Abstract
One form of carbonic anhydrase (CA) has been observed in maize (Zea mays) thylakoids and photosystem II (PSII)-enriched membranes. Here, we show that an antibody produced against a thylakoid lumen-targeted CA found in Chlamydomonas reinhardtii reacts with a single 33-kD polypeptide in maize thylakoids. With immunoblot analysis, we found that this single polypeptide could be identified only in mesophyll thylakoids and derived PSII membranes, but not in bundle sheath thylakoids. Likewise, a CA activity assay confirmed a large amount of activity in mesophyll, but not in bundle sheath membranes. Immunoblot analysis and CA activity assay showed that the maximum CA can be obtained in the supernatant of the PSII-enriched membranes washed with 1 m CaCl2, the same procedure used to remove all extrinsic lumenal proteins from PSII. Because this CA reacts with an antibody to lumen-directed CA in C. reinhardtii, and because it can be removed with 1 m CaCl2 wash, we refer to it tentatively as extrinsic CA. This is to distinguish it from another form of CA activity tightly bound to PSII membranes that remains after CaCl2 wash, which has been described previously. The function of extrinsic CA is not clear. It is unlikely to have the same function as the cytoplasmic CA, which has been proposed to increase the HCO3− concentration for phosphoenolpyruvate carboxylase and the C4 pathway. We suggest that because the extrinsic CA is associated only with thylakoids doing linear electron flow, it could function to produce the CO2 or HCO3− needed for PSII activity.
Carbonic anhydrase (CA) catalyzes the pH-dependent reversible hydration of CO2 in solution and is found in plants, animals, and bacteria (for review, see Chegwidden and Carter, 2000). Although hydration of CO2 occurs spontaneously, the uncatalyzed reaction is slow, taking place in the time range of seconds. The catalyzed reaction, in contrast, can be extremely rapid, taking place in the microsecond time range. Most CA studies have involved animal systems, where the enzyme has several important functions, e.g. assisting in the transfer of CO2/HCO3− across membranes. In plants, it is now believed that CA plays an important role in photosynthesis (for review, see Badger and Price, 1994). In C4 plants, for example, CA is located in the cytosol of mesophyll cells where it catalyzes the conversion of CO2 to HCO3− to be used as substrate for phosphoenolpyruvate carboxylase (Burnell, 2000). In C3 plants, CA is mainly found in the stroma of chloroplasts. The role of CA in C3 plants is not fully understood. Studies over the past several decades have revealed the presence of another CA firmly bound to chloroplast thylakoid membranes and denoted tCA (for review, see Stemler, 1997). Karlsson et al. (1998) have recently purified a 29.5-kD CA from Chlamydomonas reinhardtii. The enzyme was encoded by a gene-denoted cah3, and was targeted to the thylakoid lumen (Karlsson et al., 1998). Although CA activity is also associated with photosystem II (PSII) in higher plants, an enzyme corresponding to the C. reinhardtii protein has not yet been isolated.
With the realization that the oxygen-evolving mechanism in PSII, as well as later electron-transfer steps, require catalytic amounts of bicarbonate (for review, see Stemler, 1998b; Klimov and Baranov, 2001), the simultaneous presence of CA activity is all the more intriguing. The purpose of the present study was to localize and attempt to isolate the PSII CA in maize (Zea mays). We find that only mesophyll thylakoids have CA activity. Furthermore, the CA can be extracted in active form by washing PSII-enriched membranes with 1 m CaCl2. We provide additional evidence that this PSII CA is located on the lumenal side of thylakoid membranes. In the course of these experiments, it also became evident that there must be two distinct sources of CA activity in PSII.
RESULTS
Chloroplast Separation and Measures of Purity
To study the distinctive plastids of maize, various methods have been used to prepare the plastids from leaf homogenates. We used the enzymatic method of Bassi et al. (1985), which incorporated a repetition of blending and filtering steps to separate mesophyll protoplasts from bundle sheath cells from 4-week-old leaves. The light microscope examination showed no mesophyll protoplasts remained in bundle sheath strands (data not shown). Purified chloroplasts were subsequently isolated from each source, and the chlorophyll a/b ratio of bundle sheath thylakoids was 6.92 in comparison with 3.3 for mesophyll thylakoids. This result compares very well with literature values obtained by other groups (Bassi et al., 1995; Pfündel and Meister, 1996). In addition, we measured 2,6-dichlorobenzoquinone-dependent oxygen evolution in saturating light. Although mesophyll thylakoids typically showed activity of several hundred μmol O2 mg−1 chlorophyll h−1, no detectable oxygen evolution was observed in bundle sheath thylakoids (data omitted).
