Abstract
Soil represents the principal environmental reservoir of many insect-pathogenic viruses. We compared the adsorption and infectivity of one occluded and two nonoccluded viruses, Helicoverpa armigera single nucleopolyhedrovirus (HaSNPV) (Baculoviridae), Cricket paralysis virus (CrPV) (Dicistroviridae), and Invertebrate iridescent virus 6 (IIV-6) (Iridoviridae), respectively, in mixtures with a selection of soil-forming minerals. The relative infective titers of HaSNPV and CrPV were unchanged or slightly reduced in the presence of different minerals compared to their titers in the absence of the mineral. In contrast, the infective titer of IIV-6 varied according to the mineral being tested. In adsorption studies, over 98% of HaSNPV occlusion bodies were adsorbed by all the minerals, and a particularly high affinity was observed with ferric oxide, attapulgite, and kaolinite. In contrast, the adsorption of CrPV and IIV-6 differed markedly with mineral type, with low affinity to bentonites and high affinity to ferric oxide and kaolinite. We conclude that interactions between soil-forming minerals and insect viruses appear to be most important in nucleopolyhedroviruses, followed by invertebrate iridescent viruses, and least important in CrPV, which may reflect the ecology of these pathogens. Moreover, soils with a high content of iron oxides or kaolinite would likely represent highly effective reservoirs for insect-pathogenic viruses.
Soil represents the principal environmental reservoir of many insect-pathogenic viruses. As such, the transfer of virus to plant surfaces is an important part in the initiation of new cycles of infection (16). Such new infections can in turn lead to epizootics that have major impacts on the population dynamics of insect defoliators (20). The mechanisms for the transfer of virus from soil to plant surfaces have been little studied, but there are indications that the virus is transferred in association with particulate matter from soil (16, 30, 48). The interactions between soil components and virus particles are likely to be even more important for soil-dwelling insect pests such as cutworms (e.g., Agrotis spp.), leatherjackets (e.g., Tipula spp.), and white grubs (e.g., Phyllophaga spp.).
The stability of virus in the soil may also be important for the efficacy of insect-pathogenic viruses used as biological insecticides (22). Moreover, the quantification of soil-virus interactions has clear relevance to the evaluation of ecological risks posed by the use of recombinant virus insecticides that have been genetically modified for increased virulence (17, 35). As one of the main components of soils, interest has focused on clay minerals due to their very high surface area/volume ratio and their high affinity for adsorption of virus particles (2, 19).
For this study, we selected three viruses that differ in their surface structure and their ability to persist outside of the host insect. Nucleopolyhedroviruses (NPVs) (Helicoverpa armigera single nucleopolyhedrovirus [HaSNPV]; genus Nucleopolyhedrovirus; family Baculoviridae) are double-stranded DNA (dsDNA) viruses with virions that contain single or multiple nucleocapsids within a single envelope (42). The virions are occluded into large proteinaceous occlusion bodies (OBs) with a 1- to 2-μm diameter that possess an outer envelope, comprised primarily of mucopolysaccharides, which is not required for infectivity (37). Dicistroviruses (Cricket paralysis virus [CrPV]; genus Cripavirus; family Dicistroviridae) are small single-stranded RNA-containing viruses, ∼30 nm in diameter, with structural similarities to picornaviruses (7). The external surface of CrPV is formed entirely of three structural proteins of 28 to 37 kDa. Invertebrate iridescent viruses (IIVs) (Invertebrate iridescent virus 6 [IIV-6]; genus Iridovirus; family Iridoviridae) have icosahedral particles with a ∼130-nm diameter and contain a dsDNA genome (5). The capsid consists of trimers of the major capsid protein of 50 kDa (45), and a fringe of fibrilar extensions of the capsomer subunits on the exterior surface of several IIVs has been described previously (46). Although IIVs have an internal lipid envelope and are much less stable in response to organic solvents than CrPV (27), both viruses have a proteinaceous external surface that is in contrast with the carbohydrate-like external surface of NPVs.
Numerous studies have examined the role of clays and other minerals in the adsorption of bacteriophages and pathogenic viruses of humans and livestock (4, 14, 24, 25, 26, 39, 41, 44), whereas this subject remains unstudied for viruses that infect insects. To better understand the potential interactions of insect-pathogenic viruses with the soil environment, we aimed to compare the adsorption and infectivity of these three viruses in mixtures with a range of different soil-forming minerals.
MATERIALS AND METHODS
Viruses.
