Abstract
Vigorous motion can cause rodents to develop flavor aversions and show other signs of malaise. We tested whether a flavor aversion could be induced by shipping mice from an animal breeder to a test site. Boxes of 12 male C57BL/6J mice were shipped ~950 km from Bar Harbor, ME to Philadelphia, PA by truck. For some boxes, the gel provided for hydration was flavored with almond and for others it was flavored with banana. After the journey, the mice were individually housed and allowed to recover for 5 days. They then received a choice between the two flavors of gel. Contrary to expectations, mice preferred the flavor they had previously ingested during shipping. Controls given flavored gel under similar conditions but while stationary did not show a preference. These results suggest that mice find shipping or its sequelae pleasurable. If mice are travel sick this must be inconsequential relative to other components of the shipping experience.
Keywords: Conditioned flavor preference, shipping, transportation, learning, demonstrator effect, travel sickness
Recently, we conducted a series of experiments that were designed to identify optimal procedures for measuring the taste preferences of mice (20–23). These experiments revealed several methods to reduce within-strain variability but even with genetically identical mice and the most stringent experimental controls, there still remained large individual differences. This problem of heterogeneity of behavior in inbred mouse strains is not confined just to taste preferences; many phenotypes are highly variable even when genetic and environmental conditions are stringently controlled [e.g., (2,3,27)].
Casual observations made us wonder whether events occurring during shipping could be responsible for individual differences in taste preferences. Lots of mice are shipped from a breeder to another institution before they are tested. Shipping exposes the mice to many uncontrolled or poorly controlled variables, including several types of motion, loud and irregular noises, disrupted light cycles, temperature fluctuations, food and water restriction and, because mice are often shipped together, new cohorts. All of these individual environmental perturbations have the potential to be stressful, but very little is known about whether they interact or have lasting effects.
Shipping can elevate plasma corticosterone concentrations (1,10,11), suppress immune responses (1,10,11), reduce body weights [even though food and water are available; (25)], cause lung pathology (16), and compromise litter production (9). Recently, Crabbe et al (3) compared the behavioral phenotypes of mice that were either bred in-house or shipped from a supplier. They found only one significant difference related to shipping out of 7 behaviors tested, and consequently discounted shipping as an important influence on their results. However, they had very small group sizes (n = 4/strain/site) and the fact that there were no differences does not imply that shipping did not contribute to variability; merely that it did not have consistent effects on strain means.
Some of the effects of shipping could potentially be a result of travel sickness. Cats, dogs and children quite commonly get travel sick. Here, we tested whether the same is true of mice. One complication is that emesis cannot be used as a measure of gastrointestinal distress in mice because they cannot vomit. However, other measures, such as clay consumption and the acquisition of conditioned flavor aversions, indicate that vigorous motion causes sickness (6,12,14,26). Moreover, in shrews, just 10 min of fairly gentle horizontal motion (1 Hz, 4-cm displacement) is sufficient to cause emesis and a strong conditioned flavor aversion (17,24).
Flavor aversion acquisition requires (a) a novel flavor, and (b) malaise. Typically, mice are shipped with a novel flavor; it is the gel provided for hydration. The mice ingest the gel for the first time when they are placed in the shipping box, a few hours before shipping begins. This is optimal timing for a conditioned flavor aversion to develop if truck motion is a source of illness. Moreover, taste aversions may also result from, or interact with, stresses related to shipping other than motion. For example, taste aversions can be induced or exacerbated in rats by social crowding (15) or anxiety (8), which are likely to be components of shipping.
In the experiment described here, we examined whether mice acquired a conditioned flavor aversion during shipment from The Jackson Laboratory (JAX) in Bar Harbor, Maine to our institution, the Monell Chemical Senses Center (Monell), in Philadelphia, Pennsylvania, a journey of ~950 km by truck. We considered it important to use actual shipping procedures rather than simulated motion in the laboratory because our goal was to answer a practical question:- does shipping influence flavor preferences and thus increase phenotype variability? Without first answering this question it could be fruitless to attempt studies under more controlled conditions.
METHODS
Subjects
The experiment involved a total of 72 male C57BL/6J mice, aged 5 – 8 wk at the time of shipping. All mice were bred at JAX using standard procedures: In the JAX production rooms, temperature is 20 – 22 C, humidity is 44–59%, and lighting is on a 14 h on:10 h off cycle. Radios set to “talk”, soft rock, country or classical stations are played continuously at conservative volumes to provide white noise. The production rooms are specific pathogen-free (SPF) barrier facilities so animal caretakers wash and change into sterile surgical scrub suits to work in the rooms, and wear sterile facemasks, caps, socks, shoes and gloves. When mice are handled, this is accomplished using round-tipped, 10” forceps soaked in a dilute solution of Wescodyne disinfectant/detergent (Steris, Mentor, OH).
