Abstract
The oxidation of acetate to hydrogen, and the subsequent conversion of hydrogen and carbon dioxide to methane, has been regarded largely as a niche mechanism occurring at high temperatures or under inhibitory conditions. In this study, 13 anaerobic reactors and sediment from a temperate anaerobic lake were surveyed for their dominant methanogenic population by using fluorescent in situ hybridization and for the degree of acetate oxidation relative to aceticlastic conversion by using radiolabeled [2-14C]acetate in batch incubations. When Methanosaetaceae were not present, acetate oxidation was the dominant methanogenic pathway. Aceticlastic conversion was observed only in the presence of Methanosaetaceae.
Acetate is the main precursor for methane production during anaerobic digestion of organic matter. Two mechanisms for methane formation from acetate have been described. The first one is aceticlastic, being carried out by Methanosarcinaceae or Methanosaetaceae (2). Methanosarcinaceae generally have a higher acetate threshold but a higher growth rate and yield than Methanosaetaceae (2). The second mechanism encompasses a two-step reaction in which acetate is first oxidized to H2 and CO2 and, with these products, subsequently converted to methane (15). This reaction is performed by acetate-oxidizing bacteria (often Clostridium spp.) in a syntrophic association with hydrogenotrophic methanogens (often Methanomicrobiales or Methanobacteriales) (4, 10, 12).
Some important environmental factors influencing the rate of anaerobic aceticlastic activity are temperature, organic acid concentrations, and ammonia concentration (9). At temperatures between 50°C and 65°C, acetate oxidation is favored at low acetate concentrations, while aceticlastic methanogenesis is favored at high acetate concentrations (15). The dominance of acetate oxidation at lower concentrations increases with increased temperature. Syntrophic acetate oxidation is the main mechanism for acetate degradation in the presence of inhibitors, particularly ammonium and volatile fatty acids (VFAs) (13). Syntrophic acetate oxidation has been reported for natural anoxic environments in subtropical lake sediments at temperatures as low as 15°C (8).
It is relatively straightforward to detect acetate oxidation activity by measuring the production of 14CH4 and 14CO2 from acetate labeled in the methyl group (C-2). When aceticlastic methanogens degrade acetate, the labeled methyl group will form only labeled methane (2). During syntrophic acetate oxidation, both carbon atoms of acetate are converted to carbon dioxide, and some of the carbon dioxide is subsequently reduced to methane (13). Therefore, significant levels of labeled carbon dioxide from [2-14C]acetate will be formed only during the oxidation of acetate.
The diversity of environments in which syntrophic acetate oxidation has been found indicates it may also be important for commercial gas production in biogas reactors, digesting wastewater sludge and manure. Aceticlastic activity has generally been considered to be the dominant pathway, with either Methanosarcinaceae or Methanosaetaceae dominating (9, 15). If a second pathway, such as acetate oxidation, dominates, it is necessary to re-evaluate reactor operation and optimization, which are currently based on maintaining Methanosaetaceae populations. The objective of this work was to assess the degree of acetate oxidation relative to aceticlastic conversion in a wide range of industrial anaerobic digesters, fed with either manure or wastewater sludge. A low-temperature environmental sample was also evaluated.
Sampling.
Thirteen Danish full-scale anaerobic digesters digesting manure together with waste from food industries were sampled as described in reference 5. An anaerobic sediment sample was collected from a lake situated in Orholm (Sollerod municipality, Denmark) at a 0.2-m depth with a gravity corer (6).
Analysis of the samples.
The samples were analyzed for VFAs and ammonia by standard methods (1). Microbial ecology was evaluated with fluorescent in situ hybridization (FISH) using established probes (see Table S1 in the supplemental material) and a previously reported method (5). Methanogenic populations not identified by FISH were assessed using PCR-temporal temperature gradient gel electrophoresis (PCR-TGGE).
Medium.
