Abstract
In many cases, secondary plant products accumulate in the large central vacuole of plant cells. However, the mechanisms involved in the transport of secondary compounds are only poorly understood. Here, we demonstrate that the transport mechanisms for the major barley (Hordeum vulgare) flavonoid saponarin (apigenin 6-C-glucosyl-7-O-glucoside) are different in various plant species: Uptake into barley vacuoles occurs via a proton antiport and is competitively inhibited by isovitexin (apigenin 6-C-glucoside), suggesting that both flavone glucosides are recognized by the same transporter. In contrast, the transport into vacuoles from Arabidopsis, which does not synthesize flavone glucosides, displays typical characteristics of ATP-binding cassette transporters. Transport of saponarin into vacuoles of both the species is saturable with a Km of 50 to 100 μm. Furthermore, the uptake of saponarin into vacuoles from a barley mutant exhibiting a strongly reduced flavone glucoside biosynthesis is drastically decreased when compared with the parent variety. Thus, the barley vacuolar flavone glucoside/H+ antiporter could be modulated by the availability of the substrate. We propose that different vacuolar transporters may be responsible for the sequestration of species-specific/endogenous and nonspecific/xenobiotic secondary compounds in planta.
In chemical terms, the diversity among plants is reflected by the enormous interspecific variability of the products of secondary metabolism. More than 50,000 secondary plant products have been described so far and it is likely that more will be identified in the future. The enormous number of different, often species-specific, secondary metabolites results from the variability of chemical modifications of only a few main classes of compounds. The origin of this variability is a large number of enzymes modifying basal structures, each enzyme with distinct and also partially overlapping specificities. In addition to hydroxylation, acylation, and methylation, glycosylation is an important, often terminal modification of secondary products that contributes to these large structural variabilities and increases their solubility in water (Heller and Forkmann, 1994; Vogt and Jones, 2000). Furthermore, toxic xenobiotics are detoxified as glycosylated conjugates in plants (Pflugmacher and Sandermann, 1998).
A plethora of different functions have been attributed to plant secondary substances (Harborne, 1993; Dixon and Paiva, 1995). In many cases, these functions require rather high concentrations at least in the millimolar range (Saunders and Conn, 1978). On the other hand, many secondary compounds are harmful to the plant producing these substances (Matile, 1984, 1987; Wink, 1997). Therefore, the presence and synthesis of secondary plant products require a strict compartmentation of the sites of production and storage. For many glycosylated metabolites, it has been shown that they are efficiently stored within the vacuole (Wink, 1997). However, transport mechanisms for vacuolar deposition of glycosylated secondary compounds have been investigated only in a few cases. Hopp and Seitz (1987) and Matern et al. (1986) have shown that acylation is a prerequisite for vacuolar uptake of the anthocyanin produced by Daucus carota and apigenin 7-O-(6-O-malonylglucoside) synthesized in parsley (Petroselinum hortense). Addition of ATP had either no effect on the uptake of these glucosides or stimulated the transport only slightly. Abolishing the pH gradient (ΔpH) strongly inhibited the uptake of the substrates, indicating that the ΔpH generated by the two vacuolar proton pumps (Rea and Sanders, 1987) is the driving force for the uptake.
In barley (Hordeum vulgare), the 2-fold glucosylated saponarin (apigenin 6-C-glucosyl-7-O-glucoside; Fig. 1) accumulates as the major compound during primary leaf development (Seikel and Geissman, 1957; Reuber et al., 1996). Saponarin is synthesized from the precursor isovitexin (apigenin 6-C-glucoside) after the addition of Glc in the 7-O position by a soluble UDP-Glc-dependent flavone glucosyltransferase (Blume et al., 1979). Compared with saponarin, isovitexin is present only in trace amounts. The glucosylation of isovitexin in the 6-C-position but not acylation is sufficient for an efficient vacuolar uptake into barley vacuoles. Inhibition studies indicate that vacuolar uptake of isovitexin occurs by a secondary energized proton antiport mechanism (Klein et al., 1996). A completely different mechanism has been observed for the vacuolar uptake of an abiotic glucoside, hydroxyprimisulfuron glucoside, which is synthesized during the detoxification of the sul-fonylurea-type herbicide primisulfuron. Uptake was strongly stimulated by ATP. This and inhibition studies suggested the involvement of an ATP-binding cassette (ABC) protein-type transporter that directly utilizes ATP hydrolysis to drive vacuolar herbicide glucoside uptake (Klein et al., 1996). These results raise the question of which structural features determine the specificity of glucosylated compounds for their recognition either by a glucoside pump or by a secondary energized glucoside transporter. It is obvious that the Glc residue attached to the molecules is not sufficient to act as a signal.
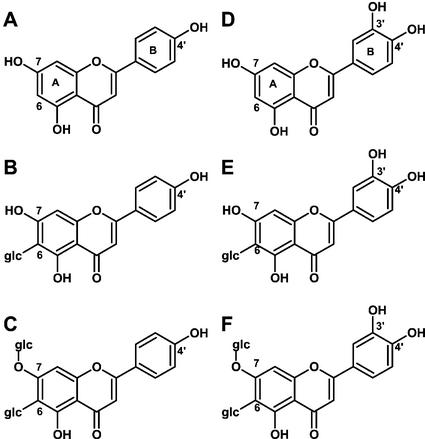
Figure 1.
Chemical structures of apigenin- (A–C) and luteolin-type flavonoids (D–F) mentioned in this study. A, Apigenin; B, isovitexin; C, saponarin; D, luteolin; E, isoorientin; F, lutonarin.
