Abstract
This paper reports the discovery of anaerobic respiration on tellurate by bacteria isolated from deep ocean (1,543 to 1,791 m) hydrothermal vent worms. The first evidence for selenite- and vanadate-respiring bacteria from deep ocean hydrothermal vents is also presented. Enumeration of the anaerobic metal(loid)-resistant microbial community associated with hydrothermal vent animals indicates that a greater proportion of the bacterial community associated with certain vent fauna resists and reduces metal(loid)s anaerobically than aerobically, suggesting that anaerobic metal(loid) respiration might be an important process in bacteria that are symbiotic with vent fauna. Isolates from Axial Volcano and Explorer Ridge were tested for their ability to reduce tellurate, selenite, metavanadate, or orthovanadate in the absence of alternate electron acceptors. In the presence of metal(loid)s, strains showed an ability to grow and produce ATP, whereas in the absence of metal(loid)s, no growth or ATP production was observed. The protonophore carbonyl cyanide m-chlorophenylhydrazone depressed metal(loid) reduction. Anaerobic tellurate respiration will be a significant component in describing biogeochemical cycling of Te at hydrothermal vents.
The metalloid tellurium (Te), a group 16 element related to oxygen and sulfur, possesses chemically stable oxidation states of +VI (tellurate), +IV (tellurite), 0 (elemental tellurium), and −II (telluride), with most Te occurring as tellurate in the hydrosphere and as tellurides of gold and silver in the lithosphere (33). The extremely low mean global abundance of Te (10−2 to 10−8 ppm) (33) conceals the fact that its distribution is highly heterogeneous, occurring at concentrations of 12 to 17 ppm in hydrothermal vents (10), 14.8 ppm in gold mines (32), and a surprising 30.6 ppt in mineral scales in some geothermal pipelines (23).
While oxidized Te is toxic to most microorganisms at concentrations as low as 1 μg/ml (28), aerobic reduction allows some species to resist K2TeO3 concentrations as high as 2,500 to 5,000 μg/ml (20, 22, 33, 34). Most reported tellurite and tellurate resistance occurs aerobically, but a few important anaerobic exceptions exist. For example, the purple bacterium Rhodobacter sphaeroides reduces tellurite at concentrations of up to 600 μg/ml to dispose of excess reducing equivalents generated during anaerobic photosynthesis (14). Resting cells of the sulfate reducer Desulfovibrio desulfuricans couple the reduction of tellurite to the oxidation of formate but cannot conserve the energy from this reaction (11). No dissimilatory electron transport to Te compounds is known to date (6, 33), even though the energetics of the TeO32−/Te redox couple (0.827 V) are more favorable for anaerobic respiration than the SO42−/HS− redox couple (−0.217 V) (11) utilized by sulfate reducers. Perhaps the toxicity of tellurite, combined with its low mean global abundance, has led to the belief that Te is biologically unimportant to microorganisms. However, tellurate is less toxic to microorganisms than is tellurite (33). Furthermore, respiration on toxic oxidized Se, As, and V is known (12, 26). Te, Se, and V are concentrated in both hydrothermal and industrial environments (10, 23, 32, 33) which select for bacteria that efficiently metabolize high concentrations of oxidized metal(loid)s.
Hydrothermal vents are geological formations that release altered seawater which has been heated, often to temperatures exceeding 400°C, by subterranean magma pockets as it circulates through the crust, mobilizing metals from basalt and acquiring elevated levels of sulfide from the reduction of sulfate by ferrous iron dissolved in the hot fluid (30). In the deep ocean, hydrothermal vents host unique communities of animals. Sulfide worms, tubeworms, and ciliates harbor bacteria with unusual metal(loid)-associated metabolisms. Alvinellid sulfide worms, such as Paralvinella sulfincola, are polychaetes that graze bacterial mats on black smoker chimneys (30). They are exposed to high concentrations of heavy metals (30) and are covered with a community of metal-resistant bacteria (8). Ridgeia piscesae is a tubeworm that forms bush-like growths near black smokers. Its internal bacterial symbionts utilize sulfide as an energy source to fix carbon (30). The proximity of worms to anoxic vent fluids suggests that they might host anaerobic metal(loid)-respiring epibiotic bacteria. One proposed basis of this association is the detoxification of invertebrate surfaces by symbiotic microorganisms (2, 7, 8). This idea is supported by the observation that bacteria isolated from hydrothermal vent fauna often produce exopolysaccharides with metal binding properties (7, 31). Our research indicates that specialized dissimilatory metabolic pathways employed by bacterial epibionts are another important detoxification strategy in such associations.
MATERIALS AND METHODS
Enumeration and isolation.
