Abstract
We set out to analyze the sequence diversity of the Bacillus thuringiensis flagellin (H antigen [Hag]) protein and compare it with H serotype diversity. Some other Bacillus cereus sensu lato species and strains were added for comparison. The internal sequences of the flagellin (hag) alleles from 80 Bacillus thuringiensis strains and 16 strains from the B. cereus sensu lato group were amplified and cloned, and their nucleotide sequences were determined and translated into amino acids. The flagellin allele nucleotide sequences for 10 additional strains were retrieved from GenBank for a total of 106 Bacillus species and strains used in this study. These included 82 B. thuringiensis strains from 67 H serotypes, 5 B. cereus strains, 3 Bacillus anthracis strains, 3 Bacillus mycoides strains, 11 Bacillus weihenstephanensis strains, 1 Bacillus halodurans strain, and 1 Bacillus subtilis strain. The first 111 and the last 66 amino acids were conserved. They were referred to as the C1 and C2 regions, respectively. The central region, however, was highly variable and is referred to as the V region. Two bootstrapped neighbor-joining trees were generated: a first one from the alignment of the translated amino acid sequences of the amplified internal sequences of the hag alleles and a second one from the alignment of the V region amino acid sequences, respectively. Of the eight clusters revealed in the tree inferred from the entire C1-V-C2 region amino acid sequences, seven were present in corresponding clusters in the tree inferred from the V region amino acid sequences. With regard to B. thuringiensis, in most cases, different serovars had different flagellin amino acid sequences, as might have been expected. Surprisingly, however, some different B. thuringiensis serovars shared identical flagellin amino acid sequences. Likewise, serovars from the same H serotypes were most often found clustered together, with exceptions. Indeed, some serovars from the same H serotype carried flagellins with sufficiently different amino acid sequences as to be located on distant clusters. Species-wise, B. halodurans, B. subtilis, and B. anthracis formed specific branches, whereas the other four species, all in the B. cereus sensu lato group, B. mycoides, B. weihenstephanensis, B. cereus, and B. thuringiensis, did not form four specific clusters as might have been expected. Rather, strains from any of these four species were placed side by side with strains from the other species. In the B. cereus sensu lato group, B. anthracis excepted, the distribution of strains was not species specific.
Bacillus thuringiensis is a gram-positive, rod-shaped, sporulating bacterium characterized at the species level by the production of a parasporal inclusion, the “crystal,” upon sporulation (2). Some B. thuringiensis strains have been shown to exhibit specific insecticidal activity against lepidopteran, dipteran, or coleopteran insect pests (6) and have been developed as biological insecticides (5). The commercial success of B. thuringiensis as a biological insecticide has driven the establishment of several screening programs throughout the world aimed at isolating novel B. thuringiensis strains expressing novel insecticidal and pesticidal activities. It is believed that by 1999, more than 50,000 B. thuringiensis strains had been isolated and were kept in various collections worldwide (16). In the early 1960s, H serotyping, the immunological reaction to the bacterial flagellar antigens, was developed as a classification tool for B. thuringiensis strains (3). By 1999, the wide diversity of B. thuringiensis strains was classified into 69 H serotypes and 82 serological varieties (7).
The bacterial flagellum is the organelle of locomotion. It is composed of three parts, a basal body, a hook, and a filament (8). The basal body anchors the flagellum to the bacterial cell wall and cell membrane. It acts as a rotary engine to enable the flagellum to propel the bacterium. The hook is the flexible coupling structure between the basal body and the filament. The filament is the rod that provides motility. It is made of multiple copies of a single protein, flagellin, which is also responsible for eliciting the immunological reaction. We report here the amplification, cloning, and determination of the nucleotide sequences of the flagellin alleles from B. thuringiensis H serotypes and from B. thuringiensis-related species in the Bacillus cereus sensu lato group. Next, all bacterial strains were positioned on a phylogenetic tree based on flagellin amino acid sequence homologies clusters were formed, and relatednesses were revealed. Finally, the flagellin amino acid sequence diversity was compared with H serotype diversity, and correlations are discussed.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The 106 bacterial strains used in this study and their sources are listed in Table 1. These include 82 Bacillus thuringiensis strains from 67 H serotypes, 5 B. cereus strains, 3 Bacillus anthracis strains, 3 Bacillus mycoides strains, 11 Bacillus weihenstephanensis strains, 1 Bacillus halodurans strain, and 1 Bacillus subtilis strain. The genomes had been fully sequenced for nine of these strains, and the gene of interest had been sequenced for a 10th strain. Their sequences are available from the GenBank database (http://www.ncbi.nlm.nih.gov/) (Table 1). Total DNAs were obtained for another nine strains. These 19 strains were not cultured here. The other 87 bacterial strains were cultured as follows. B. thuringiensis, B. cereus, and B. mycoides strains were grown in Luria-Bertani (LB) medium (15). Two B. weihenstephanensis strains, CCM 4965 and CCM 4966, were grown in 464 medium (5.0 g Bacto-tryptone, 2.5 g Bacto-yeast extract, and 1.0 g dextrose per liter, pH 7.0) (http://www.dsmz.de/microorganisms/html/media/medium000464.html). All strains were incubated at 30°C.