The C57BL/6J strain is bred using sibling trios (2 females/1 male). Litter size is typically 7 pups per litter. All mice are housed in polycarbonate cages with wire lids covered by filter bonnets. Bedding is 16–26 mm of steam-autoclaved white pine shavings. Food is autoclaved Purina 5K52 (LabDiets Inc). Hydration is provided by filtered tap water with a pH of 2.8 – 3.1 and added menadone sodium bisulfate (to replace vitamin K activity, because this is lost when the diet is autoclaved).
No special procedures were used for the mice in this experiment until they were selected from the production colony a few hours before they were shipped. All subsequent procedures were approved by the Institutional Animal Care Committees of both Monell and JAX.
General procedures
The basic design involved providing mice with flavored hydration gel during shipping and later assessing their preference for this flavor relative to a novel flavor. Controls received the same flavored gels for the same duration but when housed in shipping boxes that were stationary.
Three separate shipments were made with each consisting of two boxes of 12 mice and a third box containing a datalogger (described below). The first shipment was made on March 29th 2005. It involved 5-wk old mice that would later act as “stationary” controls. We wanted these animals to have no experience with hydration gel while being shipped yet it was important to prevent them from dehydrating. Consequently, the mice were shipped under the same conditions as subsequent shipments (see below) except instead of hydration gel, they had ~200 g wet mash on the floor of their box. The wet mash was made by mixing water 2:1 with powdered chow. This “canned diet” was used routinely by JAX before hydration gels were introduced.
The second and third shipments involved 6- and 8-wk old mice and were made 1 and 3 wk later, respectively. The third shipment was added to the original design; it was made so that we could confirm the unexpected results we found with the second shipment. For both the second and third shipments, one box of 12 mice was provided with an 8-oz sachet of almond-flavored hydration gel; the other box was provided with an 8-oz sachet of banana-flavored hydration gel.
The flavored gels were made in the following manner: Sterile unflavored gel [Napa Nectar, Systems Engineering Lab Group, Napa, CA; see ref (18)] was purchased in 4- or 8-oz (113- or 226-g) clear plastic sachets. According to the manufacturer, Napa Nectar is 99.35% water (by weight) and uses kelgum (from Kelp seaweed) as the gelling agent. The 8-oz sachets were used for training trials, when the mice were in groups, whereas the 4-oz sachets were used for tests, when the mice were individually housed. In order to provide the gel with distinctive cues, we added flavors and colors. These were commercially available products for cooking (imitation almond, imitation banana, red coloring, green coloring; all McCormick brand, McCormick, Hunt Valley, MD). The almond flavor was colored red and the banana flavor colored green by adding 0.5 ml color to a 29-ml bottle of flavor and inverting it several times to mix. Each 8-oz sachet of gel was heated in a microwave oven for ~90 sec to liquefy its contents. A 2-ml bolus of the flavor/color mixture was injected into the center of the gel using a 3-ml syringe with 23 gauge needle. Each 4-oz sachet was heated for 60 sec and then injected with 1 ml of flavor/color mixture. The sachet was then palpitated to ensure that the color was evenly distributed, and then allowed to cool and solidify overnight. We calculate that the gel contained ~0.86% flavor. Pilot work showed that C57BL/6J mice readily consume the gel and find the two flavors used here equally acceptable.
Shipping
Shipping boxes were constructed of plastic with filtered panels for air and light diffusion [see (19) for details]. The external size of a shipping box was 45 × 29 × 19 cm and the internal floor area was 37 × 20 cm. Groups of mice are sometimes separated by a plastic divider in this type of box but the divider was not used in the present experiment. The floor of each box was covered with ~1 cm of pine shavings and a few pellets of Purina 5K52 chow. A sachet of flavored hydration gel was cut open using an X pattern (from opposite diagonal corners), weighed, and placed on the floor. Then, 12 mice were placed in the box and the lid was closed. At approximately 1030 h, the boxes were placed in a transport van and taken from the mouse room to a central shipping warehouse. Here, the boxes were sorted and loaded onto a truck. The truck left JAX at ~1420 h for its trip to Philadelphia.
All mice were shipped from JAX on a Tuesday and arrived at Monell the following day. According to the delivery driver, the truck travels South, primarily on interstate highway I-95, and stops for the driver to rest between approximately midnight and 0330 h. In Linden, NJ, the mice are transferred to a second truck for local delivery, and this truck arrives at Monell at ~0900 h. Thus, the total journey is ~950 km and takes ~19 h.
