Abstract
MADS box transcription factors control diverse developmental processes in plants, metazoans, and fungi. To analyze the involvement of MADS box proteins in fruiting body development of filamentous ascomycetes, we isolated the mcm1 gene from the homothallic ascomycete Sordaria macrospora, which encodes a putative homologue of the Saccharomyces cerevisiae MADS box protein Mcm1p. Deletion of the S. macrospora mcm1 gene resulted in reduced biomass, increased hyphal branching, and reduced hyphal compartment length during vegetative growth. Furthermore, the S. macrospora Δmcm1 strain was unable to produce fruiting bodies or ascospores during sexual development. A yeast two-hybrid analysis in conjugation with in vitro analyses demonstrated that the S. macrospora MCM1 protein can interact with the putative transcription factor SMTA-1, encoded by the S. macrospora mating-type locus. These results suggest that the S. macrospora MCM1 protein is involved in the transcriptional regulation of mating-type-specific genes as well as in fruiting body development.
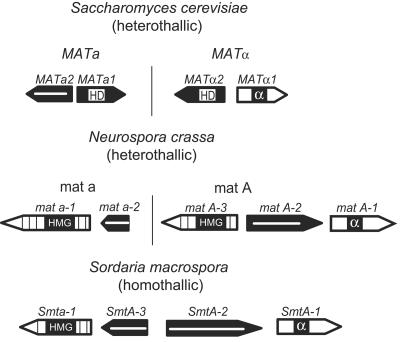
The regulation of sexual reproduction is one of the central processes in the life cycles of most fungi. Like sex chromosomes in animals and plants, the allele type at the fungal mating-type locus determines sexual compatibility between haploid individuals in fungi (19, 20). The genetic breeding mechanism of fungi in which sexual reproduction only occurs between strains of the opposite mating type is called heterothallism. Strains of heterothallic ascomycetes exist in two mating types; these are termed Matα and Mata in Saccharomyces cerevisiae and MatA and Mata in the filamentous ascomycete Neurospora crassa. The genes that determine the fungal mating type are located at the mating-type loci. In ascomycetes, alternative versions of the mating-type locus on homologous chromosomes are termed idiomorphs and are completely dissimilar in the genes they carry (11, 42, 62).
The ascomycetous fungus S. cerevisiae has the best-studied mating system. The MAT idiomorphs encode transcription factors, which in combination with other regulatory proteins are responsible for cell-type-specific gene expression, e.g., the mating-type-specific expression of pheromone and pheromone receptor genes. Each mating-type locus carries two genes: the MATα locus carries MATα1 and MATα2, and the MATa locus carries MATa1 and MATa2 (Fig. 1). The Matα1p protein has been shown to be a transcriptional activator of α-specific genes and carries the α domain as a DNA-binding motif (4). The gene product of MATα2 is a homeodomain protein and acts as a negative regulator of a-specific genes. In the MATa locus, MATa1 is the only gene to encode a functional protein, Mata1p, which is also a homeodomain transcription factor (14). However, unlike the Matα2p homeodomain protein of α cells, Mata1p does not play a role in determining the a cell type. Rather, a-specific genes are expressed because they are not repressed by Matα2p, and α-specific genes are not expressed because there is no Matα1p activator present. Mata1p does, however, have a role in diploid cells, where in conjunction with Matα2p it represses transcription of haploid-specific genes (28). To carry out their roles as transcription factors, Matα1p and Matα2p must work together with minichromosome maintenance protein 1 (Mcm1p) (4, 29). In a cells, Mcm1p activates a-specific genes, together with the homeodomain transcription factor Ste12p (28). Mcm1p is an essential sequence-specific homodimeric DNA-binding protein and a member of the MADS box transcription factor family. The MADS box is a highly conserved sequence motif characteristic of this family of transcriptional regulators. This motif was identified after sequence comparison of Mcm1p, AGAMOUS, DEFICIENS, and serum response factor (SRF), and the name MADS was derived from the “initials” of these four “founders” (63). MADS box proteins interact with diverse sequence-specific transcription factors to repress or activate different sets of genes (reviewed in reference 41). Their function in flowering plants has been studied extensively, and this protein family is a major regulator of floral organ development and controls cell differentiation as well as root architecture (2). In addition to its function in cell-type-specific gene expression, the MADS box protein Mcm1p from S. cerevisiae has a well-defined role in the control of genes that determine general metabolism (40), minichromosome maintenance (47), and the regulation of the cell cycle (36, 38).
FIG. 1.
Comparative genetic map of mating-type loci from the heterothallic ascomycete S. cerevisiae and the filamentous ascomycetes N. crassa and S. macrospora. The arrowed boxes represent the orientations and sizes of the ORFs in the mating-type loci. Black arrows with white bars indicate genes encoding proteins of unknown function; dark arrows indicate genes encoding homeodomain proteins (HD); white arrows indicate genes encoding α domain proteins (α); and striped arrows indicate genes encoding high-mobility-group domain (HMG) proteins.
The mating-type loci of the filamentous ascomycete N. crassa share some features with those of S. cerevisiae; however, the encoded proteins are different. The “A” idiomorph contains three genes, namely, mat A-1, mat A-2, and mat A-3 (18, 21). Two of them encode proteins with domains typical of eukaryotic transcription factors (17). The “a” idiomorph contains two genes, mat a-1 and mat a-2. The mat a-1 gene encodes an HMG domain protein, whereas mat a-2 encodes a protein of unknown function (53, 64) (Fig. 1). In addition to heterothallism, a second mating system, designated homothallism, can be observed in ascomycetes. Homothallic species are self-fertile. This means that either the mycelium derived from a uninucleate ascospore or a vegetative spore of a homothallic fungus is able to complete the sexual cycle without interacting with a mating partner (50). The homothallic ascomycete Sordaria macrospora is closely related to the heterothallic ascomycete N. crassa. Analyses of the mating-type locus of S. macrospora revealed that this locus contains sequences homologous to both the mat a and mat A idiomorphs of N. crassa (59). In the mating-type locus of S. macrospora, four different open reading frames (ORFs), Smta-1, SmtA-3, SmtA-2, and SmtA-1, were identified, and all of them are transcribed (53). The proteins encoded by two of these genes (Smta-1 and SmtA-1) contain domains typical of eukaryotic transcription factors (Fig. 1). SMTa-1 and SMTA-1 are able to form a heterodimer and activate transcription of reporter genes in yeast (26). Thus, they possess properties characteristic of mating-type gene-encoded transcriptional regulators of other ascomycetes. Moreover, we have recently demonstrated that the Smta-1 gene is required for fruiting body development and sexual reproduction in S. macrospora. Deletion of Smta-1 converts the self-fertile S. macrospora to a self-sterile fungus which is no longer able to produce fruiting bodies and ascospores. Microarray analyses revealed that genes affected by SMTa-1 are numerous, including the pheromone gene ppg2 and several genes encoding components of signaling cascades (58). Thus, the mating-type proteins of homothallic filamentous ascomycetes seem to control sexual reproduction by regulating a variety of essential cellular processes. To elucidate whether additional transcription factors are involved in sexual development of the homothallic ascomycete S. macrospora, we cloned and functionally characterized the S. macrospora mcm1 gene, encoding a putative homologue of the S. cerevisiae Mcm1p protein. Deletion of the mcm1 gene leads to a pleiotropic phenotype, including reduced biomass, increased hyphal branching, and reduced hyphal compartment length during vegetative growth, as well as sexual sterility. Our results revealed that S. macrospora MCM1 is able to interact with the mating-type protein SMTA-1. Thus, for filamentous ascomycetes, this study presents the first report on the interaction between a MADS box transcription factor and a mating-type protein.
MATERIALS AND METHODS
Strains and growth conditions.
Cloning and propagation of recombinant plasmids was done in Escherichia coli strain SURE under standard culture conditions (23, 61). Saccharomyces cerevisiae strain PJ69-4A was used as the host strain for the two-hybrid experiments and was cultivated as described by James et al. (27). All Sordaria macrospora strains used in this work are summarized in Table 1. The S. macrospora wild-type (wt) strain K (S48977), the fus1-1 spore color mutant (S23442), and the Δku70 strain (S66001) were derived from our laboratory collection (Department of General and Molecular Botany, Ruhr University, Bochum, Germany). S. macrospora strains were cultivated on cornmeal medium or CM medium (15, 45). S. macrospora strain K, used for the isolation of RNA, was grown in Westergaard's synthetic medium (67). S. macrospora wild-type and Δmcm1 mutant growth velocities were determined according to the method of Nowrousian and Cebula (44). Growth of mycelia was monitored by the dry cell weight. After a cultivation time of 7 days, the dry weight of each sample was estimated by vacuum filtration of a mycelium grown in 30 ml liquid culture in a petri dish. The remained cell material was dried at 60°C for 24 h and weighed. All experimental results are mean values of three independent measurements with 10 different samples each. Transformation of S. macrospora was performed as described by Nowrousian et al. (45). Transformants were selected on either nourseothricin (50 μg/ml)- or hygromycin B (110 U/ml)-containing medium.
TABLE 1.
