Abstract
The actin cytoskeleton is hypothesized to play a major role in gravity perception and transduction mechanisms in roots of plants. To determine whether actin microfilaments (MFs) are involved in these processes in stem-like organs, we studied gravitropism in Arabidopsis inflorescence stems and hypocotyls. Localization studies using Alexa Fluor-phalloidin in conjugation with confocal microscopy demonstrated a longitudinally and transversely oriented actin MF network in endodermal cells of stems and hypocotyls. Latrunculin B (Lat-B) treatment of hypocotyls caused depolymerization of actin MFs in endodermal cells and a significant reduction of hypocotyl growth rates. Actin MFs in Lat-B-treated inflorescence stems also were disrupted, but growth rates were not affected. Despite disruption of the actin cytoskeleton in these two organs, Lat-B-treated stems and hypocotyls exhibited a promotion of gravitropic curvature in response to reorientation. In contrast, Lat-B reduced gravitropic curvature in roots but also reduced the growth rate. Thus, in contrast to prevailing hypotheses, our results suggest that actin MFs are not a necessary component of gravitropism in inflorescence stems and hypocotyls. Furthermore, this is the first study to demonstrate a prominent actin MF network in endodermal cells in the putative gravity-perceiving cells in stems.
Plants can sense numerous environmental factors, and gravitropism is one of the most important directed growth responses in terms of early development. Gravitropism can be divided into three temporal phases: perception, transduction, and response (Mullen et al., 1998; Kiss, 2000). The proposed sites of gravity perception are columella cells in roots (Sack, 1997; Blancaflor et al., 1998) and endodermal cells in hypocotyls/stems (Kiss et al., 1997; Fukaki et al., 1998; Weise and Kiss, 1999). A great deal of experimental evidence supports the starch-statolith hypothesis and the importance of plastids for gravity perception in plants (Sack, 1997; Weise et al., 2000).
In addition to amyloplasts, actin microfilaments (MFs) are also considered to play a role in the early phases of gravitropism (Baluska and Hasenstein, 1997; Perbal et al., 1997). In general, actin MFs are a normal component of plant cells (Parthasarathy et al., 1985), and their dynamic structure in plants is considered to be important for cellular processes, such as cell division and elongation (Hepler et al., 1993; Baluska et al., 2001). The organization of the actin cytoskeleton in elongating cells has been studied by many researchers and appears to be composed of longitudinally oriented actin MF bundles and transversely oriented actin MFs (Parthasarathy et al., 1985; Thimann et al., 1992; Volkmann et al., 1993).
Root columella cells exhibit a different actin MF organization compared with elongating plant cells (Baluska and Hasenstein, 1997; Collings et al., 2001). Recent studies based on electron microscopy showed nonoriented actin MFs in the central region of a root columella cell in maize (Zea mays; Yoder et al., 2001) and in the statocytes of lentil (Lens culinaris) roots (Driss-École et al., 2000b). Taken together, instead of the oriented actin MF network observed in most plant cells, it has been proposed that root statocytes have a nonoriented actin MF network, and sedimented plastids are enmeshed with actin MFs (for review, see Kiss, 2000). In addition to the observation of amyloplasts surrounded by actin MFs, localization studies demonstrated that there is a domain of myosin-related proteins among and around amyloplasts in root columella cells of maize (Baluska and Hasenstein, 1997).
Statoliths in rhizoids of Chara spp. and in root statocytes of lentil and cress (Lepidium sativum) are sedimented to the distal cell wall under 1g conditions. However, under microgravity, these statoliths exhibited a basipetal displacement (upward movement from the distal end in a cell; Volkmann et al., 1991; Driss-École et al., 2000a). Moreover, the velocity of statolith basipetal displacement was decreased when cytochalasin D (an actin MF-depolymerizing drug) was applied to lentil roots (Driss-École et al., 2000a). Thus, these authors proposed that an actomyosin-based system might have caused the statolith basipetal displacement.
Results from the experiments by Kandasamy and Meagher (1999) with the actin-targeting drug latrunculin B (Lat-B) also support the interaction of actin MFs with plastids. These researchers performed experiments with Arabidopsis mesophyll cells, in which chloroplast positions are highly regulated by the actin cytoskeleton. Experiments with other actin-targeted drugs, such as cytochalasins, further support the possible interaction of actin MFs with plastids (Driss-École et al., 2000a). Taken together, many researchers have suggested that the actin MF network is involved in the mechanisms of gravitropism (Baluska and Hasenstein, 1997; Perbal et al., 1997; Kiss, 2000).
There are two major hypotheses for actin-based gravisensing in roots—the actin-tether model (Baluska and Hasenstein, 1997) and the tensegrity model (Yoder et al., 2001). The actin-tether model proposes that gravisensing occurs based on distinct connections of amyloplasts to the plasma membrane by actin MFs (Baluska and Hasenstein, 1997). In contrast, the tensegrity model proposes that gravity is sensed when a dense actin-based cytoskeleton network in the central region of a columella cell is disrupted by translocated amyloplasts and that discrete connections between amyloplasts and the plasma membrane are not necessary (Yoder et al., 2001).
Although many researchers have proposed the involvement of actin MFs in gravitropism, there have been studies on gravitropic curvature using actin MF inhibitors, which suggests that MFs are not involved in gravitropism. Staves et al. (1997) examined gravitropic curvature using cytochalasin D on roots of rice (Oryza sativa), maize, and cress. These workers did not find any reduction in root gravitropic curvature despite the disruption of actin MFs. Thus, they suggested that root gravitropism does not require an interaction of amyloplasts with MFs for gravity sensing. In addition, Blancaflor and Hasenstein (1997) performed a similar experiment on maize roots with cytochalasins and also reported no effect on gravitropism.
Although many studies have focused on the role of actin MFs in root gravitropism, there have been few investigations on the role of actin MFs in gravitropism of hypocotyls and inflorescence stems. In one of these reports, Volkmann et al. (1993) studied actin MFs in endodermal cells of cress hypocotyls and reported on the organization of the actin cytoskeleton. These authors proposed that actin MFs in endodermal cells are not directly involved in gravity sensing in hypocotyls and concluded that gravisensing occurs when the central vacuole is deformed by displacement of amyloplasts in endodermal cells (Volkmann et al., 1993).
Lat-B was chosen for the present study because of its specificity and efficiency resulting in actin MF depolymerization. Both cytochalasins and Lat-B are known as actin-targeted drugs that bind to either the barbed end of F-actin (Pendleton and Koffer, 2001) or to G-actin (Spector et al., 1999), respectively. According to Pendleton and Koffer (2001), although both cytochalasins and Lat-B target actin, only Lat-B can completely deplete cellular F-actin. In a recent study with Arabidopsis seedlings, Baluska et al. (2001) demonstrated complete actin MF depolymerization in roots and concluded that seedling dwarfism resulted from decreased cell elongation because of Lat-B-induced depolymerization.
To investigate a possible involvement of actin MFs in the mechanisms of gravitropism, we studied the effects of Lat-B on gravitropism in inflorescence stems, hypocotyls, and roots of Arabidopsis. Results from time course of curvature studies with Lat-B-treated stems and hypocotyls demonstrated a significant promotion of gravicurvature relative to the controls. By using confocal microscopy, we also show a concomitant disruption of the actin cytoskeleton in the putative gravity-perceiving endodermal cells of these organs. Therefore, in contrast to prevailing hypotheses, our results suggest that actin MFs are not a necessary component of gravitropism in inflorescence stems and hypocotyls.