To provide some information on the environmental conditions experienced during shipping, each shipment included a mouse shipping box containing an Omega Engineering Ultrashock-101 datalogger. This is a self-contained unit that measures 11 × 8 × 3 cm and weighs 425 g. The datalogger was set to record temperature, humidity, air pressure and acceleration every 10 sec. The temperature (resolution; ± 0.1 C) and humidity (resolution; ± 0.5%) data were validated at the end of each trip by comparing the recorded values with those recorded in our vivarium. Measurements of pressure appeared to be confounded by acceleration and so are not presented here. Force was measured continuously at a sampling rate of 1,000 Hz by three accelerometers, with one aligned in each dimension. The maximum acceleration experienced in each dimension was recorded every 10 sec [resolution ±0.05 g, with g referring to gravities not grams; see (13) for technical details]. To provide a frame of reference, the following values are typical: lightly tapping the monitor = 0.05 – 0.10 g, pushing the monitor ~1 cm on a smooth desktop = 0.10 – 0.20 g, shaking the monitor gently = ~0.5 g, vigorous shaking = >1 g. Note that the values obtained do not translate directly into those experienced by mice. Undoubtedly, the mice form their own microclimate by, for example, huddling together, and the recorded acceleration depends on the mass of the datalogger, which was considerably more than a mouse. Nevertheless, they provide a relative measure of the environmental conditions and changes in motion the mice experienced.
The datalogger was placed in the center of the floor of a shipping box, on pine shavings. In order to maintain the JAX pathogen barrier, the box containing the monitor started its trip in the central shipping warehouse, not the mouse room. There were no food pellets, hydration gel, or mice in this box.
At the end of each trip, data from the Ultrashock-101 were uploaded using proprietary software (Omega Engineering, OM-CP, version 2.00.54a). Because basal values of the accelerometers drifted slightly over time (i.e. days), the recorded values were corrected by subtracting the average obtained for the entire recording period. The records were then annotated using information from staff reports (e.g., “the truck left at 1420 h”), details gleaned from the local delivery driver, and comparison with ~25 other recordings we have made of this journey as part of another project.
Procedure at Monell
When mice arrived at Monell they were removed from their shipping boxes by a technician wearing disposable gloves, weighed, and housed in a vivarium under standard conditions (12:12 h light-dark cycle with lights on at 0600 h, temperature ~23 C; humidity ~45%).
The control mice were initially housed in groups of 6 in plastic tub cages, measuring 37 × 33 × 17 cm with a stainless steel grid lid and pine shavings on the floor. A hopper built into the lid provided pelleted Teklad 8604 chow and a 300-ml glass bottle with stainless steel drinking spout provided deionized water. After 5 days, at 1030 h (roughly the same time that the first group of mice was given flavored gel before shipping from JAX), each group of mice was transferred to a JAX shipping box containing pine shavings and a few pellets of Teklad 8604 chow. Instead of a water bottle, the mice were provided with a weighed sachet of either almond- or banana-flavored hydration gel. Thus, there were two boxes of 6 controls given almond-flavored gel and two boxes of 6 controls given banana-flavored gel.
When the experimental group arrived the following day at ~0900 h, the remaining contents of the sachets of flavored hydration gel were weighed after we meticulously removed bedding material stuck to them. All mice, including members of the control groups, were weighed and then individually housed in plastic “tub” cages with stainless steel grid lids. These cages had a hopper built into the lid to provide pelleted Teklad 8604 chow and a 300-ml glass bottle with stainless steel spout to provide deionized water [see (20) for details].
The mice were left undisturbed for 5 days so they could recover from any ill effects of shipping and adapt to vivarium conditions. They then received a 4-day test with a choice between 4-oz sachets of almond- and banana-flavored gel. During the test, water bottles were removed so that the only source of water the mice had available was the two gel sachets. To reduce problems with bedding material sticking to the gel, the normal pine shavings were replaced with a corrugated cardboard sheet cut to fit the cage floor. Both sachets were weighed (± 0.1 g) and placed on the cardboard sheet, with one flavor at the front of the cage and the other at the back. The position of the flavors was counterbalanced. Every 24 h, the sachets were reweighed and their position in the cage switched. All mice had their bedding sheet replaced at this time because some mice tended to shred them.
To assess the contribution of gel evaporation to “intakes”, the weights of eight sachets placed in four empty cages were recorded when measurements were made in the test involving the second set of mice given flavored gel during shipping. We found the average daily evaporation to be (mean ± SD) 3.32 ± 0.43 g (n = 32). Accordingly, we adjusted all daily intakes by subtracting this mean.