Isolate A44EB1 of HaSNPV was grown in H. armigera larvae and purified as described previously (36). The OB concentration of the virus preparation was estimated using a Petroff-Hausser counting chamber and phase-contrast microscopy. The working suspension of HaSNPV was stored at a concentration of 1 × 109 OBs/ml at 4°C. The isolate of CrPV used in this study was the cloned CrPVVIC isolate described previously by Johnson and Christian (23). The virus was grown in Drosophila line 2 (DL2) cells and stored as a semipurified preparation in aliquots at −20°C until use. The titer of the experimental preparation was 5 × 109 infectious units/ml. IIV-6 was produced by injection into third- and fourth-instar Galleria mellonella larvae as described elsewhere previously (10). The virus was semipurified, filtered through a 0.44-μm filter, and stored in aliquots at −20°C until use. The titer of the experimental preparation was 5 × 109 infectious units/ml.
Insect cell lines.
Spodoptera frugiperda cells (Sf9 cells) were maintained in Grace's medium (Sigma Cell Culture, St. Louis, Mo.) supplemented with 10% fetal calf serum (Commonwealth Serum Laboratories, Melbourne, Australia), 2 mM glutamine, penicillin (50 units/ml), and streptomycin (50 μg/ml). DL2 cells were maintained on Schneider's medium (Sigma Cell Culture) supplemented with 10% fetal calf serum, glutamine, and antibiotics as described above. Both cell lines were maintained at 27 ± 1°C and were subcultured at weekly intervals.
Soil-forming minerals.
Clay minerals for testing were selected on the basis of availability and evidence of virus binding properties in preliminary studies (A. R. Richards and P. Christian, unpublished data). The minerals selected were aluminum hydroxide {also known as gibbsite [Al(OH)3]} attapulgite (Atta) {also known as palygorskite, a hydrous magnesium aluminum silicate [(Mg,Al)5(Si,Al)8O20(OH)2 · 8H2O]}, two samples of bentonite {an impure aluminum phyllosilicate clay consisting mostly of montmorillonite [(Na,Ca)0.33(Al,Mg)2Si4O10(OH)2 · nH2O]}, two samples of ferric oxide (Fe2O3), illite (Ill) {a nonexpanding micaceous phyllosilicate, (K,H3O)(Al,Mg,Fe)2(Si,Al)4O10[(OH)2,(H2O)]}, two samples of kaolinite {a dioctahedral phyllosilicate [Al2Si2O5(OH)4]}, and talc {hydrated magnesium silicate [H2Mg3(SiO3)4 or Mg3Si4O10(OH)2]}. These minerals were obtained from commercial chemical suppliers and a soil research laboratory (Table 1).
TABLE 1.
Origin and classification of minerals used in the present study along with the relative activity of each of the three viruses in the presence of the mineral and the flocculation efficiency of the mineral
| Mineral (sample no.) | Abbreviation | Sourcea | Relative activityb
|
% Unflocculatedc | ||
|---|---|---|---|---|---|---|
| HaNPV | CrPV | IIV-6 | ||||
| Aluminum hydroxide | AlOH | Merck Pty. Ltd., Australia | 0.87 | 1.02 | 2.36 | 0.55 |
| Attapulgite | Atta | Mallina Holdings, Australia | 1.02 | 0.93 | 2.96 | 0.26 |
| Bentonite (1) | Ben-1 | American Colloid Company, Volclay premium gel | 0.39 | 1.18 | 1.23 | DNF |
| Bentonite (2) | Ben-2 | Sigma-Aldrich Chemical Co. | 0.75 | 1.39 | 0.50 | DNF |
| Ferric oxide (1) | Fer-1 | CSIRO | 1.24 | 0.76 | 0.87 | ND |
| Ferric oxide (2) | Fer-2 | BDH Chemicals, Australia | ND | ND | 1.11 | 0.61 |
| Illite | Ill | CSIRO | 0.64 | 1.13 | 2.22 | 0.36 |
| Kaolinite (1) | Kao-1 | CSIRO, Georgia cream crude | 0.36 | 0.87 | 1.08 | 0.44 |
| Kaolinite (2) | Kao-2 | Sigma-Aldrich Chemical Co. | 0.56 | 1.08 | 1.99 | 0.76 |
| Talc | Talc | Sigma-Aldrich Chemical Co. | 0.91 | 1.20 | 5.06 | 1.86 |
CSIRO Soil Laboratory, Adelaide, Australia.
Calculated as the proportion of activity in the presence of the clay relative to that without the mineral present.