RESULTS
Lat-B Results in Dose-Dependent Promotion of Gravicurvature in Hypocotyls
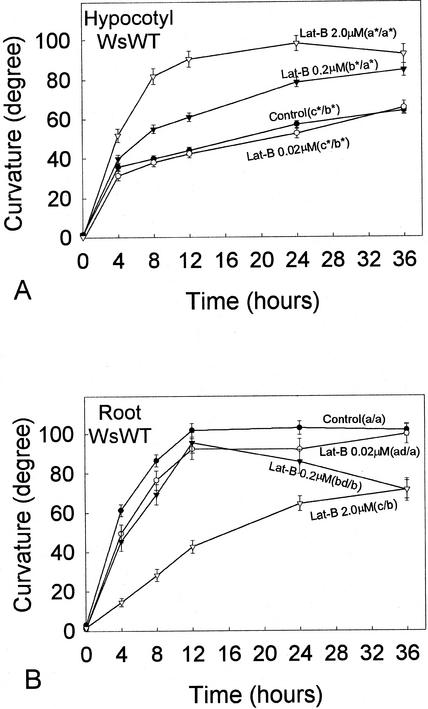
The effect of Lat-B on gravitropic curvature of Arabidopsis wild-type (WT) seedlings was examined with three different Lat-B concentrations, 0.02, 0.2, and 2.0 μm (Fig. 1). Curvature of 0.02 μm Lat-B-treated hypocotyls was not affected by Lat-B throughout the time course compared with the control (Fig. 1A). The application of 0.2 μm Lat-B significantly promoted hypocotyl curvature compared with the control, and curvature reached near the vertical position (about 80°) at 36 h after reorientation. When 2.0 μm Lat-B was applied, hypocotyl curvature was promoted to an even greater degree and reached a plateau at the vertical position after 12 h after reorientation. Statistical analyses confirmed that curvature of hypocotyls treated with 0.2 μm and 2.0 μm Lat-B (but not 0.02 μm Lat-B) was significantly greater at 24 and 36 h after reorientation (P < 0.05) compared with the control (Fig. 1A).
Figure 1.
Time course of gravitropic curvature (after reorientation) of Arabidopsis WT hypocotyls (A) and roots (B) with 0.02 μm, 0.2 μm, or 2.0 μm Lat-B. Different letters after labels in plots indicate statistical differences in curvature, which were determined by a one-way ANOVA test (P < 0.05). Where the criteria of the one-way ANOVA test were not met, an ANOVA on ranks (P < 0.05) was used (indicated by an asterisk). Dunn's method (P < 0.05) was used after either the one-way ANOVA test or the ANOVA on ranks for multiple comparisons. Left and right letters in the parentheses are results of the statistical analyses for the curvature at 24 and 36 h after reorientation, respectively. A, Hypocotyls: The application of 0.02 μm Lat-B did not affect hypocotyl curvature compared with the untreated controls; in contrast, the curvature of 0.2 or 2.0 μm Lat-B-treated hypocotyls was promoted. B, Roots: The application of 0.02 μm Lat-B did not affect root curvature compared with the untreated controls throughout the time course. Roots treated with 0.2 μm Lat-B first curved at the same rate as the control; however, after 12 h following reorientation, their curvature started decreasing. The curvature of 2.0 μm Lat-B-treated roots was significantly decreased and delayed. Black circle, Untreated control (n = 98 [A], 80 [B]). White circle, 0.02 μm Lat-B treatment (n = 57 [A], 37 [B]). Black triangle, 0.2 μm Lat-B treatment [n = 92 (A), 53 [B]). White triangle, 2.0 μm Lat-B treatment (n = 85 [A], 72 (B)]. Error bars represent se.
In the first 4 h after reorientation, the curvature rates of all Lat-B-treated hypocotyls were similar to that of the control. The promotion of curvature of Lat-B-treated hypocotyls became gradually apparent after 4 h in the time course (Fig. 1A). The control and 0.02 μm Lat-B-treated hypocotyls reduced their curvature rates at 4 h after reorientation, and they did not reach 80° within 36 h. The curvature rate of 0.2 μm Lat-B-treated hypocotyls started gradually decreasing at 4 h after reorientation; however, their curvature eventually reached almost vertical position (about 80°) at 36 h. For 2.0 μm Lat-B-treated hypocotyls, almost no decrease in curvature rate was observed until they reached near the vertical position (80°–90°) at 12 h after reorientation.
Although growth rates of 0.2 μm/2.0 μm Lat-B-treated hypocotyls were severely reduced (P < 0.05; Table I) compared with the untreated controls, their curvatures were significantly promoted. For 0.02 μm Lat-B-treated hypocotyls, the growth rate was not inhibited compared with the untreated controls (P > 0.05; Table I).
Table I.
Effects of Lat-B on hypocotyl and root growth rates of WT Arabidopsis seedlings during gravitropism experiments
| Lat-B Concentration | WsWT Growth Rate
|
|||
|---|---|---|---|---|
| Hypocotyls | Roots | |||
| mm h−1 ± se | n | mm h−1 ± se | n | |
| Control | 0.25 ± 0.01 (a*) | 98 | 0.10 ± 0.00 (a*) | 80 |
| 0.02 μM | 0.25 ± 0.01 (a*) | 57 | 0.10 ± 0.01 (a*) | 37 |
| 0.2 μM | 0.19 ± 0.01 (b*) | 92 | 0.06 ± 0.00 (b*) | 53 |
| 2.0 μM | 0.08 ± 0.00 (c*) | 85 | 0.03 ± 0.00 (c*) | 72 |
Growth was measured from the same plants that were used in time course of curvature studies. Differences among the growth rates in four treatments (no Lat-B, 0.02, 0.2, and 2.0 μm Lat-B) were determined by a one-way ANOVA test (P < 0.05) and indicated by different letters in parentheses. Where the criteria of the one-way ANOVA test were not met, an ANOVA on ranks (P < 0.05) was used (indicated by an asterisk). Dunn's method (P < 0.05) was used for multiple comparisons.
Effect of Lat-B on Roots
Similar to the effects on hypocotyls, 0.2 and 2.0 μm, but not 0.02 μm, Lat-B treatment caused significant reduction in root growth rates compared with the untreated controls (P < 0.05; Table I). However, in contrast to hypocotyls, the application of Lat-B to Arabidopsis WT roots did not promote curvature (Fig. 1B). Statistical analyses on the root curvature at 36 h after reorientation indicated that curvature of only 0.2 and 2.0 μm, but not 0.02 μm, Lat-B-treated roots was reduced compared with the untreated controls (P < 0.05; Fig. 1B). The control, 0.02 μm Lat-B-treated, and 0.2 μm Lat-B-treated roots did not differ from each other in curvature rates in the first 12 h after reorientation and reached the vertical position within 12 h after reorientation. However, 2.0 μm Lat-B-treated roots were delayed in curvature from the beginning of the time course and did not reach the vertical position by 36 h after reorientation.
Once the control hypocotyls reached a plateau at the vertical position, they maintained the vertical curvature until the end of the time course. In contrast, although curvature of 0.2 μm Lat-B-treated roots exhibited the same curvature rate as the control until they reached the vertical position, their curvature started gradually decreasing, and eventually curvature at 36 h became significantly reduced (P < 0.05) compared with that of the control.