RESULTS
Shipping environment
The three trips involving the mice used in this project had similar temperature, humidity, and acceleration profiles (Table 1), and all three trips had relatively moderate ranges of each measure compared with other trips we have monitored. For example, whereas the lowest temperature recorded here was 13.1 C, we have twice (out of 24 trips measured) recorded temperatures below 6 C. The small temperature and humidity ranges observed here most likely reflect the moderate weather experienced during the shipments, which were made in the Spring (Table 1).
Table 1.
Temperature and humidity ranges experienced by mice traveling from Bar Harbor to Philadelphia
| Temperature, C
|
Humidity, %RH
|
||||
|---|---|---|---|---|---|
| Date | Group of Mice | Min | Max | Min | Max |
| 03/29/05 | Controls | 13.8 | 25.7 | 30.5 | 44.0 |
| 04/05/05 | Experimental set 1 | 14.7 | 24.2 | 30.0 | 49.0 |
| 04/19/05 | Experimental set 2 | 13.1 | 26.8 | 16.5 | 44.0 |
Notes: Experimental set 1 and 2 traveled with flavored hydration gel in their shipping boxes.
Figure 1 provides details of the temperature, humidity, and acceleration recorded by the datalogger accompanying the first set of mice shipped with flavored gel. At 0812 h, at JAX, the monitor was loaded into its shipping box and taken to the central shipping warehouse. Here it was placed in the same truck as the mice (most likely at 1126 h). At 1420 h, the truck left JAX for its journey South. This truck had excellent climate control but there was more-or-less continuous mild vertical acceleration (i.e., shaking or bouncing). Just before midnight (2356 h) the truck stopped, presumably for the driver to take a federally mandated break, and did not continue until 0231 h. At 0353 h, the truck arrived at a distribution center (in Marlton, NJ), and the mice were transferred to a local delivery truck. This truck lacked good temperature control and judging by the higher acceleration peaks, had poorer suspension or experienced more start-stop traffic and potholes than did the first truck. The monitor and mice were delivered to Monell at 0852 h, where they were transferred to a vivarium.
Figure 1.
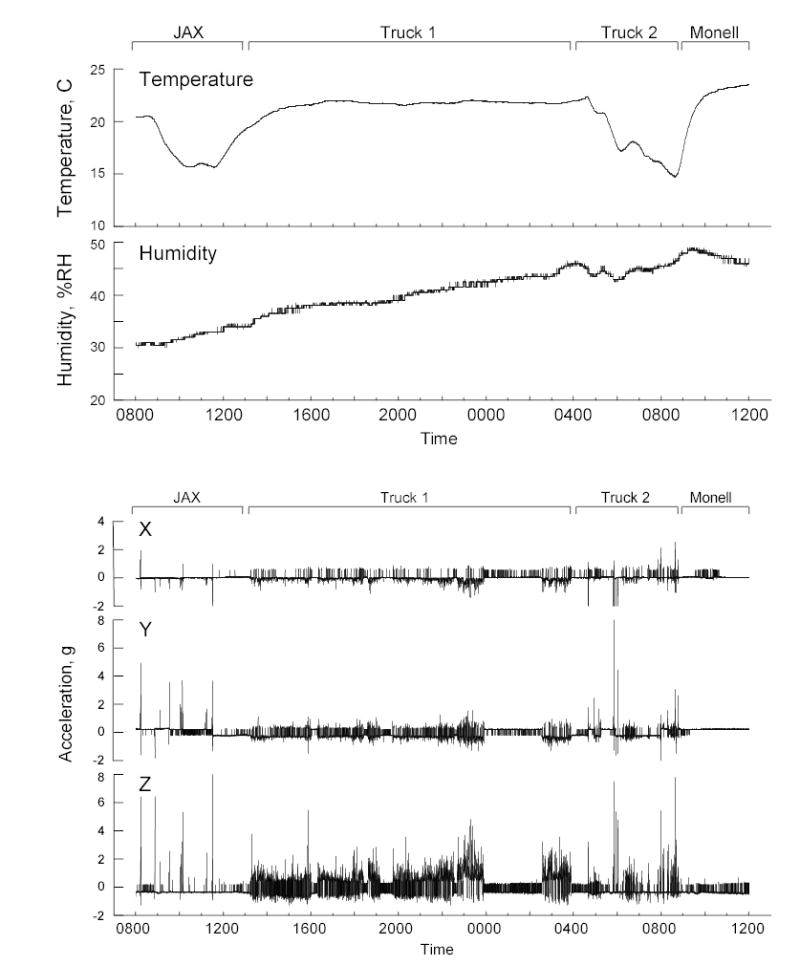
Temperature, humidity and acceleration while mice had access to flavored gel during shipment from The Jackson Laboratory (JAX) in Bar Harbor, ME to the Monell Chemical Senses Center (Monell) in Philadelphia, PA. The records show four components of the trip: 1. 0800 – 1420 h: At JAX, the monitor is loaded into the shipping box (at 0812 h) and the box is taken to the central shipping warehouse where it is placed on the appropriate truck. 2. 1420 – 0353 h: Long-distance truck journey (Truck 1), with a rest break between 2356 – 0231 h. 3. 0353 – 0852 h: Transfer and travel in local delivery truck (Truck 2). 4. 0852 h onwards: Delivery to Monell, transfer to a vivarium and recovery. Note: The two relatively large drops in temperature occurred while the box was waiting to leave JAX and while in a local delivery truck.