Elucidating the structure required for either a vacuolar antiporter or an ABC-type transporter may have some practical implications. Theoretically, directly energized, ABC transporter-driven uptake results in an approximately 106- to 108-fold higher accumulation of the glucoside within the vacuole as compared with antiport or potential-driven mechanisms, respectively, assuming published values for cytosolic ATP concentrations, the ΔpH, or the membrane potential difference across the tonoplast (Kreuz et al., 1996). Therefore, plants accumulating glucosylated metabolites in the vacuole by an ABC-type transporter are able to synthesize and store at higher concentrations compared with plants using an antiport mechanism.
In the present report, we show that saponarin is taken up by a proton antiport system into barley vacuoles and that the transport activity is strongly reduced in a mutant impaired in flavonoid biosynthesis. In contrast, saponarin was taken up by an ABC-type transporter into vacuoles from Arabidopsis, a plant that does not synthesize this class of flavonoids (Veit and Pauli, 1999). Thus, mechanistically different vacuolar transport systems exist depending on the endogenous capacity to synthesize a certain class of glucosylated compounds. We propose that specific proton antiport systems are responsible for the vacuolar transport of endogenous glucosylated compounds, whereas vacuolar ABC-type transporters are involved in the detoxification of biotic and abiotic glucosides not known to exist in a certain plant species.
RESULTS
Time-Dependent Uptake of [3H]Saponarin into Barley Vacuoles Is Not Strongly Stimulated by MgATP
In previous work, we have shown that the flavone glucoside isovitexin (Fig. 1), a minor component in barley primary leaves, is taken up into barley mesophyll vacuoles by a proton antiport mechanism, whereas the abiotic hydroxyprimisulfuron glucoside uses the hydrolysis of MgATP directly for transport into the vacuole (Klein et al., 1996). Therefore, we investigated whether saponarin, the major flavonoid of barley leaves that is synthesized from isovitexin by a glucosylation (Fig. 1), is also transported into barley mesophyll vacuoles.
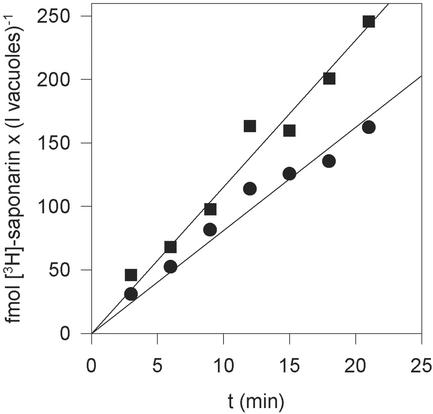
Saponarin was efficiently taken up by isolated barley cv Bakara mesophyll vacuoles irrespective of the presence of MgATP. Addition of MgATP only stimulated the uptake rate by a factor of about 1.3 (Fig. 2). In both cases, uptake was linear for at least 20 min. After this time, the vacuolar saponarin concentrations were about 150 nm in the absence and about 240 nm in the presence of MgATP (Fig. 2), exceeding the medium concentratiom (34 nm) by a factor of four to seven, respectively. Analysis of the radioactive product present in the vacuoles using reverse-phase (RP)-HPLC unequivocally demonstrated that [3H]saponarin was present and that it was not degraded or metabolized after vacuolar uptake (data not shown). This is similar to what we demonstrated for isovitexin (Klein et al., 1996).
Figure 2.
Time-dependent uptake of 34 nm [3H]-saponarin into barley cv Bakara vacuoles in the presence (▪) or absence (●) of 3 mm MgATP. Each data point represents the average of six replicates. The r2 values for the linear regression depicted in the graph are 0.970 and 0.976 for the uptake in the absence and presence of MgATP, respectively.
Two Distinct Energization Mechanisms Drive the Uptake of [3H]Saponarin into Barley or Arabidopsis Vacuoles
Vacuoles isolated from Arabidopsis cell cultures, which do not produce saponarin, were also able to take up this compound. However, in contrast to barley mesophyll vacuoles, this uptake, which was linear for at least 15 min (data not shown), could be observed only in the presence of MgATP (Table I). A comparison of the MgATP-dependent uptake of saponarin in the presence of various inhibitors showed striking differences between barley and Arabidopsis. The addition of the V-type ATPase inhibitor bafilomycin A1 slightly reduced saponarin uptake into barley vacuoles to the value observed in the absence of MgATP (Table I). In contrast, bafilomycin A1 did not affect saponarin transport in Arabidopsis. Vanadate, an inhibitor of enzymes forming phosphorylated intermediates, exhibited a low inhibition of saponarin uptake in barley vacuoles (15% inhibition) but was much more effective on the uptake in Arabidopsis vacuoles (65% inhibition). A further difference of saponarin uptake in barley and Arabidopsis could be observed in the presence of NH4Cl, which abolishes the ΔpH between the medium and the vacuolar lumen. Although uptake was almost completely inhibited in barley vacuoles (80% inhibition), only a negligible effect could be observed in Arabidopsis (Table I).
Table I.
Effect of MgATP and different inhibitors on the uptake of saponarin into isolated barley cv Bakara and Arabidopsis vacuoles
| Condition | Barley | Arabidopsis |
|---|---|---|
| % of +MgATP value | ||
| −ATP | 78.8 ± 10.3 | 0.1 ± 4.2 |
| +3 mm MgATP | 100 | 100 |
| +3 mm MgATP + NH4Cl (5 mm) | 20.2 ± 7.0 | 101.3 ± 6.2 |
| +3 mm MgATP + bafilomycin (0.1 μm) | 81.3 ± 8.9 | 111.8 ± 7.4 |
| +3 mm MgATP + vanadate (100 μm) | 85.0 ± 8.7 | 34.7 ± 1.8 |
Vacuoles were incubated in the presence of 34 nm [3H]-saponarin and inhibitors at the concentrations indicated. Uptake rates in the presence of MgATP corresponding to 100% were 12.1 ± 2.6 and 9.3 ± 2.2 fmol saponarin min−1 μL vacuolar volume−1 for barley and Arabidopsis vacuoles, respectively.