In July 2002 the submersible ROPOS was used to obtain samples for microbial culturing from two hydrothermal vent fields in the eastern Pacific Ocean. In 2003, samples of sulfide worms (P. sulfincola) and tubeworms (R. piscesae) were collected for enumeration of the external metal-resistant bacterial communities. Vent samples were acquired either by suction sampling, in which fine material is drawn into a sample jar through a hose by negative pressure and is then hermetically sealed during transport to the surface, or by placing larger pieces into a sealable acrylic box using the submersible's manipulator arm. Sampling sites are described below in Results and Discussion. For enumeration, animals were washed with sterile dilution medium. Agar deep tubes and aerobically incubated plates were inoculated in decimal dilution series on PG medium, a modification of metavanadate respiration medium (12) amended with Na-lactate (1 g/liter) and one of the metal(loid)s, K2TeO3 (300 μg/ml), K2TeO4 (300 μg/ml), Na2SeO3 (1,000 μg/ml), NaVO3 (900 μg/ml), or Na3VO4 (1,000 μg/ml). Resistance to metal(loid)s was assessed by differential counts in the absence and in the presence of the above concentrations of metal(loid). Anaerobic enrichment cultures in completely filled screw-cap tubes and in crimp-sealed tubes under a headspace of N2 were also established in PG medium at the concentrations described above. Inoculum was prepared for enrichment cultures by crushing animals by hand in aseptic plastic bags to homogenize them. Plates of PG medium were streaked from enrichments, and about 100 metal(loid)-reducing strains were purified based on visual detection of the color change indicative of metal(loid) reduction that develops in colonies during incubation. Reduction of selenite, tellurite, tellurate, metavanadate, and orthovanadate (all colorless in solution at the concentrations employed) is indicated by the appearance of bright red elemental Se, black elemental Te, bluish-green V(IV), and black V(III) (12, 22). For assessment of anaerobic metal-amended growth of purified strains on plates, cell suspensions were spread on the surface of agar containing PG medium amended with metal(loid)s at the aforementioned concentrations and incubated in GasPak anaerobic jars at room temperature.
Kinetics experiments.
Aerobically grown cells of ER-Te-48, ER-V-6, and AV-V-25 were injected into 120-ml crimp-sealed bottles containing 80 ml of PG medium amended with tellurate (0 and 300 μg/ml), metavanadate (0 or 900 μg/ml), or orthovanadate (0 or 1,000 μg/ml) under a headspace of N2. Tellurate-supplemented cultures were incubated either in the absence of added organics or in the presence of acetate (1 g/liter), lactate (1 g/liter), or yeast extract (0.2 g/liter), singly or in combination. Metavanadate-amended cultures were supplied with lactate (1 g/liter) and yeast extract (0.2 g/liter), and cultures grown with orthovanadate were supplemented with galactose (1 g/liter) and yeast extract (0.2 g/liter). Samples of tellurate-respiring cultures were collected at intervals of several days for determination of CFU/ml as an assay of growth, supplemented with microscopic analysis to verify the absence of cellular aggregation. Growth of vanadate-respiring organisms was assessed over intervals of several hours by total cell protein measurement using the Bradford method (4).
Protonophore experiments.
To investigate the effects of the protonophore and respiratory inhibitor carbonyl cyanide m-chlorophenylhydrazone (CCCP) on anaerobic metal(loid) reduction and growth, anoxic metal(loid)-amended cultures were supplemented with 0, 0.2, 0.5, 1.0, 10, 20, and 50 μM CCCP (18) and the color intensity of reduced forms of respective metal(loid)s was assessed by measuring the values of reflectance of the red, green, and blue channels using Adobe Image Ready 3.0 software applied to digital photographs of cultures (JPEG files in RGB format) obtained using an Olympus C-2020 Z digital camera. Metal(loid)s were added at levels indicated in “Enumeration and isolation,” above.
ATP experiments.
Anaerobic liquid cultures were established as described above for the kinetics experiments. Metal(loid) concentrations used were 0, 10, 100, and 300 μg/ml tellurate or 0, 300, 1,000, and 2,000 μg/ml metavanadate. Samples were collected at specified time intervals and analyzed for ATP concentration by the luciferin-luciferase bioluminescence assay (ATP bioluminescence assay kit; Sigma Chemical Company) following extraction of ATP from samples by using perchloric acid (25).
Phylogenetic analysis.
Extraction of genomic DNA, PCR-mediated amplification of the 16S rRNA gene sequences, and direct sequencing of the purified PCR products were carried out as described elsewhere (21). DNA G+C content was determined by using high-performance liquid chromatography (13, 27).
RESULTS AND DISCUSSION
Enumeration and isolation.