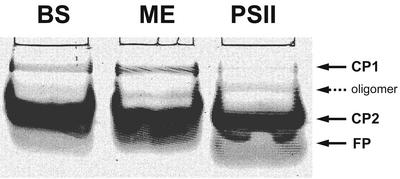
To further assess the purity of mesophyll and bundle sheath chloroplasts, native electrophoresis (Fig. 1) and immunological detection for Rubisco large subunit (rbcL) were performed (Fig. 2, A and B). In the native gel, mesophyll and bundle sheath chloroplasts show the presence of light-harvesting complexes (LHC) CP1 and CP2, although CP2 is very much reduced in bundle sheath preparations. According to the interpretation of similar findings by others (Ghirardi and Melis, 1983; Bassi et al., 1985, 1995), this result may be due to the fact that bundle sheath chloroplasts still contain a few nonfunctional PSII units. Likewise, a silver-stained SDS-PAGE gel also showed some reduced amount of LHCII in bundle sheath membranes (Fig. 3A). However, the CP2 band, which represents PSII antennae, is much more prominent in mesophyll thylakoids. For comparison, the result from a preparation of PSII-enriched membranes from mesophyll thylakoids is also shown in Figure 1. These show a clear CP2 band, but very little CP1 except for a minor band that probably represents a CP2 oligomer.
Figure 1.
Nondenaturing gel electrophoresis (Thornber gel) of isolated mesophyll, bundle sheath thylakoids, and PSII-enriched membranes. One milliliter of extraction buffer was added to membranes for each milligram of total chlorophyll. After centrifugation, a 15-μL aliquot of each supernatant, bundle sheath (BS), mesophyll (ME), and PSII were loaded onto the gel. Two major pigment-protein complexes (PPCs), CP1 and CP2, and a zone of free pigment are labeled.
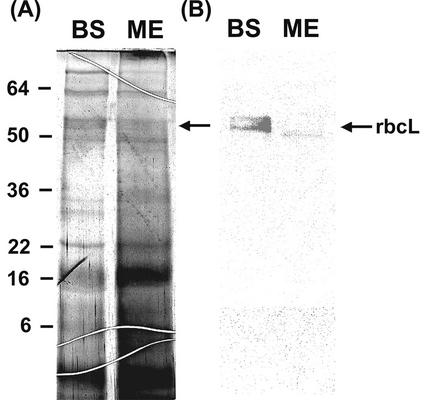
Figure 2.
SDS-PAGE and immunoblot analysis of rbcL. Separated mesophyll and bundle sheath chloroplasts were homogenized in buffered solution. After centrifuging down the insoluble fractions, 10 μL of the supernatants of bundle sheath (BS) and mesophyll (ME) were loaded onto the gel. Silver staining was used in A and anti-soybean (Glycine max) rbcL serum was used in B. The arrow indicates the presence of rbcL in the stroma of bundle sheath chloroplasts in B. The numbers indicate the molecular mass standard in kilodaltons.
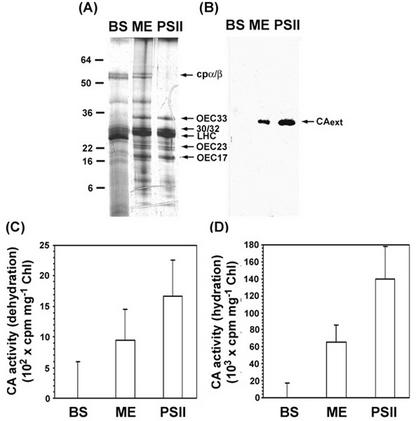
Figure 3.
SDS-PAGE, immunoblot, and CA activity analysis of extrinsic CA in bundle sheath (BS), mesophyll (ME) thylakoids, and enriched PSII membranes. After the membranes were resuspended to 1 mg chlorophyll mL−1 with denaturing buffer, three 10-μL samples were loaded onto the gel. Silver staining is shown in A and anti-C. reinhardtii CA serum is shown in B. All arrows in A indicate the positions of the various thylakoid membrane proteins among bundle sheath, mesophyll, and PSII membranes, whereas the arrow in B indicates a single polypeptide of extrinsic CA in mesophyll and PSII membranes. The numbers indicate the molecular mass standard in kilodaltons. The CA activity was measured by monitoring the 14CO2 hydration and H14CO3− dehydration in C and D. To prepare substrate, 3 μL of NaH14CO3 stock (35 mm, 2 mCi mL−1) was diluted into 1.6 mL of acidic (H2SO4, pH 2.5) and basic (NaOH, pH10) water, respectively. CA activity is normalized by total chlorophyll and is expressed as cpm per milligram of chlorophyll. Error bars represent 1 se, n = 24.