Basal anaerobic medium was used for acetic oxidation batch tests as described previously (5). The medium was dispensed anaerobically under a N2/CO2 (80%:20%) headspace in 100-ml incubation bottles, amended with labeled [2-14C]sodium acetate and nonlabeled sodium acetate. The medium was reduced with Na2S · 9H2O and supplemented aseptically with a sterilely filtered anaerobic vitamin solution as described previously (5). After inoculation with raw sample, the bottles were closed hermetically and incubated until methane production ceased. This was considered the end of the test, and analysis followed.
Radioisotope analyses.
The liquid and headspace of the bottles were sparged with approximately 2 liters of N2 through a 5 M NaOH trap to collect the 14CO2. The 14CH4 collected after trapping was combusted to 14CO2 in a tube furnace at 800°C. The 14CO2 generated in this furnace was then trapped in a carbon dioxide absorber for liquid scintillation counting (Carbosorb-E; Packard Bioscience Company). Radioactivity measurements of liquid samples were performed using a liquid scintillation counter (Tri-Carb 1600; PerkinElmer).
Simulation of methane production rates.
A simple kinetic batch model, based on Monod kinetics with zero-order lag, for conversion of acetate to methane was implemented with AQUASIM 2.1d (11). The maximum acetate removal rate and lag phase were estimated by fitting measured cumulative methane to modeled cumulative methane. The Secant method, with an objective function of residual sum of squares, was used to fit the data.
An overview of the results from the acetate oxidation survey experiment is given in Table 1.
TABLE 1.
Results from acetate oxidation survey
| Sample ID | Reactor name | Feed typea | Temp (°C) (type)b | Incubation period (days) | Dominant (nondominant) methanogenc
|
Level (mean ± SD) ofd:
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Before incubation | After incubation | VFA (g HAc liter −1) | Ammonia (g N liter−1) | Acetate removal (mM day−1) | 14CO2/14CH4 | Recovery of 14C (%) | |||||
| M1 | Nysted | Manure | 38 (M) | 9 | MS (NO) | MG (MS) | 2.7 ± 0.11d | 5.6 ± 0.12 | 0.5e± 0.1 | 2.9 ± 0.18d | 96 ± 5.2d |
| M2 | Hashøj | Manure | 37 (M) | 13 | Uncultured archaeon DQ409324 (MC) | Uncultured archaeon DQ409325 (NO) | 1.9 ± 0.07 | 4 ± 0.1 | 1.8 ± 0.1 | 2.75 ± 0.11 | 97.5 ± 0.8 |
| M3 | Lemvig | Manure | 52.5 (T) | 8 | MS (NO) | MB, MC (MS) | 0.6 ± 0.01 | 2.6 ± 0.08 | 5.3 ± 0.6 | 2.45 ± 0.15 | 92.5 ± 1 |
| M4 | Fangel | Manure | 37 (M) | 10 | MB (MC) | MB (MC) | 2.33 ± 0.12 | 4.5 ± 0.10 | 1.5 ± 0.1 | 2.2 ± 0.14 | 97.4 ± 2 |
| M5 | Studsgard | Manure | 52 (T) | 10 | UnIDd filaments (NO) | MG (MS) | 0.22 ± 0.003 | 2.20 ± 0.09 | 2.7 | 2.11 ± 0.16 | 99 ± 3 |
| M6 | Vester Hjermitslev | Manure | 37 (M) | 16 | MS (NO) | Uncultured archaeon DQ409326 (MS) | 1.81 ± 0.06 | 4.4 ± 0.11 | 0.7 ± 0.03 | 1.9 ± 0.18 | 92.2 ± 3.3 |
| M7 | Vegger | Manure | 55 (T) | 8 | MS (NO) | MS (NO) | 0.77 ± 0.04 | 2.34 ± 0.07 | 3.5 ± 0.4 | 1.61 ± 0.11 | 94 ± 3.5 |
| LS1 | Orholm | Lake sediment | 4 (P) | 49 | MB (MX, MG, MC) | MC (NO) | 0.01 | 0.03 | 1.4e± 0.2 | 1.4 ± 0.09 | 96.2 ± 4.9 |
| M8 | Sinding | Manure | 55 (T) | 8 | MS (NO) | MS (NO) | 0.41 ± 0.01 | 2.5 ± 0.06 | 3.4 | 1.1 ± 0.06 | 101 ± 4 |
| S1 | Hillerød | WW sludge | 55 (T) | 5 | MX (MS) | MX (NO) | 0.13 ± 0.001 | 1.44 ± 0.05 | 10 ± 0.5 | 0.11 ± 0.007 | 97 ± 4.2 |
| S2 | Lundtofte | WW sludge | 37 (M) | 10 | MX (NO) | MX (NO) | 0.02 | 1.00 ± 0.04 | 9.9 ± 0.6 | 0.08 ± 0.003 | 98.1 ± 2 |
| S3 | Fakse | WW sludge | 37 (M) | 6 | MX (NO) | MX (NO) | 0.04 | 1.50 ± 0.03 | 7.1 | 0.065 ± 0.004 | 91 ± 2.5 |