Sordaria macrospora strains used in this study
| Strain | Relevant genotype and phenotypea | Reference or source |
|---|---|---|
| S48977 | Wild type, homothallic | Culture collectionb |
| S23442 | fus1-1, colored spores | Culture collectionb |
| S66001 | Δku70::natrhphs | 54 |
| T1-1 | Primary Δmcm1::hphr Δku70::natr transformant, heterokaryotic, fertile, reduced ascospores | This study |
| T1-3 | Primary Δmcm1::hphr Δku70::natr transformant, homokaryotic, sterile, no ascospores | This study |
| S67718 | Single spore isolate of T1-3 × S23442 cross, Δmcm1::hphrku70+ nats fus+, sterile | This study |
| T8 | Δmcm1::hphrnatrmcm1+, fertile | This study |
natr, nourseothricin resistant; nats, nourseothricin sensitive; hphr, hygromycin resistant; hphs, hygromycin sensitive.
Department of General and Molecular Botany, Ruhr University, Bochum, Germany.
Identification of a cosmid clone carrying mcm1 and cloning of the mcm1 gene.
An indexed S. macrospora cosmid library (57) was screened by PCR with oligonucleotide primers m1 and m2 (Fig. 2; Table 2), derived from the sequence of the Neurospora crassa ORF NCU07430.2 at the Broad Institute (http://www.broad.mit.edu/annotation/fungi/fgi/). This led to the isolation of cosmid D5 from pool VI 518-614, containing the mcm1 gene of S. macrospora. Subsequently, overlapping fragments from cosmid D5 were subcloned into vector pBCKS(+) (Stratagene, La Jolla, CA). The sequences of the subcloned fragments were determined by DNA sequencing (MWG Biotech Customer Service, Ebersberg, Germany).
FIG. 2.
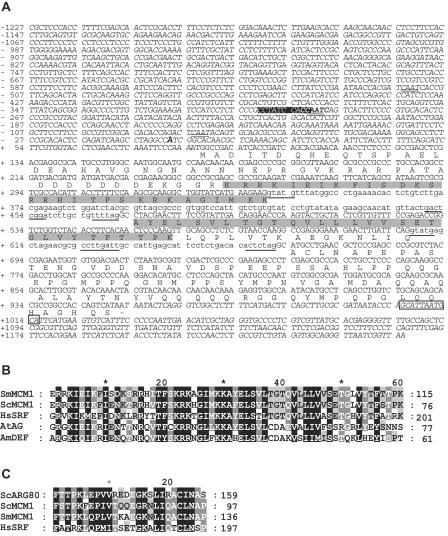
Nucleotide and deduced amino acid sequences from the S. macrospora mcm1 gene and its flanking regions and alignments with related proteins. (A) Nucleotide and deduced amino acid sequences of the S. macrospora mcm1 gene. Intron sequences are indicated with lowercase letters, and intron consensus sequences are underlined. The MADS box domain is indicated in gray. Putative CAAT box motifs are doubly underlined. A TATA box sequence is boxed in black, and the putative transcriptional start signal is marked in bold italics. A typical polyadenylation signal identified at positions 1005 to 1016 is boxed. (B) MADS box domains of S. macrospora MCM1 (SmMCM1), Mcm1p of S. cerevisiae (ScMCM1, accession no. CAA88409.1), human SRF (HsSRF, CAI13785), Arabidopsis thaliana AGAMOUS (AtAG, P17839), and Antirrhinum majus DEFICIENS (AmDEF, CAA44629), aligned to maximize similarities. Identical amino acid residues are shaded in black, and functionally similar residues are boxed in gray. (C) Alignment of SAM domains of S. macrospora MCM1 (SmMCM1), yeast Mcm1p and Arg80p (ScMCM1 and ScArg80, NP_013756.1), and human SRF (HsSRF).
TABLE 2.
Oligonucleotides used in this study
| Oligonucleotide | Sequence (5′-3′) |
|---|---|
| m1a | GAGCGCCGCAAAATCGAAATCAAG |
| m2a | AGACGCGGGCTGGGGGGCGTTTAG |
| m3(B) | GGATCCGCCGACATCACCGATCAGCACGAA |
| m8a | GGATCCTTAACCCTGCCACCTACAGACGG |
| m14 | CCATGGCCGACATCACCGATCAG |
| m16 | CCATGGTTATGACTGGTGGCCGGC |
| m33 | CAGAAGAACAGAACGACTTTGA |
| m36 | GAATTCAAGGGGCCTCCGCTGATAAT |
| m37 | GAATAACCACGATCAATCACCG |
| m41 | GCGGCCGCGAAGAAGCTATGTTTATGGCCCT |
| m42 | CGCCGGCGTGGAACTCGAAACTGAGGGAA |
| m51 | GGACAAACTCTTGAAGCACCAAG |
| m54 | CCTACAGACGGCAGAGTTGGG |
| h3 | ACTCGTCCGAGGGCAAAGGAATAG |
| tC1 | GATCCGCCTGGACGACTAAACC |
Heterologous oligonucleotides specific for Neurospora crassa NCU07430.2 (http://www.broad.mit.edu/annotation/fungi/fgi/).
Sequence analyses.
Protein sequence data were obtained from the public database NCBI Entrez (http://www.ncbi.nlm.nih.gov/entrez/) or by TBLASTP searches of the fully sequenced N. crassa genomes at the Broad Institute (http://www.broad.mit.edu/annotation/fungi/fgi/). Protein sequence alignments were performed using the ClustalX program (65). The prediction of promoter and terminator elements was done by using different “Hamming-Clustering” methods (http://www.itb.cnr.it/sun/webgene//) and the “Promoter Predictor” (http://www.fruitfly.org/seq_tools/promoter.html).
Preparation of nucleic acids, hybridization protocols, and PCR.
Isolation of S. macrospora genomic DNA was carried out as described by Pöggeler et al. (59). Southern blotting and hybridization were performed according to standard techniques (61), using 32P-labeled DNA probes. PCR amplification of S. macrospora genomic DNA or cosmid pools was performed with the HotStarTaq DNA polymerase (QIAGEN, Hilden, Germany) following the manufacturer's protocol. The different primers used for PCR experiments were synthesized by MWG Biotech (Table 2). Total RNA was isolated from S. macrospora at 3 to 7 days of growth, using the method of Hoge et al. (25). Reverse transcription-PCR was performed with the specific oligonucleotide primer pair m3(B) and m8 (Table 2) and was accomplished by the method of Mayrhofer et al. (37). mRNA was isolated from 1 mg of total RNA by using the PolyAtract mRNA isolation system IV (Promega, Mannheim, Germany). Northern blotting was carried out according to the method of Sambrook et al. (61).
Generation of S. macrospora Δmcm1 strain.
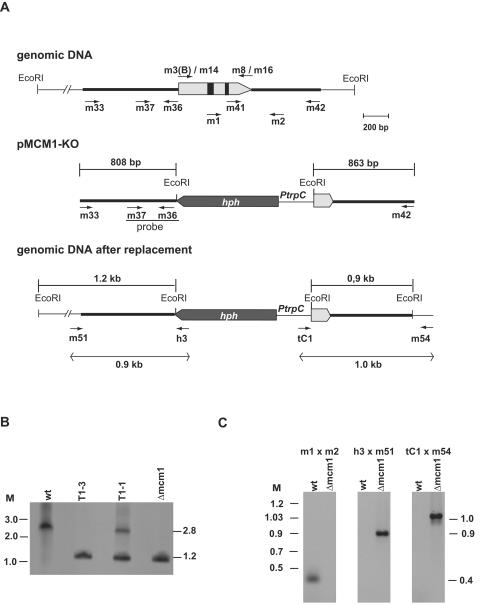
To create an mcm1 knockout construct for homologous recombination in S. macrospora, flanking regions of the mcm1 open reading frame were amplified by PCR from S. macrospora genomic DNA, using primer pair m33/m36 for the upstream region (808 bp) and primer pair m41/m42 for the downstream region (863 bp) (Table 2). Primer pair m41/m42 generated NotI ends. The PCR fragments were subcloned into vector pGEM-T (Promega) so that the 863-bp NotI fragment was fused with the 808-bp upstream fragment. The two sequences were separated by a single EcoRI restriction site which was used to introduce the 1.4-kb EcoRI hph cassette of pCB1003 (9). The resulting plasmid, pMCM1-KO, was used as a template to amplify the mcm1-hph cassette with oligonucleotides m33 and m42 as primers. The 3,029-bp PCR fragment obtained was transformed into the Δku70 strain (S66001) of S. macrospora to facilitate the knockout of the mcm1 gene by homologous recombination (54). Primary transformants were screened for homologous recombination by Southern blot analysis. Successful homologous recombination was confirmed by PCR amplification. The 5′-flanking region of the mcm1 gene was amplified with primers m54 and tC1, whereas h3 and m51 verified homologous recombination at the 3′-flanking region (see Fig. 4).
FIG. 4.
Construction of Δmcm1 strain. (A) Schematic illustration of the genomic region of the S. macrospora mcm1 gene and its flanking sequences and generation of the Δmcm1 replacement vector. The arrow represents the mcm1 coding region interrupted by two introns (black boxes). The positions of primers used to amplify the disruption construct from plasmid pMCM1-KO and to verify homologous recombination at the mcm1 locus are indicated. PtrpC, A. nidulans trpC promoter. (B) Southern analysis. The S. macrospora wt strain, primary transformants T1-3 and T1-1, and the T1-3 single-spore isolate S67718 (Δmcm1) were hybridized with the 32P-labeled probe indicated in panel A. The sizes of the hybridized fragments in wt and knockout transformants are given in panel A. (C) PCR analyses of homologous recombination from the wt and the single-spore isolate S67718 (Δmcm1). Positions of primers and sizes of amplified fragments are indicated in panel A.