The absence of Rubisco is considered the best measure of purity of mesophyll cells (Walbot and Hoisington, 1982). Only bundle sheath chloroplasts performing the reductive pentose phosphate cycle contain this enzyme. In western-blot analysis, a clear rbcL band at approximately 55 kD range is seen only with the extract of bundle sheath chloroplasts. On SDS-PAGE gels (Fig. 2B), however, bands appear at about the 55 kD range with bundle sheath and mesophyll chloroplast extracts. Nevertheless, the western-blot results indicate a clear absence of Rubisco in our mesophyll chloroplast preparations. Based on these results, we were confident of a high degree of purity of bundle sheath and mesophyll chloroplasts, which were then used for further studies.
Thylakoid Protein Composition and Tissue Location of CAext
The polypeptide composition of bundle sheath and mesophyll thylakoid membranes was determined by SDS-PAGE. Silver staining revealed a number of differences, as expected (Fig. 3A). Grana-containing mesophyll thylakoids possessed a full complement of the various polypeptides associated with PSII. In contrast, most of the components of PSII are missing in bundle sheath thylakoids. Only 30/32 kD and LHCII were still present in reduced amounts. This result confirmed the presence of a small amount of CP2 in bundle sheath thylakoids as shown on the Thornber gel (Fig. 1). However, the total lack of oxygen evolution in these thylakoids indicates that the PSII units are not completely functional.
We were particularly interested in a type of CA found associated with thylakoids in a number of different plant species (Karlsson et al., 1998; Moskvin et al., 2000; Dai et al., 2001). In recent times, a gene for an intracellular α-type CA was discovered in a green alga, C. reinhardtii (Karlsson et al., 1995). The gene product contained a thylakoid lumen targeting sequence and, subsequently, was purified to homogeneity. We have used an antibody against this enzyme to test for the presence of CA in bundle sheath and mesophyll thylakoids. We found that the anti-serum reacted with a single protein band of about 33 kD in mesophyll thylakoids, but not in bundle sheath thylakoids (Fig. 3B). The molecular size, 33 kD, contrasts with the C. reinhardtii enzyme, found to be a 29.5-kD molecule. Furthermore, a positive result was also obtained with a PSII-enriched membrane fraction derived from mesophyll chloroplasts (Fig. 3B).
We then tested mesophyll and bundle sheath thylakoids for CA activity. In agreement with our immunological studies, only the mesophyll membranes possessed CA activity (Fig. 3, C and D). In addition, this result showed approximately one-half of the CA activity in mesophyll thylakoids when compared on a chlorophyll basis with the PSII-enriched membranes. It was strongly suggested that this CA is closely associated with PSII (Stemler, 1986).
CaCl2 Washing Removes the CAext from PSII-Enriched Membranes
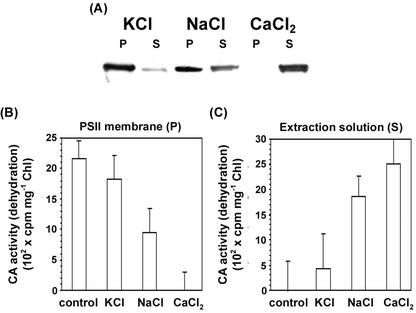
The lumen-targeted CA that was purified from C. reinhardtii is dissociated from thylakoids by treatment with 200 mm KCl (Karlsson et al., 1995). It is also known that high concentrations of CaCl2 will remove all three extrinsic PSII proteins found on the lumen side of PSII-enriched membranes. Therefore, we tested to see if salt wash would remove the CAext from PSII-enriched membranes derived from mesophyll thylakoids. The wash treatments tested were 1 m KCl, NaCl, and CaCl2.
The most effective wash solution at removing CAext from PSII membranes was 1 m CaCl2 (Fig. 4, A–C). According to the immunological assay (Fig. 4A), over 99% was removed, as was the CA activity (Fig. 4B). The CA activity subsequently appeared in the desalted wash solution (Fig. 4C). It is clear that the treatment that removes the three lumenal extrinsic PSII proteins also removes CAext from the membranes. NaCl was somewhat less effective (about 40% removal), and KCl was totally ineffective (less than 1% removal) under the same conditions. After desalting the extracts, the maximum recovery of CA activity was obtained in the CaCl2 wash solution. Less was obtained in the NaCl wash, and no significant activity was found in the KCl wash solution. These results show clearly that the CAext can be removed in toto from the PSII-enriched membranes and can function in vitro.