Flavored gel intake during shipping
Due to a technical error, we lost the initial weights of the two sachets used in the first replication of mice shipped with flavored gel. However, we weighed 12 unused sachets of gel and found very little variation in weight (mean ± SD = 234.99 ± 0.46 g) so we used this mean value (corrected for the addition of 2 ml flavor) to calculate gel intakes during shipping of this replication. For the other replications, we calculated gel intake by subtracting the weight of each sachet after access to the gel from the weight before it was provided. For the controls and two replications of mice shipped with flavored gel, intakes averaged 3.91 g, 1.05 g, and 2.66 g per mouse over the ~19 h period. Intakes of almond and banana flavor were similar (overall means; 2.73 vs. 3.03 g/mouse). We consider it inappropriate to subject these values to statistical analysis: The means for the experimental groups are based on only two boxes, there was no way to know how much gel was spilled or evaporated, and because the mice were grouped together we do not know how much each individual mouse consumed. However, the measurements establish that at least some mice in each box consumed some flavored gel, and they also suggest that mice consume less gel while being shipped than when stationary.
At the end of shipping or the equivalent period for controls, mice from the two replications that shipped with flavored gel weighed 21.0 ± 0.3 g and 21.9 ± 0.2 g, respectively and the controls weighed 21.1 ± 0.3 g (all values are mean ± SE, n = 24). The first set of mice shipped with flavored gel and the control mice had statistically indistinguishable weights. The second set of mice shipped with flavored gel weighed significantly more than did the other two groups, F(2,69) = 3.91, p = 0.0247. These weights are consistent with the difference in ages between the shipments; the control and first set of mice shipped with flavor were 6 wk; the second set of mice shipped with flavors were 8 wk.
Flavored gel intake during the choice test
The pattern of intakes of flavored gel during the two-sachet choice test were remarkably consistent from day-to-day so, for simplicity, we present values for the entire 96-h test here. To analyze these data, we first collated them according to whether each flavor was paired (i.e., given during shipping) or unpaired (i.e., novel) and then conducted a mixed-design analysis of variance to determine whether the three groups consumed different amounts of these flavor types. There was a significant group x flavor type interaction, F(2,69) = 3.38, p = 0.0396. Whereas controls consumed statistically similar amounts of the two flavor types, both groups of experimental mice consumed more of the flavor they were shipped with than the novel flavor (Fig. 2; post hoc LSD tests, 1st shipment, p = 0.0024; 2nd shipment, p = 0.0004). This effect was reflected in the preferences of individual mice: Intakes of the previously experienced flavor were higher than intakes of the novel flavor in 11 out of 24 controls, 18 out of 24 of the mice in the first group shipped with a flavor, and 20 out of 24 of the mice in the second group shipped with a flavor.
Figure 2.
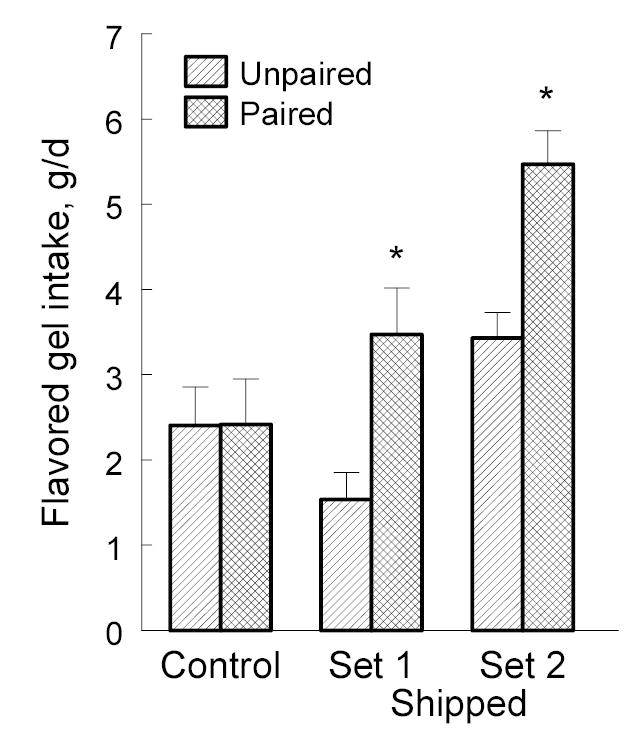
Intake of flavored hydration gel during a 96-h choice test between two flavors; one that was previously available during shipping (or an equivalent period for controls; Paired) and the other that was novel (Unpaired). *p<0.05 relative to unpaired flavor. n = 24 per group.