Conventional genetic analysis of S. macrospora was performed as described by Esser (15). For segregation of the nourseothricin and hygromycin markers, the homokaryotic Δku70 primary transformant T1-3 (ku70::nat1), carrying a deletion of the mcm1 gene (mcm1::hph), was crossed with the fus1-1 mutant (S23442), producing brown ascospores. Subsequently, we isolated from this cross the hygromycin-resistant, nourseothricin-sensitive single-spore isolate S67718 (Table 1).
To rescue the phenotype of the Δmcm1 mutant S67718, plasmid pEGFPMCM1, carrying the coding sequences of the mcm1 gene fused to the egfp gene, was cotransformed with plasmid pD-NAT1 (32), resulting in transformant T8 (Table 1). In this experiment, the nourseothricin resistance gene nat1 of plasmid pD-NAT1 was used as a selectable marker for cotransformation (30). In plasmid pD-NAT1, the nat1 gene is under the control of the gpd promoter and trpC terminator of Aspergillus nidulans (32).
Two-hybrid analyses.
For construction of the two-hybrid plasmids, the cDNA of the entire coding region of the mcm1 gene was amplified with primer pair m3(B)/m8 and subcloned into pDrive to result in plasmid pNS8 (Table 2). After being sequenced, the 975-bp BamHI mcm1 fragment of pNS8 was cloned into the yeast two-hybrid vectors pGBDU-C1 (27), containing the GAL4 DNA-binding domain (pBM1) (Table 3), and pGAD-C1 (27), containing the GAL4 activation domain (pAM1) (Table 3). Vectors pBM1d1 and pBM1d2 are derivatives of vector pBM1. They were obtained after digestion of pBM1 with PstI and ScaI, respectively, and subsequently self-ligated. Both plasmids contain 3′-truncated mcm1 coding regions. Saccharomyces cerevisiae strain PJ69-4A was used for the two-hybrid experiments. To reduce the incidence of false-positive results, this strain contains three easily assayed reporter genes under the control of different GAL4-inducible promoters (GAL2-ade2, GAL1-his3, and GAL7-lacZ) (27). Transformation of yeast cells was done by electroporation according to the method of Becker and Lundblad (3) in a Multiporator (Eppendorf, Hamburg, Germany) at 1.5 kV. For each transformation, 100 ng of plasmid DNA was used. Selection of transformants was done by screening for uracil (pGBDU derivatives) and/or leucine (pGAD derivatives) prototrophy. Two-hybrid analyses and assays for transactivation were performed as described by Jacobsen et al. (26). Activity of β-galactosidase was measured by determination of o-nitrophenylgalactoside cleavage by crude protein extracts of yeast transformants grown in liquid culture (60). The crude protein extracts were obtained by disrupting the yeast cells with glass beads (0.5-mm diameter) in the presence of the protease inhibitor phenylmethylsulfonyl fluoride. The protein concentrations in the cell extracts were determined by the method of Bradford (5), and enzyme activity was normalized to the protein concentrations in the samples.
TABLE 3.
Plasmids used in this study
| Plasmid | Vector | Insert | Reference |
|---|---|---|---|
| pNS8 | pDrive | 628-bp mcm1 cDNA, including 348-bp 3′ region of wt S. macrospora, obtained by RT-PCR with m3(B)/m8 | This study |
| pAM1 | pGAD-C1 | 976-bp BamHI mcm1 fragment of pNS8 | This study |
| pBM1 | pGBDU-C1 | 976-bp BamHI mcm1 fragment of pNS8 | This study |
| pBM1d1 | pBM1 | PstI-restricted mcm1 cDNA positions 1 to 660 | This study |
| pBM1d2 | pBM1 | ScaI-restricted mcm1 cDNA positions 1 to 465 | This study |
| pAA1 | pGAD-C1 | Full-length SmtA-1 (positions 1 to 921) | 26 |
| pAA2 | pGAD-C1 | Full-length SmtA-2 (positions 1 to 1,080) | 26 |
| pAA3 | pGAD-C1 | Full-length SmtA-3 (positions 1 to 351) | 26 |
| pAa1 | pGAD-C1 | Full-length Smta-1 (positions 1 to 864) | 26 |
| pBA1 | pGBDU-C1 | Full-length SmtA-1 (positions 1 to 921) | 26 |
| pBA1d1 | pGBDU-C1 | SmtA-1 positions 1 to 726 | 26 |
| pBA2 | pGBDU-C1 | Full-length SmtA-2 (positions 1 to 1,080) | 26 |
| pBA3 | pGBDU-C1 | Full-length SmtA-3 (positions 1 to 351) | 26 |
| pBa1 | pGBDU-C1 | Full-length Smta-1 (positions 1 to 864) | 26 |
| pC-MCM1 | pCAL-n | 976-bp BamHI mcm1 fragment of pBM, sense | This study |
| pC-MCM1i | pCAL-n | 976-bp BamHI mcm1 fragment of pBM, inverse | This study |
| pC-SMTA1 | pCAL-n | 921-bp BamHI/BglII fragment of pB-A1, sense | This study |
| pC-SMTA1i | pCAL-n | 921-bp BamHI/BglII fragment of pB-A1, inverse | This study |
| pH-MCM1 | pQE31 | 976-bp BamHI mcm1 fragment of pBM, sense | This study |
| pMCM1-KO | pPTMCM1 | 1,400-bp EcoRI hph cassette of pCB1003 | This study |
| pMCM1EGFP | pIG1783 | 869-bp NcoI fragment of wt S. macrospora, obtained by PCR with primers m14 and m16 | This study |
| pD-NAT1 | nat1 expression cassette | 32 |
Protein synthesis and purification.
A His tag fusion of MCM1 was synthesized in E. coli strain M15(pREP4) (QIAGEN). For this purpose, a 975-bp BamHI fragment of pBM1 was cloned into vector pQE31 (QIAGEN) to generate plasmid pH-MCM1 (Table 3). His-tagged proteins were purified on nickel-nitrilotriacetic acid-agarose columns (QIAGEN) according to the supplier's instructions.
Calmodulin binding protein (CBP)-tagged versions of MCM1 were generated by cloning a 975-bp BamHI fragment of pBM1 in either sense (pC-MCM1) or inverse (pC-MCM1i) orientation into vector pCAL-n (Stratagene). For synthesis of the CBP-tagged versions of SMTA-1, a 921-bp BamHI/BglII cDNA fragment of pBA-1 (26) was cloned into vector pCAL-n to generate plasmids pC-SMTA1 (sense) and pC-SMTA1i (inverse) (Table 3). Proteins were synthesized in E. coli strain BL21(DE3) (Stratagene). Expression of the genes was induced by adding 2 to 5 mM isopropyl-β-d-thiogalactopyranoside (IPTG), and the cells were harvested after incubation for 4 h or overnight at 25 to 30°C. CBP fusion proteins were purified by affinity chromatography using a 50% slurry on calmodulin affinity resin according to the instruction manual (Stratagene).
Far-Western experiments.
Far-Western experiments were conducted by using 2 μg of purified CBP or CBP fusion protein. During preparation for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), proteins were reduced and denatured via treatment with 2× Laemmli sample buffer (34). After 12.5% SDS-PAGE, proteins were transferred to a polyvinylidene difluoride membrane (Biometra, Göttingen, Germany) by using a semidry blotting system (Biometra). Subsequently, the blot was blocked in blocking buffer (QIAGEN) for 2 h and then incubated with 5 μg His-MCM1 fusion protein in 10 ml blocking buffer for 2 h at room temperature while being rocked slowly. The His-MCM1 fusion protein was discarded by washing the membrane four times for 10 min with blocking buffer. Subsequently, the membrane was incubated for 1 h at room temperature with anti-RGS-His-horseradish peroxidase (HRP) conjugate antibody (QIAGEN; 1:3,000) diluted in blocking buffer and washed four times for 5 min each with Tris-buffered saline (QIAGEN). Detection of proteins was carried out with the BM chemiluminescence Western blotting kit (Roche Diagnostics, Germany) according to the supplier's recommendations.
Fluorescence, light, and confocal laser microscopy.
For localization studies, the mcm1 coding sequence was fused to the egfp gene. Cloning was carried out by amplifying an 869-bp DNA fragment from wild-type DNA, using primers m14 and m16 (Table 2). Amplification with this primer pair generated NcoI sites at the ends. After being subcloned into vector pDrive and sequenced, the amplicon was cloned into the NcoI site of plasmid pIG1783 (56) to create plasmid pMCM1EGFP (Table 3). The desired plasmid encodes an mcm1-egfp fusion gene under control of the A. nidulans gpd promoter. The enhanced green fluorescent protein (EGFP) fluorescence emission was analyzed by confocal laser scanning microscopy using a Zeiss LSM 510 META microscopy system (Carl Zeiss, Jena, Germany) based on an Axiovert inverted microscope. EGFP was excited with the 488-nm line of an argon-ion laser. The fluorescence emission was selected with BP505-550 band-pass filters for EGFP. Staining of nuclei with Sytox orange and visualization were performed as described by Pöggeler et al. (56). Transmission images were recorded using differential interference contrast optics. Images were analyzed with Zeiss LSM 510 software.