Figure 4.
Immunoblot and CA activity analysis of extrinsic CA in three different salt-washing treatments, KCl, NaCl, and CaCl2. After being treated with 1 m KCl, NaCl, or CaCl2 (1 mg chlorophyll mL−1), the PSII-enriched membranes were centrifuged to form pellet and supernatant. Pellets (P) were washed and resuspended in a buffer that contained 0.3 m sucrose and 50 mm MES, pH 7. The supernatants (S) were desalted with Ultrafree centrifugal filters (Millipore Corp.). After denaturing, 10-μL samples were loaded onto the gel and anti-C. reinhardtii CA serum was used in A. The CA activity assay was measured as H14CO3− dehydration in B and C. Other conditions were as described in the legend of Figure 3. Error bars represent 1 se, n = 30. The control is PSII-enriched membranes treated with salt-free buffer.
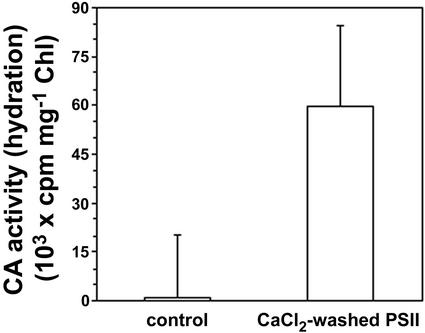
It is important to note that the dehydration activities shown in Figure 4 (B and C) were obtained under identical assay conditions characterized by low salt concentration, low pH, and no added Ca2+. As such, the CaCl2-washed membranes showed no CA activity (Fig. 4B). However, as was documented previously (Moskvin et al., 1998; Stemler, 1998a), if these CaCl2-washed membranes are assayed for hydration activity in the presence of 0.4 m NaCl and 5 mm CaCl2, CA activity is easily observed (Fig. 5). This is a second, intrinsic form of CA activity that has very different assay requirements.
Figure 5.
CA activity in CaCl2-washed PSII membranes. The activity was measured by monitoring 14CO2 hydration. The Ca+2-washed PSII was prepared as described in “Materials and Methods.” The reaction mixture contained 0.05 m Na-HEPES, pH 7.2, 0.4 m NaCl, 5 mm CaCl2, and 100 μg chlorophyll mL−1. Other conditions were as described in the legend of Figure 3. Error bars represent 1 se, n = 30.
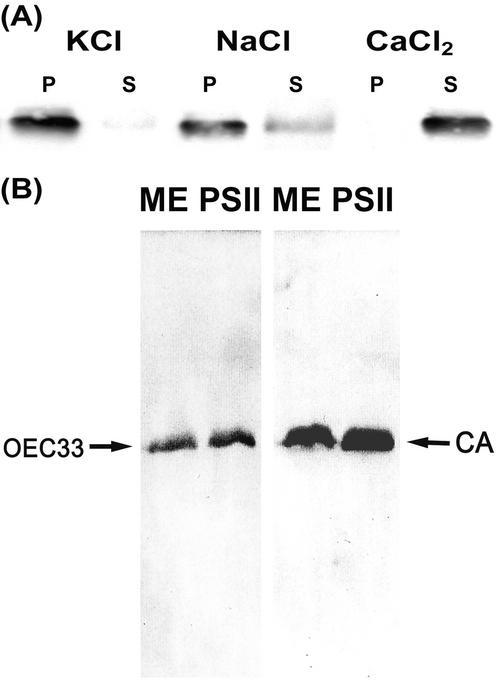
The Molecular Size of the CAext in Maize is 33 kD
In the previous experiments, we showed that only maize mesophyll thylakoids contain CAext. In addition, 1 m CaCl2 can completely extract the activity due to CAext. The immunoblot and SDS-PAGE results indicate that the CAext appears as a single band in a 32 to 33 kD range. However, it is well known that one component of the oxygen-evolving complex (OEC), an extrinsic lumenal protein denoted OEC33, is also released by washing with concentrated CaCl2 (Kuwabara et al., 1985; Ghanotakis and Yocum, 1986; Xu and Bricker, 1992) and appears in the same molecular size range. To verify that the OEC33 and CAext were comigrating on gels, we applied two different antibodies, anti-OEC33 and anti-CA from C. reinhardtii, to gels running proteins from mesophyll thylakoids and PSII-enriched membranes. The results (Fig. 6, A and B) confirmed that OEC33 and CAext have the same molecular size. The specificity of the C. reinhardtii anti-CA was also checked by challenging the antibody with other examples of the αCA family, purified human and bovine CAs. In both cases, the antibody failed to react (data not shown), indicating a high degree of specificity to the 33-kD protein in maize.