Flocculation efficiency of the mineral is expressed as the percentage of a 10-mg/ml suspension of mineral that was not flocculated by 2.5 mg/liter of Magnafloc LT25 at 25°C for 1 h. DNF, does not flocculate; ND, not determined.
Activity assays.
For CrPV and IIV-6, the titer of infectious virus was estimated using a 50% tissue culture infectious dose assay. Tenfold serial dilutions were used in all assays, and titers were calculated using the methodology of Reed and Muench (32). Larvae of H. armigera were obtained from a laboratory colony (strain AN02) reared from hatching at 25°C, with 50% relative humidity, on an artificial diet based on wheat germ, soy flour, and yeast (6). Bioassays of HaSNPV in H. armigera larvae were done by diet-surface contamination (21) and were carried out in plastic J2 jelly trays (Nu-trend containers). The artificial diet used for bioassays was formaldehyde free. After surface contamination, the diet was allowed to dry before a single 24-h-old larva was placed in each cell, sealed with Mylar film (Dupont) and perforated for ventilation. Twenty-five insects were used for each dilution. Insects were maintained at 30 ± 1°C throughout the test, and virus mortality was assessed at 10 days postinoculation. Larvae that had failed to feed by 5 days postestablishment were excluded from the assays. Each assay was performed three to five times.
Virus-mineral interactions.
For HaSNPV, 1 × 107 OBs were mixed with 10 ml of a 10-mg/ml suspension of the appropriate mineral in distilled water, and immediately after mixing, a small sample of the mineral-virus suspension was removed for assay, and the remainder was incubated at 25°C for 1 h. The clay was then flocculated using Magnafloc LT25 (Ciba Specialty Chemicals, Wyong, Australia), and a proprietary high-molecular-weight flocculant developed for the treatment of drinking water, at a final concentration of 2.5 mg/liter, and allowed to settle for 1 h. Dilutions were made from the unflocculated phase and assayed against H. armigera larvae as described above. Controls consisting of 1 × 107 OBs/ml in distilled water were treated with flocculant as described above or were left untreated and were set up alongside the mineral treatments and assayed as positive controls. Magnafloc LT25 caused no discernible flocculation of virus in the absence of the minerals.
To calculate the amount of virus in the assayed sample, only dilutions that gave mortalities of between 20% and 80% were used. The mortality was used to extrapolate an estimate of virus concentration from a standard calibration curve. As the dilution and the volume applied to each cell of the jelly tray were known, it was possible to estimate the number of OBs that remained in the original postflocculation supernatant. The standard calibration curve was generated using the following concentrations of HaSNPV: 12.98, 2.59, 0.519, and 0.130 OBs/mm2 of diet surface. A minimum of 10 replicates, each based on 25 insects/concentration, were performed for each concentration. Results were subjected to probit analysis using the PC-Polo program (LeOra Software, CA). The 50% lethal concentration of HaSNPV determined from the calibration curve was 0.47 OBs/mm2 (95% confidence interval, 0.38 to 0.57; slope ± standard error, 1.498 ± 0.069; χ2 = 2.43).
For CrPV, 100 μl of the standard preparation was mixed with 900 μl of the clay suspension at a concentration 10 mg/ml in phosphate-buffered saline. A 100-μl volume was immediately removed for titration. An untreated control consisting of the virus at the same concentration in phosphate-buffered saline was prepared and assayed alongside treated samples. The remaining clay plus the virus suspension were treated with Magnafloc LT25, incubated at room temperature for 1 h, and then centrifuged at 16,000 × g for 3 min. Tenfold dilutions were made from the unflocculated phase and titrated in DL2 cells. For IIV-6, the same procedure was followed as for CrPV, except that pelleting of the flocculated clay-and-virus complex was performed at 800 × g for 3 min. Titrations were carried out in Sf9 cells.
Estimation of clay concentrations.
Suspensions of the clay minerals (10 mg/ml) were allowed to settle for 5 min at room temperature, and the suspended mineral was collected. The weight of suspended clay particles was then estimated gravimetrically based on a 10-ml sample dried to constant weight in an oven at 60°C. A calibration curve was constructed by measuring the optical density at 600 nm of a series of diluted mineral suspensions in a Jenway 6505 spectrophotometer. To estimate the concentration of clay remaining in the unflocculated phase of a sample, the optical density at 600 nm was measured, and the mineral concentration was extrapolated from the calibration curve for that mineral.
Statistical analysis.
The loge proportion of virus activity that remained unadsorbed 1 h after incubation with clay minerals was subjected to analysis of variance using the GLIM statistical program (15). In all cases, deviations between observed and fitted values were examined using the model-checking macro present in the GLIM program. Mean values were compared for each mineral tested by t test (11). Comparisons between different viruses were not performed due to the differences in methodologies and the sensitivity of the assay techniques for each virus, as described above.