A Zeiss Axiophot or Zeiss Axio imager microscope was used for light microscopy. Pictures were captured with an AxioCam (Zeiss) or Cool SNAP HQ camera (Roper Scientific). Recorded images were edited using Adobe Photoshop CS2. Counting of fruiting bodies on agar plates was done according to the method of Mayrhofer et al. (37). To measure hyphal compartment lengths, wt and Δmcm1 strains were grown on cornmeal medium microscope slides. When adequate growth was achieved, the mycelium was stained with calcofluor white (1 μg/ml) and covered with a coverslip. Fluorescent micrographs were taken of one hypha, starting from the edge and moving 600 μm towards the interior of the colony. In doing so, 100 different hyphae each of the wt and the Δmcm1 mutant were measured.
Nucleotide sequence accession number.
The nucleotide sequence of the Sordaria macrospora mcm1 gene has been deposited in the EMBL database under accession number AM229713.
RESULTS
Sordaria macrospora possesses an mcm1 homologue.
A comparative analysis of S. macrospora and N. crassa sequences revealed that both fungi share a high degree of synteny and exhibit an average of 89.5% nucleic acid identity within exons (46). Primers m1 and m2 (Table 2) were designed according to the N. crassa open reading frame NCU07430.2, encoding a putative homologue of S. cerevisiae MCM1p. This primer pair was used to perform a high-throughput PCR screening of pooled cosmid DNAs, as described in Materials and Methods (57). The isolated cosmid was then used for subcloning and sequencing of the putative S. macrospora mcm1 coding region and its flanks. The mcm1 ORF comprised 868 bp interrupted by two introns, of 132 and 55 bp, with conserved 5′ donor and 3′ acceptor sequences (51), whose presence was confirmed by sequencing the corresponding cDNAs. The mcm1 gene encodes a putative protein of 226 amino acids (aa) with a predicted molecular mass of 24.6 kDa and a calculated isoelectric point of 5.6. The size of the S. macrospora protein is very close to that of N. crassa NCU07430.2 (24.3 kDa) but is smaller than S. cerevisiae Mcm1p (32.8 kDa) and the Homo sapiens SRF protein (51.6 kDa). Overall, the predicted S. macrospora protein shares 95.6, 32.6, and 21.9% identity with the respective proteins of N. crassa, S. cerevisiae, and H. sapiens. The putative S. macrospora MCM1 protein sequence was submitted to Superfamily (35), and the presence of the SRF-like MADS box domain (aa 55 to 115), a distinct DNA-binding motif, was recorded (Fig. 2A). This domain within S. macrospora MCM1 exhibited a high degree of sequence identity with the MADS box domains of the four founder proteins of this family (Fig. 2B). In animals and fungi, members of the family of MADS box transcription factors have been classified into two main lineages, designated SRF- and MEF2-like. Members of both lineages contain MADS boxes within their core DNA-binding domains but differ within their C-terminal extensions. The sequences of the MEF2 domain are highly homologous among members of the MEF2 subfamily but are very different from those of the SRF subfamily, such as SRF and Mcm1p, which have a 26-aa SAM (SRF, Arg80p, and Mcm1p) domain adjacent to the C terminus of the MADS box (63). Based on the sequence homology within the domain adjacent to the MADS box, the S. macrospora MCM1 protein is a member of the SRF lineage of MADS box proteins (Fig. 2C).
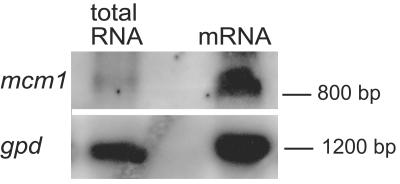
Using the program Promoter Predictor, the presumed transcriptional start site of the mcm1 gene was predicted to be 84 nucleotides upstream of the putative mcm1 translation start codon. Sequences flanking the ATG start codon show a high level of similarity to translation initiation sites in other S. macrospora genes (51). Within the promoter region, a putative TATA box was predicted by the “Hamming-Clustering method” at position −331 relative to the transcription initiation site, while three putative CAAT boxes were identified (Fig. 2). A polyadenylation signal was predicted to be 53 bp downstream of the putative mcm1 stop codon. Thus, the transcript was estimated to have a size of 808 bp. The mcm1 gene was only very weakly expressed, but a clearly visible transcript of the expected size was detected by Northern blot analysis using enriched poly(A) mRNA (Fig. 3).
FIG. 3.
Transcript analysis of S. macrospora wild type. Total RNA and mRNAs were isolated during different developmental stages of the wild type (3 to 7 days). The Northern blot was probed using an mcm1-specific probe. As a control, the blot was striped and reprobed with a gpd-specific probe.
The Δmcm1 deletion mutant exhibits a pleiotropic phenotype.
To assess the role of MCM1 in S. macrospora, we constructed a Δmcm1 deletion strain by gene replacement (Fig. 4). For this purpose, we constructed plasmid pMCM1-KO, in which part of the region encoding amino acids 1 to 84 of mcm1 and 399 bp of the mcm1 upstream region were deleted and replaced with a hygromycin resistance cassette. The 3,029-bp amplification product obtained was transformed into S. macrospora Δku70. The S. macrospora Δku70 strain is defective in the repair of DNA double-strand breaks by nonhomologous end joining and was recently demonstrated to be an ideal recipient for gene targeting of developmental genes in S. macrospora (54). Three hygromycin-resistant colonies were isolated. Among these, two strains appeared to be heterokaryotic and contained wt nuclei (corresponding to a 2.8-kb hybridizing fragment) as well as Δmcm1 nuclei (1.2-kb hybridizing fragment) (T1-1) (Fig. 4B), whereas one transformant (T1-3) contained only the Δmcm1 1.2-kb hybridizing fragment (Fig. 4B). To segregate the hph and nat1 markers of the homokaryotic transformant T1-3 and to obtain a Δmcm1 knockout mutant without the Δku70::nat1 background, we performed conventional genetic analyses. Southern blot analyses revealed a 1:1:1:1 segregation of hygromycin-resistant, nourseothricin-resistant, nourseothricin- and hygromycin-resistant, and nonresistant progeny, thereby illustrating that the mcm1 gene is a nonessential gene. The data from the Southern blot analyses were further confirmed by PCR amplification of the hygromycin-resistant/nourseothricin-sensitive single-spore isolate S67718 (Δmcm1) from primary transformant T1-3, which was selected for further analysis (Fig. 4C).
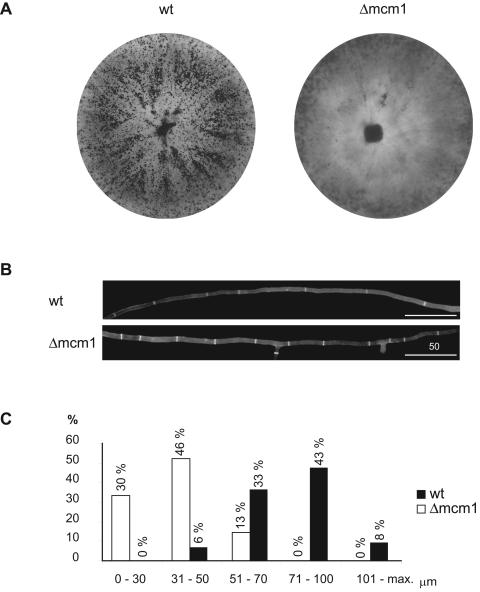
The Δmcm1 mutant showed a pleiotropic vegetative phenotype (Fig. 5). This mutant grew 15.4 (±1.5) mm/day, compared to 20.0 (±0.3) mm/day for the wt strain. Moreover, the mutant produced about 40% less mycelial mass than the wt after 7 days of growth. Furthermore, the hyphae at the peripheries of colonies of the Δmcm1 mutant showed some differences in branching frequency compared to those at the periphery of a wt colony (Fig. 5). Moreover, whereas a total of 42 branches were counted in the first 600 μm of 100 wt hyphae, the Δmcm1 mutant formed 129 branches (data not shown). In addition, hyphae of the mutant strain consisted of smaller compartments than the wt (Fig. 5B and C). For the wt strain, the mean hyphal compartment length was 74 μm (±19 μm), whereas the Δmcm1 strain exhibited a decreased hyphal compartment length of only 34 μm (±21 μm) (Fig. 5C). Thus, deletion of the mcm1 sequence resulted in reduced biomass, increased hyphal branching, and reduced hyphal compartment lengths during vegetative growth.
FIG. 5.
Vegetative growth of S. macrospora wild-type strain and Δmcm strain. (A) Wild-type S. macrospora hyphae and fruiting bodies after 7 days of growth on cornmeal medium. An mcm1 mutant grown on cornmeal medium formed highly branched and septated mycelia and no mature fruiting bodies. (B) Comparison of wild-type and Δmcm1 mutant hyphae stained with calcofluor white (1 μg/ml). Over a distance of 600 μm, wt hyphae showed seven compartments on average, and in most cases branches could not be observed. The Δmcm1 mutant contained 11 hyphal compartments over a distance of 600 μm, with two branches on average. Bar, 50 μm. (C) Measurement of hyphal compartment length. Measurements were binned into five different compartment lengths, and the number of analyzed compartments was normalized to 100%. Subsequently, compartment lengths were assigned to the segments. Dark and white bars represent compartment lengths of the wt and Δmcm1 strains, respectively.
