to 1). The experiment was repeated at least three times and a representative result is shown with the corresponding northern (inset).
Auxin Transport and Redistribution Is Necessary for Gravistimulated Responses
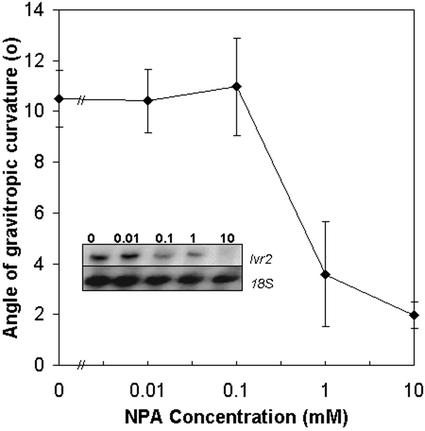
To determine whether polar auxin transport is necessary for maize stem gravitropism responses, we used a pharmacological approach. The polar auxin transport inhibitor, NPA, is the best characterized of the synthetic auxin transport inhibitors that specifically blocks auxin transport at the site of the efflux carrier (Rubery, 1990). To supply NPA to the maize pulvinus, an explant system was developed. An explant, as described in this study, encompasses a pulvinus, the associated node, the internode above, and 3 to 4 cm of the internode below. In this explant system, pharmacological inhibitors can be fed to the pulvinus via the transpiration stream. The explant was excised from the shoot immediately before each experiment. We found that explants behaved in a similar manner to intact maize stems in response to gravistimulation (data not shown). A range of NPA concentrations was supplied to the pulvinus prior to and throughout gravistimulation. After 24 h, curvature was measured and Ivr2 transcript accumulation was determined. The results are shown in Figure 3. NPA concentrations below 0.1 μm had little effect on curvature, whereas concentrations greater than 0.1 μm NPA decreased gravistimulated curvature. A representative northern blot of total RNA probed with Ivr2 cDNA is shown as an inset in Figure 3. The decrease in accumulation of Ivr2 transcripts in the presence of NPA concentrations greater than 1 μm correlates well with the inhibition of curvature.
Figure 3.
Concentration-dependent effect of NPA on gravistimulated curvature and Ivr2 transcript accumulation in maize stem pulvini. Maize explants were gravistimulated with the indicated concentration of NPA. After 24 h, angle of gravitropic curvature was measured and whole pulvini were harvested. Total RNA was isolated from pulvini tissue and was hybridized with an Ivr2 cDNA probe, radioactivity stripped, and the membrane was rehybridized with 18S rRNA probe. Mean values for gravitropic curvature (±se) from three independent experiments and a representative northern from one experiment (inset) are shown.
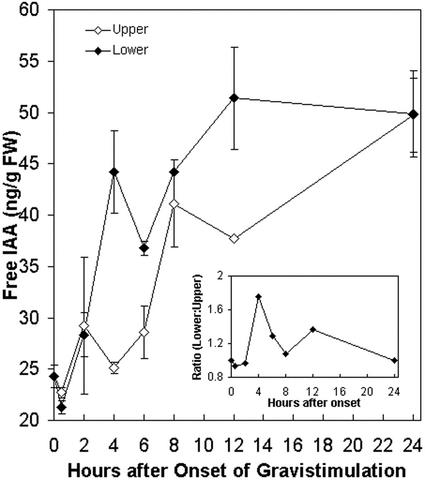
The effect of NPA shown in Figure 3 strongly suggests that auxin transport and redistribution is an important factor during maize pulvini gravitropism. Therefore, we measured total free IAA concentration in upper and lower halves of pulvini during the first 24 h of gravistimulation in intact plants by GC-MS. Free IAA is reported as a function of time after gravity stimulation in Figure 4. There was initially a slight decrease in free IAA levels in upper and lower halves by 30 min. However, there was subsequently a rapid increase in free IAA levels only in the lower one-half, which resulted in a nearly 2-fold increase in free IAA levels by 4 h. In contrast, in upper halves of pulvini there remained little change in free IAA levels until after 6 h of stimulus, whereupon free IAA levels slowly increased to about 2-fold by 24 h.
Figure 4.
Time course of free IAA concentration and distribution in maize stem pulvini during gravistimulation. Maize plants were gravistimulated for the time indicated, and upper and lower halves were harvested. Free IAA concentration was measured by GC-MS-selected ion monitoring (SIM). Mean values from two independent experiments (±se) are shown. The upper one-half sample at 12 h after onset has no error bar as we were only able to include one data point for this sample. Ratio of free IAA levels in the lower compared with the upper halves was calculated from the mean values (inset).
A plot of the ratio of free IAA between upper and lower halves after gravity stimulation is shown for the first 24 h in the inset of Figure 4. The ratio of free IAA in the lower one-half compared with the upper one-half remained unchanged over the first 2 h, and then increased over the next 2 h to peak at the 4-h time point. After this time, as free IAA levels increased in the upper one-half, the ratio of free IAA in the lower one-half compared with the upper one-half began to decrease. These dynamics over the entire 24-h period are perceived as a transient gradient of free IAA across the pulvinus early on in the response.
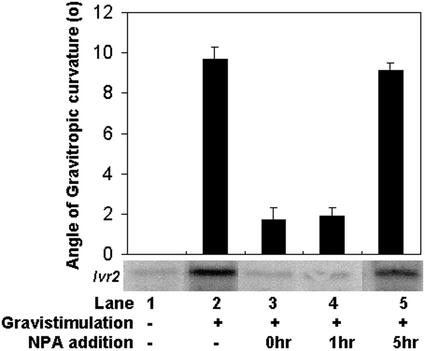
To determine whether the transient gradient in free IAA concentration across the gravity-stimulated pulvinus at 4 h was physiologically significant, we tested whether application of an inhibitory concentration of NPA would still inhibit gravitropic responses if applied after this gradient-free IAA peak. The explant system was used to supply NPA at different time points after gravity stimulation, and the resulting gravitropic bending and Ivr2 message level at 24 h is reported in Figure 5. NPA was applied immediately prior to gravistimulation (lane 3), 1 h after gravistimulation (lane 4), or 5 h after gravistimulation (lane 5). The 5-h time point was chosen to be sure that NPA was applied after the peak in auxin gradient. For comparison, vertical and untreated gravity-stimulated samples are shown in lanes 1 and 2. Application of NPA inhibited curvature and Ivr2 expression when applied prior to or 1 h after gravistimulation. However, NPA had no effect if applied at 5 h after gravistimulation.