RESULTS
The titer of each of the viruses was assessed in the presence of each of the minerals and expressed relative to that of the untreated control (Table 1). For HaSNPV, the relative activity of the virus in the presence of the mineral was usually close to 1.0, except in the case of bentonite sample 1 (Ben-1), kaolinite sample 1 (Kao-1), and Kao-2, where the relative activity was reduced, indicating an inhibition of HaSNPV activity in the presence of the mineral. Similarly, for CrPV, the relative activity of the virus in the presence of all minerals was close to 1.0. In contrast, for IIV-6, the relative activity of the virus was lowered in the presence of Ben-2, close to 1.0 for Fer-1, Fer-2, and Kao-1, and close to or greater than 2.0 for the remaining minerals (AlOH, Atta, Ben-1, Ill, Kao-2, and talc), indicating increased activity in the presence of these compounds.
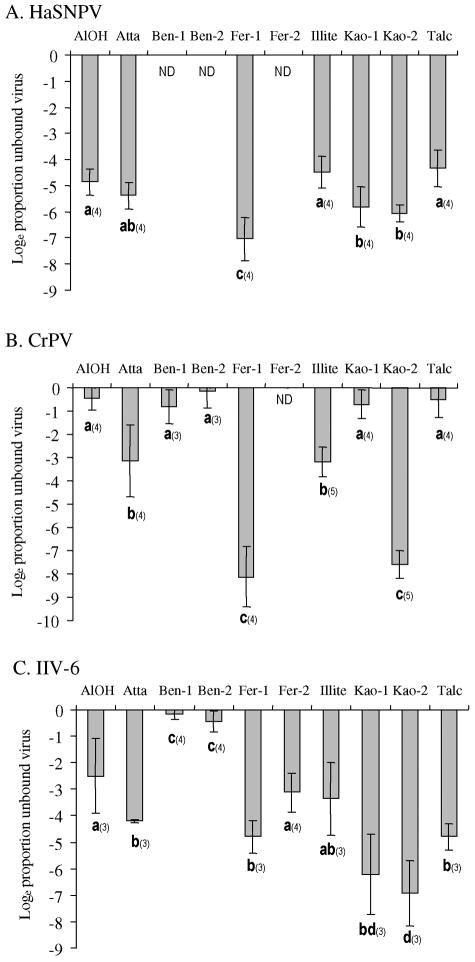
In the adsorption studies, for HaSNPV, the proportion of virus activity that remained in the suspension ranged from 0.12 to 1.83%, indicating that over 98% of the virus was adsorbed by all the minerals. Clearly, HaNPV showed a high affinity for all the minerals tested, particularly Fer-1, Atta, Kao-1, and Kao-2 (Fig. 1A). A slightly lower affinity for AlOH, Ill, and talc was observed. Overall, mineral type accounted for 72% of the total variance in nonadsorbed HaSNPV activity (F = 11.7; df = 6, 28; P < 0.001).
FIG. 1.
Mean (±standard deviation) loge proportion of virus remaining nonadsorbed to different minerals after 1 h of incubation for (A) HaSNPV, (B) CrPV, and (C) IIV-6. In all cases, unbound virus activity was compared to mineral-bound virus activity and found to correspond precisely to the reciprocal of the unbound fraction. Column headings with the same letter are not significantly different for comparisons of each virus incubated with different minerals (t test in GLIM, P > 0.05). Numbers in parentheses indicate the number of replicate observations for each mineral tested. A single outlier was eliminated from the analysis of CrPV binding to talc. ND, not determined.
In contrast, the adsorption of CrPV differed markedly with mineral type (Fig. 1B), which accounted for 94% of the variance in unbound CrPV activity (F = 50.1, df = 8, 26; P < 0.001). Adsorption of CrPV to AlOH, Ben-1, and Ben-2, talc, and Kao-1 was low, with the mean proportion of unbound virus representing between 53% (Ben-1) and 103% (Ben-2) of the original virus activity. In contrast, adsorption to Fer-1 and Kao-2 was extremely high, approaching the limits of detection of the assay system. Affinity to Ill and Atta was intermediate, with a mean of 5.1 and 8.6% of the original virus activity remaining unbound, respectively (Fig. 1B).