Figure 6.
Immunological analysis of OEC33 and extrinsic CA. A, After treatment with 1 m KCl, NaCl, and CaCl2 at 1 mg chlorophyll mL−1, PSII-enriched membranes were centrifuged. Pellet (P) and supernatant (S) were treated in the same procedure as described in Figure 4. The samples were loaded onto the gel. Anti-pea (Pisum sativum) OEC33 serum was used. B, Mesophyll thylakoids (ME) and PSII-enriched membranes were denatured at 1 mg chlorophyll mL−1 and 10 μL was loaded onto the gel. Anti-pea OEC33 and anti-C. reinhardtii CA serums were used. The left arrow indicates the positions of OEC33, whereas the right arrow indicates extrinsic CA.
Localization of CAext in Maize
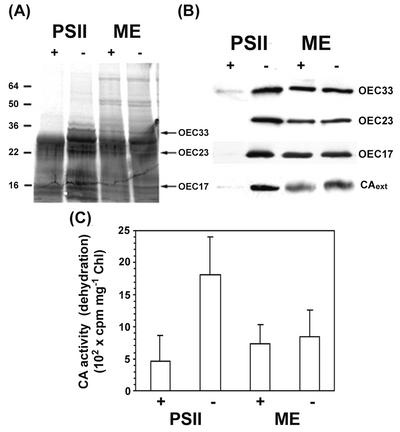
It has been shown that the CA in question exists as a lumenal protein in C. reinhardtii (Karlsson et al., 1998). To verify that the CAext is also lumenal in maize, thylakoid and PSII-enriched membranes were treated with trypsin in an attempt to digest the enzyme. The assumption was that the enzyme, if lumenal, would be more vulnerable to attack in PSII-enriched membranes than in thylakoids. An immunoblot (Fig. 7B) showed that the CAext band was seriously degraded only in PSII-enriched membranes, scarcely at all in thylakoids. Likewise, the activity of the CA was reduced 88% in trypsin-treated PSII membranes, but only 10% in trypsin-treated thylakoids (Fig. 7B). These results are consistent with a lumenal location of the CAext in maize. As a control, similar digestion was observed with the OEC33, 23, and 17 proteins using specific antibodies to them (Fig. 7, A and B).
Figure 7.
Immunoblot analysis and CA activity of trypsin-treated mesophyll thylakoids (ME) and PSII-enriched membranes. A 10-μL aliquot of trypsin stock (1 mg mL−1) was added to both resuspended samples (0.5 mL, 3 mg chlorophyll mL−1) that contained 0.3 m sucrose and 50 mm HEPES, pH 7. Samples were incubated for 30 min and were then centrifuged at 27,000g for 10 min. The pellet was resuspended and denatured, and 10-μL samples were loaded onto the gel. Silver stain was used in A, and anti-pea OEC33, 23, and 17, and anti-C. reinhardtii CA in B. Plus signs indicate trypsin treatment, whereas minus signs indicate controls. C, CA activity was measured as H14CO3− dehydration. To prepare substrate, 3 μL of 14C stock (35 mm) was diluted into 1.6 mL of basic water, pH 10. Error bars represent 1 se, n = 24.
DISCUSSION
CAs are widely distributed among photosynthetic organisms. In plants, most are highly soluble and are present in the cytoplasm, as in C4 plants, or in the chloroplast stroma, as in C3 plants (Sültemeyer et al., 1993; Burnell, 2000). However, another form of CA associated with thylakoid membranes is well documented, but not yet purified from higher plants (for review, see Stemler, 1997). Moreover, its function has not been determined. The purpose of this study was to further localize the thylakoid CA in maize and to obtain clues as to its function. We chose maize as a source of thylakoid CA because of past reports (Burnell, 1990) that the chloroplast stroma in maize was free of CA, which could contaminate our preparations. Second, due to the structural and functional differentiation between mesophyll and bundle sheath chloroplasts (Bassi et al., 1985), localization of the thylakoid CA would provide information regarding its possible function, at least in higher plants. Here, we have separated mesophyll and bundle sheath chloroplasts. By chlorophyll a/b ratios, oxygen evolution activity, immunoblot tests for rbcL, and other criteria, we established that our preparations were pure. We find that the thylakoid CA activity is associated exclusively with mesophyll thylakoids and PSII-enriched membranes. Because mesophyll chloroplasts alone have active PSII, but do not have Rubisco or a complete reductive pentose phosphate cycle, our results support the suggestion made several decades ago by Vaklinova et al. (1982) that the thylakoid CA functions to form CO2 or HCO3−, a necessary cofactor for PSII activity. An attempt at a more detailed model is complicated by the apparent presence of two distinct forms of CA activity, a 33-kD CAext described here and another source intrinsic to the membrane (Fig. 5; Moskvin et al., 1998; Stemler, 1998a).