Alexa Fluor-Phalloidin Labeling of Actin MFs in Hypocotyl Endodermal Cells
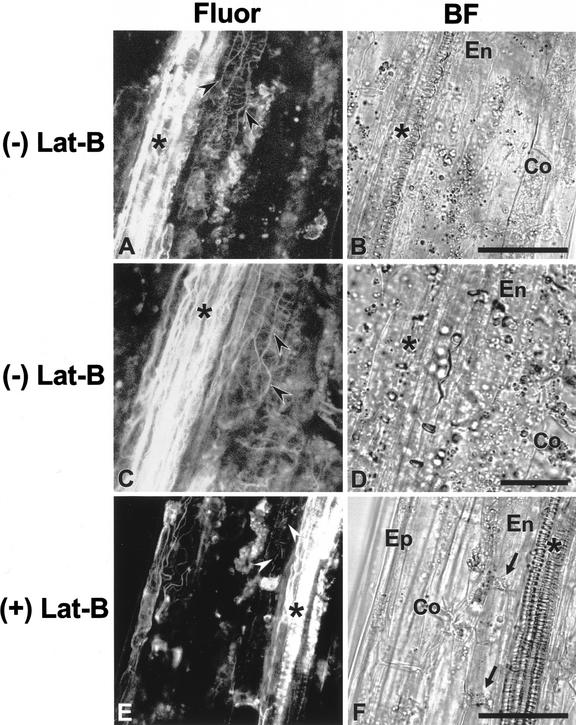
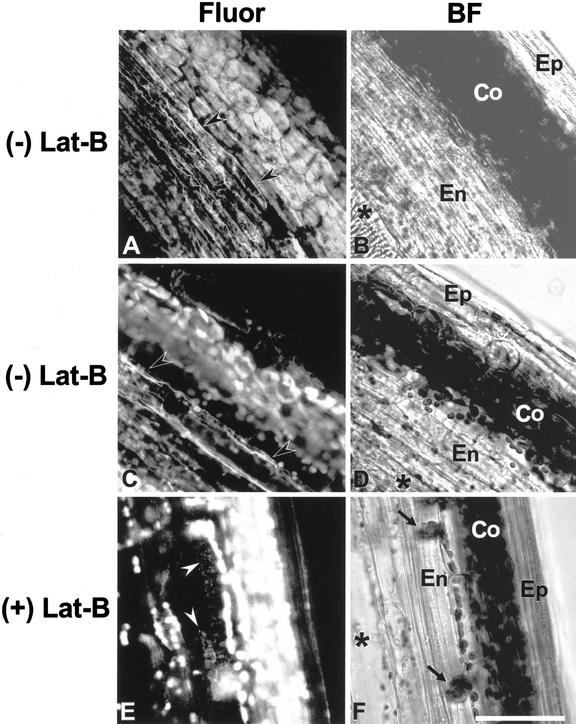
To examine actin MF organization in endodermal cells, we stained WT hypocotyls with Alexa Fluor-phalloidin (Fig. 2, A–D). In sections of hypocotyls, vascular bundles were easily recognized because they exhibited a strong fluorescence signal (Fig. 2, A and C; see also Blancaflor and Hasenstein, 1997). Endodermal cells are found between vascular bundles and the wide cortical cells (Fig. 2, B, D, and F; Fig. 3, B and D) and were recognized by their distinctive morphology (i.e. having a relatively greater longitudinal compared with transverse length). Actin MFs were observed in endodermal cells and were arranged longitudinally and transversely (Fig. 2, A and C). Longitudinally oriented actin MFs were thick bundles, whereas transversely oriented actin MFs were thinner relative to longitudinal bundles. These latter actin MFs appeared to be connected to each other and appeared to form a net-like structure (Fig. 2, A and C). Because the transverse actin MFs emanated a very weak signal, the photomultiplier tube (PMT) gain value had to be increased to visualize them when samples were scanned. As a consequence, some undesirable noise was captured in the background.
Figure 2.
Confocal and brightfield images of endodermal cells in hypocotyls. Demonstration of actin MF organization in endodermal cells (A and C), and disruption of actin MF network in endodermal cells after an application of Lat-B (E). On the right of each fluorescence image, a corresponding brightfield image is shown (B, D, and F). After hand sectioning of hypocotyls, actin MFs were fixed with MBS and then stained with Alexa Fluor 488-phalloidin. The two fluorescence images showing the actin MF network in endodermal cells are representative of about 15 different hypocotyl specimens. The fluorescence image of Lat-B-treated hypocotyls is representative of about 10 different Lat-B-treated specimens. Actin MFs (arrowheads) were observed in endodermal cells (En), which are located between vascular bundles (asterisk) and cortical cells (Co). A through D, Fluorescence images (A and C) and corresponding brightfield images (B and D) of hypocotyls without Lat-B treatment. The actin MF network (arrowheads) that is composed of thick longitudinal and thin transverse actin MFs was clearly stained. E and F, A fluorescence image (E) and a brightfield image (F) of a hypocotyl treated with 2.0 μm Lat-B before hand sectioning. Actin MFs were disrupted but not completely depolymerized, and fragments of actin MFs were observed in the endodermal cells. Epidermal cell (Ep). Scale bar = 50 μm, except for C and D, where bar = 25 μm.
Figure 3.
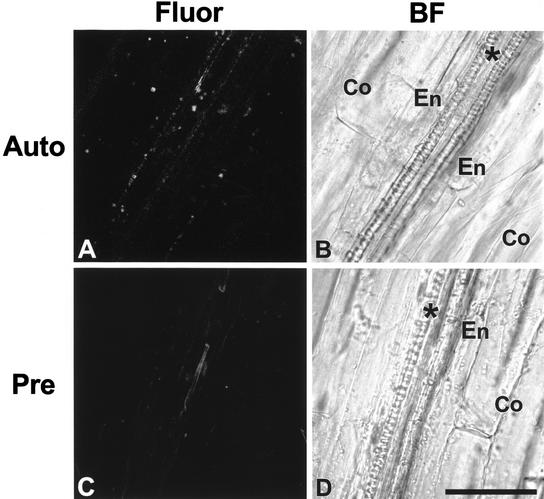
Control experiments to demonstrate autofluorescence of hypocotyls (A) and Alexa Fluor-phalloidin specificity (C). On the right of each fluorescence image, a corresponding brightfield image is shown (B and D). Endodermal cells (En) are located between vascular bundles (asterisk) and cortical cells (Co). A and B, A fluorescence image (A) and a corresponding brightfield image (B) of a hypocotyl that was prepared without MBS fixation and without Alexa Fluor-phalloidin staining. No actin MFs were detected in the fluorescence image. C and D, A fluorescence image (C) and a brightfield image (D) of a hypocotyl that was incubated with 1.0 μm unconjugated phalloidin before Alexa Fluor-phalloidin staining. A weak fluorescence signal was detected from the vascular bundles. Scale bar = 50 μm.
Examination of actin MF organization in root columella cells was attempted by using similar Alexa Fluor-phalloidin procedures but was not successful. It was difficult to prepare root samples as we did for hypocotyls and stems because roots are too small to section longitudinally with a scalpel. These problems have been reported by numerous other researchers (e.g. Collings et al., 2001).