TABLE 1.
Bacterial strains and species used in this study
| PCR primer pair | Species | H antigena | Source or strainb | GenBank accession no. | Gene name in GenBank |
|---|---|---|---|---|---|
| Bthag-F1/-R1 | B. thuringiensis serovar thuringiensis | 1 | IEBC-T01 001 | DQ377245 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar finitimus | 2 | IEBC-T02 001 | DQ377243 | hag |
| B. thuringiensis serovar alesti | 3a,3c | Bt5 | X67138 | flaA | |
| Bthag-F1/-R1 | B. thuringiensis serovar kurstaki | 3a,3b,3c | IEBC-T03A001 | DQ377225 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar sumiyoshiensis | 3a,3d | IEBC-T03B001 | DQ377246 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar fukuokaensis | 3a,3d,3e | IEBC-T03C001 | DQ377247 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar sotto | 4a,4b | IEBC-T04 001 | DQ377248 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar kenyae | 4a,4c | IEBC-T04B001 | DQ377249 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar galleriae | 5a,5b | IEBC-T05 001 | DQ377225 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar canadensis | 5a,5c | IEBC-T05A001 | DQ377227 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar entomocidus | 6 | IEBC-T06 001 | DQ377250 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar aizawai | 7 | IEBC-T07 001 | DQ377228 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar morrisoni | 8a,8b | IEBC-T08 001 | DQ377229 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar ostriniae | 8a,8c | IEBC-T08A001 | DQ377251 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar nigeriensis | 8b,8d | IEBC-T08B001 | DQ377252 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar tolworthi | 9 | IEBC-T09 001 | DQ377253 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar darmstadiensis | 10a,10b | IEBC-T10 001 | DQ377242 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar londrina | 10a,10c | IEBC-T10A001 | DQ377230 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar toumanoffi | 11a,11b | IEBC-T11 001 | DQ377254 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar kyushuensis | 11a,11c | IEBC-T11A001 | DQ377255 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar thompsoni | 12 | IEBC-T12 001 | DQ377294 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar pakistani | 13 | IEBC-T13 001 | DQ377256 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar israelensis | 14 | IEBC-T14 001 | DQ377244 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar dakota | 15 | IEBC-T15 001 | DQ377231 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar indiana | 16 | IEBC-T16 001 | DQ377257 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar tohokuensis | 17 | IEBC-T17 001 | DQ377258 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar kumamotoensis | 18a,18b | IEBC-T18 001 | DQ377293 | hag |
| Bthag-F2/-R2 | B. thuringiensis serovar yosoo | 18a,18c | IEBC-T18A001 | DQ377300 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar tochigiensis | 19 | IEBC-T19 001 | DQ377259 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar yunnanensis | 20a,20b | IEBC-T20 001 | DQ377260 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar pondicheriensis | 20a,20c | IEBC-T20A001 | DQ377261 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar colmeri | 21 | IEBC-T21 001 | DQ377262 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar shandongiensis | 22 | IEBC-T22 001 | DQ377263 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar japonensis | 23 | IEBC-T23 001 | DQ377264 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar neoleonensis | 24a,24b | IEBC-T24 001 | DQ377265 | hag |
| Bthag-F2/-R2 | B. thuringiensis serovar novosibirsk | 24a,24c | IEBC-T24A001 | DQ377317 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar coreanensis | 25 | IEBC-T25 001 | DQ377232 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar silo | 26 | IEBC-T26 001 | DQ377266 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar mexicanensis | 27 | IEBC-T27 001 | DQ377267 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar monterrey | 28a,28b | IEBC-T28 001 | DQ377233 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar jegathesan | 28a,28c | IEBC-T28A001 | DQ377234 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar amagiensis | 29 | IEBC-T29 001 | DQ377268 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar medellin | 30 | IEBC-T30 001 | DQ377269 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar toguchini | 31 | IEBC-T31 001 | DQ377270 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar cameroun | 32 | IEBC-T32 001 | DQ377271 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar leesis | 33 | IEBC-T33 001 | DQ377272 | hag |
| B. thuringiensis serovar konkukian | 34 | 97-27 | YP_035879 | hag | |