Kinetic Determinants and Competitive Inhibition of Vacuolar [3H]Saponarin Uptake
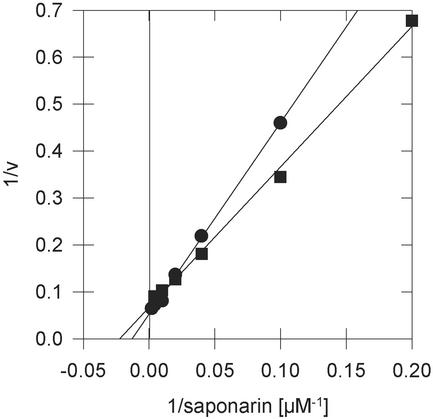
The uptake of saponarin into barley and Arabidopsis vacuoles exhibited Michaelis-Menten-type saturation kinetics (Fig. 3). Despite the different inhibition profiles arguing for distinct mechanisms involved in flavone glucoside uptake in the two plants, comparable Km values were observed in barley and in Arabidopsis vacuoles (50–100 μm). The Vmax values for both species varied from preparation to preparation, but were in the same order of magnitude (5–18 pmol μL vacuolar volume−1 min−1).
Figure 3.
Uptake of [3H]-saponarin into mesophyll vacuoles of barley cv Bakara (●) or into cell culture vacuoles of Arabidopsis (▪) displays Michaelis-Menten-type saturation kinetics. Representative saturation experiments are illustrated as a Lineweaver-Burk plot. Linear regression analysis gives r2 values of 0.998 for each of the two lines depicted. Km values measured in three independent experiments ranged between 50 and 100 μm for vacuoles from both plant species.
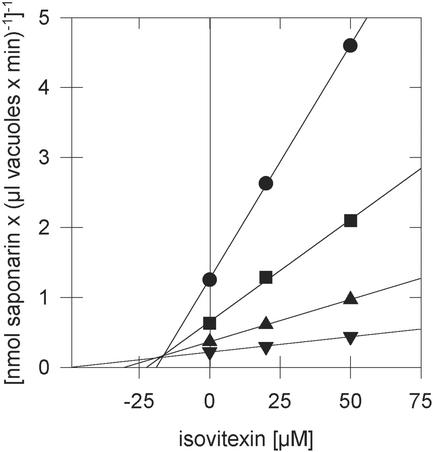
To analyze whether other glucosides of plant secondary compounds may cross the vacuolar membrane of barley by the same transporter as saponarin, we monitored uptake of this compound in the presence of other glucosides (Table II). The two apigenin derivatives, apigenin 7-O-glucoside and isovitexin, were the strongest inhibitors of saponarin uptake. The inhibition of saponarin uptake by isovitexin was competitive and exhibited a Ki value of approximately 20 μm (Fig. 4). This result and the observation that isovitexin uptake into barley vacuoles is strongly inhibited by saponarin (Klein et al., 1996) argue for the fact that apparently the same vacuolar transporter accepts both flavone glucosides. Isoorientin and the glucosylated flavanone naringin also inhibited saponarin uptake, although not as strongly (60% to 70% inhibition). Isoorientin is a C-6-gluco-sylated luteolin derivative that is hydroxylated at the 3′ and 4′ position of the B-ring (Fig. 1). It is the precursor for the diglucoside lutonarin [luteolin (6-C-glucosyl-7-O-glucoside)] and accumulates in minor amounts in barley vacuoles (Reuber et al., 1996). In contrast, non-flavonoid-type glucosides like coumarins (esculin), simple phenolics (arbutin), phenolic acids (rosmarinic acid), glucosinolates (sinigrin), or glucosylated terpenoids (α-solanin) inhibited saponarin uptake only 20% to 40%.
Table II.
Influence of potential competitive inhibitors including flavone glucosides and other glucosylated secondary compounds on the uptake of saponarin into isolated barley cv Bakara vacuoles
| Compound | Substance Class | Control Value |
|---|---|---|
| % | ||
| Control | – | 100 |
| Rosmarinic acid | Phenolic Acid | 90.8 ± 4.3 |
| Sinigrin | Glucosinolate | 91.5 ± 2.7 |
| Esculin | Coumarin | 72.8 ± 7.1 |
| Arbutin | Simple Phenol | 83.0 ± 3.1 |
| α-Solanin | Terpenoid | 64.8 ± 10.0 |
| Isoorientin | Flavone | 34.8 ± 8.4 |
| Naringin | Flavanone | 37.6 ± 7.7 |
| Isovitexin | Flavone | 21.9 ± 8.7 |
| Apigenin 7-O-glucoside | Flavone | 6.7 ± 5.6 |
Vacuoles were incubated with 34 nm [3H]-saponarin and 3 mm MgATP in the absence (control) or presence of 0.2 mm of the competitors indicated. For 100% value, see Table 1.
Figure 4.
Dixon plots of the competitive inhibition of [3H]-saponarin by its precursor isovitexin. Barley cv Bakara mesophyll vacuoles were incubated in the presence of MgATP with 20 (●), 50 (▪), 100 (▴), and 200 μm (▾) of saponarin and the competitor concentrations indicated. A representative of three independent experiments is illustrated (six replicates per condition).