Figure 5.
Effect of NPA at different times after onset of gravistimulation. Maize explants were held vertical in control buffer (lane 1), gravistimulated (lane 2), or gravistimulated with NPA applied before onset (lane 3), 1 h after onset (lane 4), or 5 h after onset (lane 5). After 24 h, the angle of gravitropic curvature was measured and whole pulvini were harvested. Total RNA was isolated from pulvini tissue and was hybridized with an Ivr2 cDNA probe, radioactivity stripped, and the membrane was rehybridized with an 18S rRNA probe. The experiment was repeated three times with similar results and a representative experiment is shown. Mean values for angle of gravitropic curvature (±sd) and a representative northern are shown.
IAA Stimulates Ivr2 Transcript Accumulation
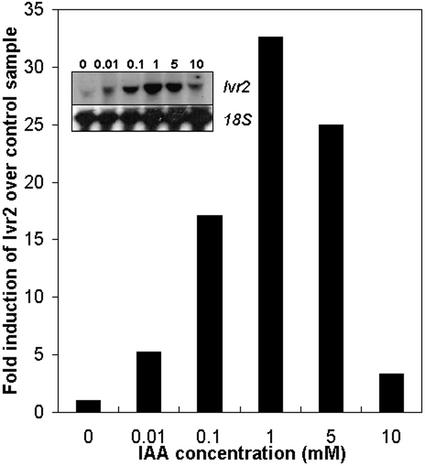
The results shown above suggested that the asymmetric increase in free IAA levels in the pulvinus could directly regulate Ivr2 transcript abundance. To determine whether Ivr2 transcripts can be up-regulated by auxin alone, vertically held maize stem explants were treated with increasing concentrations of the biologically active auxin, IAA. Ivr2 transcript abundance after 24 h was determined by northern analysis, and results from a representative experiment are shown in Figure 6. Addition of 10 μm IAA increased Ivr2 transcript accumulation 5-fold, and 100 μm IAA increased Ivr2 transcripts approximately 17-fold. Maximal induction occurred with 1 mm IAA, which increased transcript abundance over 30-fold. The IAA-response curve of Ivr2 transcripts displays a typical auxin bell-shaped curve, peaking at 1 mm, with higher concentrations inhibiting this response. We also confirmed that transcript accumulation resulted in an increase in acid invertase activity (data not shown). Kinetic studies of IAA-stimulated Ivr2 transcript accumulation showed that exogenous IAA began to increase Ivr2 approximately 4 h after addition of IAA (data not shown).
Figure 6.
IAA induces Ivr2 transcript accumulation in pulvini of vertical stem explants. Maize explants were held vertically in buffer with freshly prepared IAA at the concentration indicated. Pulvini were harvested 24 h later. Total RNA was isolated and hybridized with an Ivr2 cDNA probe, radioactivity stripped, and the membrane was rehybridized with an 18S rRNA probe. Ivr2 and 18S hybridization signals were quantified using a densitometer, and Ivr2 expression levels in each treatment were normalized to the 18S loading control. Transcript accumulation in IAA-treated samples is expressed relative to the vertical control (set to 1). The experiment was repeated at least three times and a representative result is shown with the corresponding northern (inset).
Protein Synthesis Is Required for Gravity- and Auxin-Stimulated Ivr2 Transcript Accumulation
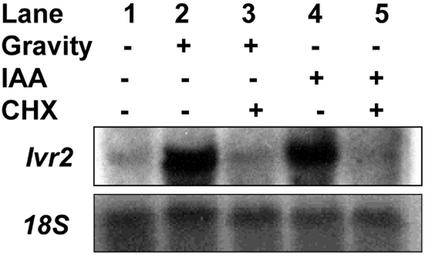
The identification of auxin responsive promoters and transcription factors is currently an area of intense research (Guilfoyle, 1999). Studies on auxin-regulated gene expression have classified genes as primary- (or early) responsive or secondary-responsive genes. Primary-responsive genes are induced within minutes of auxin application and, typically, this response does not require de novo protein synthesis. To determine if protein synthesis is required for the gravity and auxin response, the effect of the specific protein synthesis inhibitor, cycloheximide, was examined. As shown in Figure 7, pretreatment with cycloheximide abolished the ability of gravity and auxin to stimulate Ivr2 transcript accumulation. The graviresponse (bending) was also blocked by cycloheximide (data not shown). These results suggest that signal transduction pathways are required for auxin-regulated Ivr2 expression, and that Ivr2 is not a primary auxin-induced gene.
Figure 7.
Cycloheximide inhibits gravity- and IAA-induced Ivr2 transcript accumulation. Maize explants were kept vertical (lane 1), gravistimulated (lane 2), or gravistimulated with 10 μm cycloheximide (lane 3), treated with 1 mm IAA (lane 4), or treated with 1 mm IAA and 10 μm cycloheximide (lane 5). Pulvini were harvested 24 h later. Total RNA was isolated and hybridized with an Ivr2 cDNA probe, radioactivity stripped, and the membrane was rehybridized with an 18S rRNA probe. Experiment was repeated three times with similar results and a representative northern is shown.