| Bthag-F1/-R1 | B. thuringiensis serovar seoulensis | 35 | IEBC-T35 001 | DQ377273 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar malaysiensis | 36 | IEBC-T36 001 | DQ377235 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar andaluciensis | 37 | IEBC-T37 001 | DQ377236 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar oswaldocruzi | 38 | IEBC-T38 001 | DQ377274 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar brasiliensis | 39 | IEBC-T39 001 | DQ377237 | hag |
| Bthag-F2/-R2 | B. thuringiensis serovar huazhongensis | 40 | IEBC-T40 001 | DQ377320 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar sooncheon | 41 | IEBC-T41 001 | DQ377275 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar jinghongiensis | 42 | IEBC-T42 001 | DQ377238 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar guiyangiensis | 43 | IEBC-T43 001 | DQ377239 | hag |
| Bthag-F2/-R2 | B. thuringiensis serovar higo | 44 | IEBC-T44 001 | DQ377301 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar roskildiensis | 45 | IEBC-T45 001 | DQ377276 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar chanpaisis | 46 | IEBC-T46 001 | DQ377240 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar wratislaviensis | 47 | IEBC-T47 001 | DQ377277 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar balearica | 48 | IEBC-T48 001 | DQ377297 | hag |
| Bthag-F2/-R2 | B. thuringiensis serovar muju | 49 | IEBC-T49 001 | DQ377302 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar navarrensis | 50 | IEBC-T50 001 | DQ377278 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar xiaguangiensis | 51 | IEBC-T51 001 | DQ377279 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar kim | 52 | IEBC-T52 001 | DQ377280 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar asturiensis | 53 | IEBC-T53 001 | DQ377241 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar poloniensis | 54 | IEBC-T54 001 | DQ377281 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar palmanyolensis | 55 | IEBC-T55 001 | DQ377282 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar rongseni | 56 | IEBC-T56 001 | DQ377283 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar pirenaica | 57 | IEBC-T57 001 | DQ377295 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar argentinensis | 58 | IEBC-T58 001 | DQ377284 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar iberica | 59 | IEBC-T59 001 | DQ377285 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar pingluosensis | 60 | IEBC-T60 001 | DQ377286 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar sylvestriensis | 61 | IEBC-T61 001 | DQ377287 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar zhaodongensis | 62 | IEBC-T62 001 | DQ377288 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar bolivia | 63 | IEBC-T63 001 | DQ377289 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar azorensis | 64 | IEBC-T64 001 | DQ377296 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar pulsiensis | 65 | IEBC-T65 001 | DQ377290 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar graciosensis | 66 | IEBC-T66 001 | DQ377291 | hag |
| Bthag-F1/-R1 | B. thuringiensis serovar vazensis | 67 | IEBC-T67 001 | DQ377292 | hag |
| Bthag-F1/-R1 | B. thuringiensis subsp. wuhanensis | UN | BGSC 4T1 | DQ377298 | hag |
| Bthag-F1/-R1 | B. thuringiensis | UN | M15 | DQ377303 | hag |
| B. cereus | ATCC 14579 | NP_831434 | Flagellin | ||
| B. cereus | ATCC 10987 | NP_978100 | Flagellin | ||
| B. cereus | E33L | YP_083130 | fliC | ||
| Bthag-F1/-R1 | B. cereus | BGSC 6A17 | DQ377304 | hag | |
| Bthag-F1/-R1 | B. cereus | BGSC 6A18 | DQ377305 | hag | |
| B. anthracis | Ames ancestor | YP_018345 | Flagellin | ||
| B. anthracis | Ames | NP_844143 | Flagellin | ||
| B. anthracis | Sterne | YP_027849 | Flagellin | ||
| Bthag-F1/-R1 | B. mycoides | ATCC 6462 | DQ377299 | hag | |
| Bthag-F1/-R1 | B. mycoides | BGSC 6A13 | DQ377306 | hag | |
| Bthag-F1/-R1 | B. mycoides | BGSC 6A20 | DQ377307 | hag | |
| Bthag-F1/-R1 | B. weihenstephanensis | WSBC10001 | DQ377308 | hag | |
| Bthag-F1/-R1 | B. weihenstephanensis | WSBC10045 | DQ377309 | hag | |
| Bthag-F1/-R1 | B. weihenstephanensis | WSBC10067 | DQ377318 | hag | |
| Bthag-F1/-R1 | B. weihenstephanensis | WSBC10090 | DQ377310 | hag | |
| Bthag-F1/-R1 | B. weihenstephanensis | WSBC10204 | DQ377311 | hag | |
| Bthag-F1/-R1 | B. weihenstephanensis | WSBC10295 | DQ377312 | hag | |
| Bthag-F1/-R1 | B. weihenstephanensis | WSBC10296 | DQ377313 | hag | |
| Bthag-F3/-R3 | B. weihenstephanensis | WSBC10363 | DQ377319 | hag | |
| Bthag-F1/-R1 | B. weihenstephanensis | WSBC10365 | DQ377314 | hag | |
| Bthag-F1/-R1 | B. weihenstephanensis | CCM 4965 | DQ377315 | hag | |
| Bthag-F1/-R1 | B. weihenstephanensis | CCM 4966 | DQ377316 | hag | |
| B. subtilis | 168 | NP_391416 | hag | ||
| B. halodurans | C-125 | NP_244483 | hag |
UN, unserotypeable.