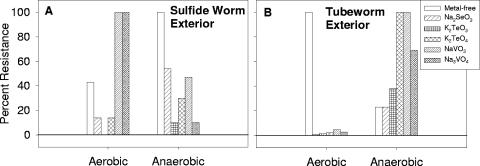
Enumeration of the cultivable metal(loid)-resistant aerobic and anaerobic bacteria inhabiting sulfide worms and tubeworms from the main Endeavor vent field of the Juan de Fuca Ridge (47°54′N, 129°6′W) suggested that the occurrence of anaerobic metal(loid) reduction among bacterial epibionts depends on the animal species with which they are associated (Fig. 1). The color of the colonies in agar deep cultures indicated that bacteria were not only resistant to but also reducing the metals to which they were exposed. Between 10 and 54% of the anaerobic epibionts of sulfide worms were resistant to selenite, tellurite, and tellurate, but a smaller proportion expressed aerobic resistance, suggesting the existence of anaerobic respiration in this bacterial community (Fig. 1A). In contrast, levels of metavanadate and orthovanadate resistance were dramatically greater aerobically than anaerobically, and more growth occurred in the presence of vanadates than in their absence (Fig. 1A). This observation suggests that sulfide worm epibionts may benefit from V aerobically, just as Azotobacter sp. utilizes V in place of Mo in nitrogenases (17). The tubeworm epibiont enumeration revealed that anaerobic metal(loid) reduction is more prevalent in association with this animal than with sulfide worms. Anaerobically, 23 to 100% of the total tubeworm epibionts appeared in the presence of metal(loid)s, while only 23% could grow in their absence (Fig. 1B). The lower counts on metal-free medium may be the result of the inability of the bacteria to find alternate electron acceptors for anaerobic respiration. This preferential growth on metal(loid)s is strongly suggestive that metal(loid)-respiring bacteria are well-represented in the epibiotic microflora of tubeworms. Furthermore, a much smaller proportion of aerobic organisms were resistant to any of the metal(loid)s (Fig. 1B). Dissolved sulfide in the vent fluids that bathe the animals might chemically remove enough oxygen from the surface of tubeworms to force attached bacteria to shift from aerobic to anaerobic respiration on metal(loid)s. Tubeworms rely on mutualistic sulfide-oxidizing chemoautotrophic endosymbionts to provide them with fixed forms of carbon, requiring them to more consistently expose their branchial plumes to sulfide-rich fluids than sulfide worms, which lack mutualistic endosymbionts (30). Hence, the tubeworm integument should be a more oxygen-free environment, and this would explain why the anaerobic metal(loid)-reducing bacterial population was more pronounced in tubeworm samples than in sulfide worm samples.
FIG. 1.
Percent resistance of cultivable aerobic and anaerobic epibiotic bacterial communities to Na2SeO3 (1,000 μg/ml), K2TeO3 (300 μg/ml), K2TeO4 (300 μg/ml), NaVO3 (900 μg/ml), and Na3VO4 (1,000 μg/ml). Samples were from sulfide worms (A) and tubeworms (B). All populations were normalized to the highest CFU value measured for each animal and oxygenation treatment, which was assumed to represent 100% of the bacterial population under those conditions. Values provided are the means calculated from three samples each.
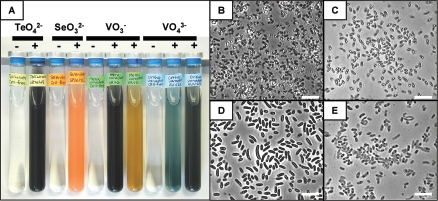
We isolated over 100 strains of facultatively anaerobic metal(loid)-resistant bacteria from metal(loid)-amended anaerobic enrichment cultures from hydrothermal vent fauna and chose four strains for detailed physiological work. Both tellurate-reducing strain ER-Te-48 and selenite-reducing strain ER-Se-17L originated from P. sulfincola organisms from the Lucky Find site within the Explorer Ridge vent field (49°45′38"N, 130°15′23"W; 1,791 m depth). Orthovanadate-reducing strain AV-V-25 was isolated from P. sulfincola from Hell vent, within the Axial Volcano caldera (45°56′00"N, 130°00′51"W; 1,543 m depth). Metavanadate-reducing strain ER-V-6 was isolated from a ciliate-rich blue-colored mat-like growth from the site called Tubeworm, within the Explorer Ridge vent field (49°45′35"N, 130°15′26"W; 1,781 m depth). All four strains were motile rods (Fig. 2B to E).
FIG. 2.