DISCUSSION
Gravistimulated Changes in Invertase Activity and Gene Expression in the Maize Pulvinus
The mechanisms by which plants perceive and transduce signals in response to changes in reorientation of the plant to the gravity vector are poorly understood. The gravitropic response of grasses such as maize are excellent model systems for studying these responses, and in some cases are more suitable for some cellular and biochemical analysis than the model system of Arabidopsis. Although we are beginning to learn more about some of the very early events in maize gravity signaling events, there is a need to also understand the downstream responses and how they are regulated by these early signaling events.
Cell elongation requires cell wall loosening (Cosgrove, 1999) and accumulation of intracellular solutes, for example, soluble sugars or K+. Asymmetrical accumulation of hexose sugars and K+ has been implicated in gravitropism (Kaufman et al., 1995; Philippar et al., 1999). In the maize pulvinus, we observed an asymmetrical accumulation of hexose sugars upon gravistimulation (Fig. 1B), which closely follows the growth curve (Fig. 1A). Although there was also an overall increase in pulvinus K+ content, an obvious asymmetry in accumulation was less obvious. We propose that in maize pulvinus, asymmetrical hexose accumulation plays an important role in differential water uptake and cell elongation.
Hexose sugars can be generated as a result of starch breakdown or Suc hydrolysis. In the maize pulvinus, starch content did not decrease during gravitropism (H. Winter and S.C. Huber, unpublished data), suggesting that starch mobilization is not involved. It is interesting that Chang et al. (2001) recently demonstrated that starch levels within the oat shoot pulvini initially increase in response to gravistimulation during the first 8 h. This is followed by a decline in starch content over the remainder of gravitropic curvature. Therefore, it appears that maize and oat pulvini differ in the mobilization of starch during gravitropism.
However, differential accumulation of hexose sugars was correlated with an asymmetrical increase in acid invertase activity (Fig. 1D). Soluble acid invertase activity has been shown to be closely correlated with growth and cell expansion in a number of systems (Sturm and Tang, 1999), including differential growth responses involved in oat shoot gravitropism (Gibeaut et al., 1990) and apical hook opening in sunflower (Helianthus annuus) hypocotyls (Rabe and Kutschera, 1998).
The observed gravistimulated increase in acid invertase activity in maize pulvini was correlated with the increased accumulation of Ivr2 transcripts (Fig. 1E). In this study, we found that Ivr2 transcripts accumulated in response to a gravity signal after approximately 6 h, peaking maximally at around 24 h (Fig. 2). In maize, invertase activity is regulated by transcript availability in response to such diverse environmental signals as sugars (Xu et al., 1996), water stress (Pelleschi et al., 1999), and anoxia (Zeng et al., 1999). The strong correlation between invertase activity and Ivr2 transcripts in maize pulvini suggests that one of the underlying mechanisms for the increased accumulation of hexose sugars driving cell elongation and upward curvature of the maize stem is differential regulation of the vacuolar acid invertase gene, Ivr2.
Pharmacological and Biochemical Evidence for Gravistimulated Changes in Polar Auxin Transport and a Transient IAA Gradient
Although maize shoot gravitropism is becoming increasingly well described, direct studies on the role of auxin, and measurements of auxin dynamics across the pulvinus in response to a gravity signal, had not been previously reported. In this study, we describe several lines of evidence that polar auxin transport and a transient IAA gradient across the pulvinus is necessary for mediating gravitropic curvature and Ivr2 expression. First, pretreatment of maize pulvini with the polar auxin transport inhibitor, NPA, inhibited gravitropic curvature and Ivr2 expression (Fig. 3). Second, gravistimulation resulted in a rapid, but transient, asymmetry in free IAA across the pulvinus (Fig. 4). Furthermore, we found that treatment with auxin transport inhibitors after this transient gradient no longer abolished gravitropic bending (Fig. 5).
It is interesting that this time period when the transient IAA gradient is set up and peaks roughly corresponds to another important phase in maize gravitropism—the period required for “commitment to bend” (Perera et al., 1999). If a gravistimulated plant is returned to a vertical position within the first few hours after gravistimulation (prior to the time when the transient auxin gradient is established), the plant will not respond by bending to the initial gravity signal. However, once the plant has been continuously stimulated for a longer time period (a time that roughly coincides with the transient auxin gradient), the plant will bend even if it returned to the vertical position. We propose that once the transient auxin gradient is established, the plant is committed to bend.
Auxin Regulation of Suc Metabolism in Maize Pulvini?
One of the main goals of this study was to determine what effect, if any, auxin has on Suc metabolism in the maize pulvinus. As a first attempt to address this question, we examined the effect of IAA on Ivr2 transcript accumulation. We found that exogenous IAA stimulated Ivr2 transcript accumulation in ungravistimulated maize pulvini in a dose-response curve typical for IAA responses (Fig. 6). Although the optimum response for Ivr2 transcript accumulation was with a relatively high concentration of IAA compared with other systems, we would expect that this does not reflect the concentration of IAA actually in the pulvinus, but may reflect limited IAA uptake by the explants. The actual concentration would depend on how much IAA entered the tissue. Despite potential problems with IAA stability and uptake, we chose to use IAA because it is the most abundant auxin in plant tissue, and because our studies have shown that free IAA increases during gravistimulation.
In addition to our studies, acid invertase activity has been shown to be stimulated by auxin in Phaseolus vulgaris internode tissue (Morris and Arthur, 1984), strawberry (Fragaria spp.) fruits (Pooviah and Veluthambi, 1985), and in eggplant (Solanum melongena) fruit growth (Lee et al., 1997). There has also been one other report of an auxin-regulated invertase gene (Tymowska-Lalanne and Kreis, 1998b). These authors reported an increase in Arabidopsis vacuolar invertase transcript abundance in mature plants treated with the synthetic auxin, 1-naphthaleneacetic acid. In addition, transcript accumulation of genes encoding extracellular invertases have been shown to be stimulated by other plant hormones, including cytokinin (Ehness and Roitsch, 1997), jasmonic acid and abscisic acid (Zhang et al., 1996), ethylene (Linden et al., 1996), gibberellin (Wu et al., 1993c), and brassinosteroid (Goetz et al., 2000).