| S4 | Sydkyst | WW sludge | 37 (M) | 8 | MX (NO) | MX (NO) | 0.13 | 0.50 ± 0.001 | 4.8e± 0.4 | 0.04 ± 0.002 | 92 ± 3.4 |
| S5 | Helsingør | WW sludge | 37 (M) | 6 | MX (MB, MG) | MX (NO) | 0.06 | 0.84 ± 0.03 | 6.1 ± 0.7 | 0.025 ± 0.001 | 95.4 ± 3.2 |
WW, wastewater.
P, psychrophilic (<20°C); M, mesophilic (35°C to 40°C); T, thermophilic (>50°C).
MS, Methanosarcinaceae; MX, Methanosaetaceae; MB, Methanobacteriales; MG, Methanomicrobiales; MC, Methanococcales; NO, not observed; UnIDd, unidentified (by both FISH and TGGE). Dominant methanogen, more than 90% of the total number of methanogenic cells (archaea responding to ARC915); nondominant methanogen, 1 to 10% of the total number of methanogenic cells. Cells in 20 fields were counted.
Standard deviation is based on triplicate analysis.
Acetate removal is given as the maximum rate of removal. The long lag phase was observed before acetate removal.
Rates of methane production and acetate removal.
Methane production rates varied considerably, with fast samples (such as Lundtofte and Hillerød) stopping methane production in 3 days and slow samples (e.g., Nysted and Vegger) requiring more than 10 days. The anaerobic lake sediment sample (Orholm) had a lag phase of 31.5 ± 0.8 days. Acetate removal rates also varied within a factor of approximately 10 (Table 1). These rates were higher (>4 mM · day−1) for cultures with a low degree of acetate oxidation than for cultures with a high degree of acetate oxidation (acetate utilization rates lower than 4 mM · day−1). Our rates compare with acetate removal rates in pure culture for mesophilic (12) and thermophilic (4) acetate-oxidizing cultures.
Anaerobic acetate conversion pathways and environmental conditions.
In all cases, populations dominated by Methanosaetaceae had low levels of acetate oxidation (14CO2/14CH4 < 0.1), while populations dominated by other methanogenic Archaea and without Methanosaetaceae had high levels of acetate oxidation (14CO2/14CH4 > 1) (Table 1). Results obtained clearly showed a strong correlation between the absence of Methanosaetaceae and the involvement of the acetate oxidation pathway. Other factors (e.g., source and inoculum temperature) had no influence. Acetate cleavage has been generally regarded as a bimodal system, dominated by Methanosarcinaceae at high acetate concentrations and by Methanosaetaceae at low acetate concentrations (2, 14). From the data presented here, we propose instead a different bimodal system in mixed cultures, with aceticlastic methanogenesis in the presence of Methanosaetaceae and acetate oxidation in their absence. The absence of this methanogenic phylogenetic group has been previously investigated in the systems analyzed here and was linked to the presence of high ammonia and VFA levels (5). Most probably, the high ammonia concentrations inhibit the aceticlastic methanogens much more than the hydrogenotrophic methanogens, and methane is formed mainly by hydrogen-utilizing methanogens. This idea is supported by previous studies (3) indicating that acetate-utilizing methanogens are more sensitive to ammonia than are hydrogenotrophic methanogens. The high degree of acetate oxidation in digested manure at high ammonia and VFA levels is also in agreement with other results (13). However, a large potential for syntrophic acetate oxidation was also observed at low acetate concentrations (in the Orholm sample). It is likely that inhibition or other factors prevent growth of Methanosaetaceae and allow dominance by acetate oxidation by default.