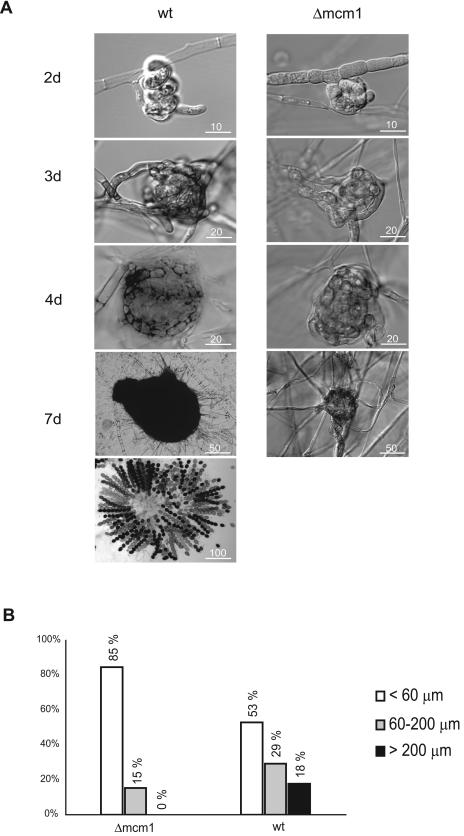
In addition to vegetative growth defects, the Δmcm1 mutant strain failed to complete the sexual cycle (Fig. 6). The development of sexual reproductive structures was analyzed on fructification medium. Although the mutant strain showed the formation of ascogonia and protoperithecia, it was unable to perform the transition to mature fruiting bodies (Fig. 6). When grown on fructification medium, the Δmcm1 strain produced 244 protoperithecia/cm2, similar to the wt strain (231 protoperithecia/cm2). The mean diameters of protoperithecia in the mutant and wt were 41 and 46 μm, respectively. However, the fertile fruiting bodies (>200 μm, with a neck and ascospores) that were formed in the wt (39 perithecia/cm2) were never observed in the Δmcm1 mutant, even after an extended incubation time. Thus, the mutant is completely sterile. All morphological changes of the phenotype cosegregated with the knockout hph marker in crosses between Δmcm1 and wt strains, indicating that deletion of the mcm1 gene was responsible for the pleiotropic mutant phenotype.
FIG. 6.
Sexual developmental stages of wild-type and Δmcm1 mutant strains. (A) Strains of S. macrospora were grown on fructification medium for 9 days. Note the complete absence of perithecia in the mutant strain compared with the wt strain. Differential interference contrast microscopy identified ascogonia (wt and Δmcm1), protoperithecia (wt and Δmcm1), young perithecia (only wt strain), perithecia (only wt strain), and ascospores (only wt strain). Strains were grown on fructification medium and examined after growth at 25°C for days, as indicated. Bars represent sizes (μm) as indicated. (B) Distribution of protoperithecia and perithecia produced by the S. macrospora wild type and the Δmcm1 strain. Percentages of small protoperithecia (<60 μm, white bars), protoperithecia (60 to 200 μm, gray bars), and perithecia (>200 μm, black bars) were calculated from 250 fruiting bodies from three different plates.
The MCM1 protein localizes to the nucleus.
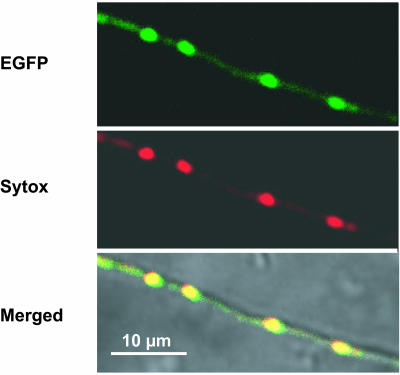
To examine MCM1 localization and to prove that the mutation can be complemented by a wt mcm1 copy, we generated strains expressing an mcm1-egfp fusion gene under control of the strong constitutive gpd promoter of A. nidulans (see Materials and Methods). Mutant strains expressing the fusion gene displayed wt hyphal growth and fruiting body development, thus indicating that the wt mcm1 gene can complement the mutant defects and that the MCM1-EGFP fusion protein is biologically active (Fig. 7).
FIG. 7.
Fluorescence microscopic analysis of Δmcm1 strains expressing mcm1-egfp. The images show a hypha of S. macrospora carrying the chimeric mcm1-egfp gene. (Top) EGFP fluorescence; (middle) Sytox orange staining of nuclei; (bottom) merged image of top and middle panels and the differential interference contrast micrograph.
As shown in Fig. 7, the MCM1-EGFP fusion protein was targeted to the nuclei, where it produced a fluorescence pattern which coincided with the staining pattern of the Sytox orange nucleic acid dye. As a control for localization, we used plasmid pIG1783, containing the egfp gene under control of the A. nidulans gpd promoter (56). In S. macrospora transformants carrying this plasmid, fluorescence appeared uniformly distributed throughout the hyphae, and EGFP fluorescence was not extensively concentrated in the nuclei (data not shown). This result showed that staining of the nuclei was due to the S. macrospora MCM1 protein fused to the EGFP fluorescence marker.
The S. macrospora MCM1 protein interacts with the mating-type protein SMTA-1.
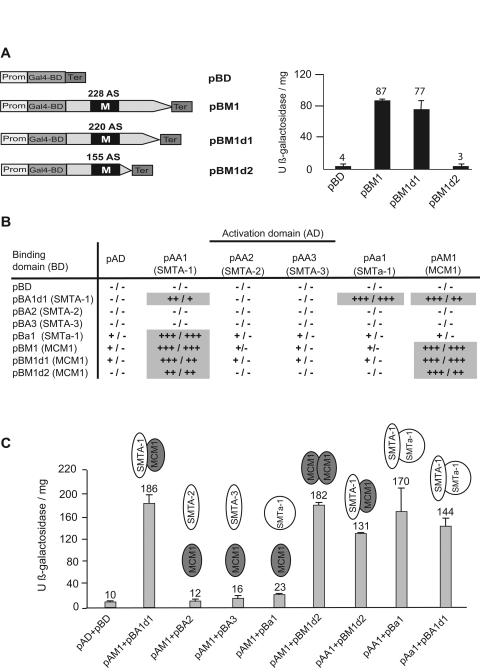
In the yeast S. cerevisiae, the MCM1 gene encodes an essential DNA-binding protein that, in cooperation with Matα1p, Ste12p, and the repressor Matα2p, confers mating specificity to haploid yeast cells (24, 39). To initially screen for in vivo interactions between the S. macrospora MCM1 and mating-type proteins, we performed a two-hybrid analysis by using the yeast two-hybrid system with the DNA-binding domain (BD) and activation domain (AD) derived from the GAL4 system (27). Sequence analysis of the S. macrospora MCM1 protein revealed that it contains a MADS box domain typical for DNA binding. This finding implies that the S. macrospora MCM1 protein might act as a transcriptional activator in yeast. To identify sequences of the MCM1 protein involved in transcriptional activation, the S. macrospora mcm1 full-length cDNA (pBM1) (Fig. 8A) and two versions carrying deletions of the 3′ end of the mcm1 coding region (pBM1d1 and pBM1d2) (Fig. 8A) were fused to sequences encoding the DNA-binding domain of the S. cerevisiae GAL4 protein (GAL4-BD). These constructs were transformed into the yeast strain PJ69-4A, which contains three reporter genes under the control of different GAL4-inducible promoters (27). PJ69-4A yeast cells, transformed with the recombinant pGBDU derivatives carrying full-length or truncated versions of the mcm1 gene and the empty pGBDU vector, were first tested for self-activation of reporter gene expression. The GAL4-BD-MCM1 fusion proteins encoded by pBM1 and pBM1d1 led to self-activation of reporter gene expression. A 20-fold increase in β-galactosidase activation compared to that of the negative control (pGBDU) was observed in cells carrying either pBM1 or pBM1d1. In contrast, the 73-aa C-terminally truncated version of MCM1, encoded by pBM1d2, exhibited no measurable expression of reporter genes (Fig. 8A). Thus, the MCM1 protein seems to contain a C-terminal domain that can activate the transcription of reporter genes in yeast.
FIG. 8.
Two-hybrid analyses of MCM1 and mating-type proteins. (A) Localization of MCM1 transcriptional activation domain. The numbers above the ORFs correspond to the lengths of the MCM1 derivatives. Black boxes represent the MADS box domain (M). β-Galactosidase activity was measured in triplicate for each transformant and is reported in units per milligram of protein. (B) Yeast transformants carrying different combinations of pB and pA derivatives (Table 3) were examined for growth on −Leu, −Ura, −His medium plus 20 mM 3-AT (left) or −Leu, −Ura, −Ade medium (right). −, no growth; +, slow growth; ++, growth; +++, strong growth. Measurements of interactions are highlighted in gray. (C) The β-galactosidase activities of the transformants carrying the GAL4-AD and -BD fusion proteins are given in units per milligram of protein.