We found that IAA increased Ivr2 transcript abundance in maize pulvini approximately 4 h after addition to the buffer (data not shown). We also know that increased Ivr2 transcript abundance in maize pulvini by gravity stimulation also requires at least 6 h (Fig. 2). In addition, we clearly show here that gravity- and auxin-induced Ivr2 expression is sensitive to cycloheximide (Fig. 7). Therefore, de novo protein synthesis is necessary. Taken together, we propose that auxin signaling pathways are required for the gravity-stimulated Ivr2 expression, Suc metabolism, and cell elongation in maize pulvini. There has been significant progress in the area of auxin signaling in plants recently (for review, see Estelle, 1999), and future research will identify the underlying mechanisms involved.
A Two-Phase Model for Maize Stem Gravitropism
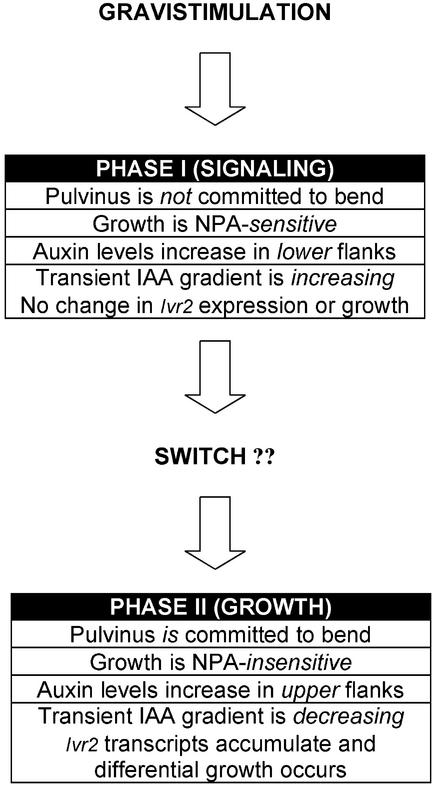
Several important issues still remain to be elucidated for maize pulvini gravitropism. Most notably absent is detailed evidence for the mechanism of graviperception in maize pulvini. IN addition, asymmetric growth has been shown to be due to asymmetric changes in the regulation of a number of cellular processes in other model systems, including cell wall loosening enzymes (Cosgrove, 1999). How these cellular processes are regulated during maize pulvini gravitropism has yet to be determined. However, based on the results presented in this study, we propose a two-phase model describing possible relationships between gravitropic curvature, IAA distribution, and Ivr2 expression during maize stem gravitropism, illustrated schematically in Figure 8. It is important to note that for reasons of clarity, the model does not attempt to include other important events in maize pulvini, such as graviperception and cell wall loosening, which have not yet been fully characterized in maize pulvini. In addition, although there are striking similarities between maize pulvini and oat pulvini, including the up-regulation of invertase gene expression during gravitropism, there are differences between the kinetics of the response (this study; Wu et al., 1993a, 1993b). Therefore, the model shown in Figure 8 is representative of studies in maize pulvini only.
Figure 8.
Two-phase model of gravistimulated auxin redistribution and growth responses during the first 24 h in maize pulvini. Upon gravistimulation of maize plants, the stem internodal pulvinus tissue enters Phase I. Growth does not occur during this phase; instead, Phase I can generally be thought of as a signaling phase. During Phase I, increases in free IAA in the lower one-half of the pulvinus result in IAA asymmetry increasing. In addition, Phase I can be inhibited by NPA. Gravistimulation of maize plants leads to a number of other changes within the pulvinus that not shown here, which would be expected to occur during Phase I. These include MAP kinase signaling, pH changes, gene expression changes, and inositol signaling (see text). A continuous gravity signal is required during phase I and, if disrupted, progression will halt. Successful progression through Phase I with a continuous gravity signal leads to an as-yet-unidentified “signal,” perhaps build-up of a sufficient IAA gradient across the pulvinus, indicated here as a “switch.” Once Phase II is entered, the pulvinus is now committed to bend, and a continuous signal is no longer necessary for cell elongation. Phase II is longer than Phase I, and can be thought of as the growth phase. Events that occur include changes in IAA redistribution and the disappearance of a gradient across the pulvinus. Invertase gene, Ivr2, is up-regulated and acid invertase activity is increased.
In this model, Phase I can be considered the signaling phase, and Phase II can be considered the growth phase. In Phase I, gravistimulation results in increased accumulation of free IAA in the lower one-half of the pulvinus shortly after gravistimulation. This is most likely due to polar auxin transport, as this phase is sensitive to NPA. As a result of the increase in free IAA in the lower pulvinus one-half, a gradient of free IAA begins to build across the pulvinus. During the early stages of Phase I, there is a lag of approximately 2 h before changes in auxin in the lower pulvinus are evident. This lag strongly suggests that auxin redistribution is not the first event in gravity responses, but more rapid changes in signaling molecules likely occur. Several recent studies have focused on such early changes in signaling events within the maize pulvinus. A rapid, transient increase in inositol 1,4,5-trisphosphate is observed within minutes of maize gravistimulation (Perera et al., 1999). Rapid changes in cytosolic pH have been shown to occur in response to gravistimulation of maize pulvinal cells (Johannes et al., 2001). Other early signaling events in maize pulvini include a differential increase in transcripts of calmodulin and calreticulin genes (I. Heilmann, J. Shin, J. Huang, I.Y. Perera, and E. Davies, unpublished data) and an early involvement of MAP kinases (A.M. Clore and R.W. Whetten, unpublished data). Perhaps some of these events are involved in regulating auxin transport. It is also possible that the actin cytoskeleton is involved in early events regulating the auxin transporter in response to a gravity signal (Muday, 2000). During Phase I, there is no change in Ivr2 expression or growth. In addition, the pulvinus is not committed to bend at this stage. In other words, if the plant is returned to the vertical position at any time during Phase I, differential growth will not occur (Perera et al., 1999). However, an uninterrupted gravity signal throughout the duration of Phase I results in some unknown change in the pulvinus, indicated in Figure 8 as a “switch.” This switch triggers events in Phase II, the growth phase. The pulvinus is now committed to bend.