It is known that most extrinsic lumenal proteins can be extracted from PSII-enriched membranes with concentrated salt solutions (Kuwabara et al., 1985; Xu and Bricker, 1992). Karlsson et al. (1995) have shown that the lumen-directed CA in C. reinhardtii could be extracted in buffered solution containing 200 mm KCl. We find that even 1 m KCl was almost totally ineffective in removing the CAext in maize. A more rigorous extraction in 1 m CaCl2 was necessary, the same treatment that removes all extrinsic PSII proteins associated with the oxygen-evolving mechanism. That the CA in maize is lumenal is supported by our protease digestion experiment. Only by exposing the lumenal proteins in PSII-enriched membranes was trypsin effective in attacking the CA.
We were able to determine the molecular size of the CAext in maize. With immunoblot analysis, we detected a single band in the size range of 32 to 33 kD in maize. This is identical to one of the known extrinsic PSII proteins, OEC33. With two different antibodies, one against OEC33 and the other against CA, we showed that the two proteins comigrate on SDS-PAGE gels (Fig. 6). This is important for quantitative and stoichiometric determinations because it was not previously suspected that the OEC33 band is actually made up of two different proteins. However, this conclusion can be avoided with another interpretation of the results. It could be proposed that the OEC33 is the CAext. This interpretation requires that the antibody against C. reinhardtii CA crossreacts with the OEC33 in maize. However, we have examined the OEC33 and have found that it has less than 5% similarity to the C. reinhardtii CA in sequence alignment analysis, making crossreactivity improbable. The lack of primary sequence homology to any known CA would, in itself, make the OEC33 a poor candidate for CAext. In addition, the OEC33 is not known to bind zinc, a universal cofactor in all CAs. Rather, it is suggested to protect the manganese cluster in PSII (Kuwabara et al., 1985). Therefore, although it is doubtful that the OEC33 and CAext are the same protein, the results presented here do not rule out this possibility and we will continue to test this hypothesis as we further purify the CAext.
A sequence analysis in Arabidopsis was done to identify genes that could correspond to the maize CAext. The search revealed several putative αCA sequences exist on each of the Arabidopsis chromosomes, thus yielding a surprisingly large number of candidates. In addition, several cDNA sequences in the maize expressed sequence tag database showed similarities to the C. reinhardtii αCA gene, but no positive identification can be made at this time.
MATERIALS AND METHODS
Maize (Zea mays) was grown in a greenhouse as previously described (Moubarak-Milad and Stemler, 1994). Bundle sheath and mesophyll cells and chloroplasts from 1-month-old plants were separated enzymatically as described in Bassi et al. (1985, 1995). The purity of preparations was monitored by light microscopy, chlorophyll a/b ratio, native gel electrophoresis (Thornber and Highkins, 1974), and by the presence of rbcL.
For experiments that did not require extreme purity, mesophyll thylakoids were isolated from 3-week-old plants as previously described (Moubarak-Milad and Stemler, 1994). PSII-enriched membranes were prepared by the procedure of Kuwabara et al. (1985) as modified in Xu and Bricker (1992). To remove extrinsic PSII proteins, a modified high-salt washing treatment was used (Ghanotakis and Yocum, 1986). PSII membranes were suspended for 30 min at 4°C in a wash medium with 50 mm Na-HEPES (N-[2-hydroxyethyl]piperazine-N′-[2-ethanesulfonic acid]), pH 7, and 1 m KCl, NaCl, or CaCl2. All of the incubations were followed by a 30-min centrifugation at 100,000g and the supernatants were further dialyzed in 50 mm Na-MES (2[N-morpholino]ethanesulfonic acid), pH 6, overnight. Each dialyzed sample was concentrated in a 50-mL concentrator by expression through a 5-kD cut-off filter (Millipore). N2 gas was applied to a pressure of 60 psi. All thylakoids, treated and untreated PSII membranes, and dialyzed supernatants were stored at −80°C for further use.