Total gel consumption (i.e., the sum of unpaired and paired flavor intakes) was similar for the control group (4.8 ± 0.6 g/d) and first set of mice shipped with flavored gel (5.0 ± 0.6 g/d). Consistent with their older age and higher body weight, the second set of mice shipped with flavored gel had significantly higher total gel intakes than did the other two groups [9.2 ± 0.5 g/d, F(2,69) = 25.5, p<0.0001].
We conducted separate analyses to examine the extent to which the following contributed to the results: (a) whether the paired flavor was almond or banana, (b) whether the paired flavor was available first at the front or back of the cage, and (c) whether total intakes differed from day-to-day. The mice showed no preference for almond or banana [overall, n = 72; almond intake = 3.0 ± 0.4 g/d, banana intake = 3.4 ± 0.3 g/d]. They consumed equal amounts of gel whether it was placed at the front or back of their cage (overall, n = 72; front = 3.2 ± 0.2 g/d, back = 3.2 ± 0.2 g/d), and they consumed less on the first day of the test than on subsequent days (overall, n = 72, first day = 4.8 ± 0.5 g/d, second day = 7.1 ± 0.4 g/d, third day = 8.3 ± 0.6 g/d, fourth day = 7.4 ± 0.5 g/d).
DISCUSSION
Mice that were shipped with an arbitrarily flavored hydration gel acquired a preference for this flavor relative to a flavor they had never consumed before. Control mice that were given the same exposure to flavored gel while under similar conditions but remained stationary had equal preferences for the two flavors. These findings argue that (a) some aspect of shipping induces a flavor preference in mice, and (b) this is not simply because the mice are familiar with one of the flavors or had consumed it recently. Given that the mice traveling with flavored gel developed a preference for it, we conclude that the motion associated with shipping by truck is not a significant source of malaise. If shipping does cause malaise its effects to produce a flavor aversion must be offset or obscured by stronger acquired preferences.
We can think of several possible reasons why mice acquire flavor preferences during shipping. First, they may enjoy gentle motion, in much the same way that many humans take pleasure from amusement park rides or babies are soothed by rocking. Second, perhaps ingesting the kelgum gelling agent or another component of the flavored gel dispels gastrointestinal distress in the same way as does kaolin consumption. Third, perhaps the act of gel consumption reduces anxiety, which can be a cause of taste aversion (8). Fourth, perhaps the mice are too scared, anxious, or excited to drink when first placed in the shipping container so they become thirsty; when they overcome this initial reluctance the gel satisfies their thirst and consequently increases the hedonic value of its flavor. There is no compelling evidence for any of these possibilities, nor can we rule any of them out.
Initially, we thought it possible that an uncontrolled difference in social experience may have been responsible for the flavor preference shown by “shipped” but not “stationary” mice. This idea was based on work showing that rats given a choice between two flavors, one of which they have previously eaten alone and the other they have eaten together with another rat, prefer the flavor eaten with the stranger (4). Because the experimental mice were placed into groups immediately before shipping, it was possible that the excitement of meeting strangers might mediate their flavor preference. The controls were maintained in the same groups for 6 days before being given flavors, so their group-mates were no longer novel. To determine whether experience with a new stranger could influence flavor preferences, we conducted a follow-up experiment in which four groups of four mice received flavored gel in stationary shipping boxes, following identical procedures to those used with the control group in the experiment outlined here. Two of the four groups consisted of mice that were housed together for 6 days whereas the other two groups consisted of two “friends” (housed together for 6 days) and two strangers (introduced at the same time flavored gel was given). When, later, the mice were individually housed and given a choice between the two flavors, mice from the groups comprised solely of “friends” and those from groups containing strangers had similar intakes of both flavors, and these did not deviate significantly from indifference (i.e., 50% preference). Thus, we do not think that differences in social experience can account for the differences in flavor preferences observed here.
Perfect control is impractical if not impossible to achieve in studies involving transportation. We used controls that were shipped a week before they received the flavored gels (i.e., a US-CS control). To avoid giving the controls more experience than the experimental groups with flavored gel, the controls were shipped with wet mash. Despite this precaution, the controls consumed more of the flavored gel during training than did the experimental groups. This could introduce a confound but, fortunately, the amount of flavor consumed during training is not a very critical determinant of subsequent preferences or aversions in flavor conditioning experiments. During the week while the control mice were waiting before being given access to flavored gel, they were group housed, and as discussed above, this may have provided a confounding source of experience. However, we note that even if the data from controls are disregarded, the finding of flavor preferences in two independent experimental groups makes a strong case that mice do not form an aversion to the gel they consume during shipping.