Shortly after entering Phase II, free IAA begins to increase in the upper one-half, and the transient IAA gradient begins to decrease. IAA transport is no longer necessary, as Phase II is insensitive to NPA. IAA signal transduction pathways, including de novo protein synthesis, differentially stimulate Ivr2 transcripts across the pulvinus. We propose that the differential increase in invertase transcripts lead to differential acid invertase activity and accumulation of hexose sugars. Toward the end of Phase II, differential cell elongation across the pulvinus occurs.
However, this model does not satisfactorily explain long-term differential Ivr2 expression and growth, as seen in Figure 1. By the end of Phase II, the IAA gradient is no longer evident. Therefore, continued curvature must require additional events across the pulvinus to maintain differential responses. A potential candidate could be changes in auxin sensitivity across the pulvinus. Changes in auxin sensitivity have been proposed to occur differentially across tissue in response to gravitropism (Evans, 1990). Perhaps during Phase I, changes in auxin sensitivity across the pulvinus are initiated. More detailed studies in this area will be necessary to fully explain maize pulvini gravitropism.
In general, gravity stimulations experienced by plants can be classified as temporary, intermittent stimulations, such as caused by touch or wind, and permanent, continuous stimulations, such as plant lodging or reorientation (Perera et al., 1999). Only the latter class of stimulation would require a differential growth response. The ability of the plant to differentiate between these two different gravity signals is certainly important and could prevent an unnecessary, and metabolically expensive, growth response. A two-phase gravitropism response could potentially be important in this process.
MATERIALS AND METHODS
Plant Growth and Experimental Treatments
Maize (Zea mays L. cv Pioneer 3183, Des Moines, IA) plants were grown in 20-cm pots, four per pot, in a greenhouse. During the winter months, the photoperiod was extended to 14 h, with supplemental lighting in the absence of sunlight. Plants were fertilized three times weekly with modified Hoagland solution. In all experiments, maize plants were typically 4 to 6 weeks old (spring-summer) or 6 to 8 weeks old (fall-winter). In these plants, the pulvinus associated with the first node above the soil and the two pulvini above are gravitropically competent. The first pulvinus above the soil was typically used in all the studies.
Gravistimulation of maize plants was carried out in the greenhouse by displacing the maize pot onto its side so that the maize stems were reoriented 90° from a vertical to a horizontal position. The pulvinus was excised from the stem at the time indicated in the figure, divided into upper and lower halves, and harvested into liquid nitrogen.
Explants of maize stems as described in this study, encompass the pulvinus and its associated node, the internode above, and 3 to 4 cm of the internode below. Explants were excised from the stem and placed in a beaker containing an explant buffer of 100 mm Suc, 5 mm MES [2-(N-morpholino)ethanesulfonic acid]-NaOH, pH 5.5, with the basal end of the explant in contact with the buffer, taking care to maintain a vertical gravity vector at all times. Buffer level was maintained below the pulvinus. Explants were then transferred to a controlled-environment growth chamber (25°C) for the remainder of the treatment with a 12-h light:12-h dark cycle with light supplied from above and below. After a 2-h pretreatment, explants were transferred to fresh buffer (for vertical controls) or were gravistimulated.
To gravistimulate the explants, a 6-cm balloon filled with explant buffer was placed over basal end on the explant and the explant was placed horizontally onto a glass plate. A rubber band was used to secure the explant to the glass plate without interfering with gravitropic bending. A syringe needle was inserted into the balloon and was connected with a buffer reservoir through Tygon tubing. In this manner, a continuous supply of buffer can be delivered to the pulvinus via the transpiration stream. Pulvinus tissue was excised from the stem at the time indicated and was harvested into liquid nitrogen.
Pharmacological inhibitors, NPA and cycloheximide, were supplied to pulvinus tissue using the explant system described above, except that explants were pretreated vertically in buffer containing the inhibitor for 2 h, and were gravistimulated with a continuous supply of buffer with inhibitor. NPA (Chemical Services, West Chester, PA) and cycloheximide (Sigma, St. Louis) were prepared as 10 mm stocks in dimethyl sulfoxide. Water-soluble IAA (Sigma) was supplied to pulvinus tissue also using the explant system, but without gravistimulation. Vertically held explants were prepared as described above, and were pretreated vertically in buffer alone without IAA. After 2 h, explants were transferred to fresh buffer with IAA at the required concentration.
In each experiment with intact plants or explants, typically six to 10 whole pulvini or pulvini halves were harvested and pooled for each sample. Tissue was stored at −80°C.
Measurement of Bending Angle
The bending angle was defined as the angle formed between the internodes above and below the graviresponsive pulvinus. Curvature was measured by tracing the angle formed by the internodes onto paper, and measuring this angle with a protractor.
Hexose, K+, and Acid Invertase Assay
One gram (approximately) of frozen pulvini tissue was homogenized in two volumes of ice-cold enzyme extraction buffer (100 mm MOPS [3-(N-morpholino)propanesulfonic acid], pH 7.5, 10 mm MgCl2, 1 mm EDTA, 5 mm dithiothreitol, 20 mm NaF, 0.1% [v/v] Triton X-10, and 1 mm phenylmethylsulfonyl fluoride). All further manipulations were carried out at 4°C. The homogenate was filtered through three layers of Miracloth (Calbiochem, San Diego) followed by centrifugation at 10,000g at 4°C for 10 min. An aliquot of the supernatant was removed, boiled for 5 min, and analyzed enzymatically for hexose concentration as described by Huber (1984) and K+ concentration by emission spectroscopy using a 500 atomic absorption spectrophotometer (PerkinElmer, Norwalk, CT).