IIV-6 showed intermediate or high affinity for all the minerals tested, with the exception of the bentonites (Ben-1 and Ben-2) (Fig. 1C). Mineral type accounted for 89% of the variance in unbound virus activity (F = 20.9, df = 9, 23; P < 0.001). The highest affinity was observed in the case of the kaolinites (Kao-1 and Kao-2), followed by Fer-1, talc, and Atta. Binding to Ill, Fer-2, and AlOH was lower, with 5.3, 5.8, and 13.1% of the virus activity remaining unbound, respectively. Affinity to the bentonites (Ben-1 and Ben-2) was particularly weak, with 69 to 87% of the original virus activity remaining nonadsorbed after 1 h.
DISCUSSION
The three viruses used in the current study provided a selection of the structural paradigms found among the insect-pathogenic viruses. HaSNPV is a representative of the occluded viruses, which also include the cypoviruses (Reoviridae) and entomopoxviruses (Poxviridae), whereas CrPV and IIV-6 are nonoccluded viruses with an outer capsid composed entirely of proteins. The only category not represented was the enveloped insect viruses that have an outer lipid membrane, such as rhabdoviruses (e.g., sigma virus of Drosophila melanogaster) or the taxonomically unassigned nudiviruses (3).
Assays of virus activity in the presence of mineral suspensions revealed that certain minerals altered the infectivity of the nucleopolyhedrovirus and the iridovirus but did not affect CrPV activity. Why this should have occurred is unclear. A comparison of Kao-2 and talc, which reduced HaSNPV activity but increased IIV-6 activity, indicates that these interactions are both mineral and virus specific. For example, increased and decreased activities of IIV-6 were observed in the presence of Ben-1 and Ben-2, respectively. Altered infectivity was not correlated with the prevalence of adsorption for particular minerals for any of the viruses tested. Previous studies with mammalian viruses also reported increased virus infectivity in the presence of the mineral. The authors of those studies speculated that virus adsorbed to clays may result in improved presentation to cells either by improved proximity to the host cell (39) or because virus binding to charged surfaces induces conformational changes in surface proteins, resulting in altered infectivity (25). Others previously suggested that cells may acquire infection by phagocytosis of virus-contaminated clay particles (9) or that mineral electrostatic forces may disperse virus aggregates, resulting in higher effective titers (25).
Patterns of adsorption to the minerals studied differed markedly for each type of virus. The NPV displayed a consistently high affinity for all the minerals tested, whereas the IIV displayed a moderate to high affinity for all minerals except the bentonites. In contrast, CrPV showed a low affinity for about half of the minerals tested but very high affinity for ferric oxide and for one of the kaolinites used but not the other kaolinite. This indicates that minor differences in clay composition may strongly affect the magnitude of virus adsorption.
Interestingly, both CrPV and IIV-6 showed low levels of adsorption to both of the bentonites used in the study. Unfortunately, it was not possible to test these minerals with HaSNPV because they did not flocculate with the reagent employed or with several other flocculants tested (data not shown). Moreover, these clays could not be removed from suspension by centrifugation because both bentonites had a particle size/buoyant density similar to that of the nucleopolyhedrovirus OBs; i.e., unbound virus and mineral would pellet together.
Most clay minerals have a negative surface charge at a nearly neutral pH and would hence be expected to adsorb biomolecules such as viruses that carry a positive surface charge. However, while this was found to be the overall case in the present study, there were important anomalies inasmuch as the bentonites did not adsorb either of the nonoccluded viruses that we tested. This is surprising considering that several other studies reported bentonite adsorption to other viruses, including picornaviruses, coronaviruses, and reoviruses (9, 28, 29). These are all nonoccluded viruses with a naked (unenveloped) proteinaceous coat. Such results confirm that there is not a simple relationship between the net surface charge of the mineral (receptor) and the virus (acceptor) and that complex electrostatic forces (4) and physicochemical characteristics, such as the pH of the medium and the presence of cations, play a crucial role in virus adsorption to clays (24, 40, 41). Because of this, our experiments were performed at a nearly neutral pH, typical of the pH of many agricultural soils.