The lower flavored gel intakes of shipped than stationary mice could potentially be a sign of illness. This finding is consistent with previous work showing that mice and rats lose body weight during shipping even though food and water are available (5,10,11,25). However, this does not necessarily imply that motion sickness is responsible. Simply transferring mice from a laboratory cage to a transit box can cause weight loss (25). Moreover, eating with a conspecific reduces intake of flavored food, at least in rats (4).
An impetus for this study was to understand why mice of inbred strains often display marked phenotypic variability, even when experimental procedures are rigorously controlled [e.g., (3)]. Shipping is arguably the most poorly controlled event in the early life of most laboratory mice, and so it seems a likely potential source of phenotypic variability. We note that mice shipped with flavored gel showed a wide range of preferences for the flavor they were shipped with (i.e., 30 – 86%). This may reflect differences in the amount ingested or exposure to the flavor during shipping, the time the gel was first eaten, the degree of malaise or anxiety experienced, and social hierarchy. In most cases, it would be easier to breed animals in-house than attempt to control for all these factors.
Acquired flavor preferences and aversions are usually long-lasting, if not permanent [e.g., (7)], so changes in food acceptance produced by shipping are likely to persist well into adult life. Preferences for a specific flavor generalize to other flavors, so shipping conditions may influence preferences for, and consumption of, a variety of foods and drinks. Moreover, in situations where flavor preferences develop mice most likely acquire preferences for other aspects of their environment including their bedding and the walls of their box (i.e., place conditioning). This could potentially influence several adult phenotypes such as odor recognition, open field behavior and elevated maze performance. At the very least, the results of this study remind us that mice are not tabulae rasae when they arrive in the laboratory. Their early life experiences can have profound, latent and, as in this case, unexpected effects on later phenotypes.
Acknowledgments
We thank several staff of The Jackson Laboratory for help arranging and conducting this project. Rosalie Robidoux coordinated the project at JAX. Jennifer Littlefield mixed the flavored gels and gave them to the appropriate mice. Dawn Jellison, Laura Mathews, and Karen Palosky arranged for shipping of the datalogger that measured shipping motion and environment. Kitty Barbee-Ellsworth shepherded the request to conduct this study through JAX administration and animal care approval. Jay Palmer provided much useful information about husbandry conditions of C57BL/6J mice at JAX.
Several Monell staff provided useful advice about the design of this experiment, including Drs. Alexander Bachmanov, Gary Beauchamp, Mark Friedman, Arla Hile, Yanina Pepino and Danielle Reed. Funding was provided by institutional funds from the Monell Chemical Senses Center.
References
- 1.Aguila HN, Pakes SP, Lai WC, Lu YS. The effect of transportation stress on splenic natural killer cell activity in C57BL/6J mice. Lab Anim Sci. 1988;38:148–151. [PubMed] [Google Scholar]
- 2.Burcelin R, Crivelli V, Dacosta A, Roy-Tirelli A, Thorens B. Heterogeneous metabolic adaptation of C57BL/6J mice to high-fat diet. Am J Physiol Endocrinol Metab. 2002;282:E834–842. doi: 10.1152/ajpendo.00332.2001. [DOI] [PubMed] [Google Scholar]
- 3.Crabbe JC, Wahlsten D, Dudek BC. Genetics of mouse behavior: interactions with laboratory environment. Science. 1999;284:1670–1672. doi: 10.1126/science.284.5420.1670. [DOI] [PubMed] [Google Scholar]
- 4.Duncan HJ, Buxbaum A, Tordoff MG. Rats eating together prefer the taste of their food. Ann NY Acad Sci. 1987;510:263–264. [Google Scholar]
- 5.Dymsza HA, Miller SA, Maloney JF, Foster HL. Equilibration of the laboratory rat following exposure to shipping stresses. Lab Anim Sci. 1963;13:61–65. [Google Scholar]
- 6.Fox RA, Lauber AH, Daunton NG, Phillips M, Diaz L. Off-vertical rotation produces conditioned taste aversion and suppressed drinking in mice. Aviat Space Environ Med. 1984;55:632–635. [PubMed] [Google Scholar]
- 7.Galef BG., Jr Enduring social enhancement of rats’ preferences for the palatable and the piquant. Appetite. 1989;13:81–92. doi: 10.1016/0195-6663(89)90106-2. [DOI] [PubMed] [Google Scholar]
- 8.Guitton MJ, Dudai Y. Anxiety-like state associates with taste to produce conditioned taste aversion. Biol Psychiatry. 2004;56:901–904. doi: 10.1016/j.biopsych.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 9.Hayssen V. Effect of transatlantic transport on reproduction of agouti and nonagouti deer mice, Peromyscus maniculatus. Lab Anim. 1998;32:55–64. doi: 10.1258/002367798780559482. [DOI] [PubMed] [Google Scholar]
- 10.Landi, M.; Bowman, T. A.; Campbell, S. Effects of handling and transportation stress on rodents. In: Beynen, A. C.; Solleveld, H. A., eds. New developments in biosciences: their implications for laboratory animal science. Dordrecht: Martinus Nijhoff; 1987:449–457.