To screen for homodimerization of MCM1 as well as for the ability of MCM1 to interact with mating-type proteins of S. macrospora, we tested pGBDU and pGAD derivatives of the mcm1 gene in combination with pGBDU and pGAD derivatives carrying S. macrospora mating-type genes. During previous analyses, we were able to demonstrate that the mating-type protein SMTA-1 activates transcription of the yeast reporter genes HIS3, ADE2, and LACZ (26). Therefore, plasmid pBA1d1, harboring a truncated version of the SmtA-1 gene and exhibiting no transcriptional activation of the yeast reporter genes, was used in this study. Similarly, we used plasmid pBM1d2, encoding a truncated version of MCM1. Our previous two-hybrid analyses demonstrated the ability of SMTA-1 to form a homodimer and to interact with SMTa-1 (26). These interactions were used as a positive control in the two-hybrid analyses. Analyses of reporter gene activities of yeast transformants carrying different combinations of pGBDU and pGAD derivatives revealed strong positive interactions of MCM1 and SMTA-1 and homodimerization of MCM1 (Fig. 8B and C). Similar to the case with the positive controls (pAA1 plus pBa1 [SMTA-1 plus SMTa-1]/pAa1 plus pBA1d1 [SMTa-1 plus truncated version of SMTA-1]), a >10-fold increase in β-galactosidase activation compared to the negative control (pAD plus pBD) was observed in cells carrying pAM1 plus pBA1d1 (MCM1 plus truncated version of SMTA-1), pAM1 plus pBM1d2 (MCM1 plus truncated version of MCM1), or pAA1 plus pBM1d2 (SMTA-1 plus truncated version of MCM1). However, MCM1 of S. macrospora seemed not to interact with the mating-type proteins SMTA-2, SMTA-3, and SMTa-1 (Fig. 8C).
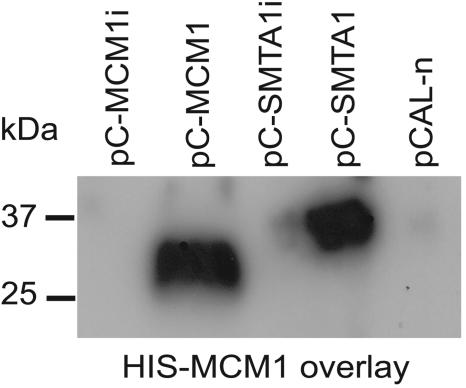
To confirm our results from two-hybrid assays, we examined interactions of MCM1 and SMTA-1 and the homodimerization of MCM1 by far-Western blot analysis (Fig. 9). After induction with IPTG, CBP-tagged fusion proteins were isolated, separated in gels, and analyzed by far-Western blotting. Filters were incubated with a purified His-MCM1 fusion protein, which was detected by the anti-RGS-His-HRP conjugate antibody (Fig. 9). As clearly shown in Fig. 9, both the CBP-MCM1 and CBP-SMTA-1 fusion proteins, corresponding to 28 and 34 kDa, respectively, were detected, thus indicating specific positive interactions between CBP-MCM1 and His-MCM1 as well as between CBP-SMTA-1 and His-MCM1.
FIG. 9.
Far-Western analysis of MCM1 homodimerization and MCM1 binding to SMTA-1. CBP-tagged MCM1 (pC-MCM1) and SMTA-1 (pC-SMTA1) fusion proteins, the corresponding inverse fusion constructs (pC-MCMi and pC-SMTAi), and a calmodulin binding protein tag (pCAL-n) were purified, separated by SDS-PAGE, blotted, and overlaid with His-MCM1 fusion protein. Bonded His-MCM1 was detected by the anti-RGS-His-HRP conjugate antibody.
To check that there was no nonspecific interaction between His-MCM1 and the CBP tag, we separated the CBP tag produced by the nonrecombinant pCal-n plasmid as a negative control. In addition, products of the antisense constructs pC-SMTA1i and pC-MCM1i were used to confirm that the observed interactions were not artifacts from nonspecific cross reactions. The positive interactions from the far-Western blot analyses were confirmed by means of pull-down experiments (data not shown). Thus, these data corroborate the yeast two-hybrid data indicating that the S. macrospora MCM1 protein is able to form a homodimer and to interact with the α1 domain mating-type protein SMTA-1.
DISCUSSION
In this study, we describe the cloning and characterization of the mcm1 gene from the homothallic ascomycete Sordaria macrospora. S. macrospora MCM1 displays significant similarities to the MADS box protein Mcm1p of Saccharomyces cerevisiae. Like S. cerevisiae Mcm1p (66), the MCM1 protein of S. macrospora also localizes to the nucleus. MADS box proteins are characterized by the conserved MADS box domain, which is required for DNA binding and dimerization (63). Based on a domain adjacent to the MADS box domain, the S. macrospora MCM1 protein can be classified as an SRF-type MADS box protein. This group of MADS box proteins is an evolutionarily conserved subfamily of MADS box transcription factors and includes animal SRF proteins as well as yeast Mcm1p and Arg80p (1). To our knowledge, the S. macrospora MCM1 protein is the first SRF-type protein characterized for a filamentous ascomycete.
Recently, Damveld et al. (12) described the isolation and characterization of the RLMA protein of Aspergillus niger, which is a member of the MEF2-type subfamily of MADS box proteins. A. niger RLMA was shown to have an important role in regulating and activating gene expression in response to cell wall stress (12). In contrast to the ascomycetous yeasts S. cerevisiae and Schizosaccharomyces pombe, which carry four and three MADS box proteins, respectively, we identified two MADS box genes within the available fungal genomes of the filamentous ascomycetes N. crassa, Aspergillus fumigatus, Aspergillus nidulans, Chaetomium globosum, Fusarium graminearum, and Podospora anserina (data not shown). One of these two genes encodes a MEF2-type RlmAp homologue, while the other encodes an SRF-type Mcm1p homologue. An exception is Magnaporthe grisea, which encodes only one SRF-type MADS box protein (12). The close relationship between S. macrospora and N. crassa suggests that S. macrospora also possesses a second MEF2-type MADS box protein, in addition to the SRF-type MCM1 protein.
Although S. cerevisiae carries four MADS box proteins in total, S. cerevisiae Mcm1p is an essential protein, and complete deletion of MCM1 results in a loss of viability of yeast cells. Thus, in S. cerevisiae, mcm1Δ defects cannot be restored by the other MADS box proteins (6, 48). In contrast, our results revealed that deletion of the mcm1 gene did not affect viability in S. macrospora. Therefore, the essential function of Mcm1p in S. cerevisiae might be a specific feature of this species, since deletion of the Mcm1p gene homologues map1 from the ascomycetous yeast S. pombe and umc1 from the basidiomycete Ustilago maydis showed that they are not essential (31, 43, 68). For S. macrospora, despite being viable, the Δmcm1 strain was affected in vegetative growth and fertility. This strain grew more slowly than the wt and displayed increased hyphal branching of hyphae with reduced compartment lengths. The vegetative phenotype of S. macrospora might be explained by the failure of interactions of MCM1 with transcriptional regulators playing conserved roles in regulating cell cycle processes. In S. cerevisiae, Mcm1p and the forkhead transcription factor Fkh2p act in a DNA-bound complex to control G2/M-specific transcription (33). Deletion of FKH2 in S. cerevisiae leads to a 10% extended generation time compared to that described for wild-type cells (49). In S. pombe, the forkhead transcription factor Fkh2p is required for correct timing, positioning, and contraction of the division septum. Deletion of the fkh2 gene in S. pombe leads to branched cells with multiple septa (7, 8). Indeed, a gene encoding a homologue of the yeast Fkh2p protein is present in all fungal genomes available (data not shown). Therefore, it seems likely that S. macrospora also has an FKH2 homologue. Thus, the phenotypic defects observed in the Δmcm1 mutant might be explained by the inability of S. macrospora FKH2 to bind to MCM1.
In addition to the vegetative defects observed in the Δmcm1 mutant, deletion of mcm1 dramatically affected the sexual fertility of S. macrospora. The Δmcm1 mutant was only capable of producing protoperithecia and was unable to form either ascospores or perithecia. Our two-hybrid analyses revealed that MCM1 may physically interact with itself and with the α domain mating-type protein SMTA-1. In addition, we showed that the N-terminal 155 amino acids of MCM1 containing the MADS box domain are sufficient for homodimerization and interaction with SMTA-1. Similarly, the conserved MADS box domain of S. cerevisiae Mcm1p was shown to be sufficient for dimerization and DNA binding as well as for interaction with different cofactors (6, 10).
In S. cerevisiae, Mcm1p activates α-specific genes, e.g., α-specific pheromone and pheromone receptor genes, together with the α domain transcription factor Matα1p, and represses a-specific genes (e.g., a-specific pheromone and pheromone receptor genes), together with the homeodomain transcription factor Matα2p (4, 24). In this study, we found an interaction of S. macrospora MCM1 with SMTA-1. However, no positive interactions between MCM1 and other proteins encoded by the S. macrospora mating-type locus were detected. This result does not come as a surprise, since unlike that of S. cerevisiae, the mating-type locus of S. macrospora does not encode a Matα2p-like homeodomain transcription factor.
In S. macrospora, the α domain protein SMTA-1 has previously been shown to interact with the mating-type locus-encoded HMG domain protein SMTa-1. Thus, the HMG domain protein SMTa-1 may be recruited via SMTA-1 into a complex which contains, among others, the MCM1 protein. Interestingly, with respect to fruiting body and ascospore development, the phenotype of a ΔSmta-1 mutant resembles that of the Δmcm1 mutant. Similar to Δmcm1, a ΔSmta-1 mutant was shown to be sterile and to produce only protoperithecia (58).