Lat-B Disrupts Actin MFs in Hypocotyl Endodermal Cells
The effects of Lat-B on actin MFs in hypocotyl endodermal cells were examined with the confocal microscope after staining of 2.0 μm Lat-B-treated hypocotyls with Alexa Fluor-phalloidin (Fig. 2, E and F). In contrast to the fluorescence images of untreated hypocotyls (Fig. 2, A and C), neither continuous actin MF bundles nor continuous transverse actin MFs were detected in treated endodermal cells; instead, several short fragmented actin MFs and punctate fluorescence were observed (Fig. 2E). Hypocotyls treated with 0.2 μm Lat-B also exhibited disruption of the actin MF network (not shown), but to a lesser degree compared with the 2.0 μm Lat-B treatment. In addition, a strong fluorescence signal was detected around the area of the sedimented plastids in endodermal cells of Lat-B-treated hypocotyls (Fig. 2, E and F). Such a strong signal from the area around sedimented plastids was not observed in the control fluorescence images (Fig. 2, A and C).
An MF network was present in cortical cells, but it appeared less extensive compared with endodoermal cells (Fig. 2, A and C). The MF network in these cortical cells also was disrupted by Lat-B treatment (Fig. 2E). Actin MFs also were observed in epidermal cells, but these were resistant to Lat-B (Fig. 2E).
The fluorescence image of Lat-B-treated hypocotyls (Fig. 2E) exhibited a lower background noise compared with the two images of untreated hypocotyls (Fig. 2, A and C) because a lower PMT gain value was used when samples were scanned. Even when a high PMT gain value was used for scanning Lat-B-treated hypocotyls, neither continuous thick actin MF bundles nor transverse actin MFs were observed (not shown). Therefore, the lower PMT gain value was used for Lat-B-treated hypocotyls to minimize the background noise.
Control Experiments with Hypocotyls Demonstrate the Binding Specificity of Alexa Fluor-Phalloidin to Actin MFs
Control experiments were performed to examine the fluorescence in hypocotyls incubated without 3-maleimidobenzoyl-N-hydroxy-succinimide ester (MBS) and without Alexa Fluor-phalloidin (Fig. 3, A and B). In such a treatment (Fig. 3A), a weak fluorescence signal was detected in vascular bundles and cortical and endodermal cells, but no distinguishable filaments were observed. Hypocotyls prepared with MBS and without Alexa Fluor-phalloidin resulted in a similar fluorescence image to Figure 3A (data not shown). In addition to the above control, autofluorescence from hypocotyls was examined and did not show any distinguishable filaments (data not shown).
The binding specificity of Alexa Fluor-phalloidin to actin MFs was examined by using unconjugated phalloidin (Fig. 3, C and D). Preincubation of hypocotyls with unconjugated phalloidin saturated the binding sites on actin MFs for Alexa Fluor-phalloidin and resulted in no detection of a specific signal (Fig. 3C).
Control Experiments Demonstrate That Lat-B Does Not Disrupt Phototropic Curvature
In an effort to determine which part of the gravitropism pathway is affected by Lab-B disruption of the actin cytoskeleton, we also studied the effects of this drug on phototropism in hypocotyls (Fig. 4). In contrast to the Lat-B promotion of hypocotyl gravicurvature (Fig. 1A), there were no significant effects (P > 0.05) on phototropic curvature at the concentration of 0.02 or 0.2 μm compared with the control, and the higher concentration of 2.0 μm Lat-B resulted in inhibition of curvature. Furthermore, Lat-B did not affect growth at concentrations of 0.02 or 0.2 μm but did reduce growth at 2.0 μm (Table II). Thus, because Lat-B promotes gravitropic curvature while having no effect on phototropic curvature, it is unlikely that this drug affects the response phase of gravitropism but rather Lat-B may affect the earlier phases (i.e. perception or transduction) of gravitropism.
Figure 4.
Time course of phototropic curvature of hypocotyls with 0.02, 0.2, or 2.0 μm Lat-B. Different letters after labels in plots indicate statistical differences in curvature, and statistical analyses were performed as described in the legend to Figure 1. The application of 0.02 and 0.2 μm Lat-B did not affect curvature compared with the untreated controls throughout the time course. Curvature of 2.0 μm Lat-B-treated roots was significantly decreased and delayed. Black circle, untreated control (n = 51). White circle, 0.02 μm Lat-B treatment (n = 53). Black triangle, 0.2 μm Lat-B treatment (n = 49). White triangle, 2.0 μm Lat-B treatment (n = 47). Error bars represent se.
Table II.
Effects of Lat-B on hypocotyl growth rates of WT seedlings during phototropism experiments
| Lat-B Concentration | Growth Rate of WsWT Hypocotyls | |
|---|---|---|
| mm h−1 ± se | n | |
| Control | 0.17 ± 0.02 (a*) | 51 |
| 0.02 μM | 0.17 ± 0.01 (a*) | 51 |
| 0.2 μM | 0.13 ± 0.02 (a*) | 50 |
| 2.0 μM | 0.05 ± 0.00 (b*) | 46 |
Statistical methods and other details are as described in Table I.
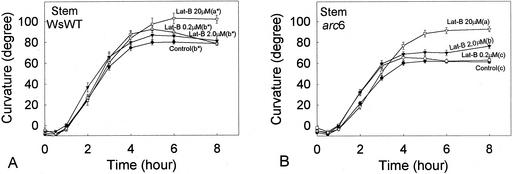
Lat-B Also Causes a Promotion of Gravicurvature in Stems
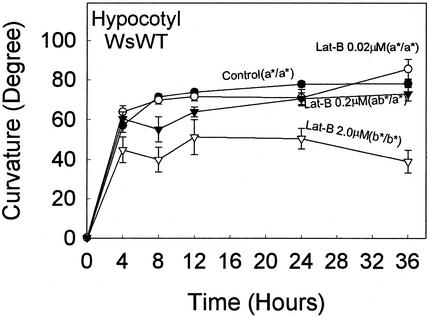
Because the application of Lat-B resulted in promoting hypocotyl curvature (Fig. 1A), the effect of Lat-B on curvature of inflorescence stems also was examined in WT Arabidopsis (Fig. 5A). The curvature rates of Lat-B-treated (0.2, 2.0, and 20 μm) stems did not appear to be different from the untreated controls in the first 4 h after reorientation. None of the growth rates of Lat-B treated stems were affected by Lat-B compared with the control (P < 0.05; Table III). However, 20 μm, but not 0.2 and 2.0 μm, Lat-B treatment significantly promoted the stem curvature (P < 0.05) at 8 h after reorientation compared with the control.
Figure 5.
Time course of gravitropic curvature (after reorientation) of inflorescence stems with 0.2, 2.0, or 20 μm Lat-B. Different letters after labels in plots indicate statistical differences in curvature at 8 h, and statistical analyses were performed as described in the legend to Figure 1. A, WsWT: The application of 0.2 and 2.0 μm Lat-B did not affect stem curvature compared with the untreated controls. In contrast, the curvature of 20 μm Lat-B-treated stems was significantly promoted. B, arc6: The application of 0.2 μm Lat-B did not affect stem curvature compared with the untreated control. However, the curvature of 2.0 μm Lat-B-treated stems was significantly promoted compared with the untreated controls, and the curvature of 20 μm Lat-B-treated stems was promoted to a greater degree compared with 2.0 μm Lat-B-treated stems. Black circle, Untreated control (n = 101 [A], 105 [B]). Black circle, 0.2 μm Lat-B treatment (n = 42 [A], 48 [B]). Black triangle, 2.0 μm Lat-B treatment (n = 52 [A], 58 [B]). White triangle, 20 μm Lat-B treatment (n = 39 [A], 50 [B]). Error bars represent se.