- 11.Landi MS, Kreider JW, Lang CM, Bullock LP. Effects of shipping on the immune function in mice. Am J Vet Res. 1982;43:1654–1657. [PubMed] [Google Scholar]
- 12.McCaffrey RJ. Appropriateness of kaolin consumption as an index of motion sickness in the rat. Physiol Behav. 1985;35:151–156. doi: 10.1016/0031-9384(85)90329-4. [DOI] [PubMed] [Google Scholar]
- 13.Omega Engineering. OM-CP-ULTRASHOCK101 Humidity, Temperature, Pressure and Tri-Axial Shock Datalogger. http://www.omega.com/ppt/pptsc.asp?ref=OM-CP-ULTRASHOCK101 2004.
- 14.Ossenkopp KP, Bettin MA, Kavaliers M. The effects of naloxone on body rotation-induced analgesia and anorexia in male mice. Pharmacol Biochem Behav. 1989;34:317–320. doi: 10.1016/0091-3057(89)90318-3. [DOI] [PubMed] [Google Scholar]
- 15.Pilcher CW, Jones SM. Social crowding enhances aversiveness of naloxone in rats. Pharmacol Biochem Behav. 1981;14:299–303. doi: 10.1016/0091-3057(81)90394-4. [DOI] [PubMed] [Google Scholar]
- 16.Slanetz CA, Fratta I, Crouse CW, Jones SC. Stress and transportation of animals. Proc Anim Care Panel. 1957;7:278–289. [Google Scholar]
- 17.Smith JE, Friedman MI, Andrews PL. Conditioned food aversion in Suncus murinus (house musk shrew) - a new model for the study of nausea in a species with an emetic reflex. Physiol Behav. 2001;73:593–598. doi: 10.1016/s0031-9384(01)00538-8. [DOI] [PubMed] [Google Scholar]
- 18.Systems Engineering Lab Group Inc. Napa nectar. http://www.selabgroup.com/napanectar.htm 2002.
- 19.The Jackson Laboratory. JAX mice shipping container. http://jaxmice.jax.org/orders/shipping_container.html 2004.
- 20.Tordoff, M. G.; Bachmanov, A. A. Monell mouse taste phenotyping project. Monell Chemical Senses Center. www.monell.org/MMTPP 2001.
- 21.Tordoff MG, Bachmanov AA. Influence of test duration on the sensitivity of the two-bottle choice test. Chem Senses. 2002;27:759–768. doi: 10.1093/chemse/27.9.759. [DOI] [PubMed] [Google Scholar]
- 22.Tordoff MG, Bachmanov AA. Mouse taste preference tests: Why only two bottles? Chem Senses. 2003;28:315–324. doi: 10.1093/chemse/28.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tordoff MG, Pilchak DM, Williams JA, McDaniel AH, Bachmanov AA. The maintenance diets of C57BL/6J and 129X1/SvJ mice influence their taste solution preferences: Implications for large-scale phenotyping projects. J Nutr. 2002;132:2288–2297. doi: 10.1093/jn/132.8.2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ueno S, Matsuki N, Saito H. Suncus murinus as a new experimental model for motion sickness. Life Sci. 1988;43:413–420. doi: 10.1016/0024-3205(88)90520-6. [DOI] [PubMed] [Google Scholar]
- 25.Wallace ME. Effects of stress due to deprivation and transport in different genotypes of house mouse. Lab Anim. 1976;10:335–347. doi: 10.1258/002367776781035260. [DOI] [PubMed] [Google Scholar]
- 26.Wang Z, Liu L, Jiang B. Motion sickness in mice and conditioned taste aversion. Chin Med J (Engl) 1994;107:712–714. [PubMed] [Google Scholar]
- 27.Wolfer DP, Litvin O, Morf S, Nitsch RM, Lipp HP, Wurbel H. Laboratory animal welfare: cage enrichment and mouse behaviour. Nature. 2004;432:821–822. doi: 10.1038/432821a. [DOI] [PubMed] [Google Scholar]