CA activity was determined with the assay procedure previously described by Stemler (1993) and modified as detailed in Moubarak-Milad and Stemler (1994). The samples were suspended in reaction mixture: CO2 hydration was measured in 50 mm Na-Tris, pH 8.0, and 20 mm NaCl; and HCO3− dehydration was in 50 mm Na-MES, pH 5.5, and 20 mm NaCl. All samples with membranes were assayed in the dark. Each reaction tube that contained 0.3 mL of reaction mixture was injected with 50 μL of the diluted substrate to a final concentration of 12 μm 14CO2 or H14CO3−. The CA activity was determined with a scintillation counter and was expressed in relative terms as cpm. Buffered reaction media were routinely boiled or ultrafiltrated to eliminate CA-containing microorganisms.
Chlorophyll concentration was determined spectrophotometrically by the method of Porra et al. (1989), and the concentration of protein by the method of Bradford (1976). Bovine serum albumin was used as a standard.
PAGE was carried out as in Laemmli (1970), with the modification that a 12% (w/v) acrylamide resolving gel was used. Immunoblotting was performed as described in the protocol from enhanced chemiluminescence western-blotting kit (Amersham Biosciences, Piscataway, NJ). For some control experiments, bovine CA and human CA were purchased (Sigma) and dissolved at a concentration of 1 mg mL−1 in 50 mm Na-HEPES, pH 7. The different antibodies used in the analyses were as follows: against rbcL of soybean, OEC33, 23, and 17 of pea (provided by Dr. Steven Theg, University of California, Davis), and αCA of C. reinhardtii.
ACKNOWLEDGMENTS
The authors thank Dr. Göran Samuelsson (Department of Plant Physiology, Umeå University, Sweden) for anti-C. reinhardtii αCA serum, Dr. Terence Murphy (Section of Plant Biology, University of California, Davis) for anti-Soybean rbcL serum, and Dr. Steven Theg (Section of Plant Biology, University of California, Davis) for anti-Pea OEC33, 23, and 17 serums. We also thank emeritus professors Paul Castelfranco and Bruce Bonner (University of California, Davis) for helpful advice and encouragement.
Footnotes
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010643.
LITERATURE CITED
- Badger MR, Price GD. The role of carbonic anhydrase in photosynthesis. Annu Rev Plant Physiol. 1994;45:369–393. [Google Scholar]
- Bassi R, Marquardt J, Lavergne J. Biochemical and functional properties of photosystem II in agranal membranes from maize mesophyll and bundle sheath chloroplasts. Eur J Biochem. 1995;233:709–719. doi: 10.1111/j.1432-1033.1995.709_3.x. [DOI] [PubMed] [Google Scholar]
- Bassi R, Peruffo AB, Barbato R, Ghisi R. Difference in chlorophyll-protein complexes and composition of polypeptides between thylakoids from bundle sheath and mesophyll cells in maize. Eur J Biochem. 1985;146:589–595. doi: 10.1111/j.1432-1033.1985.tb08692.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burnell JN. Immunological study of carbonic anhydrase in C3 and C4plants using antibodies to maize cytosolic and spinach chloroplasts carbonic anhydrase. Plant Cell Physiol. 1990;31:423–427. [Google Scholar]
- Burnell JN. Carbonic anhydrases of higher plants: an overview. In: Chegwidden WR, Carter ND, Edwards YH, editors. The Carbonic Anhydrases. Basel, Switzerland: New Horizons; 2000. pp. 501–518. [DOI] [PubMed] [Google Scholar]
- Chegwidden WR, Carter ND. Introduction to the carbonic anhydrases. In: Chegwidden WR, Carter ND, Edwards YH, editors. The Carbonic Anhydrases. Basel, Switzerland: New Horizons; 2000. pp. 13–28. [Google Scholar]
- Dai XB, Yu Y, Zhang RX, Yu XJ, He PM, Xu CH. Relationship among photosystem II carbonic anhydrase, extrinsic polypeptides and manganese cluster. Chinese Sci Bull. 2001;46:406–408. [Google Scholar]
- Ghanotakis DF, Yocum CF. Purification and properties of an oxygen-evolving reaction center complex from photosystem II membranes: a simple procedure utilizing a non-ionic detergent and elevated ionic strength. FEBS Lett. 1986;197:244–248. [Google Scholar]
- Ghirardi ML, Melis A. Localization of photosynthetic electron transport components in mesophyll and bundle sheath chloroplasts of Zea mays. Arch Biochem Biophys. 1983;224:19–28. doi: 10.1016/0003-9861(83)90186-8. [DOI] [PubMed] [Google Scholar]