Table III.
Effects of Lat-B on inflorescence stem growth rates of WT and arc6 seedlings during gravitropism experiments
| Lat-B Concentration | Stem
|
|||
|---|---|---|---|---|
| Growth rate of WsWT | Growth rate of arc6 | |||
| mm h−1 ± se | n | mm h−1 ± se | n | |
| Control | 0.21 ± 0.02 (ab*) | 101 | 0.19 ± 0.02 (a*) | 105 |
| 0.2 μM | 0.28 ± 0.03 (a*) | 42 | 0.22 ± 0.02 (a*) | 48 |
| 2.0 μM | 0.16 ± 0.02 (b*) | 52 | 0.21 ± 0.02 (a*) | 58 |
| 20.0 μM | 0.25 ± 0.02 (a*) | 39 | 0.24 ± 0.02 (a*) | 50 |
Statistical methods and other details are as described in Table I.
Curvature of 20 μm Lat-B-treated stems began overshooting (curvature exceeding 90°) at 5 h after reorientation and maintained the overshoot for about 7 h (data not shown) compared with an overshoot of about 1 to 2 h in untreated WT inflorescence stems (Yamamoto et al., 2002).
Previous studies have demonstrated that Arabidopsis arc6 mutants, which have one to two large plastids in the endodermal cells, were useful in studying the mechanisms of gravitropism (Yamamoto et al., 2002). These authors suggested that alteration of the plastid phenotype in endodermal cells caused the arc6 stem curvature to decrease compared with that of the WT. Application of 2.0 μm Lat-B to arc6 stems (Fig. 5B) significantly promoted curvature compared with the untreated arc6 controls (P < 0.05) and resulted in a similar curvature to the untreated controls of WT stems (Fig. 5A; WsWT control) during the time course. The application of 20 μm Lat-B to arc6 stems resulted in greater magnitude of curvature compared with 2.0 μm Lat-B (P < 0.05).
Although no overshooting was observed for arc6 stems in untreated arc6 stems (Yamamoto et al., 2002), 20 μm Lat-B-treated arc6 stems exhibited overshooting (i.e. curvature greater than 90°) after 6 h after reorientation and maintained for about 6 h (data not shown). There were no differences in arc6 stem growth rates between the untreated control and all Lat-B-treated stems (P > 0.05; Table III).
Alexa Fluor-Phalloidin Labeling of Actin MFs in Inflorescence Stem Endodermal Cells
The organization of actin MFs was examined in endodermal cells of WT inflorescence stems after labeling with Alexa Fluor-phalloidin (Fig. 6, A–D). In both fluorescence (Fig. 6, A and C) and brightfield images (Fig. 6, B and D), the cortical cells were easily recognized because of their distinctive morphology (containing more plastids compared with other cells in the stem) and a strong fluorescence signal (autofluorescence) from chloroplasts. More than one layer of endodermal cells was visible (Fig. 6, B and D) in these relatively thick sections. The organization of the actin MF system in stem endodermal cells was similar to that in hypocotyl endodermal cells. There were distinct, longitudinal actin MF bundles in addition to thinner transversely oriented actin MFs, and they appeared to be connected to form a network (Fig. 6, A and C).
Figure 6.
Confocal and brightfield images of endodermal cells in inflorescence stems. Demonstration of actin MF organization in endodermal cells (A and C) and depolymerization of actin MF network in endodermal cells after an application of Lat-B (E). On the right of each fluorescence image, a corresponding brightfield image is shown (B, D, and F). After stem sections were prepared with a vibratome, actin MFs were stained with Alexa Fluor 488-phalloidin after MBS fixation. The two fluorescence images showing the actin MF network in endodermal cells are representative of about 15 different stem sections. The fluorescence image of the Lat-B-treated stem is representative of about 10 different Lat-B-treated stem sections. Actin MFs (arrowheads) were observed in endodermal cells (En), which are located between vascular bundles (asterisks) and cortical cells (Co). A through D, Fluorescence images (A and C) and corresponding brightfield images (B and D) of stems without Lat-B treatment. Actin MF networks (arrowheads) that were composed of thick longitudinal and thin transverse actin MFs were clearly stained. E and F, A fluorescence image (E) and a corresponding brightfield image (F) of a stem treated with 20 μm Lat-B before sectioning with a vibratome. The actin MF network in the endodermal cells was disrupted. Note the autofluorescence of chlorophyll from the chloroplasts in cortical cells (Co). Epidermal cell (Ep). Scale bar = 50 μm.
Lat-B Results in Disruption of Actin MFs in Inflorescence Stem Endodermal Cells
The effect of Lat-B on the actin MF network was confirmed by observing Lat-B treated inflorescence stems with the confocal microscope after staining with Alexa Fluor-phalloidin (Fig. 6, E and F). In contrast to the untreated specimens (Fig. 6, A and C), no continuous actin MFs were visualized in the endodermal cells of Lat-B-treated stems; instead, diffused nonspecific punctate fluorescence and fragmented MFs were observed (Fig. 6E). Controls similar to those performed for hypocotyls, including preabsorption experiments, indicated the specificity of binding of Alexa Fluor-phalloidin to MFs in endodermal cells of stems (not shown).
DISCUSSION
Endodermal Cells Have a Well-Developed Actin MF Network
There has been little research on the actin network in the putative gravity-perceiving cells in stem-like organs. In one of the few reports, Volkmann et al. (1993) observed numerous transversely oriented, but few longitudinally oriented, actin MFs in endodermal cells of cress hypocotyls. In contrast, the organization of actin MFs in root columella cells has been well characterized (Baluska and Hasenstein, 1997; Driss-École et al., 2000b; Collings et al., 2001; Yoder et al., 2001) compared with that of endodermal cells. A key characteristic of actin MF organization in columella cells is the nonspecific orientation of filaments, which was suggested based on confocal and electron microscopic studies of roots of lentil, flax (Linum usitatissimum), and maize (Driss-École et al., 2000b; Collings et al., 2001; Yoder et al., 2001).
To our knowledge, this is the first report to characterize the actin cytoskeleton in endodermal cells in stems. We have shown by Alexa Fluor-phalloidin labeling that shoot endodermal cells contain a longitudinally and transversely oriented actin MF network (Fig. 2, A and C; Fig. 6, A and C). Because the longitudinally oriented MFs were thicker relative to the transverse strands, they are likely to be composed of a greater number of individual actin MFs compared with the thinner transverse bundles.
An Intact Actin Cytoskeleton Is Not Required for Gravitropism in Hypocotyls and Inflorescence Stems
Incubation of hypocotyls and stems in Lat-B resulted in a severe disruption of actin MFs in endodermal cells (Figs. 2 and 6). At the same time, although growth was reduced, Lat-B promoted gravitropic curvature in hypocotyls and stems (Figs. 1 and 5). These results clearly demonstrate that an intact actin cytoskeleton is not required for gravitropism in these organs and do not support current hypotheses that suggest an essential role of the cytoskeleton in gravity perception (for review, see Kiss, 2000).