Metal(loid) reduction and morphology of deep ocean bacteria. (A) Anaerobic cultures of strains respiring metal(loid)s. −, cell-free control containing the metal(loid); +, cultures containing the indicated metal(loid). For metavanadate, the black and the rust-colored cultures contained 0.3 and 2 g/liter organics, respectively. For orthovanadate, the blue and the black cultures contained 0.7 and 2.2 g/liter organics, respectively. (B to E) Phase-contrast microscopy of anaerobic metal(loid)-amended ER-Se-17L, ER-Te-48, ER-V-6, and AV-V-25, respectively.
Range of metal(loid) reduction.
When cultured anaerobically in liquid medium with tellurite or tellurate, ER-Te-48 reduced a clear solution of either oxyanion to black elemental Te that accumulated intracellularly as refractile crystallites, which previous research has firmly established as Te(0) (Fig. 2A and C) (14, 33). In the presence of selenite, ER-Se-17L reduced a clear oxyanionic solution to a reddish-orange suspension of elemental Se, accumulating extracellular refractive globules of Se(0) (Fig. 2A and B). Neither of the V reducers initially accumulated any precipitates when cultured on vanadates (Fig. 2D and E). However, ER-V-6 altered the color of the medium from a clear solution characteristic of metavanadate to black and then to rusty brown, which finally precipitated as an unidentified material (Fig. 2A). Formation of a black colloidal in an initially clear-to-yellowish solution in vanadate-respiring Pseudomonas isachenkovii indicates the reduction of V(V) to V(III) (12). Anaerobic cultures of AV-V-25 turned deep bluish-green or black (Fig. 2A), denoting the presence of the +IV and +III oxidation states of V (12). Cell-free control medium did not change color in the presence of any of the metal(loid)s, confirming that abiological metalloid reduction did not occur. Furthermore, the rate of color change associated with metal(loid) reduction was faster and more intense in cell suspensions of higher density.
On aerobic agar-containing medium, only the colonies of tellurate and selenite reducers accumulated persistent color. Vanadate-reducing colonies initially turned the surrounding agar bluish-black, but the color was only transient, clearing after a few days. Strains also grew on anaerobic plates and in agar deeps amended with metal(loid)s, forming colonies with intense color indicative of the reduced metal(loid)s. In fact, reduction was more extensive anaerobically than aerobically, supporting the argument for anaerobic respiration. Under anoxic conditions, the color generated by vanadate reducers was persistent, and prolonged growth led to the development of rust-colored deposits.
All strains exhibited various degrees of high-level resistance to and reduction of several different metal(loid)s tested singly (tellurite and tellurate at 300 μg/ml, selenite and orthovanadate at 1,000 μg/ml, and metavanadate at 900 μg/ml), both aerobically and anaerobically (Table 1). Aerobically, ER-Se-17L was resistant to all metalloids but only reduced selenite strongly. Anaerobically, it was resistant to and reduced selenite, orthovanadate and, weakly, tellurate. Strain ER-Te-48 grew on all metalloids aerobically and reduced all except the vanadates. It reduced tellurite even more effectively than tellurate. Anaerobically, it was resistant to and reduced only tellurate, tellurite and, weakly, selenite. Strain ER-V-6 grew on all metalloids aerobically and visibly reduced both Te oxyanions. Anaerobically, it survived on and reduced metavanadate and tellurate. Finally, AV-V-25, the fastest grower, survived on vanadates and selenite aerobically but was sensitive to Te compounds and reduced only selenite. In the absence of oxygen, it was resistant to and reduced selenite and vanadates but not tellurate.
TABLE 1.
Resistance and reduction of metal(loid)s in vent strains
| Strain | Resistance to (and reduction of)a:
|
||||
|---|---|---|---|---|---|
| Na2SeO3 | K2TeO3 | K2TeO4 | NaVO3 | Na3VO4 | |
| Aerobic conditions | |||||
| ER-Se-17L | + (+) | W (W) | + (W) | + (−) | + (W) |
| ER-Te-48 | + (+) | + (+) | + (W) | + (−) | + (−) |
| ER-V-6 | + (+) | + (+) | + (+) | + (−) | + (−) |
| AV-V-25 | + (+) | − (−) | W (W) | + (−) | + (−) |
| Anaerobic conditions | |||||
| ER-Se-17L | + (+) | NA | W (W) | − (−) | + (+) |
| ER-Te-48 | W (W) | NA | + (+) | − (−) | − (−) |
| ER-V-6 | − (−) | NA | + (+) | + (+) | − (−) |
| AV-V-25 | + (+) | NA | − (−) | + (+) | + (+) |
+, good growth or reduction; W, weak growth or reduction; −, no growth or reduction; NA, not applicable.
The diversity of metal(loid)s that each strain reduced is consistent with an encounter of multiple metals by bacteria in hydrothermal vents, where resistance to just one metal(loid) would not aid in survival against the other metal(loid)s simultaneously present. Multiple resistance makes vent isolates particularly useful in bioremediation either as whole organisms or as a source of appropriate genetic elements.