[3H]Saponarin Uptake Was Strongly Decreased in a Barley Ant Mutant Synthesizing Only Very Low Amounts of Flavone Glucosides
Synthesis of secondary compounds such as simple phenolics, flavonoids, or stilbenes may be modulated according to environmental conditions and stress (Dixon and Paiva, 1995). It is unknown whether “late” biosynthetic steps of secondary substances like glycosylations or even transport steps are regulated or modulated in response to environmental challenges or whether their activity is constitutive. Therefore, we analyzed whether the vacuolar saponarin transport activity is modulated by the availability of its substrates in planta using the barley mutant ant 310 which synthesizes only negligible amounts of flavonoids. The mutant ant 310 was identified by screening the Carlsberg collection of proantho-cyanidin-free barley lines for mutants lacking flavone glucosides (Jende-Strid, 1988; Reuber et al., 1997). Ant 310 exhibited the same typical changes in the composition of phenylpropanoid compounds as already described for other ant mutants of the Ant 30 complementation group: (a) Only traces of all flavonoids are synthesized in the mutant, and (b) the chalcone glucoside isosalipurposide accumulates, suggesting a putative defect in the chalcone isomerase gene (Reuber et al., 1996, 1997).
We investigated the uptake of unlabeled isovitexin into vacuoles isolated from the parent barley variety of the mutant, Ca 33787. As seen in Figure 5A, isovitexin transport into Ca 33787 mesophyll vacuoles could be observed using the same RP-HPLC detection system as previously described for uptake into the barley variety Bakara (Klein et al., 1996). Uptake of isovitexin was linear with time for at least 20 min and stimulated by MgATP by a factor of about 1.5 (data not shown). However, uptake of unlabeled isovitexin or saponarin into vacuoles isolated from the ant 310 mutant was almost not detectable by HPLC analysis. Furthermore, when [3H]saponarin was used as the substrate, uptake into vacuoles isolated from ant 310 was strongly reduced when compared with the corresponding parent var. Ca 33787, both in the absence as well as in the presence of MgATP (Fig. 5B). It should be mentioned that despite the lower uptake activity of vacuoles isolated from ant 310, inhibition by bafilomycin, NH4Cl, and vanadate was comparable with that observed with vacuoles isolated from the parent variety or cv Bakara (Table I; data not shown).
Figure 5.
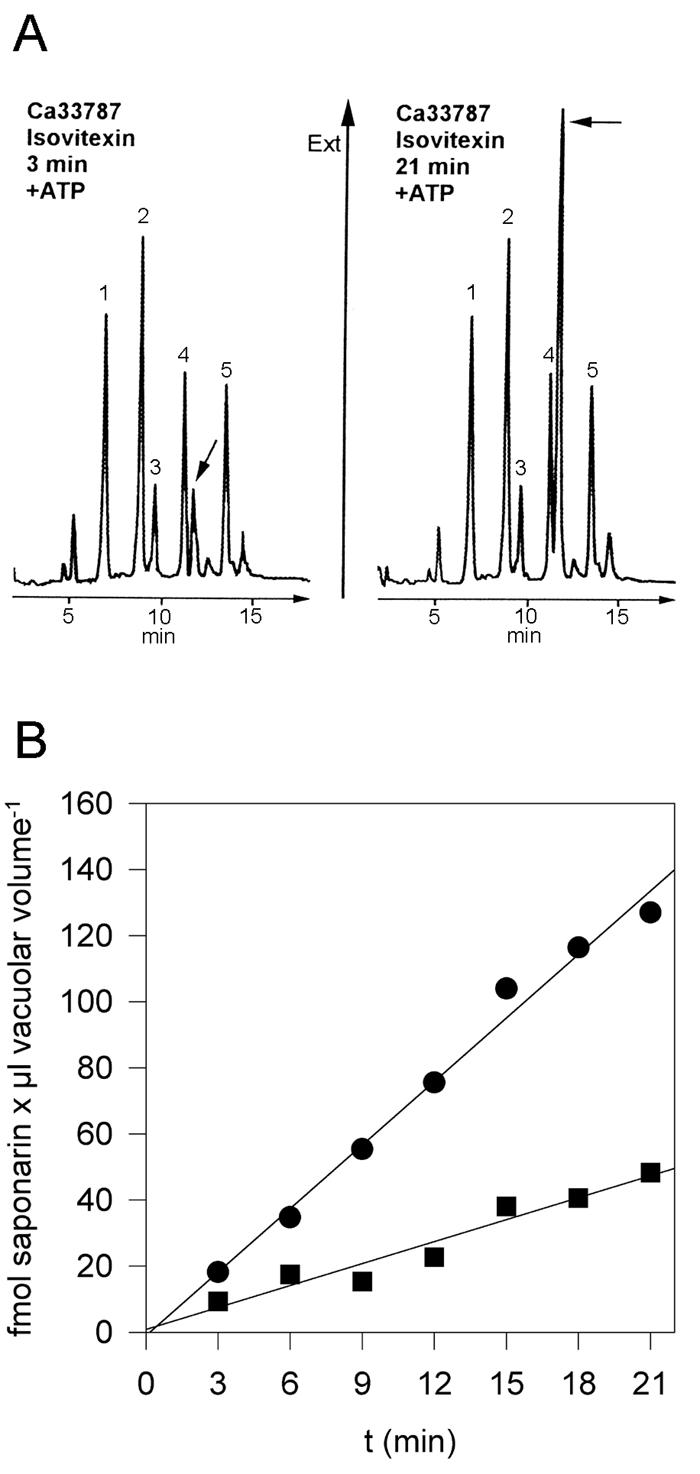
The uptake of saponarin is strongly reduced in vacuoles of the barley mutant ant 310. A, HPLC profile of vacuolar supernatants of the parent variety Ca 33787 recovered after 3- (left) or 21- (right) min (right) with 0.1 mm isovitexin in the presence of MgATP. The isovitexin peak (arrows) is strongly increased after 21 min. Extinction was recorded at 340 nm. The numbered peaks have been identified by co-elution with authentic substances or by spectral analysis: 1, lutonarin; 2, saponarin; 3, lutonarin 4-methylether; and 4 and 5, flavones. B, Time-dependent uptake of [3H]saponarin, initial concentration 34 nm into vacuoles isolated from Ca 33787 (●) and ant 310 (▪) in the presence of MgATP. The r2 values for the linear regression depicted in the graph are 0.987 and 0.936 for the uptake into vacuoles isolated from Ca 33787 and ant 310, respectively.