IEBC, International Entomopathogenic Bacillus Centre, Institut Pasteur, Paris, France; BGSC, Bacillus Genetic Stock Center, The Ohio State University, Columbus, OH; ATCC, American Type Culture Collection, Rockville, MD; WSBC, Weihenstephanen Bacillus Collection, Institute of Microbiology, FML Weihenstephanen, Germany; CCM, Czech Collection of Microorganisms, Masaryk University, Brno, Czech Republic.
Escherichia coli strain TOP10 (Invitrogen Canada, Burlington, Ontario, Canada) was used for cloning of PCR fragments. It was cultured on LB agar plates to select transformants or in LB broth, with shaking at 180 to 200 rpm at 37°C, overnight. When necessary, kanamycin was added to the medium at a final concentration of 50 μg/ml.
Amplification and cloning of internal sequences of the hag alleles.
Total DNAs of Bacillus species and strains and recombinant plasmid from the E. coli strain were isolated as described previously (21), with some exceptions. The total DNAs from nine B. weihenstephanensis strains, WSBC10001, WSBC10045, WSBC10067, WSBC10090, WSBC10204, WSBC10295, WSBC10296, WSBC10363, and WSBC10365, were kindly provided by Monika Ehling-Schulz, Technical University of Munich, Germany.
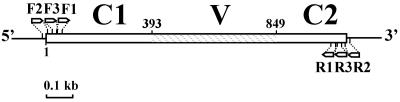
The hag allele internal sequences were amplified with primer pairs Bthag-F1 (5′-AGTACATGCGCCAAAACCAAG) and Bthag-R1 (5′-GTTTGCTTGAGAAAGCATGCT), Bthag-F2 (5′-GGGGTTCTTAATCATGAGAA) and Bthag-R2 (5′-TAACTCAAATGGCTTATTGT), or Bthag-F3 (5′-AAYATTAAYAGCATGCGTAC) and Bthag-R3 (5′-TTTGTGGWGTTTGGTTWGCT) (Table 1). Positions of the three pairs of primers, with respect to the B. thuringiensis serovar konkukian strain 97-27 hag allele, are shown in Fig. 1. B. thuringiensis serovar konkukian strain 97-27 was chosen as the standard because its genome, including the hag gene, had been fully sequenced and was freely available from the GenBank database (accession no. AE017355). PCR with primer pair Bthag-F1/Bthag-R1 (Bthag-F1/-R1) was run for 30 cycles at a denaturing temperature of 95°C for 15 s, annealing at 54°C for 30 s, and extension at 72°C for 1.5 min. PCR with the other primer pairs differed only in annealing temperatures: 48°C for Bthag-F2/-R2 and 44°C for Bthag-F3/-R3.
FIG. 1.
Map of the Bacillus thuringiensis serovar konkukian flagellin (hag) gene. The number 1 refers to the first nucleotide of the hag gene coding region. Arrowheads indicate the orientations and positions of the three primer pairs used for amplification. They are F1 and R1 at nucleotide positions 41 to 61 and 1068 to 1048, respectively; F2 and R2 (positions −13 to 7 and 1117 to 1098, respectively); and F3 and R3 (positions 16 to 35 and 1043 to 1062, respectively). C1 and C2 correspond to conserved regions 1 and 2, respectively. The shaded box corresponds to the variable, V, region between nucleotides positions 394 and 849.
The amplified DNAs were cloned into the pCR2.1-TOPO cloning vector using the TOPO TA cloning kit (Invitrogen Canada) according to the manufacturer's instructions. Transformants were selected on LB agar plates supplemented with kanamycin (50 μg/ml) and X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) (40 μg/ml). Recombinant plasmids were isolated using the alkaline lysis method (1) with some modifications (19, 21), digested with EcoRI, and visualized on agarose gels to confirm the presence of an inserted fragment.