However, the results in this study do not exclude the possibility that the actin cytoskeleton still plays some role in plant gravitropism. Although we have shown that disruption of the actin network promotes gravicurvature, an alternate interpretation of these data is that accelerated gravitropism with actin depolymerization actually highlights the role of actin in the normal gravisensing system. Such a scenario might include increased plastid sedimentation after disruption of the actin cytoskeleton (see below).
Endodermal cells of inflorescence stems and hypocotyls are considered to be involved in gravity sensing (Fukaki and Tasaka, 1999; Tasaka et al., 1999). Thus, because the most severe disruption was observed in these cells, Lat-B may have a more specific effect on the earlier stages of gravitropism (i.e. perception) rather than the latter (response) phase. The results of the phototropism experiments support this view because Lat-B does not affect phototropic curvature, although it promotes gravitropic curvature in hypocotyls. Because both phototropic and gravitropic curvature result from asymmetrical growth during the response phase, it is unlikely that Lat-B affects the latter events of gravitropism.
An interesting result of our studies is that asymmetrical gravitropic growth in hypocotyls is promoted (Fig. 1), whereas linear growth is inhibited (Table I). These data show that there is little correlation between linear growth rate and rate of differential growth during gravitropism. Thus, regulation of these two different growth phenomena may occur by distinct mechanisms.
Increased Plastid Movement May Explain Lat-B-Based Promotion of Gravicurvature
In the present study, the application of 2.0 μm Lat-B promoted inflorescence stem curvature only in arc6 (Fig. 5B), but, unexpectedly, not stem curvature in the WT (Fig. 5A). In a recent study with inflorescence stems (Yamamoto et al., 2002), we proposed that the morphologically altered large plastids in endodermal cells of arc6 were limited in translocation during gravitropism, which consequently resulted in reduction of gravicurvature.
If the application of 2.0 μm Lat-B caused plastid translocation in endodermal cells, then this treatment may not have promoted curvature in the WT because, unlike the large plastids in arc6, the (typical) small plastids in the WT already move more readily (MacCleery and Kiss, 1999). However, if the application of 2.0 μm Lat-B to arc6 stems affected something other than plastid translocation, then this treatment should have promoted the stem curvature in the WT as well. These arguments are made based on the hypothesis that there are no major morphological and physiological differences between the WT and arc6 stems other than the plastid phenotype (arc6 has a defect only in proplastid and plastid divisions compared with the WT; Robertson et al., 1995). Thus, a possible explanation for promotion of inflorescence stem curvature by Lat-B is the improvement of plastid translocation/movement in endodermal cells because of the disruption of the actin MF network.
In contrast to the lower concentrations of the drug, the application of 20 μm Lat-B promoted stem curvature in the WT as well as in arc6. If the above assumption regarding plastid translocation is correct, the promotion of WT stem curvature by higher concentrations of the drug can be explained by a further movement of smaller (WT) plastids because of more severe disruption of actin MFs in endodermal cells.
Root Gravitropism
Although most of these studies have focused on stems and hypocotyls, our results have implications for root gravitropism as well. The decrease in gravicurvature of Lat-B-treated WT roots (Fig. 1B) appears to have been caused primarily by decrease in growth. For example, the growth rate of 2.0 μm Lat-B-treated roots was about 30% of that of the control (Table I), and curvature of 2.0 μm Lat-B-treated roots in the first 12 h after reorientation was about 30% of that of the control (Fig. 1B). Because the inhibition of gravitropic curvature was the same as the decrease in growth, it appears that Lat-B does not have a significant specific effect on root gravitropism. These results are consistent with the studies by Staves et al. (1997) and Blancaflor and Hasenstein (1997), who found that cytochalasins do not affect root gravitropism of rice, maize, and cress.
However, there is another potential explanation for the observation that there were dramatic effects of the drug Lat-B on hypocotyl/stem gravitropism, whereas there were only relatively minimal effects on root gravitropism (although growth was inhibited). These results could be explained by the possibility that Lat-B penetrated into all parts of the root except for the root cap region. Because the root cap is involved in gravisensing (Blancaflor et al., 1998), if there were limited penetration by the drug into the cap, there would not be a great effect on gravitropic curvature. This scenario is raised because several researchers have found that it is difficult to obtain adequate penetration by various chemical reagents into root caps (e.g. White and Sack, 1990).
Yoder et al. (2001) recently proposed a new, tensegrity-based model for gravity sensing in roots, which suggested that graviperception occurs when the dense actin-based cytoskeleton network in the central region of a columella cell is disrupted by displacing amyloplasts and that these plastids do not need to be connected to actin MFs. In contrast, the actin-tether model (i.e. Baluska and Hasenstein, 1997) proposes that gravisensing occurs based on a distinct connection of amyloplasts to the plasma membrane by actin MFs. As Yoder et al. (2001) propose, the actin-tether model suggests that gravitropism is more sensitive to actin-disrupting drugs because the model requires discrete connections between amyloplasts and the plasma membrane by actin MFs. Based on the above discussion, we believe that further studies are needed to evaluate the tensegrity hypothesis for gravity perception in both roots and stem-like organs of plants.
CONCLUSIONS
Endodermal cells of inflorescence stems and hypocotyls exhibit an actin cytoskeleton composed of longitudinal and transverse MFs. Depolymerization of the actin MF network with Lat-B in endodermal cells resulted in promotion of gravitropic curvature for both stems and hypocotyls but possibly not in roots. Similar levels of latrunculin did not significantly affect phototropic curvature. Thus, we conclude that an intact actin MF network is not required for gravisensing in stems and hypocotyls. These results will lead us to continue to reevaluate paradigms regarding the role of the cytoskeleton in the signal transduction pathways of plant gravitropism.
MATERIALS AND METHODS
Plant Materials and Culture Conditions
WT Arabidopsis (geographic race Wassilewskija) and the arc6 mutant (Pyke et al., 1994; Robertson et al., 1995) were used in these experiments. Seeds were surface sterilized with 30% (v/v) commercial bleach and 0.002% (v/v) Triton X-100 for 15 to 20 min, and then washed four times with sterilized distilled water. Seeds were sown onto presterilized cellophane that was placed on top of a 1.2% (w/v) agar with Arabidopsis growth medium (AGM; described by Kiss et al., 1996) and 1% (w/v) Suc at pH 5.5 in a square (100- × 100- × 15-mm) petri dish. A light stimulus (at approximately 70 to 100 μmol m−2 s−1) was given to seeds in vertically placed petri dishes for a period of 24 h to enhance germination. After the light stimulus, seeds were placed in the dark and grown at 20°C to 22°C for about 2.5 d.
For studies of inflorescence stems, surface-sterilized seeds were sown on rockwool cubes and grown as described by Weise and Kiss (1999). Inflorescence stems were grown until they were 6 cm in length, approximately 23 to 25 d after seeds were sown.
Preparation of Lat-B
A stock solution of 2 mm Lat-B (Calbiochem, La Jolla, CA) was prepared in dimethyl sulfoxide. For experiments with seedlings, the desired concentration of Lat-B was obtained by diluting the stock solution in liquefied, sterile 1.2% (w/v) agar with AGM (pH 7.3) and 1% (w/v) Suc when the agar temperature was about 55°C, and, then, Lat-B containing agar was poured into square petri dishes before it solidified. The plates with Lat-B-containing agar were prepared immediately before their use in time course of curvature experiments.