DISCUSSION
Although a few studies have addressed the transport processes of secondary metabolites across the vacuolar membrane, our knowledge of the mechanisms involved is still scarce (Martinoia et al., 2000). In the present study, we demonstrated that at least two different transport mechanisms exist for the major barley flavone glucoside saponarin (Fig. 1). We propose that the secondary energized flavone glucoside/H+-antiporter catalyzing transport of apigenin-type glucosides across barley vacuolar membranes is unique to species synthesizing flavone glucosides. The energization of saponarin uptake into barley vacuoles was similar to that observed for isovitexin. It was strongly inhibited by NH4Cl but only weakly by vanadate, indicating that at least the major portion of saponarin uptake into barley vacuoles occurs by a saponarin/H+-antiport mechanism. Comparison of these properties with those observed for saponarin uptake into Arabidopsis vacuoles showed striking differences. Saponarin uptake into Arabidopsis vacuoles is almost completely inhibited by vanadate but only negligibly affected by bafilomycin A1 or NH4Cl (Table I). Thus, Arabidopsis in which flavone glucosides have not been identified (Veit and Pauli, 1999) possesses a directly energized ABC-type vacuolar glucoside transport system sharing characteristic features with the glucoside ATPase described for hydroxyprimisulfuron-glucoside (Klein et al., 1996). To our knowledge, this is the first demonstration that a plant-born glucoside is taken up by an ABC-type transporter in plants. From these observations, we propose that plants contain proton antiport transporters for species-specific or closely related substances, whereas “foreign” glucosylated molecules are recognized and transported by ABC-type transporters. In species that lack a certain class of endogenous, glucosylated secondary compounds, such glucosides are recognized as a modified or foreign substance that has to be detoxified. As a consequence, ABC-type transporters with rather broad substrate specificity may be responsible for the transfer of these Glc conjugates into the vacuole.
Considering the energy used for vacuolar accumulation, it is unclear why it is favorable to use proton antiport mechanisms for endogenous compounds even when ABC transporters could be used. ABC transporters allow a much higher accumulation of a solute compared with an antiport-driven uptake (Kreuz et al., 1996). If the energy consumption of the vacuolar proton pumps is taken into account, the additional energy required for solute accumulation by an ABC-type transporter is insignificant (assuming 2H+ translocated for one ATP hydrolyzed and a stochiometry of one proton per saponarin molecule translocated by the proton antiport mechanism).
Saponarin is taken up into barley mesophyll vacuoles with a similar Km value and at rates comparable with those observed for its precursor, isovitexin (Klein et al., 1996). This is surprising because in planta saponarin is the major flavonoid (Fig. 5A) and isovitexin is present only in low amounts. Therefore, it must be postulated that either glucosylation of isovitexin is a channeled process or that the corresponding glucosyltransferase has a much higher affinity for isovitexin than the vacuolar transporter, resulting in more efficient conversion of isovitexin to saponarin prior to vacuolar deposition. Competition experiments indicate that the transport of isovitexin and saponarin into barley vacuoles occurs by the same transporter (Fig. 4). Furthermore, from these data it is tempting to speculate that apigenin derivatives in general are recognized by a secondary energized glucoside transporter (Table II). However, the relatively low inhibition of saponarin transport by other flavonoids, especially flavone glucosides derived from luteolin, indicates that the vacuolar saponarin transporter is not a general “flavonoid” or “glucoside” permease. This hypothesis is supported by earlier experiments that showed that orientin (luteolin 8-C-glucoside) is not taken up by barley vacuoles, arguing for the importance of the B-ring hydroxylation pattern in substrate recognition (Klein et al., 1996).
The analysis of the vacuolar uptake of the luteolin-type flavone glucuronides that can be found in only a limited number of plants argues for the existence of a plant MRP (multidrug resistance-associated protein)-type ABC transporter (Cole et al., 1992) for negatively charged flavonoids, which is present also in species not synthesizing glucuronidated compounds (Klein et al., 2000, 2001). Several MRP-like plant ABC transporters have been identified and characterized in detail with regard to their substrate specificity with negatively charged organic anions like glutathione conjugates (Liu et al., 1997, 1998, 2001; Tommasini et al., 1998). However, the transport of uncharged glucosylated substrates has not been investigated in heterologous expression systems. Therefore, it will be interesting to examine which of the Arabidopsis ABC transporters is responsible for the transport of glucosides like saponarin observed in this study. Debeaujon et al. (2001) recently characterized the tt12 (transparent testa12) mutation in Arabidopsis, which affects seed coat pigmentation due to a strong reduction of the deposition of proanthocyanidins in the vacuoles of endothelial cells. The cloned TT12 gene encoded a protein with 12 transmembrane-spanning segments exhibiting similarity to the novel multidrug and toxic compound extrusion family (Brown et al., 1999). Although biochemical evidence via transport experiments is still lacking, these data raise the possibility that membrane proteins belonging to the multidrug and toxic compound extrusion family may be responsible for the transport of glucosylated phenolics such as isovitexin or saponarin across the barley vacuolar membrane by an H+-antiport mechanism.