In heterothallic filamentous ascomycetes, the expression of pheromone and pheromone receptor genes is supposed to be controlled directly by transcription factors encoded by the mating-type genes (13). The mating-type-dependent expression of pheromone and receptor genes might be assisted by an MCM1 MADS box protein. However, the homothallic fungus S. macrospora has no genetically defined mating type and carries one mating-type locus that incorporates homologues of genes of both mating types of related heterothallic species (59). Thus, which functions does the MCM1 protein have during the sexual development of homothallic S. macrospora? In heterothallic filamentous ascomycetes, the mating-type-dependent expression of pheromone and receptor genes regulates the following two important steps in sexual reproduction: (i) the initial fertilization event, mediated by pheromone-dependent chemoattraction between the reproductive structures of two compatible partners; and (ii) prior to karyogamy, the paired migration of nuclei of opposite mating types into the ascogenous hyphae (11, 62). In S. macrospora, fruiting body development is an apandrous process and therefore lacks the cooperative interaction of two opposite mating-type strains. After autogamous fertilization, i.e., pairwise fusion of nuclei present within the ascogonium without cell fusion having taken place, the protoperithecia differentiate into inner ascus initials and an outer pigmented peridial tissue of the perithecium. Meiosis and a postmeiotic division lead to eight meiotically derived ascospores in each ascus (15, 16). Thus, although the fertilization process does not involve two genetically distinct nuclei, like it does in heterothallic relatives, S. macrospora must ensure that karyogamy occurs between two nuclei only. Therefore, it has been proposed that individual nuclei of homothallic fungi could be functionally heterothallic in order to allow nucleus recognition (11, 22). This might be achieved through differential expression of mating-type genes, which then should lead to differential expression of pheromone and receptor genes (53). Indeed, S. macrospora transcriptionally expresses two pheromone genes (ppg1 and ppg2) and two pheromone receptor genes (pre1 and pre2). The proteins encoded by these genes are similar to α-factor-like and a-factor-like pheromones and to G-protein-coupled pheromone receptors of the yeast S. cerevisiae (52, 55). Moreover, pheromones and receptors have recently been demonstrated to play an important role in fruiting body development and ascosporogenesis of S. macrospora. Similar to its function in basidiomycetes, where pheromones regulate nuclear migration and clamp-cell fusion in the dikaryotic mycelium, the pheromone system of S. macrospora seems to promote crozier formation and ensure the stability of the dikaryon (37). Furthermore, we found that the S. macrospora SMTa-1 protein has a direct or indirect impact on the activation of expression of the a-factor-like pheromone gene ppg2 and, in addition, regulates the expression of a variety of genes involved in different cellular processes (58). Likewise, it is conceivable that SMTA-1, along with MCM1, might be involved in the activation of the α-factor-like pheromone gene ppg1 and numerous other genes involved in fruiting body and ascospore formation in S. macrospora.
Taken together, the pleiotropic phenotype of the Δmcm1 mutant of S. macrospora reveals that the SRF-like MADS box proteins of filamentous ascomycetes play a role in a wide range of functions, including those controlling vegetative growth as well as sexual reproduction. Further studies are necessary to elucidate the molecular mechanism underlying the complex MCM1-dependent regulatory network of filamentous ascomycetes.
Acknowledgments
We thank Gisela Isowitz, Silke Nimtz, Susanne Schlewinski, and Regina Ricke for excellent technical assistance and Ulrich Kück (Ruhr University of Bochum) for laboratory resources.
This work was funded by Deutsche Forschungsgemeinschaft grant PO523/3-1 (Bonn, Germany) and by the Ruhr University of Bochum. N.N. was kindly supported by a Ph.D. grant from the Konrad-Adenauer Stiftung e.V. (Bonn, Germany).
REFERENCES
- 1.Alvarez-Buylla, E. R., S. Pelaz, S. J. Liljegren, S. E. Gold, C. Burgeff, G. S. Ditta, L. Ribas de Pouplana, L. Martinez-Castilla, and M. F. Yanofsky. 2000. An ancestral MADS-box gene duplication occurred before the divergence of plants and animals. Proc. Natl. Acad. Sci. USA 97:5328-5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Becker, A., and G. Theissen. 2003. The major clades of MADS-box genes and their role in the development and evolution of flowering plants. Mol. Phylogenet. Evol. 29:464-489. [DOI] [PubMed] [Google Scholar]
- 3.Becker, D. M., and V. Lundblad. 1994. Introduction of DNA into yeast cells, p. 13.7.1-13.7.10. In F. M. Ausubel, R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.), Current protocols in molecular biology. Wiley, New York, N.Y. [DOI] [PubMed]
- 4.Bender, A., and G. F. Sprague, Jr. 1987. MAT alpha 1 protein, a yeast transcription activator, binds synergistically with a second protein to a set of cell-type-specific genes. Cell 50:681-691. [DOI] [PubMed] [Google Scholar]
- 5.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 6.Bruhn, L., J. J. Hwang-Shum, and G. F. Sprague. 1992. The N-terminal 96 residues of MCM1, a regulator of cell type-specific genes in Saccharomyces cerevisiae, are sufficient for DNA binding, transcription activation, and interaction with α-1. Mol. Cell. Biol. 14:2534-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buck, V., S. S. Ng, A. B. Ruiz-Garcia, K. Papadopoulou, S. Bhatti, J. M. Samuel, M. Anderson, J. B. Millar, and C. J. McInerny. 2004. Fkh2p and Sep1p regulate mitotic gene transcription in fission yeast. J. Cell Sci. 117:5623-5632. [DOI] [PubMed] [Google Scholar]
- 8.Bulmer, R., A. Pic-Taylor, S. K. Whitehall, K. A. Martin, J. B. Millar, J. Quinn, and B. A. Morgan. 2004. The forkhead transcription factor Fkh2 regulates the cell division cycle of Schizosaccharomyces pombe. Eukaryot. Cell 3:944-954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carroll, A. M., J. A. Sweigard, and B. Valent. 1994. Improved vectors for selecting resistance to hygromycin. Fungal Genet. Newslett. 41:22. [Google Scholar]
- 10.Christ, C., and B. K. Tye. 1991. Functional domains of the yeast transcription/replication factor MCM1. Genes Dev. 5:751-763. [DOI] [PubMed] [Google Scholar]
- 11.Coppin, E., R. Debuchy, S. Arnaise, and M. Picard. 1997. Mating types and sexual development in filamentous ascomycetes. Microbiol. Mol. Biol. Rev. 61:411-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Damveld, R. A., M. Arentshorst, A. Franken, P. A. Van Kuyk, F. M. Klis, C. A. Van den Hondel, and A. F. J. Ram. 2005. The Aspergillus niger MADS-box transcription factor RlmA is required for cell wall reinforcement in response to cell wall stress. Mol. Microbiol. 58:305-319. [DOI] [PubMed] [Google Scholar]
- 13.Debuchy, R. 1999. Internuclear recognition: a possible connection between euascomycetes and homobasidiomycetes. Fungal Genet. Biol. 27:218-223. [DOI] [PubMed] [Google Scholar]
- 14.Dolan, J. W., and S. Fields. 1991. Cell-type-specific transcription in yeast. Biochim. Biophys. Acta 1088:155-169. [DOI] [PubMed] [Google Scholar]
- 15.Esser, K. 1982. Cryptogams—cyanobacteria, algae, fungi, lichens. Cambridge University Press, London, United Kingdom.