The nucleotide sequences were determined by the dideoxynucleotide chain termination method (17) using a capillary array automated DNA sequencer (ABI3730xl DNA analyzer; Applied Biosystems, Foster City, CA). The sequences were determined for both strands.
hag gene sequences for B. thuringiensis serovar alesti; B. thuringiensis serovar konkukian; B. cereus strains ATCC 14579, ATCC 10987, and E33L; all three B. anthracis strains; B. halodurans; and B. subtilis were retrieved directly from GenBank.
Sequence analysis.
The hag internal nucleotide sequences from 82 B. thuringiensis strains, 5 B. cereus strains, 3 B. anthracis strains, 3 B. mycoides strains, 11 B. weihenstephanensis strains, 1 B. halodurans strain, and 1 B. subtilis strain were translated into amino acid sequences using the “traduc” software at Infobiogen (http://www.infobiogen.fr/services/analyseq/cgi-bin/traduc_in.pl) or retrieved directly from GenBank when available. The amino acid sequences were aligned, and a neighbor-joining tree was constructed (14) and bootstrapped using 1,000 random samples of sites from the alignment, all using the Clustal W software (20) at the DNA Data Bank of Japan (DDBJ) (http://www.ddbj.nig.ac.jp/search/clustalw-e.html). Two bootstrapped neighbor-joining trees were constructed: a first one generated from the alignment of the flagellin (Hag) amino acid sequences, and a second one generated from the alignment of the flagellin variable central region amino acid sequences. TreeView (version 1.6.6) (10, 11) was used to display and print the trees.
Nucleotide sequence accession numbers.
Sequence data from this study have been deposited in the GenBank database under accession no. DQ377225 to DQ377320.
RESULTS
Flagellin (Hag protein) polymorphism.
The internal sequences of the flagellin (hag) alleles were amplified from 80 Bacillus thuringiensis strains and 16 strains from the B. cereus sensu lato group, for a subtotal of 96 bacterial strains. Three pairs of primers were used. A first pair was sufficient to amplify the internal sequences of the flagellin alleles from 90 bacterial strains. Six strains showed no amplification products. A second pair of primers was designed and proved sufficient to amplify the internal sequences of the flagellin alleles for five additional B. thuringiensis serovars, serovars yosoo, novosibirsk, huazhongensis, higo, and muju. Finally, a third pair was designed and used successfully for the last bacterial strain assayed, B. weihenstephanensis strain WSBC10363. The flagellin allele nucleotide sequences for 10 additional strains were retrieved from GenBank, for a total of 106 Bacillus species and strains used in this study.
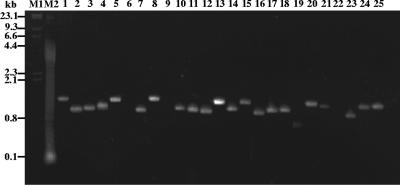
Most of the 96 bacterial strains assayed yielded a single major amplified band. The amplified products ranged in size from 700 bp to 1.9 kb. A subset of the amplification products for selected B. thuringiensis strains is shown in Fig. 2.
FIG. 2.
Agarose gel electrophoresis of hag gene amplification products in selected B. thuringiensis serovars using the F1/R1 primer pair. Lane 1, B. thuringiensis serovar cameroun; lane 2, B. thuringiensis serovar leesis; lane 3, B. thuringiensis serovar seoulensis; lane 4, B. thuringiensis serovar oswaldocruzi; lane 5, B. thuringiensis serovar sooncheon; lane 6, B. thuringiensis serovar higo; lane 7, B. thuringiensis serovar roskildiensis; lane 8, B. thuringiensis serovar wratislaviensis; lane 9, B. thuringiensis serovar muju; lane 10, B. thuringiensis serovar navarrensis; lane 11, B. thuringiensis serovar xiaguangiensis; lane 12, B. thuringiensis serovar kim; lane 13, B. thuringiensis serovar poloniensis; lane 14, B. thuringiensis serovar palmanyolensis; lane 15, B. thuringiensis serovar rongseni; lane 16, B. thuringiensis serovar argentinensis; lane 17, B. thuringiensis serovar iberica; lane 18, B. thuringiensis serovar pingluosensis; lane 19, B. thuringiensis serovar sylvestriensis; lane 20, B. thuringiensis serovar zhaodongensis; lane 21, B. thuringiensis serovar bolivia; lane 22, B. thuringiensis serovar azorensis; lane 23, B. thuringiensis serovar pulsiensis; lane 24, B. thuringiensis serovar graciosensis; lane 25, B. thuringiensis serovar vazensis. No amplification products were detected with the F1/R1 primer pair in lanes 6, 9 and 22. Molecular weight markers are shown in lane M1, lambda DNA digested with HindIII, and lane M2, a 100-bp ladder.