Little is known about the ability of vacuolar membrane transporters to respond to physiological or environmental changes. Therefore, we were interested to see whether the transport activity of the saponarin/H+ antiporter is modulated by the presence or absence of its substrate(s) using the barley mutant ant 310. Ant 310 is putatively devoid of chalcone isomerase activity (Reuber et al., 1997; G. Weissenböck, unpublished data), which leads to a large reduction in the levels of the specific flavone glucosides. Compared with the parent variety, vacuolar saponarin transport activity was greatly decreased in the mutant (Fig. 5B). This result supports the idea that vacuolar transport activities are modulated by the amount of substrate available in the cell. However, on the basis of our experiments we cannot clearly define whether (a) the substrate saponarin itself or any other intermediate induces the transcription of the transporter, or (b) whether in response to substrate availability, the transport activity is posttranslationally regulated either by increasing the turnover of the transporter in absence of the substrate or by modulation of its activity. This modulation could be via allosteric activation by intermediates, phosphorylation, or protein-transporter interactions.
In conclusion, we have shown that the apigenin-type flavone diglucoside saponarin is taken up by distinct mechanisms in barley and Arabidopsis vacuoles. The fact that the transport activity is down-regulated in barley mutants synthesizing only low amounts of flavonoids indicates that uptake activity is modulated by an unknown feedback mechanism. The comparison of protein composition or transport activities of parent and mutant barley may allow us to identify the saponarin antiporter using a proteomic approach.
MATERIALS AND METHODS
Tritiation of Saponarin and HPLC Analysis
Saponarin was obtained from Extrasynthese (Genay, France). Random custom tritiation of saponarin was performed by SibTech Inc. (Newington, CT). After purification via RP-HPLC using Nucleosil C18 materials, specific activities ranged between 25 and 30 Ci mmol−1. The radiochemical purity of the product which exceeded 95% was verified by the supplier and by our laboratory via analytical RP-HPLC (see below) and by two-dimensional thin-layer chromatography on cellulose (Merck, Darmstadt, Germany; first dimension CHCl3/CH3COOH 3:2, almost water saturated; second dimension water:CH3COOH, 85:15 [v/v]) followed by autoradiography. The identity of the tritiated product was confirmed by co-elution of the radioactive product with unlabeled pure saponarin via RP-HPLC using conditions previously described (Klein et al., 1996). Furthermore, the identity of the tritiated product taken up by the vacuoles was verified by RP-HPLC of the vacuolar contents following the procedure described for flavone glucuronides (Klein et al., 2000) but using the HPLC conditions for barley (Hordeum vulgare) flavonoids (see below; data not shown).
Plant Materials and Growth Conditions
Barley var. Bakara, var. Ca 33787, and var. mutant ant 310 (Jende-Strid, 1988, 1993; Reuber et al., 1996) were grown on vermiculite for 8 d in a growth cabinet with 12 h of fluorescent light (100 μmol m−2 s−1) at 20°C, 70% relative humidity, and were watered daily with Hoagland solution. For the isolation of Arabidopsis vacuoles, a cell suspension culture (ecotype Columbia, cell line T87) was used (Axelos et al., 1992). Every week, 300 mL of fresh Gamborg B5 medium containing 2,5 μm 2,4-dichloro-phenoxyacetic acid were inoculated with 5 g of 7-d-old cell suspension culture. Cells were grown in continuous light at 20°C and 70% relative humidity by gentle shaking (120 rpm).
Isolation of Mesophyll Protoplasts and Vacuoles from Barley Primary Leaves
Barley protoplasts and vacuoles were prepared following published procedures (Rentsch and Martinoia, 1991) with a minor modification: All media used for vacuole isolation after protoplast purification contained 30 mm KCl instead of K gluconate. Contamination of barley vacuoles with other cell constituents was less than 3% as measured by marker enzyme activities.
Isolation of Vacuoles from an Arabidopsis Cell Suspension Culture
Digestion of the cells and isolation of protoplasts were performed essentially as previously described (Nagy and Maligy, 1976). The protoplasts were lysed by adding two volumes of prewarmed medium A {0.2 m mannitol, 10% [w/v] Ficoll 400, 20 mm EDTA, 5 mm HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid]-KOH, pH 8, 150 μg mL−1 bovine serum albumin, and 1 mm dithiothreitol [DTT], 42°C} followed by a 5-min incubation at room temperature. Vacuoles were purified and concentrated by centrifugation (20 min, 1,500g) using a step gradient as follows: lower phase, one volume of lysed protoplast suspension; middle phase, two volumes of a 1:1 mixture of medium A and medium B (0.4 m betaine, 20 mm HEPES-KOH, pH 7.5, 30 mm KCl, 15 mg mL−1 bovine serum albumin, and 1 mm DTT) resulting in a final concentration of 5% (w/v) Ficoll; and upper phase, one volume of medium B. Vacuoles were collected from the interface between the 5% (w/v) Ficoll solution and medium B.