Kinetics experiments.
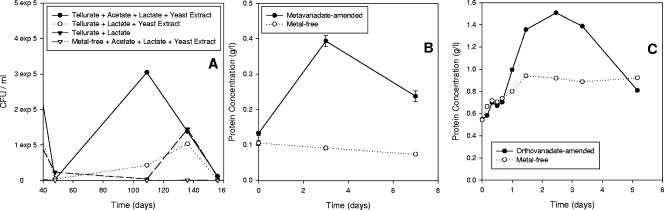
The strongest evidence for anaerobic respiration on a particular metal(loid) is an increase in cellular biomass coupled with the reduction of the metal(loid). In anoxic liquid cultures amended with tellurate, we noted first a drop in colony count, followed by an increase of nearly 2 orders of magnitude, to 105 to 106 CFU/ml (Fig. 3A). This transition may involve the initial induction of enzymes in a specific metalloid respiratory pathway. Tellurate-free cultures, by contrast, dropped to and remained at 103 CFU/ml or less. The initial drop in CFU may indicate (i) the manifestation of toxic effects of tellurate prior to the induction of enzymes that allowed for energetic utilization of tellurate and (ii) the depletion of energy reserves of cells that had no alternate metabolic strategy available prior to induction of tellurate respiration enzymes. Because cultures in the absence of tellurate continued to lack an alternate electron acceptor, they never recovered from the initial drop. Cultures with the most extensive growth also showed the highest degree of tellurate reduction, evident from the dark color of the medium. When cultured on a single carbon source, reduction and growth were most extensive in the presence of lactate. Amendment of cultures with various combinations of lactate, acetate, and yeast extract indicated that lactate and yeast extract stimulated growth and reduction, with the latter probably acting as a growth factor, while acetate did not serve as a source of reducing power for bioreduction.
FIG. 3.
Anaerobic respiratory growth kinetics. (A) ER-Te-48 with various organics, with and without tellurate. (B) ER-V-6 with and without metavanadate. (C) AV-V-25 with and without orthovanadate. Metal(loid) concentrations are as described for Fig. 1.
Anaerobic cultures of ER-V-6 and AV-V-25 showed an appreciable increase in biomass only in the presence of metavanadate and orthovanadate, respectively (Fig. 3B and C), distinguishing this process from electron disposal strategies that simply maintain redox poise, such as in anaerobic phototrophs (14). Unlike tellurate-reducing ER-Te-48, they showed no initial decrease in biomass and development was much faster, reaching stationary phase within 2 to 3 days. Thus, metal respiratory systems in both strains either are induced much faster than in the tellurate reducer or are constitutively expressed. Such a trait would be useful to bacteria utilizing vanadate for respiration at deep ocean hydrothermal vents, as levels of V are often very high in this environment (33). The distribution of Te, on the other hand, is more variable (33), and constitutive expression of tellurate respiration systems may be wasteful to cells.
Protonophore experiments.
The inhibitory effects of protonophores on metal(loid) reduction may indicate anaerobic respiration, because protonophores collapse the transmembrane pH gradient that is established during respiration to generate ATP from ADP, a reaction which in sulfate-reducing bacteria is involved in a preparatory step for the reduction of sulfate to sulfite (15, 24). We investigated the effects of the protonophore CCCP on anaerobic reduction of tellurate, selenite, metavanadate, and orthovanadate. Anaerobic metal(loid)-amended cultures supplemented with 10 and 50 μM CCCP resulted in no growth or metal(loid) reduction, while CCCP-free cultures reduced metal(loid)s. Oremland et al. (18) reported similar results for cultures of selenate-respiring strain SES-3. At such high concentrations, CCCP also inhibited growth aerobically. At 1.0 μM, CCCP did not arrest aerobic growth, but anaerobically it had increasingly inhibitory effects on ER-Se-17L, ER-Te-48, and ER-V-6 as the CCCP concentration increased from 0 to 0.2, 0.5, and 1.0 μM. AV-V-25, the fastest-growing strain, showed no difference in performance between 0 and 1.0 μM. We interpret these results to mean that the proton gradient in anaerobically slowly growing strains is more easily quenched by the ionophore than in fast growers. Contrary to previously published short-duration experiments (a few minutes) on resting cells in which an initial increase in the reduction of the terminal electron acceptor was typically observed (1), our long-term growth experiments (several days in duration) focused on preventing growth (and therefore metal[loid] reduction) by the only method of metabolism available for exploitation under the conditions provided. Under these conditions, growth and metal(loid) reduction in metal(loid)-respiring strains should be inhibited concurrently by a protonophore.
ATP experiments.