The remainder of the supernatant was used for acid invertase assays. Extracts were desalted immediately prior to assay by centrifugal filtration through two successive 3-mL Sephadex G-25 columns prewashed with two volumes of desalting buffer (10 mm MOPS, pH 7.5, 5 mm MgCl2, 0.1 mm CaCl2, and 2 mm dithiothreitol). Enzyme activity was assayed in a reaction mixture containing 100 μL of desalted extract, 20 μL of 1 m Suc, and 80 μL of 50 mm sodium acetate buffer, pH 4.5, for acid invertase in a total volume of 200 μL in a 30°C water bath for 30 min. Reactions were terminated by immersion of tubes in a 95°C water bath for 10 min. Hexose concentration was analyzed enzymatically as above (Huber, 1984). Protein was quantified using the Bradford microassay according to the manufacturer's instructions (Bio-Rad, Richmond, CA).
RNA Extraction and Northern Analysis
Total RNA was extracted from pulvini tissue using the FastRNA kit (Bio 101, Vista, CA) according to the manufacturer's instructions. RNA was separated on a denaturing formaldehyde gel and was blotted onto a Hybond-XL nylon membrane (Amersham plc, Little Chalfont, Buckinghamshire, UK) essentially as described (Sambrook et al., 1987). Ivr1 and Ivr2 cDNA probes used in this study were obtained from Dr. Karen Koch (University of Florida, Gainesville). The 18S ribosomal DNA was obtained from PCR amplification of Arabidopsis genomic DNA using specific primers (forward: 5′-GAATTCAGACTGTGAAACTGCG-3′; reverse: 5′-ATTCCTCGTTGAAGACCAACAA-3′) and Arabidopsis genomic DNA was isolated essentially as described (Chen and Dellaporta, 1994). Probe DNA was labeled with α-32P dCTP (New England Nuclear, Boston) using the Rediprime II labeling reaction (Amersham plc) according to the manufacturer's instructions. Membranes were prehybridized in buffer (10 mg mL−1 bovine serum albumin, 0.5 m Na2HPO4, pH 7.2, and 7% [w/v] SDS) at 55°C prior to the addition of radiolabeled probe DNA and hybridization overnight. Transcript accumulation was determined using a densitometer.
IAA Measurements
The GC-SIM-MS analysis was essentially as described by Chen et al. (1988) with the following modifications. Maize pulvinus tissue (0.2–0.3 g) was ground to a fine powder in liquid nitrogen and was homogenized in 3 mL of 0.2 m imidazole buffer (pH 7.0) containing 3 μL of [3H]IAA (200,000 dpm 10 μL−1) and 40 μL of [13C6]-IAA (1 ng μL−1). The homogenate was incubated for 1 h at 4°C, followed by centrifugation for 5 min at 10,000g. The supernatant was diluted with 25 mL of double-distilled water. Diluted extract was applied to a conditioned amino anion-exchange column that had been pre-equilibrated sequentially with 4 mL each of hexane, acetonitrile, double-distilled water, 0.2 m imidazole buffer (pH 7.0), and 10 mL double-distilled water. The column was then washed sequentially with 2 mL each of hexane, ethyl acetate, acetonitrile, and methanol. The column was eluted with 3 mL of methanol containing 5% (v/v) acetic acid. The acidic methanol eluate was evaporated to near dryness using a rotary evaporator. The residue was resuspended in 50% (v/v) methanol (3 × 40 μL) for HPLC (5 μm C18-HPLC column, 12.5 cm × 4.6 mm) purification. HPLC fractions were collected for 20 min (flow rate at 1 mL min−1; 1 mL fraction−1). Fractions eluting from 12 to 20 min were counted using a liquid scintillation counter. The radioactive fractions collected from HPLC were pooled together and taken to near dryness using a rotary evaporator. The residue was resuspended in 120 μL of 100% (v/v) methanol, methylated using ethereal diazomethane, and resuspended in 20 μL of ethyl acetate for GC-SIM-MS analysis.
ACKNOWLEDGMENTS
We thank Dr. Karen Koch (University of Florida, Gainesville) for the Ivr1 and Ivr2 cDNA clones used in this study. For the IAA measurements in this study, we thank Dr. Jerry Cohen (University of Minnesota, St. Paul) for the use of equipment and laboratory space, for technical advice, and for helpful discussions, and Nebojsa Ilic for technical assistance. Finally, we would like to thank Drs. Heike Winter (University of Osnabrueck, Germany) and Joan Huber (Horticulture Science, North Carolina State University) for developing the explant system used in this study, and the members of the National Aeronautics and Space Administration Specialized Center of Research and Training in Gravitational Biology for stimulating discussions and helpful advice.
Footnotes
This work was supported by a National Aeronautics and Space Administration Specialized Center of Research and Training in Gravitational Biology grant (no. NAGW–4984).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010579.