These results have a number of practical implications, particularly in ecological terms. First, the NPV appears likely to be adsorbed strongly by almost any type of soil. Strong adsorption and temporal stability of NPV OBs in different types of soils have been reported for a number of baculoviruses, both nucleopolyhedroviruses, and granuloviruses (22). In this respect, soil pH, temperature, and microbial activity are known to be important factors that affect the rate of deactivation of NPVs (13, 31). Second, CrPV-soil interactions appear to be of a lower magnitude than those of NPV, except for the minerals Fer-1 and kaolinite. Little is known about the role of soil in the survival of CrPV, although CrPV infections can occasionally devastate populations of soil-dwelling crickets (33). Third, IIV-6 interactions are likely to depend on soil type. Studies on IIV-6 survival in a sandy-loam soil (17% clay of unknown type) indicated that soil moisture was the principal determinant of virus persistence; the half-life in moist and wet soils was about 5 days, but the virus remained readily detectable 3 months after the start of the experiment (34). Previous studies have reported enhanced survival of human, fish, and bacterial viruses in the presence of clay minerals (18, 38, 40, 47). Clays can effectively protect viruses from inactivation by solar UV light (1, 43), a major limitation in the successful use of insect viruses as biological pesticides (12). More importantly, for all viruses tested, the presence of iron oxides is likely to be highly influential on virus survival. Iron oxides have been identified as binding strongly to noninsect viruses (8, 28, 29), and the collection of field samples for analysis is greatly facilitated by the brick-red color that these compounds impart to the soil.
In conclusion, interactions between soil-forming minerals and virus appear to be most important in NPVs, followed by IIVs, and least important in CrPV. This is likely to reflect the ecology of the viruses and the nature of the habitats of their respective insect hosts. From the results of this study, we hypothesize that soils with a high content of iron oxides or kaolinite would likely form reservoirs for insect-pathogenic viruses that are more effective than soils rich in bentonites and those lacking iron oxides.
Acknowledgments
We are grateful to Paul Scotti (Horticulture Research, Auckland, New Zealand) for the Excel program used to calculate tissue culture infectious dose values and to Jock Churchman (CSIRO Soil, Australia) for samples of ferric oxide, illite, and kaolinite. We thank Anne-Marie Wilkes for insect rearing and bioassays and Teresa Scoggins for calibration of suspended clay concentrations.
Andrew Richards was funded by the Cotton Co-operative Research Centre, Australia.
REFERENCES
- 1.Batista-Fihlo, A., S. B. Alves, N. T. Augusto, R. M. Pereira, and L. F. A. Alves. 2001. Stability and persistence of two formulations containing Anticarsia gemmatalis nuclear polyhedrovirus (AgMNPV). Neotrop. Entomol. 30:411-416. [Google Scholar]
- 2.Bitton, G. 1975. Adsorption of viruses onto surfaces in soil and water. Water Res. 9:473-484. [Google Scholar]
- 3.Burand, J. P. 1998. Nudiviruses, p. 69-90. In L. K. Miller and L. A. Ball (ed.), The insect viruses. Plenum, New York, N.Y.
- 4.Chattopadhyay, S., and R. W. Puls. 1999. Adsorption of bacteriophages on clay minerals. Environ. Sci. Technol. 33:3609-3614. [Google Scholar]
- 5.Chinchar, V. G., S. Essbauer, J. G. He, A. Hyatt, T. Miyazaki, V. Seligy, and T. Williams. 2005. Iridoviridae, p. 163-175. In C. M. Fauquet, M. A. Mayo, J. Maniloff, U. Desselberger, and L. A. Ball (ed.), Virus taxonomy: VIII report of the International Committee on the Taxonomy of Viruses. Elsevier, London, United Kingdom.
- 6.Christian, P. D., N. Gibb, A. B. Kasprzak, and A. Richards. 2001. A rapid method for the identification and differentiation of Helicoverpa nucleopolyhedroviruses (NPV Baculoviridae) isolated from the environment. J. Virol. Methods 96:51-65. [DOI] [PubMed] [Google Scholar]
- 7.Christian, P., E. Carstens, L. Domier, J. Johnson, K. Johnson, N. Nakashima, P. Scotti, and F. van der Wilk. 2005. Dicistroviridae, p. 783-788. In C. M. Fauquet, M. A. Mayo, J. Maniloff, U. Desselberger, and L. A. Ball (ed.), Virus taxonomy: VIII report of the International Committee on the Taxonomy of Viruses. Elsevier, London, United Kingdom.
- 8.Chu, Y., Y. Jin, T. Baumann, and M. V. Yates. 2003. Ground water quality: effect of soil properties on saturated and unsaturated virus transport through columns. J. Environ. Qual. 32:2017-2025. [DOI] [PubMed] [Google Scholar]
- 9.Clark, K. J., A. B. Sarr, P. G. Grant, T. D. Phillips, and G. N. Woode. 1998. In vitro studies on the use of clay, clay minerals and charcoal to adsorb bovine rotavirus and bovine coronavirus. Vet. Microbiol. 63:137-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Constantino, M., P. Christian, C. F. Marina, and T. Williams. 2001. A comparison of techniques for detecting Invertebrate iridescent virus 6. J. Virol. Methods 98:109-118. [DOI] [PubMed] [Google Scholar]
- 11.Crawley, M. J. 1993. GLIM for ecologists. Blackwell, Oxford, United Kingdom.