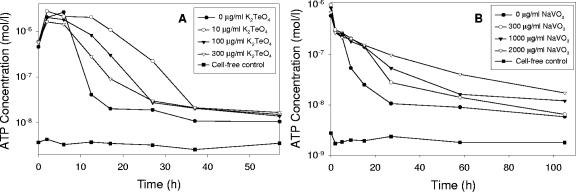
Organisms that carry on anaerobic respiration generate ATP from the coupling of the oxidation of a carbon source with the reduction of a terminal electron acceptor. In our case, metal(loid)s served the purpose of this acceptor. The rate of ATP generation should increase with metal(loid) concentration to a maximum at some optimum concentration and then decrease with further increases in metal(loid) concentration as toxic effects of the element outweigh its utility as an electron acceptor. We measured the intracellular ATP levels of cell suspensions in the presence of various metal(loid) concentrations for the tellurate and metavanadate reducers. As cells of ER-Te-48 aged, ATP declined much more quickly in the dying metal-free cultures than in cells in the presence of 10, 100, or 300 μg/ml of K2TeO4, which serves as a terminal electron acceptor to support metabolism in cell suspensions. At a point when dead tellurate-free cells contained very low ATP levels (only 20 nM), cultures amended with 10 μg/ml of K2TeO4 possessed 50 times more ATP (1,068 nM). The greatest ATP regeneration was at 10 μg/ml of K2TeO4 (Fig. 4A), comparable to Te concentrations reported for deep ocean hydrothermal vents (10). Cells of the metavanadate reducer ER-V-6 showed a similar trend, although ATP was regenerated most efficiently in medium amended with the highest concentration of NaVO3, 2,000 μg/ml (Fig. 4B), a concentration of V sometimes encountered in the environment (33). The rapid ATP decline in the absence of metalloids indicated that cells could not oxidize lactate because of the lack of an appropriate terminal electron acceptor. While our experiments did not track exponentially growing cultures, addition of tellurate or metavanadate to resting cell suspensions greatly increased the levels and duration of these levels of ATP in metal(loid)-amended cultures relative to metal(loid)-free cultures, demonstrating the ability of cells to conserve energy from electron transport to metal(loid)s.
FIG. 4.
Intracellular ATP concentrations. (A) ER-Te-48 with and without tellurate. (B) ER-V-6 with and without metavanadate.
Phylogenetic analysis.
Partial sequencing of 16S rRNA revealed that strains ER-Se-17L, ER-Te-48, and ER-V-6 were 99.3 to 99.8% similar to the type strain of Shewanella frigidimarina, isolated from Antarctic ice and capable of anaerobic Fe(III) respiration (3). Members of Shewanella are among the most metabolically versatile bacteria in their diversity of metal resistance and anaerobic respiration (16). Shewanella oneidensis was recently shown to be capable of metavanadate respiration (5). However, ER-V-6 is the first metavanadate-respiring Shewanella relative isolated from deep ocean hydrothermal vents. Furthermore, unlike S. frigidimarina, ER-V-6 was cultured from invertebrates, implying a possible symbiotic association with vent polychaetes. This hypothesis is theoretically sound, on the basis of a detoxification-nutrient acquisition mutualism (2, 7, 8). Associations between metal-respiring bacteria and invertebrates are known from nonvent systems (12). Metavanadate respiration has been reported for Pseudomonas species (12) and for Geobacter metallireducens (19), none of which come from hydrothermal vents. While respiration on selenite is known for microorganisms from hydrothermal systems, it has only been documented for the archaean Pyrobaculum, and only from terrestrial solfatara (33). Even more interesting is the demonstration of orthovanadate respiration in AV-V-25, which was 99.8% similar to Vibrio pomeroyi isolated from larvae of the bivalve Nodopecten nodosus from southern Brazil (29). Bacteria of this genus are not well known for their metal resistance, much less for dissimilatory reduction of toxic metal oxyanions. Our strain is also the first known organism capable of respiring on orthovanadate rather than the usual form of pentavalent V (metavanadate) utilized in microbiological experiments. Anaerobic reduction of tellurite occurs in Desulfovibrio desulfuricans (11), Rhodobacter sphaeroides (14), and Shewanella oneidensis (9), but in none is it reported to be dissimilatory in nature, and no reduction of Te oxyanions has previously been reported for S. frigidimarina.
Genetic affinities were further supported by G+C contents of 42.4, 42.4, 41.0, and 43.1 mol% for ER-Se-17L, ER-Te-48, ER-V-6, and AV-V-25, respectively. However, despite the high sequence similarities, phenotypic characterization must be performed for proper identification. Detailed taxonomic characterization of isolated strains is forthcoming.