Uptake Experiments with Plant Vacuoles
Transport studies with barley mesophyll and Arabidopsis cell culture vacuoles were performed as previously described (Rentsch and Martinoia, 1991) using the silicone oil centrifugation technique. Unless stated otherwise, for each time point and condition six polyethylene microcentrifugation tubes were prepared containing 70 μL of 23% (v/v) Percoll, 0.4 m sorbitol, 30 mm KCl, 20 mm MES [2-(N-morpholino)-ethanesulfonic acid]/BTP[1,3-bis(tris[hydroxymethyl]methylamino) propane, pH 7.2, 0.12% (w/v) bovine serum albumin, 1 mm DTT, and the substrate (34 nm [3H]saponarin [0.05 μCi] or 0.1 mm unlabeled isovitexin) including 1 mm MgCl2 or 4 mm MgCl2 and 3 mm Na2ATP for experiments in the absence or presence of MgATP, respectively. Uptake was started by adding 30 μL of concentrated vacuole suspension. The reaction mixture was rapidly over-layered with 200 μL of silicone oil AR 200 and 60 μL of water. The incubation was terminated by floatation of the vacuoles (10,000g for 15 s). For [3H]saponarin uptake, the level of radioactivity in the aqueous phase (50 μL) was determined by liquid scintillation counting using 3 mL of scintillation cocktail (Readysafe, Beckman Coulter, Inc., Fullerton, CA). All counts were corrected for background and quenching. The amount of vacuolar isovitexin after uptake of the unlabeled substrate was analyzed by separation of the vacuolar supernatants on a Kontron HPLC system using the following conditions: Nucleosil RP-18 column (125 × 4.6 mm; 5-μm grain size; CS Chromatographie, Langerwehe, Germany); 1 mL min−1 flow rate; solvent A, HPLC grade water containing 1% (v/v) H3PO4; solvent B, acetonitrile; and steps (all linear) 13% to 25% (v/v) B in 12 min, 25% to 40% (v/v) B in 3 min, 40% to 100% (v/v) B in 2 min, and re-equilibration to 13% (v/v) B in 7 min. Spectrophotometric detection and quantification of the phenolic compounds was performed at 340 nm. The vacuolar volume was calculated by the addition of 0.05 μCi of 3H2O that rapidly equilibrated between the medium and the vacuolar lumen. For [3H]saponarin uptake, the vacuolar volume was determined in separate tubes from those used for saponarin. Unless stated otherwise, uptake rates were calculated by subtracting the radioactivity measured after 2 min of incubation from corresponding 20-min values. The accumulation of [3H]saponarin against a concentration gradient was calculated by dividing the determined vacuolar concentration of saponarin by the exterior substrate concentration present in the transport experiment at a given time.
Analysis of Phenolic Compounds in Barley Primary Leaves
The composition of methanolic extracts of primary leaves of the barley varieties Ca 33787 and the mutant ant 310 was analyzed as described (Reuber et al., 1996) using the conditions for RP-HPLC mentioned above. Absorption spectra between 220 and 370 nm were recorded during the HPLC elution. The identities of most of the peaks were verified by co-elution with authentic substances or by spectral analysis (data not shown).
ACKNOWLEDGMENTS
The authors thank Aurélie Pedezert (Institut de Botanique, Université de Neuchâtel, Neuchâtel, Switzerland) and Anne-Claire Flamant (Institut de Botanique, Université de Neuchâtel) for technical assistance. Drs. Freddie Theodoulou (IACR-RES, UK) and Susannah Gal (Binghamton University, NY) are gratefully acknowledged for critical reading of the manuscript.
Footnotes
This work was supported by the Schweizer Nationalfonds (grants to T.E., E.M., and M.K.), by the European Union Biotech Program (grant no. BBW 97.0570 to N.F. and E.M.), and by the Deutsche Forschungsgemeinschaft (to G.W.). M.K. was a Feodor-Lynen Fellow supported by the Alexander-von-Humboldt Stiftung, Germany.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010590.
LITERATURE CITED
- Axelos M, Curie C, Mazzolini L, Bardet C, Lescure B. A protocol for transient gene expression in Arabidopsis thalianaprotoplasts isolated from cell suspension cultures. Plant Physiol Biochem. 1992;30:123–128. [Google Scholar]
- Blume DE, Jaworski JG, McClure JW. Uridinediphosphate-glucose: isovitexin 7-O-glucosyltransferase from barley protoplasts: subcellular localization. Planta. 1979;146:199–202. doi: 10.1007/BF00388232. [DOI] [PubMed] [Google Scholar]
- Brown MH, Paulsen IT, Skurray RA. The multidrug efflux protein NorM is a prototype of a new family of transporters. Mol Microbiol. 1999;31:393–395. doi: 10.1046/j.1365-2958.1999.01162.x. [DOI] [PubMed] [Google Scholar]
- Cole SPC, Bhardwaj G, Gerlach JH, Mackie JE, Grant CE, Almquist KC, Stewart AJ, Kurz EU, Duncan AMV, Deeley RC. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science. 1992;258:1650–1654. doi: 10.1126/science.1360704. [DOI] [PubMed] [Google Scholar]
- Debeaujon I, Peeters AJ, Leon-Kloosterziel KM, Koornneef M. The transparent testa12 gene of Arabidopsis encodes a multidrug secondary transporter-like protein required for flavonoid sequestration in vacuoles of the seed coat endothelium. Plant Cell. 2001;13:853–872. doi: 10.1105/tpc.13.4.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon RA, Paiva NL. Stress-induced phenylpropanoid metabolism. Plant Cell. 1995;7:1085–1097. doi: 10.1105/tpc.7.7.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harborne J. Introduction to Ecological Biochemistry. Ed 4. London: Academic Press; 1993. [Google Scholar]
- Heller W, Forkmann G. Biosynthesis of flavonoids. In: Harborne JB, editor. The Flavonoids, Advances in Research since 1986. London: Chapman & Hall; 1994. pp. 499–535. [Google Scholar]