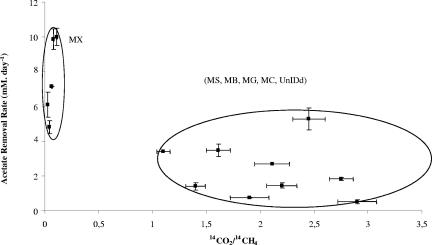
The bimodality of the system is also highlighted in Fig. 1, which shows two distinct groups, with hydrogen-utilizing methanogens (Methanobacteriales, Methanococcales, Methanomicrobiales, possibly Methanosarcinaceae, uncultured archaea [Hashøj and Lemvig], and unidentified archaea [Studsgard]) in syntrophic cooperation with acetate oxidizers at low maximum acetate removal rates and the strict aceticlastic methanogen Methanosaetaceae at maximum acetate removal rates. The presence of Methanosarcinaceae as a hydrogen-utilizing syntrophic partner in the acetate-oxidizing cultures is not surprising. In contrast to the Methanosaetaceae species, which are strict aceticlastic methanogens, most Methanosarcinaceae species are mixotrophic, utilizing not only acetate but also hydrogen and carbon dioxide, methanol, and methylamines (2). In addition, Methanosarcinaceae are capable themselves of acetate oxidation (7) and could be therefore be mediating the entire process of acetate oxidation, and subsequent methanogenesis, rather than acting as an acetate sink via aceticlastic reaction.
FIG. 1.
Distribution of the dominant methanogens observed in the samples as a function of the degree of acetate oxidation versus acetate removal rate. Error bars indicate standard deviations. MX, Methanosaetaceae; MS, Methanosarcinaceae; MB, Methanobacteriales; MG, Methanomicrobiales; MC, Methanococcales; UnIDd, unidentified.
Methanogenic populations.
The FISH observations showed that dominant methanogenic populations (see Figure S1 in the supplemental material) of wastewater sludge samples were consistently Methanosaetaceae, as previously reported (5), while manure samples were phylogenetically more diverse. In every case, dominance of specific groups as observed by FISH was clear, and they constituted more than 90% of the archaeal population, as described previously (5). Diversity in subdominant methanogens was limited, except in the Orholm sample (sediment sample), where archaea belonging to Methanosaetaceae, Methanomicrobiales, and Methanococcales were observed. Methanogenic population changes were observed during growth on acetate in the incubations. For the samples in which Methanosaetaceae were dominant, the only change observed during incubation was the elimination of subdominant populations. In the other samples, there was a shift to known hydrogen consumers (Methanobacteriales, Methanomicrobiales, or Methanococcales) or uncultured archaea (samples M2 and M6).
Methanogenic communities in several samples (M2 and M5 before incubation and M2 and M6 after incubation) were not identified by FISH. This was due to the limitations of visual in situ hybridization. FISH is very convenient for the rapid analysis of a large number of environmental samples but is limited when carried beyond the limits of oligonucleotide probes. ARC915 is an effective general probe, and order-level probes have been used in a wide range of systems; however, in complicated systems, such as manure, they might fail to detect all methanogens. Therefore, unidentified methanogens were phylogenetically characterized by PCR-TGGE. Samples not identified by FISH (e.g., M5, Studsgard before inoculation) were found by PCR-TGGE to be far outside known phylogenetic groupings for methanogens. It is likely that these microbes are still methanogens, since bacterial methanogenesis is unknown. These unknown microbial groups are interesting scientifically and deserve further investigation.