Acknowledgments
We acknowledge the support of sampling cruise chief and cochief scientists Bob Embley, John Delaney, and Deborah Kelley, officers and crew of the R/V T.G. Thompson of the University of Washington, the ROV ROPOS team of the Canadian Scientific Submersible Facility, and Kim Juniper of UQAM, head of CanRidge. This research was supported by an NSERC CRO grant (K. Juniper, principal applicant) and an NSERC operating grant held by V. Yurkov and by an NSERC Postgraduate Scholarship B awarded to J. T. Csotonyi.
REFERENCES
- 1.Aguilaniu, H., L. Gustafsson, M. Rigoulet, and T. Nyström. 2001. Protein oxidation in G0 cells of Saccharomyces cerevisiae depends on the state rather than rate of respiration and is enhanced in pos9 but not yap1 mutants. J. Biol. Chem. 276:35396-35404. [DOI] [PubMed] [Google Scholar]
- 2.Alayse-Danet, A. M., D. Desbruyères, and F. Gaill. 1987. The possible nutritional or detoxification role of the epibiotic bacteria of Alvinellid polychaetes: review of current data. Symbiosis 4:51-62. [Google Scholar]
- 3.Bowman, J. P., S. A. McCammon, D. S. Nichols, J. H. Skerratt, S. M. Rea, P. D. Nichols, and T. A. McMeekin. 1997. Shewanella gelidimarina sp. nov. and Shewanella frigidimarina sp. nov., novel Antarctic species with the ability to produce eicosapentanoic acid (20:5ω3) and grow anaerobically by dissimilatory Fe(III) reduction. Int. J. Syst. Bacteriol. 47:1040-1047. [DOI] [PubMed] [Google Scholar]
- 4.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 5.Carpentier, W., L. De Smet, J. Van Beeumen, and A. Brige. 2005. Respiration and growth of Shewanella oneidensis MR-1 using vanadate as the sole electron acceptor. J. Bacteriol. 187:3293-3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ehrlich, H. L. 2002. Geomicrobiology, 4th ed. Marcel Dekker, Inc, New York, N.Y.
- 7.Holmström, C., and S. Kjelleberg. 1999. Marine Pseudoalteromonas species are associated with higher organisms and produce biologically active extracellular agents. FEMS Microbiol. Ecol. 30:285-293. [DOI] [PubMed] [Google Scholar]
- 8.Jeanthon, C., and D. Prieur. 1990. Susceptibility to heavy metals and characterization of heterotrophic bacteria isolated from two hydrothermal vent polychaete annelids, Alvinella pompejana and Alvinella caudata. Appl. Environ. Microbiol. 56:3308-3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klonowska, A., T. Heulin, and A. Vermeglio. 2005. Selenite and tellurite reduction by Shewanella oneidensis. Appl. Environ. Microbiol. 71:5607-5609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knott, R., A. E. Fallick, D. Rickard, and H. Backer. 1995. Mineralogy and sulfur isotope characteristics of a massive sulphide boulder, Galapagos Rift, 85°55′W, p. 207-222. In L. M. Parson, D. R. Dixon, and C. W. Walker (ed.), Hydrothermal vents and processes. Geological Society, London, United Kingdom.
- 11.Lloyd, J. R., A. N. Mabbett, D. R. Williams, and L. E. Macaskie. 2001. Metal reduction by sulphate-reducing bacteria: physiological diversity and metal specificity. Hydrometallurgy 59:327-337. [Google Scholar]
- 12.Lyalikova, N. N., and N. A. Yurkova. 1992. Role of microorganisms in vanadium concentration and dispersion. Geomicrobiol. J. 10:15-26. [Google Scholar]
- 13.Mesbah, M., and W. B. Whitman. 1989. Measurement of deoxyguanosine/thymidine ratios in complex mixtures by high-performance liquid chromatography for determination of the mole percentage guanosine + cytosine of DNA. J. Chromatogr. 479:297-306. [DOI] [PubMed] [Google Scholar]
- 14.Moore, M. D., and S. Kaplan. 1994. Members of the family Rhodospirillaceae reduce heavy-metal oxyanions to maintain redox poise during photosynthetic growth. ASM News 60:17-23. [Google Scholar]
- 15.Myers, C. R., and K. H. Nealson. 1988. Bacterial manganese reduction and growth with manganese oxide as the sole electron acceptor. Science 240:1319-1321. [DOI] [PubMed] [Google Scholar]