- 16.Esser, K., and J. Straub. 1958. Genetische Untersuchungen an Sordaria macrospora Auersw.: Kompensation und Induktion bei genbedingten Entwicklungsdefekten. Z. Vererbungsl. 89:729-746. [PubMed] [Google Scholar]
- 17.Ferreira, A. V., Z. An, R. L. Metzenberg, and N. L. Glass. 1998. Characterization of mat A-2, mat A-3 and deltamatA mating-type mutants of Neurospora crassa. Genetics 148:1069-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferreira, A. V., S. Saupe, and N. L. Glass. 1996. Transcriptional analysis of the mtA idiomorph of Neurospora crassa identifies two genes in addition to mtA-1. Mol. Gen. Genet. 250:767-774. [DOI] [PubMed] [Google Scholar]
- 19.Fraser, J. A., and J. Heitman. 2005. Chromosomal sex-determining regions in animals, plants and fungi. Curr. Opin. Genet. Dev. 15:645-651. [DOI] [PubMed] [Google Scholar]
- 20.Fraser, J. A., and J. Heitman. 2004. Evolution of fungal sex chromosomes. Mol. Microbiol. 51:299-306. [DOI] [PubMed] [Google Scholar]
- 21.Glass, N. L., J. Grotelueschen, and R. L. Metzenberg. 1990. Neurospora crassa A mating-type region. Proc. Natl. Acad. Sci. USA 87:4912-4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glass, N. L., R. L. Metzenberg, and N. B. Raju. 1990. Homothallic Sordariaceae from Nature: the absence of strains containing only the a mating type sequence. Exp. Mycol. 14:274-289. [Google Scholar]
- 23.Greener, A. 1990. New competent cells for highest transformation efficiencies. Strategies 3:5-6. [Google Scholar]
- 24.Herskowitz, I. 1989. A regulatory hierarchy for cell specialization in yeast. Nature 342:749-757. [DOI] [PubMed] [Google Scholar]
- 25.Hoge, J. H. C., J. Springer, B. Zantige, and J. G. H. Wessels. 1982. Absence of differences in polysomal RNA from vegetative monokaryotic and dikaryotic cells of the fungus Schizophyllum commune. Exp. Mycol. 6:225-232. [Google Scholar]
- 26.Jacobsen, S., M. Wittig, and S. Pöggeler. 2002. Interaction between mating-type proteins from the homothallic fungus Sordaria macrospora. Curr. Genet. 41:150-158. [DOI] [PubMed] [Google Scholar]
- 27.James, P., J. Halladay, and E. A. Craig. 1996. Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics 144:1425-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson, A. D. 1995. Molecular mechanisms of cell-type determination in budding yeast. Curr. Opin. Genet. Dev. 5:552-558. [DOI] [PubMed] [Google Scholar]
- 29.Keleher, C. A., C. Goutte, and A. D. Johnson. 1988. The yeast cell-type-specific repressor alpha 2 acts cooperatively with a non-cell-type-specific protein. Cell 53:927-936. [DOI] [PubMed] [Google Scholar]
- 30.Krugel, H., G. Fiedler, C. Smith, and S. Baumberg. 1993. Sequence and transcriptional analysis of the nourseothricin acetyltransferase-encoding gene nat1 from Streptomyces noursei. Gene 127:127-131. [DOI] [PubMed] [Google Scholar]
- 31.Krüger, J., C. Aichinger, R. Kahmann, and M. Bölker. 1997. A MADS-box homologue in Ustilago maydis regulates the expression of pheromone-inducible genes but is nonessential. Genetics 147:1643-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kück, U., and B. Hoff. 2006. Application of the nourseothricin acetyltransferase gene (nat1) as dominant marker for the transformation of filamentous fungi. Fungal Genet. Newsl. 53:9-11. [Google Scholar]
- 33.Kumar, R., D. M. Reynolds, A. Shevchenko, S. D. Goldstone, and S. Dalton. 2000. Forkhead transcription factors, Fkh1p and Fkh2p, collaborate with Mcm1p to control transcription required for M-phase. Curr. Biol. 10:895-906. [DOI] [PubMed] [Google Scholar]
- 34.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 35.Madera, M., C. Vogel, S. K. Kummerfeld, C. Chothia, and J. Gough. 2004. The SUPERFAMILY database in 2004: additions and improvements. Nucleic Acids Res. 32:D235-D239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maher, M., F. Cong, D. Kindelberger, K. Nasmyth, and S. Dalton. 1995. Cell cycle-regulated transcription of the CLB2 gene is dependent on Mcm1 and a ternary complex factor. Mol. Cell. Biol. 15:3129-3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mayrhofer, S., J. M. Weber, and S. Pöggeler. 2006. Pheromones and pheromone receptors are required for proper sexual development in the homothallic ascomycete Sordaria macrospora. Genetics 172:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McInerny, C. J., J. F. Partridge, G. E. Mikesell, D. P. Creemer, and L. L. Breeden. 1997. A novel Mcm1-dependent element in the SWI4, CLN3, CDC6, and CDC47 promoters activates M/G1-specific transcription. Genes Dev. 11:1277-1288. [DOI] [PubMed] [Google Scholar]
- 39.Mead, J., A. R. Bruning, M. K. Gill, A. M. Steiner, T. B. Acton, and A. Vershon. 2002. Interactions of the Mcm1 MADS box protein with cofactors that regulate mating in yeast. Mol. Cell. Biol. 22:4607-4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Messenguy, F., and E. Dubois. 1993. Genetic evidence for a role for MCM1 in the regulation of arginine metabolism in Saccharomyces cerevisiae. Mol. Cell. Biol. 13:2586-2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Messenguy, F., and E. Dubois. 2003. Role of MADS box proteins and their cofactors in combinatorial control of gene expression and cell development. Gene 316:1-21. [DOI] [PubMed] [Google Scholar]
- 42.Metzenberg, R. L., and N. L. Glass. 1990. Mating type and mating strategies in Neurospora. Bioessays 12:53-59. [DOI] [PubMed] [Google Scholar]
- 43.Nielsen, O., T. Friies, and S. Kjaerulff. 1996. The Schizosaccharomyces pombe map1 gene encodes an SRF/MCM1-related protein required for P-cell specific gene expression. Mol. Gen. Genet. 253:387-392. [DOI] [PubMed] [Google Scholar]
- 44.Nowrousian, M., and P. Cebula. 2005. The gene for a lectin-like protein is transcriptionally activated during sexual development, but is not essential for fruiting body formation in the filamentous fungus Sordaria macrospora. BMC Microbiol. 5:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nowrousian, M., S. Masloff, S. Pöggeler, and U. Kück. 1999. Cell differentiation during sexual development of the fungus Sordaria macrospora requires ATP citrate lyase activity. Mol. Cell. Biol. 19:450-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nowrousian, M., C. Würtz, S. Pöggeler, and U. Kück. 2004. Comparative sequence analysis of Sordaria macrospora and Neurospora crassa as a means to improve genome annotation. Fungal Genet. Biol. 41:285-292. [DOI] [PubMed] [Google Scholar]
- 47.Passmore, S., R. Elble, and B. K. Tye. 1989. A protein involved in minichromosome maintenance in yeast binds a transcriptional enhancer conserved in eukaryotes. Genes Dev. 3:921-935. [DOI] [PubMed] [Google Scholar]
- 48.Passmore, S., G. T. Maine, R. Elble, C. Christ, and B. K. Tye. 1988. Saccharomyces cerevisiae protein involved in plasmid maintenance is necessary for mating of MAT alpha cells. J. Mol. Biol. 204:593-606. [DOI] [PubMed] [Google Scholar]
- 49.Pic, A., F. L. Lim, S. J. Ross, E. A. Veal, A. L. Johnson, M. R. Sultan, A. G. West, L. H. Johnston, A. D. Sharrocks, and B. A. Morgan. 2000. The forkhead protein Fkh2 is a component of the yeast cell cycle transcription factor SFF. EMBO J. 19:3750-3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pöggeler, S. 2001. Mating-type genes for classical strain improvements of ascomycetes. Appl. Microbiol. Biotechnol. 56:589-601. [DOI] [PubMed] [Google Scholar]
- 51.Pöggeler, S. 1997. Sequence characteristics within nuclear genes from Sordaria macrospora. Fungal Genet. Newslett. 44:41-44. [Google Scholar]
- 52.Pöggeler, S. 2000. Two pheromone precursor genes are transcriptionally expressed in the homothallic ascomycete Sordaria macrospora. Curr. Genet. 37:403-411. [DOI] [PubMed] [Google Scholar]
- 53.Pöggeler, S., and U. Kück. 2000. Comparative analysis of the mating-type loci from Neurospora crassa and Sordaria macrospora: identification of novel transcribed ORFs. Mol. Gen. Genet. 263:292-301. [DOI] [PubMed] [Google Scholar]
- 54.Pöggeler, S., and U. Kück. Highly efficient generation of signal transduction knockout mutants using a fungal strain deficient in the mammalian ku70 ortholog. Gene, in press. [DOI] [PubMed]
- 55.Pöggeler, S., and U. Kück. 2001. Identification of transcriptionally expressed pheromone receptor genes in filamentous ascomycetes. Gene 280:9-17. [DOI] [PubMed] [Google Scholar]
- 56.Pöggeler, S., S. Masloff, B. Hoff, S. Mayrhofer, and U. Kück. 2003. Versatile EGFP reporter plasmids for cellular localization of recombinant gene products in filamentous fungi. Curr. Genet. 43:54-61. [DOI] [PubMed] [Google Scholar]
- 57.Pöggeler, S., M. Nowrousian, S. Jacobsen, and U. Kück. 1997. An efficient procedure to isolate fungal genes from an indexed cosmid library. J. Microbiol. Methods 29:49-61. [Google Scholar]
- 58.Pöggeler, S., M. Nowrousian, C. Ringelberg, J. Loros, J. Dunlap, and U. Kück. 2006. Microarray and real-time PCR analyses reveal mating type-dependent gene expression in a homothallic fungus. Mol. Genet. Genomics 275:492-503. [DOI] [PubMed] [Google Scholar]
- 59.Pöggeler, S., S. Risch, U. Kück, and H. D. Osiewacz. 1997. Mating-type genes from the homothallic fungus Sordaria macrospora are functionally expressed in a heterothallic ascomycete. Genetics 147:567-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rose, M., and D. Botstein. 1983. Construction and use of gene fusions to lacZ (β-galactosidase) that are expressed in yeast. Methods Enzymol. 101:167-180. [DOI] [PubMed] [Google Scholar]
- 61.Sambrook, J., E. F. Fritsch, and T. Maniatis. 2001. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 62.Shiu, P. K., and N. L. Glass. 2000. Cell and nuclear recognition mechanisms mediated by mating type in filamentous ascomycetes. Curr. Opin. Microbiol. 3:183-188. [DOI] [PubMed] [Google Scholar]
- 63.Shore, P., and A. D. Sharroks. 1995. The MADS-box family of transcription factors. Eur. J. Biochem. 229:1-13. [DOI] [PubMed] [Google Scholar]
- 64.Staben, C., and C. Yanofsky. 1990. Neurospora crassa a mating-type region. Proc. Natl. Acad. Sci. USA 87:4917-4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 24:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wagner, P., and M. N. Hall. 1993. Nuclear protein transport is functionally conserved between yeast and higher eukaryotes. FEBS Lett. 321:261-266. [DOI] [PubMed] [Google Scholar]
- 67.Westergaard, M., and H. K. Mitchell. 1947. Neurospora. V. A synthetic medium favoring sexual reproduction. Am. J. Bot. 34:573-577. [Google Scholar]
- 68.Yabana, N., and M. Yamamoto. 1996. Schizosaccharomyces pombe map1+ encodes a MADS-box-family protein required for cell-type-specific gene expression. Mol. Cell. Biol. 16:3420-3428. [DOI] [PMC free article] [PubMed] [Google Scholar]