LITERATURE CITED
- Bridges IG, Wilkins MB. The role of reducing sugars in the geotropic response of the wheat node. Planta. 1974;117:243–250. doi: 10.1007/BF00388397. [DOI] [PubMed] [Google Scholar]
- Brock TG, Burg J, Ghosheh NS, Kaufman PB. The role of calcium in growth induced by indole-3-acetic acid and gravity in the leaf-sheath pulvinus of oat (Avena sativa) J Plant Growth Regul. 1992;11:99–103. doi: 10.1007/BF00198021. [DOI] [PubMed] [Google Scholar]
- Brock TG, Kapen EH, Ghosheh NS, Kaufman PB. Dynamics of auxin movement in the gravistimulated leaf-sheath pulvinus of oat (Avena sativa) J Plant Physiol. 1991;138:57–62. doi: 10.1016/s0176-1617(11)80730-3. [DOI] [PubMed] [Google Scholar]
- Chang SC, Cho MH, Kang BG, Kaufman PB. Changes in starch content in oat (Avena sativa) shoot pulvini during the gravitropic response. J Exp Bot. 2001;52:1029–1040. doi: 10.1093/jexbot/52.358.1029. [DOI] [PubMed] [Google Scholar]
- Chang SC, Kaufman PB. Effects of staurosporine, okadaic acid and sodium fluoride on protein phosphorylation in graviresponding oat shoot pulvini. Plant Physiol Biochem. 2000;38:315–323. doi: 10.1016/s0981-9428(00)00745-2. [DOI] [PubMed] [Google Scholar]
- Chen J, Dellaporta SL. Urea-based plant DNA miniprep. In: Freeling M, Walbot V, editors. The Maize Handbook. New York: Springer-Verlag; 1994. pp. 526–527. [Google Scholar]
- Chen KH, Miller AN, Patterson GW, Cohen JD. A rapid and simple procedure for purification of indole-3-acetic-acid prior to GC-SIM-MS analysis. Plant Physiol. 1988;86:822–825. doi: 10.1104/pp.86.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Rosen E, Masson PH. Gravitropism in higher plants. Plant Physiol. 1999;120:343–350. doi: 10.1104/pp.120.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collings DA, Winter H, Wyatt SE, Allen NS. Growth dynamics and cytoskeleton organization during stem maturation and gravity-induced stem bending in Zea maysL. Planta. 1998;207:246–258. doi: 10.1007/s004250050480. [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ. Enzymes and other agents that enhance cell wall extensibility. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:391–417. doi: 10.1146/annurev.arplant.50.1.391. [DOI] [PubMed] [Google Scholar]
- Ehness R, Roitsch T. Coordinated induction of mRNAs for extracellular invertase and a glucose transporter in Chenopodium rubrumby cytokinins. Plant J. 1997;11:539–548. doi: 10.1046/j.1365-313x.1997.11030539.x. [DOI] [PubMed] [Google Scholar]
- Estelle M. Auxin perception and signal transduction. In: Hooykaas PJJ, Hall MA, Libbenga KR, editors. Biochemistry and Molecular Biology of Plant Hormones. New York: Elsevier Science; 1999. pp. 411–421. [Google Scholar]
- Evans ML. Gravitropism: interaction of sensitivity modulation and effector distribution. Plant Physiol. 1990;95:1–5. doi: 10.1104/pp.95.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibeaut DM, Karuppiah N, Chang SR, Brock TG, Vadlamudi B, Kim D, Ghosheh NS, Rayle DL, Carpita NC, Kaufman PB. Cell wall and enzyme changes during the graviresponse of leaf-sheath pulvinus of oat (Avena sativa) Plant Physiol. 1990;94:411–416. doi: 10.1104/pp.94.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz M, Godt DE, Roitsch T. Tissue-specific induction of the mRNA for an extracellular invertase isoenzyme of tomato by brassinosteroids suggests a role for steroid hormones in assimilate partitioning. Plant J. 2000;22:515–522. doi: 10.1046/j.1365-313x.2000.00766.x. [DOI] [PubMed] [Google Scholar]
- Guilfoyle TJ. Auxin-regulated genes and promoters. In: Hooykaas PJJ, Hall MA, Libbenga KR, editors. Biochemistry and Molecular Biology of Plant Hormones. New York: Elsevier Science; 1999. pp. 423–459. [Google Scholar]
- Huber SC. Biochemical basis for effects of K-deficiency on assimilate export rate and accumulation of soluble sugars in soybean leaves. Plant Physiol. 1984;76:424–430. doi: 10.1104/pp.76.2.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannes E, Collings DA, Rink JC, Allen NS. Cytoplasmic pH dynamics in Zea mayspulvinal cells induced by gravity vector changes. Plant Physiol. 2001;127:119–130. doi: 10.1104/pp.127.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada M, Fujii N, Aizawa S, Kamigaichi S, Mukai C, Shimazu T, Takahashi H. Control of gravimorphogenesis by auxin: accumulation pattern of CS-IAA1mRNA in cucumber seedlings grown in space and on the ground. Planta. 2000;211:493–501. doi: 10.1007/s004250000321. [DOI] [PubMed] [Google Scholar]
- Kaufman PB, Wu LL, Brock TG, Kim D. Hormones and the orientation of growth. In: Davies PJ, editor. Plant Hormones: Physiology, Biochemistry and Molecular Biology. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 547–571. [Google Scholar]
- Kim D, Kaufman PB. Basis for changes in the auxin-sensitivity of Avena sativa(oat) leaf-sheath pulvini during the gravitropic response. J Plant Physiol. 1995;145:113–120. doi: 10.1016/s0176-1617(11)81856-0. [DOI] [PubMed] [Google Scholar]
- Kiss JZ. Mechanisms of the early phases of plant gravitropism. Crit Rev Plant Sci. 2000;19:551–573. doi: 10.1080/07352680091139295. [DOI] [PubMed] [Google Scholar]
- Lee TH, Sugiyama A, Ofosu-Anim J, Takeno K, Ohno H, Yamaki S. Activation of sucrose-metabolizing enzymes and stimulation of sucrose uptake by auxin and sucrose in eggplant (Solanum melongenaL.) J Plant Physiol. 1997;150:297–301. [Google Scholar]
- Li Y, Hagen G, Guilfoyle TJ. An auxin-responsive promoter is differentially induced by auxin gradients during tropisms. Plant Cell. 1991;3:1167–1175. doi: 10.1105/tpc.3.11.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden JC, Ehness R, Roitsch T. Ethylene regulation of apoplastic invertase expression in autotrophic cells of Chenopodium rubrum. Plant Growth Regul. 1996;19:219–222. [Google Scholar]