For experiments with inflorescence stems, the desired concentration of Lat-B was obtained by diluting the 2 mm Lat-B stock solution in liquefied, sterile, 1.0% (w/v) Phytagel (Sigma, St. Louis) with one-quarter strength AGM (pH 7.3) when the temperature of the gel was about 55°C, and then the Lat-B-containing gel was poured into 1.5-mL microcentrifuge tubes. The tubes were covered with Parafilm and prepared immediately before their use in time course of curvature experiments.
Gravitropism Experiments
Before reorientation of seedlings, the cellophane with seedlings (3.5 d old) was lifted from normal AGM agar (pH 5.5) and transferred to AGM agar (pH 7.3) with 0.02, 0.2, or 2.0 μm Lat-B in square petri dishes (see above). The transfer of the cellophane took less than 20 s per plate and was conducted in the dark. After the transfer, the square petri dishes were sealed with Parafilm, and seedlings were incubated for about 1.5 to 2 h in a vertical position in the dark before use in curvature studies.
For data collection, photographs were taken with a 35-mm camera equipped with a macro lens using Technical Pan film at ISO 50 after reorientation of square petri dishes. After reorientation, seedlings remained in the dark to avoid phototropic effects. A dim green light (from a 15-W incandescent lamp filtered through green Plexiglas; fluence rate of 0.8 μmol m−2 s−1) was used for photography. Images were digitally captured from the film with the program Photoshop (version 4.0; Adobe, San Jose, CA) on a PC computer, and measurements of angles and length were made with the image analysis program Image Pro Plus (version 3.0; Media Cybernetics, Silver Spring, MD). The gravity vector (down) and anti-parallel gravity vector (up) was defined as 90° for roots and hypocotyls, respectively, and 0° was defined as the horizontal.
Each experiment was repeated at least three times, and values are reported as the mean ± se. Statistical significance was determined by using a one-way ANOVA test (P < 0.05). Where the criteria of the one-way ANOVA test were not met, an ANOVA on ranks (P < 0.05) followed by a Dunn's method (P < 0.05) was used for multiple comparisons. All statistical analyses were performed with a PC with Sigma Stat software (version 2.0, SPSS, Chicago).
Time course of curvature studies on Lat-B-treated inflorescence stems were conducted as follows. Stems that were 3 to 6 cm in length were excised at the base and inserted into 1.5-mL microcentrifuge tubes with a Lat-B-containing Phytagel prepared as described above. For control experiments, the Phytagel without Lat-B was used. The tubes with stems were loaded into a microcentrifuge tube rack and placed in a clear plastic box (100 × 120 × 180 mm). Excised stems in the plastic box were incubated under the constant light overnight (12 to 14 h) before time course of curvature studies. After reorientation, data collection was performed in the same way as seedlings (described above).
Phototropism Experiments
Seedlings were illuminated from the side (90° from the vertical) with blue light obtained by passing light from fluorescent bulbs through a Plexiglas filter. The fluence rate through the blue filter (Rohm and Haas no. 2424; Dayton Plastics, Columbus, OH) was 12 to 14 μmol m−2 s−1 with a transmission maximum (determined with a LI-COR LI-1800 spectroradiometer) of 490 nm. Time course studies were performed and hypocotyl curvature was measured and analyzed as described above.
Confocal Microscopy
The novel procedures described by Collings et al. (2001) were used to optimize actin MF localization via fluorescently labeled phalloidin. Briefly, hypocotyls of 3.5-d-old seedlings were cut in half (longitudinally) with a scalpel. Inflorescence stems were cut into pieces about 2 to 3 mm in length in PME (50 mm 1,4-piperazinediethanesulfonic acid [PIPES], 4 mm MgSO4, 10 mm EGTA) buffer (pH 6.9) and embedded in 4% (w/v) agar block-containing PME buffer. After the embedment, inflorescence segments in the agar were cut longitudinally with a Vibratome Series 1000 Sectioning System (Technical Products International, Inc., St. Louis) using a razor blade, and longitudinal sections (70-mm thick) were collected. After the above preparation, hypocotyl and inflorescence stem sections were immediately immersed in PME buffer containing 300 μm MBS (3-maleimidobenzoyl-N-hydroxy-succinimide ester) and incubated for 30 min. The sections were incubated for 15 to 20 min in the PME buffer containing 0.1 μm Alexa Fluor 488 phalloidin (Molecular Probes, Eugene, OR), 0.3 m mannitol, and 2% (v/v) glycerol. Before their observation with a confocal microscope, sections were washed with PME buffer briefly and mounted on a slide with a drop of PME buffer. Preparation of samples of Lat-B-treated hypocotyls and stems for confocal studies was also conducted as described above.
Images were captured with a Nikon PCM-2000 laser scanning confocal microscope using a 40× (numerical aperture = 0.75) objective and a 50-μm pinhole. Each image was scanned five to 10 times and averaged. To obtain the higher signal, 4× slow scanning speed was sometimes used. An argon ion laser at 488 nm was used to excite the Alexa Fluor 488, and the emission between 500 to 530 nm was collected. Corel Photo Paint (version 8; Corel Corporation, Ottawa, Ontario, Canada) was used to process images.
Several controls were performed to examine (a) autofluorescence, (b) fluorescence of hypocotyls prepared without MBS and without Alexa Fluor-phalloidin, (c) fluorescence of hypocotyls prepared with MBS and without Alexa Fluor-phalloidin, and (d) Alexa Fluor-phalloidin specificity to actin MF. For the latter control, hypocotyls were preabsorbed against 1.0 μm unconjugated phalloidin (Molecular Probes) before labeling with Alexa Fluor-phalloidin.
ACKNOWLEDGMENTS
We thank Richard E. Edelmann for providing critical comments on the manuscript and Melanie J. Correll for assistance with the phototropism experiments. We also thank Kevin A. Pyke for supplying the arc6 mutant and Lori G. Isaacson for use of the Vibratome in these studies.
Footnotes
This work was supported by the National Aeronautics and Space Administration (grant no. NCC2–1200) and the National Institutes of Health (grant no. 1R15GM57806–01).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010804.