- 12.Dougherty, E. M., K. P. Guthrie, and M. Shapiro. 1996. Optical brighteners provide baculovirus activity enhancement and UV radiation protection. Biol. Contr. 7:71-74. [Google Scholar]
- 13.England, L. S., S. B. Holmes, and J. T. Trevors. 1998. Persistence of viruses and DNA in soil. World J. Microbiol. Biotechnol. 14:163-169. [Google Scholar]
- 14.Filder, P., and D. Kay. 1963. The conditions which govern the adsorption of a tryptophan-dependent bacteriophage to kaolin and bacteria. J. Gen. Microbiol. 30:183-191. [DOI] [PubMed] [Google Scholar]
- 15.Francis, B., M. Green, and C. Payne (ed.). 1993. The GLIM system: release 4 manual. Clarendon Press, Oxford, United Kingdom.
- 16.Fuxa, J. R., and A. R. Richter. 2001. Quantification of soil-to-plant transport of recombinant nucleopolyhedrovirus: effects of soil type and moisture, air currents, and precipitation. Appl. Environ. Microbiol. 67:5166-5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuxa, J. R., M. M. Matter, A. Abdel-Rahman, S. Micinski, A. R. Richter, and J. L. Flexner. 2001. Persistence and distribution of wild-type and recombinant nucleopolyhedrovirus in soil. Microb. Ecol. 41:222-232. [DOI] [PubMed] [Google Scholar]
- 18.Gantzer, C., F. Quignon, and L. Schwartzbrod. 1994. Poliovirus-1 adsorption onto and desorption from montmorillonite in seawater. Survival of the adsorbed virus. Environ. Technol. 15:271-278. [Google Scholar]
- 19.Gerba, C. P. 1984. Applied and theoretical aspects of virus adsorption to surfaces. Adv. Appl. Microbiol. 30:133-168. [DOI] [PubMed] [Google Scholar]
- 20.Hochberg, M. E. 1989. The potential role of pathogens in biological control. Nature 337:262-265. [DOI] [PubMed] [Google Scholar]
- 21.Ignoffo, C. M., A. H. McIntosh, and C. García. 1983. Susceptibility of larvae of Heliothis zea, H. virescens and H. armigera (Lep. Noctuidae) to 3 baculoviruses. Entomophaga 28:1-8. [Google Scholar]
- 22.Jaques, R. P. 1985. Stability of insect viruses in the environment, p. 285-360. In K. Maramorosch and K. E. Sherman (ed.), Viral insecticides for biological control. Academic Press, San Diego, Calif.
- 23.Johnson, K. N., and P. D. Christian. 1996. A molecular taxonomy for cricket paralysis virus including two new isolates from Australian populations of Drosophila (Diptera: Drosophilidae). Arch. Virol. 141:1509-1522. [DOI] [PubMed] [Google Scholar]
- 24.Lipson, S. M., and G. Stotzky. 1983. Adsorption of reovirus to clay minerals: effects of cation-exchange capacity, cation saturation, and surface area. Appl. Environ. Microbiol. 46:673-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lipson, S. M., and G. Stotzky. 1984. Effect of proteins on reovirus adsorption to clay minerals. Appl. Environ. Microbiol. 48:525-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lipson, S. M., and G. Stotzky. 1985. Specificity of virus adsorption to clay minerals. Can. J. Microbiol. 31:50-53. [DOI] [PubMed] [Google Scholar]
- 27.Martínez, G., P. Christian, C. F. Marina, and T. Williams. 2003. Sensitivity of Invertebrate iridescent virus 6 to organic solvents, detergents, enzymes and temperature treatment. Virus Res. 91:249-254. [DOI] [PubMed] [Google Scholar]
- 28.Meschke, J. S., and M. D. Sobsey. 1998. Comparative adsorption of Norwalk virus, poliovirus 1 and F+ RNA coliphage MS2 to soils suspended in treated wastewater. Water Sci. Technol. 38(12):187-189. [PubMed] [Google Scholar]
- 29.Moore, R. S., D. H. Taylor, L. S. Sturman, M. M. Reddy, and G. W. Fuhs. 1981. Poliovirus adsorption by 34 minerals and soils. Appl. Environ. Microbiol. 42:963-975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olofsson, E. 1988. Dispersal of the nuclear polyhedrosis virus on Neodiprion sertifer from soil to pine foliage with dust. Entomol. Exp. Appl. 46:181-186. [Google Scholar]