Nucleotide sequence accession numbers.
Sequence data for microbes identified in this study have been submitted to the GenBank database under accession numbers DQ409324 to DQ409326.
Supplementary Material
Acknowledgments
We thank Lene Kirstejn Jensen, Birthe Ebert, and Hector Garcia for technical assistance with the experiments.
This work was supported by a Danish Government Scholarship and the Danish Research Programme (EFP 05).
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.American Public Health Association. 1985. Standard methods for the examination of waste and wastewater. American Public Health Association, Washington, D.C.
- 2.Ferry, J. 1993. Fermentation of acetate, p. 305-334. In J. G. Ferry (ed.), Methanogenesis. Ecology, physiology, biochemistry and genetics. Chapman and Hall, New York, N.Y.
- 3.Garcia, J. L., B. K. C. Patel, and B. Ollivier. 2000. Taxonomic, phylogenetic, and ecological diversity of methanogenic Archaea. Anaerobe 6:205-226. [DOI] [PubMed] [Google Scholar]
- 4.Hattori, S., Y. Kamagata, S. Hanada, and H. Shoun. 2000. Thermacetogenium phaeum gen. nov., sp. nov., a strictly anaerobic, thermophilic, syntrophic acetate-oxidizing bacterium. Int. J. Syst. Evol. Microb. 50:1601-1604. [DOI] [PubMed] [Google Scholar]
- 5.Karakashev, D., D. J. Batstone, and I. Angelidaki. 2005. Influence of environmental conditions on methanogenic compositions in anaerobic biogas reactors. Appl. Environ. Microbiol. 71:331-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelts, K. U., K. Briegel, K. Ghilardi, and K. Hsu. 1986. The limnogeology-ETH coring system. Schweitz. Z. Hydrol. 48:104-116.
- 7.Lovley, D. R., and J. G. Ferry. 1985. Production and consumption of H2 during growth of Methanosarcina spp. on acetate. Appl. Environ. Microbiol. 49:247-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nusslein, B., K.-J. Chin, W. Eckert, and R. Conrad. 2001. Evidence for anaerobic syntrophic acetate oxidation during methane production in the profundal sediment of subtropical Lake Kinneret (Israel). Environ. Microbiol. 3:460-470. [DOI] [PubMed] [Google Scholar]
- 9.Pavlostathis, S. G., and E. Giraldo-Gomez. 1991. Kinetics of anaerobic treatment: a critical review. Crit. Rev. Environ. Control 21:411-490. [Google Scholar]
- 10.Petersen, S., and B. Ahring. 1991. Acetate oxidation in a thermophilic anaerobic sewage-sludge digestor: the importance of non-aceticlastic methanogenesis from acetate. FEMS Microbiol. Ecol. 86:149-158. [Google Scholar]
- 11.Reichert, P. 1994. AQUASIM: a tool for simulation and data analysis of aquatic systems. Water Sci. Technol. 30:21-30. [Google Scholar]
- 12.Schnurer, A., B. Svensson, and B. Schink. 1997. Enzyme activities in energetics of acetate metabolism by the mesophilic syntrophically acetate-oxidizing anaerobe Clostridium ultunense. FEMS Microbiol. Lett. 154:331-336. [Google Scholar]
- 13.Schnurer, A. G., G. Zellner, and B. Svensson. 1999. Mesophilic syntrophic acetate oxidation during methane formation in biogas reactors. FEMS Microbiol. Ecol. 29:249-261. [Google Scholar]
- 14.Speece, R. E. 1996. Anaerobic biotechnology for industrial wastewaters. Archae Press, Nashville, Tenn.
- 15.Zinder, S., and M. Koch. 1984. Non-aceticlastic methanogenesis from acetate: acetate oxidation by a thermophilic syntrophic coculture. Arch. Microbiol. 138:263-272. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.