- Lomax TL. Molecular genetic analysis of plant gravitropism. Grav Space Biol Bull. 1997;10:75–82. [PubMed] [Google Scholar]
- McClure BA, Guilfoyle TJ. Rapid redistribution of auxin-regulated RNAs during gravitropism. Science. 1989;243:91–93. doi: 10.1126/science.11540631. [DOI] [PubMed] [Google Scholar]
- Momonoki YS. Asymmetric distribution of glucose and indole-3-acetyl-myo-inositol in geostimulated Zea maysseedlings. Plant Physiol. 1988;87:751–756. doi: 10.1104/pp.87.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris DA, Arthur ED. Invertase and auxin-induced elongation in internodal segments of Phaseolus vulgaris. Phytochemistry. 1984;23:2163–2167. [Google Scholar]
- Muday GK. Interactions between the actin cytoskeleton and an auxin transport protein. In: Staiger CJ, Baluska F, Volkmann D, Barlow P, editors. Actin: A Dynamic Framework for Multiple Plant Cell Functions. Dordrecht, The Netherlands: Kluwer Academic Press; 2000. pp. 541–571. [Google Scholar]
- Pelleschi S, Guy S, Kim JY, Pointe C, Mahe A, Barthes L, Leonardi A, Prioul JL. Ivr2, a candidate gene for a QTL of vacuolar invertase activity in maize leaves: gene-specific expression under water stress. Plant Mol Biol. 1999;39:373–380. doi: 10.1023/a:1006116310463. [DOI] [PubMed] [Google Scholar]
- Perera IY, Heilmann I, Boss WF. Transient and sustained increases in inositol 1,4,5-bisphosphate precede the differential growth response in gravistimulated maize pulvini. Proc Natl Acad Sci USA. 1999;96:5838–5843. doi: 10.1073/pnas.96.10.5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera IY, Heilmann I, Chang SC, Boss WF, Kaufman PB. A role for inositol 1,4,5-trisphosphate in gravitropic signaling and the retention of cold-perceived gravistimulation of oat shoot pulvini. Plant Physiol. 2001;125:1499–1507. doi: 10.1104/pp.125.3.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippar K, Fuchs I, Luthen H, Hoth S, Bauer CS, Haga K, Thiel G, Ljung K, Sandberg G, Bottger M et al. Auxin-induced K+channel expression represents an essential step in coleoptile growth and gravitropism. Proc Natl Acad Sci USA. 1999;96:12186–12191. doi: 10.1073/pnas.96.21.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooviah BW, Veluthambi K. Auxin-related invertase activity in strawberry fruits. J Am Soc Hort Sci. 1985;110:258–261. [Google Scholar]
- Rabe C, Kutschera U. Sucrose metabolism during apical hook opening in sunflower hypocotyls. Plant Physiol Biochem. 1998;36:389–394. [Google Scholar]
- Rashotte AM, DeLong A, Muday GK. Genetic and chemical reductions in protein phosphatase activity alter auxin transport, gravity response, and lateral root growth. Plant Cell. 2001;13:1683–1697. doi: 10.1105/TPC.010158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubery PH. Phytotropins: receptors and endogenous ligands. Soc Exp Biol Symp. 1990;44:119–146. [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Ed 2. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1987. [Google Scholar]
- Sturm A, Tang GQ. The sucrose cleaving enzymes of plants are crucial for development. Trends Plant Sci. 1999;4:401–406. doi: 10.1016/s1360-1385(99)01470-3. [DOI] [PubMed] [Google Scholar]
- Tymowska-Lalanne Z, Kreis M. The plant invertases: physiology, biochemistry and molecular biology. Adv Bot Res. 1998a;28:71–117. [Google Scholar]
- Tymowska-Lalanne Z, Kreis M. Expression of the Arabidopsis thalianainvertase gene family. Planta. 1998b;207:259–265. doi: 10.1007/s004250050481. [DOI] [PubMed] [Google Scholar]
- Winter H, Huber JL, Huber SC. Membrane association of sucrose synthase: changes during the graviresponse and possible control by protein phosphorylation. FEBS Lett. 1997;420:151–155. doi: 10.1016/s0014-5793(97)01506-8. [DOI] [PubMed] [Google Scholar]
- Winter H, Huber SC. Regulation of sucrose metabolism in higher plants: localization and regulation of activity of key enzymes. Crit Rev Plant Sci. 2000;19:31–67. doi: 10.1080/10409230008984165. [DOI] [PubMed] [Google Scholar]
- Wu LL, Song I, Karuppiah N, Kaufman PB. Kinetic induction of oat shoot pulvinus invertase mRNA by gravistimulation and partial cDNA cloning by the polymerase chain reaction. Plant Mol Biol. 1993a;21:1175–1179. doi: 10.1007/BF00023613. [DOI] [PubMed] [Google Scholar]
- Wu LL, Song I, Kim D, Kaufman PB. Molecular basis of the increase in invertase activity elicited by gravistimulation of oat-shoot pulvini. J Plant Physiol. 1993b;142:179–183. doi: 10.1016/s0176-1617(11)80960-0. [DOI] [PubMed] [Google Scholar]
- Wu LL, Mitchell JP, Cohn NS, Kaufman PB. Gibberellin (GA3) enhances cell wall invertase activity and mRNA levels in elongating dwarf pea (Pisum sativum) shoots. Int J Plant Sci. 1993c;154:280–289. doi: 10.1086/297108. [DOI] [PubMed] [Google Scholar]
- Xu J, Avigne WT, McCarty DR, Koch KE. A similar dichotomy of sugar modulation and developmental expression affects both paths of sucrose metabolism: evidence from a maize invertase gene family. Plant Cell. 1996;8:1209–1220. doi: 10.1105/tpc.8.7.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, Wu Y, Avigne WT, Koch KE. Rapid repression of maize invertases by low oxygen: invertase/sucrose synthase balance, sugar signaling potential, and seedling survival. Plant Physiol. 1999;121:599–608. doi: 10.1104/pp.121.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Cohn NS, Mitchell JP. Induction of a cell wall invertase gene by wounding and its localized expression in phloem. Plant Physiol. 1996;112:1111–1117. doi: 10.1104/pp.112.3.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]