- 31.Peng, F., J. R. Fuxa, A. R. Richter, and S. J. Johnson. 1999. Effects of heat-sensitive agents, soil type, moisture, and leaf surface on persistence of Anticarsia gemmatalis (Lepidoptera: Noctuidae) nucleopolyhedrovirus. Environ. Entomol. 28:330-338. [Google Scholar]
- 32.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 33.Reinganum, C., G. T. O'Loughlin, and T. W. Hogan. 1970. A nonoccluded virus of the field crickets Teleogryllus oceanicus and T. commodus. J. Invertebr. Pathol. 16:214-220. [Google Scholar]
- 34.Reyes, A., P. Christian, J. Valle, and T. Williams. 2004. Persistence of Invertebrate iridescent virus 6 in soil. BioControl 49:433-440. [Google Scholar]
- 35.Richards, A., M. Matthews, and P. Christian. 1998. Ecological considerations for the environmental impact evaluation of recombinant baculovirus insecticides. Annu. Rev. Entomol. 43:493-517. [DOI] [PubMed] [Google Scholar]
- 36.Richards, A. R., and P. D. Christian. 1999. A rapid bioassay screen for quantifying nucleopolyhedroviruses (Baculoviridae) in the environment. J. Virol. Methods 82:63-75. [DOI] [PubMed] [Google Scholar]
- 37.Rohrmann, G. F. 1992. Baculovirus structural proteins. J. Gen. Viol. 73:749-761. [DOI] [PubMed] [Google Scholar]
- 38.Rossi, P., and M. Aragno. 1999. Analysis of bacteriophage inactivation and its attenuation by adsorption onto colloidal particles by batch agitation techniques. Can. J. Microbiol. 45:9-17. [Google Scholar]
- 39.Schaub, S. A., and B. P. Sagik. 1975. Association of enteroviruses with natural and artificially introduced colloidal solids in water and infectivity of solids-associated virions. Appl. Microbiol. 30:212-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sobsey, M. D., C. H. Dean, M. E. Knuckles, and R. A. Wagner. 1980. Interactions and survival of enteric viruses in soil materials. Appl. Environ. Microbiol. 40:92-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taylor, D. H., R. S. Moore, and L. S. Sturman. 1981. Influence of pH and electrolyte composition on adsorption of poliovirus by soils and minerals. Appl. Environ. Microbiol. 42:976-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Theilmann, D. A., G. W. Blissard, B. Bonning, J. Jehle, D. R. O'Reilly, G. F. Rorhmann, S. Theim, and J. M. Vlak. 2005. Baculoviridae, p. 177-185. In C. M. Fauquet, M. A. Mayo, J. Maniloff, U. Desselberger, and L. A. Ball (ed.), Virus taxonomy: VIII report of the International Committee on the Taxonomy of Viruses. Elsevier, London, United Kingdom.
- 43.Vettori, C., E. Gallori, and G. Stotzky. 2000. Clay minerals protect bacteriophage PBS1 of Bacillus subtilis against inactivation and loss of transducing ability by UV radiation Can. J. Microbiol. 46:770-773. [PubMed] [Google Scholar]
- 44.Vilker, V. L., G. C. Meronek, and P. C. Butler. 1983. Interactions of poliovirus with montmorillonite clay in phosphate-buffered saline. Environ. Sci. Technol. 71:631-634. [DOI] [PubMed] [Google Scholar]
- 45.Williams, T., V. Barbosa-Solomieu, and V. G. Chinchar. 2005. A decade of advances in iridovirus research. Adv. Virus Res. 65:173-248. [DOI] [PubMed] [Google Scholar]
- 46.Yan, X., N. H. Olson, J. L. Van Etten, M. Bergoin, M. G. Rossmann, and T. S. Baker. 2000. Structure and assembly of large lipid-containing dsDNA viruses. Nat. Struct. Mol. Biol. 7:101-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoshinaka, T., M. Yoshimizu, and Y. Ezura. 2000. Adsorption and infectivity of infectious hematopoietic necrosis virus with various solids. J. Aq. Anim. Health 12:64-68. [DOI] [PubMed] [Google Scholar]
- 48.Young, S. Y., and W. C. Yearian. 1986. Movement of a nuclear polyhedrosis virus from soil to soybean and transmission to Anticarsia gemmatalis (Lepidoptera: Noctuidae) populations on soybean. Environ. Entomol. 15:573-580. [Google Scholar]