- 16.Myers, J. M., W. E. Antholine, and C. R. Myers. 2004. Vanadium(V) reduction by Shewanella oneidensis MR-1 requires menaquinone and cytochromes from the cytoplasmic and outer membranes. Appl. Environ. Microbiol. 70:1405-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nies, D. H. 1999. Microbial heavy-metal resistance. Appl. Microbiol. Biotechnol. 51:730-750. [DOI] [PubMed] [Google Scholar]
- 18.Oremland, R. S., J. Switzer Blum, C. W. Culbertson, P. T. Visscher, L. G. Miller, P. Dowdle, and F. E. Strohmaier. 1994. Isolation, growth, and metabolism of an obligately anaerobic, selenate-respiring bacterium, strain SES-3. Appl. Environ. Microbiol. 60:3011-3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ortiz-Bernad, I., R. T. Anderson, H. A. Vrionis, and D. R. Lovley. 2004. Vanadium respiration by Geobacter metallireducens: novel strategy for in situ removal of vanadium from groundwater. Appl. Environ. Microbiol. 70:3091-3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pearion, C. T., and P. E. Jablonski. 1999. High level, intrinsic resistance of Natronococcus occultus to potassium tellurite. FEMS Microbiol. Lett. 174:19-23. [Google Scholar]
- 21.Rainey, F. A., N. Ward-Rainey, R. M. Kroppenstedt, and E. Stackebrandt. 1996. The genus Nocardiopus represents a phylogenetically coherent taxon and a distinct actinomycete lineage: proposal of Nocardiopsaceae fam. nov. Int. J. Syst. Bacteriol. 46:1088-1092. [DOI] [PubMed] [Google Scholar]
- 22.Rathgeber, C., N. Yurkova, E. Stackebrandt, J. T. Beatty, and V. Yurkov. 2002. Isolation of tellurite- and selenite-resistant bacteria from hydrothermal vents of the Juan de Fuca Ridge in the Pacific Ocean. Appl. Environ. Microbiol. 68:4613-4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reyes, A. G., W. J. Trompetter, K. Britten, and J. Searle. 2002. Mineral deposits in the Rotokawa geothermal pipelines. N. Z. J. Volcanol. Geotherm. Res. 119:215-239. [Google Scholar]
- 24.Singleton, R., Jr. 1993. The sulfate-reducing bacteria: an overview, p. 1-20. In J. M. Odom and R. Singleton, Jr. (ed.), The sulfate-reducing bacteria: contemporary perspectives. Springer-Verlag, New York, N.Y.
- 25.Stanly, P. E., and S. G. Williams. 1969. Use of the liquid scintillation spectrometer for determining adenosine triphosphate by the luciferase enzyme. Anal. Biochem. 29:381-392. [DOI] [PubMed] [Google Scholar]
- 26.Stolz, J. F., and R. S. Oremland. 1999. Bacterial respiration of arsenic and selenium. FEMS Microbiol. Rev. 23:615-627. [DOI] [PubMed] [Google Scholar]
- 27.Tamaoka, J., and K. Komagata. 1984. Determination of DNA base composition by reversed-phase high-performance liquid chromatography. FEMS Microbiol. Lett. 25:125-128. [Google Scholar]
- 28.Taylor, D. E. 1999. Bacterial tellurite resistance. Trends Microbiol. 7:111-115. [DOI] [PubMed] [Google Scholar]
- 29.Thompson, F. L., C. C. Thompson, Y. Li, B. Gomez-Gil, J. Vandenberghe, B. Hoste, and J. Swings. 2003. Vibrio kanaloae sp. nov., Vibrio pomeroyi sp. nov. and Vibrio chagasii sp. nov., from sea water and marine animals. Int. J. Syst. Evol. Microbiol. 53:753-759. [DOI] [PubMed] [Google Scholar]
- 30.Van Dover, C. L. 2000. The ecology of deep-sea hydrothermal vents. Princeton University Press, Princeton, N.J.
- 31.Vincent, P., P. Pignet, F. Talmont, L. Bozzi, B. Fournet, J. Guezennec, C. Jeanthon, and D. Prieur. 1994. Production and characterization of an exopolysaccharide excreted by a deep-sea hydrothermal vent bacterium isolated from the polychaete annelid Alvinella pompejana. Appl. Environ. Microbiol. 60:4134-4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wray, D. S. 1998. The impact of unconfined mine tailings and anthropogenic pollution on a semi-arid environment—an initial study of the Rodalquilar mining district, southeast Spain. Environ. Geochem. Health 20:29-38. [Google Scholar]
- 33.Yurkov, V. V., and J. T. Csotonyi. 2003. Aerobic anoxygenic phototrophs and heavy metalloid reducers from extreme environments. Recent Res. Dev. Bacteriol. 1:247-300. [Google Scholar]
- 34.Yurkov, V., J. Jappe, and A. Vermeglio. 1996. Tellurite resistance and reduction by obligately aerobic photosynthetic bacteria. Appl. Environ. Microbiol. 62:4195-4198. [DOI] [PMC free article] [PubMed] [Google Scholar]