The nucleotide sequences were translated into amino acid sequences using the reading frame that codes for a protein homologous to the B. thuringiensis serovar konkukian flagellin (Hag) amino acid sequence. The amino acid sequences translated from the amplified internal nucleotide sequences ranged in length from 231 to 433 amino acids. The amino acid sequences were aligned. Gaps were introduced when necessary to maximize the homology. The final alignment was 557 amino acids and gaps in length for the 106 Bacillus species and strains under study. The sequence corresponds to amino acids 21 to 349 of the B. thuringiensis serovar konkukian flagellin (Hag) amino acid sequence (see the supplemental material). The first 111 and the last 66 amino acids were highly conserved not only among the 82 B. thuringiensis strains but also among the 22 B. cereus sensu lato strains and, perhaps surprisingly, among B. halodurans and B. subtilis strains. They were referred to as the conserved C1 and C2 regions, respectively. The central region, however, was highly variable, included most gaps introduced to maximize homology, and is referred to as the variable, V, region. The sequence corresponds to amino acids 132 to 283 of the B. thuringiensis serovar konkukian flagellin (Hag) amino acid sequence. (The amino acid sequence alignment of the V region is also presented in the supplemental material.)
Two bacterial strains deserve specific comments. B. thuringiensis serovar yosoo was unusual in that its nucleotide sequence showed a thymine deletion at nucleotide position 138 (with reference to the B. thuringiensis serovar konkukian hag gene sequence), which thereby changed the reading frame and created several stop codons downstream. However, another possible initiation codon, ATG, was located a few nucleotides downstream from the thymine deletion at position 153, perhaps making the synthesis of a truncated flagellin protein possible. This truncated sequence was used in the alignment. Likewise, B. weihenstephanensis CCM 4965 showed an adenine deletion at nucleotide position 102, which thereby changed the reading frame and created several stop codons downstream. However, here also, another possible initiation codon was located a few nucleotides downstream from the adenine deletion at position 153, perhaps making the synthesis of a truncated flagellin protein possible. Here also, the truncated sequence was used in the alignment.
Phylogenetic analysis of flagellins (Hag proteins).
Two bootstrapped neighbor-joining trees were generated, a first one from the alignment of the amino acid sequences of the amplified internal sequences of the flagellin (hag) alleles and a second one from the alignment of the V region amino acid sequences. They are presented in Fig. 3 and 4, respectively.
FIG. 3.
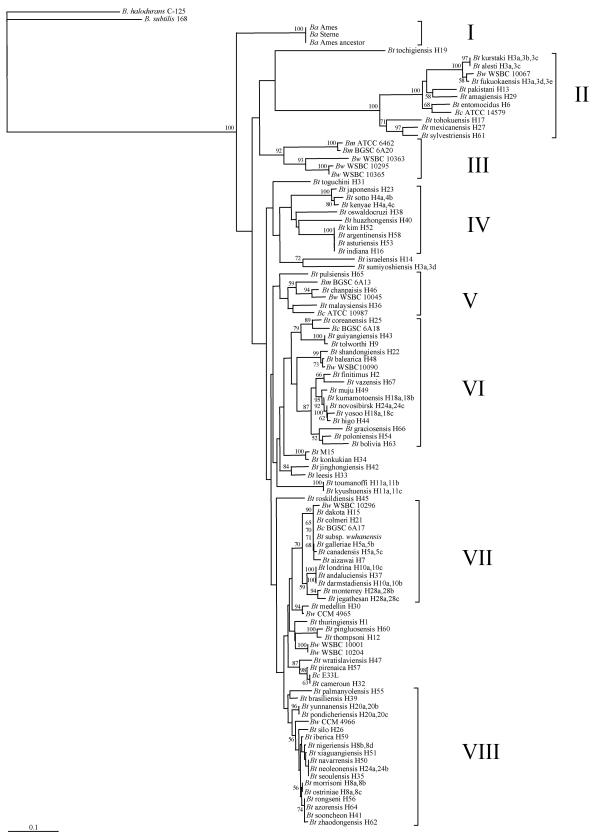
Bootstrapped neighbor-joining tree of Bacillus thuringiensis (Bt) generated from the alignment of flagellin amino acid sequences. Clusters are indicated in roman numerals. Bootstrap values higher than 50% are indicated. The horizontal bar represents 10% differences in amino acids. Ba, B. anthracis; Bw, B. weihenstephanensis; Bm, B. mycoides; Bc, B. cereus.
FIG. 4.