LITERATURE CITED
- Baluska F, Hasenstein KH. Root cytoskeleton: its role in perception of and response to gravity. Planta. 1997;203:S69–S78. doi: 10.1007/pl00008117. [DOI] [PubMed] [Google Scholar]
- Baluska F, Jasik J, Edelmann HG, Salajova T, Volkmann D. Latrunculin B-induced plant dwarfism: Plant cell elongation is F-actin-dependent. Dev Biol. 2001;231:113–124. doi: 10.1006/dbio.2000.0115. [DOI] [PubMed] [Google Scholar]
- Blancaflor EB, Fasano JM, Gilroy S. Mapping the functional roles of cap cells in the response of Arabidopsis primary roots to gravity. Plant Physiol. 1998;116:213–222. doi: 10.1104/pp.116.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blancaflor EB, Hasenstein KH. The organization of the actin cytoskeleton in vertical and graviresponding primary roots of maize. Plant Physiol. 1997;113:1447–1455. doi: 10.1104/pp.113.4.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collings DA, Zsuppan G, Allen NS, Blancaflor EB. Demonstration of prominent actin filaments in the root columella. Planta. 2001;212:392–403. doi: 10.1007/s004250000406. [DOI] [PubMed] [Google Scholar]
- Driss-École D, Jeune B, Prouteau M, Julianus P, Perbal G. Lentil root statoliths reach a stable state in microgravity. Planta. 2000a;211:396–405. doi: 10.1007/s004250000298. [DOI] [PubMed] [Google Scholar]
- Driss-École D, Vassy J, Rembur J, Guivarc'h A, Prouteau M, Dewitte W, Perbal G. Immunolocalization of actin in root statocytes of Lens culinarisL. J Exp Bot. 2000b;51:521–528. doi: 10.1093/jexbot/51.344.521. [DOI] [PubMed] [Google Scholar]
- Fukaki H, Tasaka M. Gravity perception and gravitropic response of inflorescence stems in Arabidopsis thaliana. Adv Space Res. 1999;24:763–770. doi: 10.1016/s0273-1177(99)00410-x. [DOI] [PubMed] [Google Scholar]
- Fukaki H, Wysocka-Diller J, Kato T, Fujisawa H, Benfey PN, Tasaka M. Genetic evidence that the endodermis is essential for shoot gravitropism in Arabidopsis thaliana. Plant J. 1998;14:425–430. doi: 10.1046/j.1365-313x.1998.00137.x. [DOI] [PubMed] [Google Scholar]
- Hepler PK, Cleary AL, Gunning BES, Wadsworth P, Wasteneys GO, Zhang DH. Cytoskeletal dynamics in living plant cells. Cell Biol Int. 1993;17:127–142. [Google Scholar]
- Kandasamy MK, Meagher RB. Actin-organelle interaction: association with chloroplast in Arabidopsisleaf mesophyll cells. Cell Motil Cytoskel. 1999;44:110–118. doi: 10.1002/(SICI)1097-0169(199910)44:2<110::AID-CM3>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Kiss JZ. Mechanisms of the early phases of plant gravitropism. Crit Rev Plant Sci. 2000;19:551–573. doi: 10.1080/07352680091139295. [DOI] [PubMed] [Google Scholar]
- Kiss JZ, Guisinger MM, Miller AJ, Stackhouse KS. Reduced gravitropism in hypocotyls of starch-deficient mutants of Arabidopsis. Plant Cell Physiol. 1997;38:518–525. doi: 10.1093/oxfordjournals.pcp.a029199. [DOI] [PubMed] [Google Scholar]
- Kiss JZ, Wright JB, Caspar T. Gravitropism in roots of intermediate-starch mutants of Arabidopsis. Physiol Plant. 1996;97:237–244. doi: 10.1034/j.1399-3054.1996.970205.x. [DOI] [PubMed] [Google Scholar]
- MacCleery SA, Kiss JZ. Plastid sedimentation kinetics in roots of wild-type and starch-deficient mutants of Arabidopsis. Plant Physiol. 1999;120:183–192. doi: 10.1104/pp.120.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen JL, Ishikawa H, Evans ML. Analysis of changes in relative elemental growth rate patterns in the elongation zone of Arabidopsisroots upon gravistimulation. Planta. 1998;206:598–603. doi: 10.1007/s004250050437. [DOI] [PubMed] [Google Scholar]
- Parthasarathy MV, Perdue TD, Witztum A, Alvernaz J. Actin network as a normal component of the cytoskeleton in many vascular plant cells. Am J Bot. 1985;72:1318–1323. [Google Scholar]
- Pendleton A, Koffer A. Effects of latrunculin reveal requirements for the actin cytoskeleton during secretion from mast cells. Cell Motil Cytoskel. 2001;48:37–51. doi: 10.1002/1097-0169(200101)48:1<37::AID-CM4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Perbal G, Driss-École D, Tewinkel M, Volkmann D. Statocyte polarity and gravisensitivity in seedling roots grown in microgravity. Planta. 1997;203:S57–S62. doi: 10.1007/pl00008115. [DOI] [PubMed] [Google Scholar]
- Pyke KA, Rutherford SM, Robertson EJ, Leech RM. arc6, a fertile Arabidopsismutant with only two mesophyll cell chloroplasts. Plant Physiol. 1994;106:1169–1177. doi: 10.1104/pp.106.3.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson EJ, Pyke KA, Leech RM. arc6, an extreme chloroplast division mutant of Arabidopsisalso alters proplastid proliferation and morphology in shoot and root apices. J Cell Sci. 1995;108:2937–2944. doi: 10.1242/jcs.108.9.2937. [DOI] [PubMed] [Google Scholar]
- Sack FD. Plastids and gravitropic sensing. Planta. 1997;203:S63–S68. doi: 10.1007/pl00008116. [DOI] [PubMed] [Google Scholar]
- Spector I, Braet F, Shochet NR, Bubb MR. New anti-actin drugs in the study of the organization and function of the actin cytoskeleton. Microsc Res Tech. 1999;47:18–37. doi: 10.1002/(SICI)1097-0029(19991001)47:1<18::AID-JEMT3>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Staves MP, Wayne R, Leopold AC. Cytochalasin D does not inhibit gravitropism in roots. Am J Bot. 1997;84:1530–1535. [PubMed] [Google Scholar]
- Tasaka M, Kato T, Fukaki H. The endodermis and shoot gravitropism. Trends Plant Sci. 1999;4:103–107. doi: 10.1016/s1360-1385(99)01376-x. [DOI] [PubMed] [Google Scholar]
- Thimann KV, Reese K, Nachmias VT. Actin and the elongation of plant cells. Protoplasma. 1992;171:153–166. [Google Scholar]
- Volkmann D, Buchen B, Hejnowicz Z, Tewinkel M, Sievers A. Oriented movement of statoliths studied in a reduced gravitational field during parabolic flights of rockets. Planta. 1991;185:153–161. [PubMed] [Google Scholar]
- Volkmann D, Winn-Borner U, Waberzeck K. Graviresponsiveness of cress seedlings and structural status of presumptive statocytes from the hypocotyl. J Plant Physiol. 1993;142:710–716. [Google Scholar]
- Weise SE, Kiss JZ. Gravitropism of inflorescence stems in starch-deficient mutants of Arabidopsis. Int J Plant Sci. 1999;160:521–527. doi: 10.1086/314142. [DOI] [PubMed] [Google Scholar]
- Weise SE, Kuznetsov OA, Hasenstein KH, Kiss JZ. Curvature in Arabidopsisinflorescence stems is limited to the region of amyloplasts displacement. Plant Cell Physiol. 2000;41:702–709. doi: 10.1093/pcp/41.6.702. [DOI] [PubMed] [Google Scholar]
- White RG, Sack FD. Actin microfilaments in presumptive statocytes of root caps and coleoptiles. Am J Bot. 1990;77:17–26. [PubMed] [Google Scholar]
- Yamamoto K, Pyke KA, Kiss JZ (2002) Reduced gravitropism in inflorescence stems and hypocotyls, but not roots, of Arabidopsis mutants with large plastids. Physiol Plant (in press) [DOI] [PubMed]
- Yoder TL, Zheng H, Todd P, Staehelin LA. Amyloplast sedimentation dynamics in maize columella cells support a new model for the gravity-sensing apparatus of roots. Plant Physiol. 2001;125:1045–1060. doi: 10.1104/pp.125.2.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]