- Hopp W, Seitz HU. The uptake of acylated anthocyanin into isolated vacuoles from a cell suspension culture of Daucus carota. Planta. 1987;170:74–85. doi: 10.1007/BF00392383. [DOI] [PubMed] [Google Scholar]
- Jende-Strid B. Co-ordinator's report: anthocyanidin genes: stock list of antmutants kept at the Carlsberg Laboratory. Barley Genet Newslett. 1988;18:734–779. [Google Scholar]
- Jende-Strid B. Genetic control of flavonoid biosynthesis in barley. Hereditas. 1993;119:187–204. [Google Scholar]
- Klein M, Martinoia E, Hoffmann-Thoma G, Weissenböck G. A membrane-potential dependent, ABC-like transporter mediates the vacuolar uptake of rye flavone glucuronides. Plant J. 2000;21:289–304. doi: 10.1046/j.1365-313x.2000.00684.x. [DOI] [PubMed] [Google Scholar]
- Klein M, Martinoia E, Hoffmann-Thoma G, Weissenböck G. The ABC-like vacuolar transporter for rye mesophyll flavone glucuronides is not species-specific. Phytochemistry. 2001;56:153–159. doi: 10.1016/s0031-9422(00)00377-0. [DOI] [PubMed] [Google Scholar]
- Klein M, Weissenböck G, Dufaud A, Gaillard C, Kreuz K, Martinoia E. Different energization mechanisms drive the vacuolar uptake of a flavonoid glucoside and a herbicide glucoside. J Biol Chem. 1996;271:29666–29671. doi: 10.1074/jbc.271.47.29666. [DOI] [PubMed] [Google Scholar]
- Kreuz K, Tommasini R, Martinoia E. Old enzymes for a new job: herbicide detoxification in plants. Plant Physiol. 1996;111:349–353. doi: 10.1104/pp.111.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Sánchez-Fernández R, Li ZS, Rea PA. Enhanced multispecifity of Arabidopsis vacuolar MRP-type ABC transporter, AtMRP2. J Biol Chem. 2001;276:8648–8656. doi: 10.1074/jbc.M009690200. [DOI] [PubMed] [Google Scholar]
- Lu YP, Li ZS, Drozdowicz Y, Hortensteiner S, Martinoia E, Rea PA. AtMRP2, an Arabidopsis ATP binding cassette transporter able to transport glutathione S-conjugates and chlorophyll catabolites: functional comparisons with AtMRP1. Plant Cell. 1998;10:267–282. doi: 10.1105/tpc.10.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YP, Li ZS, Rea PA. AtMRP1 gene of Arabidopsis encodes a glutathione-S-conjugate pump: isolation and functional definition of a plant ABC-transporter gene. Proc Natl Acad Sci USA. 1997;94:8243–8248. doi: 10.1073/pnas.94.15.8243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinoia E, Klein M, Geisler M, Sánchez-Fernández R, Rea PA. Vacuolar transport of secondary metabolites and xenobiotics. In: Robinson D, Rogers J, editors. Vacuolar Compartments: Annual Plant Reviews. Vol. 5. Sheffield, UK: Sheffield Academic Press; 2000. pp. 221–253. [Google Scholar]
- Matern U, Reichenbach C, Heller W. Efficient uptake of flavonoids into parsley (Petroselinum hortense) vacuoles requires acylated glycosides. Planta. 1986;167:183–189. doi: 10.1007/BF00391413. [DOI] [PubMed] [Google Scholar]
- Matile P. Das toxische kompartiment der pflanzenzelle. Naturwissenschaften. 1984;71:18–24. [Google Scholar]
- Matile P. The sap of plant cells. New Phytol. 1987;105:1–26. doi: 10.1111/j.1469-8137.1987.tb00107.x. [DOI] [PubMed] [Google Scholar]
- Nagy JI, Maligy P. Callus induction and plant regeneration from mesophyll protoplasts of Nicotiana sylvestris. Z Pflanzenphysiol. 1976;78:453–455. [Google Scholar]
- Pflugmacher S, Sandermann H., Jr Taxonomic distribution of plant glucosyltransferases acting on xenobiotics. Phytochemistry. 1998;49:507–511. doi: 10.1016/s0031-9422(00)00116-3. [DOI] [PubMed] [Google Scholar]
- Rea PA, Sanders D. Tonoplast energization: two H+pumps, one membrane. Physiol Plant. 1987;71:131–141. [Google Scholar]
- Rentsch D, Martinoia E. Citrate transport into barley mesophyll vacuoles: comparison with malate-uptake activity. Planta. 1991;184:532–537. doi: 10.1007/BF00197903. [DOI] [PubMed] [Google Scholar]
- Reuber S, Bornman JF, Weissenböck G. A flavonoid mutant of barley (Hordeum vulgareL.) exhibits increased sensitivity to UV-B radiation in the primary leaf. Plant Cell Environ. 1996;19:593–601. [Google Scholar]
- Reuber S, Jende-Strid B, Wray V, Weissenböck G. Accumulation of the chalcone isosalipurposide in primary leaves of barley flavonoid mutants indicates a defective chalcone isomerase. Physiol Plant. 1997;101:827–832. [Google Scholar]
- Saunders JA, Conn EE. Presence of the cyanogenic glucoside dhurrin in isolated vacuoles from Sorghum. Plant Physiol. 1978;61:154–157. doi: 10.1104/pp.61.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seikel MK, Geissman TA. The flavonoid constituents of barley (Hordeum vulgare): I. Saponarin. Arch Biochem Biophys. 1957;71:17–30. doi: 10.1016/0003-9861(57)90004-8. [DOI] [PubMed] [Google Scholar]
- Tommasini R, Vogt E, Fromenteau M, Hörtensteiner S, Matile P, Amrhein N, Martinoia E. An ABC-transporter of Arabidopsis thaliana has both glutathione-conjugate and chlorophyll catabolite transport activity. Plant J. 1998;13:773–780. doi: 10.1046/j.1365-313x.1998.00076.x. [DOI] [PubMed] [Google Scholar]
- Veit M, Pauli GF. Major flavonoids from Arabidopsis thalianaleaves. J Nat Prod. 1999;62:1301–1303. doi: 10.1021/np990080o. [DOI] [PubMed] [Google Scholar]
- Vogt T, Jones P. Glycosyltransferases in plant natural product synthesis: characterization of a supergene family. Trends Plant Sci. 2000;5:380–386. doi: 10.1016/s1360-1385(00)01720-9. [DOI] [PubMed] [Google Scholar]
- Wink M. Compartmentation of secondary metabolites and xenobiotics in plant vacuoles. In: Leigh RA, Sanders D, Callow JA, editors. The Plant Vacuole: Advances in Botanical Research. Vol. 25. London: Academic Press; 1997. pp. 141–170. [Google Scholar]