- Karlsson J, Clarke AK, Chen ZY, Hugghins SY, Park YL, Husic HD, Moroney JV, Samuelsson G. A novel α-type carbonic anhydrase associated with the thylakoid membrane in Chlamydomonas reinhardtii is required for growth at ambient CO2. EMBO J. 1998;17:1208–1216. doi: 10.1093/emboj/17.5.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson J, Hiltonen T, Husic HD, Ramazanov Z, Samuelsson G. Intracellular carbonic anhydrase of Chlamydomonas reinhardtii. Plant Physiol. 1995;109:533–539. doi: 10.1104/pp.109.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimov VV, Baranov SV. Bicarbonate requirement for the water-oxidizing complex of photosystem II. Biochim Biophys Acta. 2001;1503:187–196. doi: 10.1016/s0005-2728(00)00222-x. [DOI] [PubMed] [Google Scholar]
- Kuwabara T, Miyao M, Murata T, Murata N. The function of 33-kDa protein in the photosynthetic oxygen-evolution system studied by reconstitution experiments. Biochim Biophys Acta. 1985;806:283–289. [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Moskvin OV, Ivanov BN, Ignatova LK, Kollmeier MA. Light-induced stimulation of carbonic anhydrase activity in pea thylakoids. FEBS Lett. 2000;470:375–377. doi: 10.1016/s0014-5793(00)01328-4. [DOI] [PubMed] [Google Scholar]
- Moskvin OV, Razguliayeva AY, Shutova TV, Khristin MS, Ivanov BN, Klimov VV. Carbonic anhydrase activity of different photosystem II preparations. In: Garab G, editor. Photosynthesis: Mechanisms and Effects. II. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1998. pp. 1201–1204. [Google Scholar]
- Moubarak-Milad M, Stemler A. Oxidation-reduction potential dependence of photosystem II carbonic anhydrase in maize thylakoids. Biochemistry. 1994;33:4432–4438. doi: 10.1021/bi00180a042. [DOI] [PubMed] [Google Scholar]
- Pfündel E, Meister A. Flow cytometry of mesophyll and bundle sheath chloroplasts thylakoids of maize (Zea maysL.) Cytometry. 1996;23:97–105. doi: 10.1002/(SICI)1097-0320(19960201)23:2<97::AID-CYTO2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Porra RJ, Thompson WA, Kriedemann PE. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and bextracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta. 1989;975:384–394. [Google Scholar]
- Stemler A. Carbonic anhydrase associated with thylakoids and photosystem II particles from maize. Biochim Biophys Acta. 1986;850:97–107. [Google Scholar]
- Stemler A. An assay for carbonic anhydrase activity and reactions that produce radiolabeled gases or small uncharged molecules. Anal Biochem. 1993;210:328–331. doi: 10.1006/abio.1993.1203. [DOI] [PubMed] [Google Scholar]
- Stemler A. Photosystem II carbonic anhydrase activity depends on Cl− and Ca++ In: Garab G, editor. Photosynthesis: Mechanisms and Effects. II. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1998a. pp. 1193–1196. [Google Scholar]
- Stemler AJ. The case for chloroplast thylakoid carbonic anhydrase. Physiol Plant. 1997;99:348–353. [Google Scholar]
- Stemler AJ. Bicarbonate and photosynthetic oxygen evolution: an unwelcome legacy of Otto Warburg. Indian J Exp Biol. 1998b;36:841–848. [Google Scholar]
- Sültemeyer D, Schmidt C, Fock HP. Carbonic anhydrases in higher plants and aquatic microorganisms. Physiol Plant. 1993;88:179–190. [Google Scholar]
- Thornber JP, Highkins HR. Composition of the photosynthetic apparatus of normal barley leaves and a mutant lacking chlorophyll b. Eur J Biochem. 1974;41:109–116. doi: 10.1111/j.1432-1033.1974.tb03250.x. [DOI] [PubMed] [Google Scholar]
- Vaklinova SG, Goushtina LM, Lazova GN. Carboanhydrase activity in chloroplasts and chloroplast fragments. CR Acad Bulg Sci. 1982;35:721–1724. [Google Scholar]
- Walbot V, Hoisington DA. Isolation of mesophyll and bundle sheath chloroplasts from maize. In: Edelman M, Hallick RB, Chua N-H, editors. Methods in Chloroplast Molecular Biology. Amsterdam: Elsevier Biomedical Press; 1982. pp. 211–219. [Google Scholar]
- Xu Q, Bricker TM. Structural organization of proteins on the oxidizing side of photosystem II. J Biol Chem. 1992;267:25816–25821. [PubMed] [Google Scholar]