Bootstrapped neighbor-joining tree of Bacillus thuringiensis (Bt) generated from the alignment of the variable central region (V region) flagellin amino acid sequences. Clusters are indicated in roman numerals followed by a prime sign to distinguish them from the clusters in Fig. 3. Bootstrap values higher than 50% are indicated. The horizontal bar represents 10% differences in amino acids. Bc, B. cereus; Bw, B. weihenstephanensis; Bm, B. mycoides; Ba, B. anthracis.
Figure 3 shows the phylogenetic relationships between the flagellin amino acid sequences for all 106 bacterial species and strains studied. Briefly, whereas some species, namely, B. halodurans, B. subtilis, and B. anthracis, form specific branches that are clearly well separated from others, the other four species, all in the B. cereus sensu lato group, B. mycoides, B. weihenstephanensis, B. cereus, and B. thuringiensis, do not form four specific clusters as might have been expected. Rather, strains from any of these four species are placed side by side with strains from other species. The distribution of strains is not species specific. At least eight distinct clusters, clusters I to VIII, are revealed. With the exceptions of the homogenous B. anthracis cluster (cluster I), and cluster IV, which contains a subset of B. thuringiensis serovars, all other clusters comprised strains from at least two, and sometimes three, species of the B. cereus sensu lato group. It is worth noting, however, that B. thuringiensis serovars from same H serotypes are most often found clustered together, with exceptions.
The positioning of the species, and strains within species, was examined in detail. Bacillus halodurans and B. subtilis are each well separated from all 104 B. cereus sensu lato strains. The three B. anthracis strains share identical amino acid sequences, are undistinguishable, and form the single homogeneous cluster, cluster I, exclusive of other species. Two of the three B. mycoides strains, ATCC 6462 and BGSC 6A20, are grouped together, close to three B. weihenstephanensis strains, to form cluster III. The third B. mycoides strain, BGSC 6A13, is more distant and is found in cluster V. The 11 B. weihenstephanensis strains are separated in eight different locations, six different clusters, on the phylogram. Three strains, WSBC10363, WSBC10295, and WSBC10365, are found in cluster III along with two B. mycoides strains. Five other B. weihenstephanensis strains, WSBC10067, WSBC10045, WSBC10090, WSBC10296, and CCM 4966 are scattered in five different clusters on the phylogram, clusters II, V, VI, VII, and VIII, with each strain close to B. thuringiensis strains. Two other B. weihenstephanensis strains, WSBC10001 and WSBC10204 (the type strain), share identical amino acid sequences, are undistinguishable, and are found outside the eight defined clusters. The last B. weihenstephanensis strain, CCM 4965, is also found outside the eight defined clusters, close to a B. thuringiensis strain. The five B. cereus strains are scattered in five different locations, four different clusters, on the phylogram. B. cereus ATCC 14579, B. cereus ATCC 10987, and B. cereus BGSC 6A18 are found in clusters II, V, and VI, respectively, each relatively close to B. thuringiensis serovars, namely, serovars entomocidus, malaysiensis, and coreanensis, respectively. B. cereus BGSC 6A17 is found in cluster VII, very close to B. thuringiensis serovar dakota, B. thuringiensis serovar colmeri, and B. thuringiensis subsp. wuhanensis (4), with which its flagellin shares nearly identical amino acid sequences. On the phylogram, they appear to be undistinguishable. They are close to B. weihenstephanensis WSBC 10296 and B. thuringiensis serovar galleria, B. thuringiensis serovar canadensis, and B. thuringiensis serovar aizawai. B. cereus E33L is found outside the eight defined clusters, grouped with B. thuringiensis serovar cameroun, with which its flagellin shares nearly identical amino acid sequences. On the phylogram, they appear to be undistinguishable. Both are close to B. thuringiensis serovar pirenaica. The B. thuringiensis strains are scattered throughout the phylogram, interspersed with B. weihenstephanensis, B. cereus, and B. mycoides strains. B. thuringiensis strains are absent in clusters I and III. Most B. thuringiensis serovars, which belong to same H serotype, are grouped together. Three H3 serovars, serovars kurstaki (H3a,3b,3c), alesti (H3a,3c), and fukuokaensis (H3a,3d,3e), are found closely grouped together, in cluster II. Perhaps surprisingly at first glance, the B. weihenstephanensis WSBC10067 flagellin amino acid sequence shares extensive identities with the ones of the three H3 serovars and is grouped with them. The fourth H3 serovar, B. thuringiensis serovar sumiyoshiensis (H3a,3d), is located elsewhere on the phylogram, relatively distant from the three other H3 serovars, outside the eight defined clusters. The two H4 serovars, serovars sotto (H4a,4b) and kenya (H4a,4c), are found closely grouped together, in cluster IV. The two H18 serovars, serovars kumamotoensis (H18a,18b) and yosoo (H18a,18c), are grouped together along with some B. thuringiensis serovars, serovars muju, novosibirsk, and higo, in cluster VI. The two H11 serovars, B. thuringiensis serovars toumanoffi (H11a,11b) and kyushuensis (H11a,11c), are found closely grouped together, outside the eight defined clusters. They share nearly identical flagellin amino acid sequences, with only four differences. On the phylogram, they appear to be undistinguishable. The two H5 serovars, B. thuringiensis serovars galleriae (H5a,5b) and canadensis (H5a,5c), are found closely grouped together along with B. weihenstephanensis WSBC10296, B. thuringiensis serovar dakota, B. thuringiensis serovar colmeri, B. cereus BGSC 6A17, B. thuringiensis subsp. wuhanensis, and B. thuringiensis serovar aizawai in cluster VII. The two H10 serovars, B. thuringiensis serovars londrina (H10a,10c) and darmstadiensis (H10a,10b), are found closely grouped together along with B. thuringiensis serovar andaluciensis in cluster VII. Their flagellins share several identical amino acid sequences, with some differences. On the phylogram, all three appear to be undistinguishable. The two H28 serovars, B. thuringiensis serovars monterrey (H28a,28b) and jegathesan (H28a,28c), are grouped together in cluster VII. The two H20 serovars, B. thuringiensis serovars yunnanensis (H20a,20b) and pondicheriensis (H20a,20c), are grouped together in cluster VIII. Their flagellins share several identical amino acid sequences, with nine differences. On the phylogram, both appear to be undistinguishable. The three H8 serovars are found in cluster VIII. B. thuringiensis serovars morrisoni (H8a,8b) and ostriniae (H8a,8c), are found closely grouped together. Their flagellins share several identical amino acid sequences with six differences. On the phylogram, both appear to be undistinguishable. The third H8 serovar, B. thuringiensis serovar nigeriensis (H8b,8d), is located in the vicinity, close to B. thuringiensis serovars iberica, xiaguangiensis, navarrensis, neoleonensis, and seoulensis. All three H8 serovars are close to B. thuringiensis serovars rongseni, azorensis, sooncheon, and zhaodongensis. The two H24 serovars, B. thuringiensis serovars novosibirsk (H24a,24c) and neoleonensis (H24a,24b), are not grouped together but, rather, are found relatively distant from each other in clusters VI and VIII, respectively.
Several different H serotypes are grouped together and appear to be undistinguishable. This is the case for B. thuringiensis serovar kim, B. thuringiensis serovar argentinensis, B. thuringiensis serovar asturiensis, and B. thuringiensis serovar indiana in cluster IV. Indeed, the last three subspecies share identical flagellin amino acid sequences. They also share identical nucleotide sequences. Likewise, B. thuringiensis serovar dakota, B. thuringiensis serovar colmeri, B. cereus BGSC 6A17, B. thuringiensis subsp. wuhanensis, B. thuringiensis serovar galleriae, and B. thuringiensis serovar canadensis are grouped together in cluster VII. B. thuringiensis subsp. wuhanensis and B. thuringiensis serovar galleriae share identical flagellin amino acid sequences. They also share identical nucleotide sequences. The B. thuringiensis serovar canadensis flagellin protein differs from these two by three amino acids. The flagellin amino acid sequences for the others are more divergent. As indicated above, B. thuringiensis serovar londrina, B. thuringiensis serovar andaluciensis, and B. thuringiensis serovar darmstadiensis are grouped together and appear to be undistinguishable. Their flagellin proteins, however, differ in some positions. Likewise, B. cereus E33L and B. thuringiensis serovar cameroun are grouped together and appear to be undistinguishable. Their flagellin proteins, however, differ in some positions. This is also true for a subgroup formed by B. thuringiensis serovar navarrensis, B. thuringiensis serovar neoleonensis, and B. thuringiensis serovar seoulensis in cluster VIII. Finally, B. thuringiensis serovar rongseni, B. thuringiensis serovar azorensis, and B. thuringiensis serovar sooncheon in cluster VIII are also grouped together and appear to be undistinguishable. B. thuringiensis serovar rongseni and B. thuringiensis serovar azorensis share identical flagellin amino acid sequences. They also share identical nucleotide sequences. The B. thuringiensis serovar sooncheon flagellin protein differs by some amino acids.
Figure 4 shows the relationships between the flagellin highly variable central region amino acid sequences for all 106 bacterial species and strains studied. Its percentage of amino acid sequence divergence is up to threefold higher than the one shown in Fig. 3 with the entire flagellin amino acid sequence. Clearly, the central region can be used to obtain deeper levels of discrimination between serovars. Of the eight clusters revealed in Fig. 3, seven are present in Fig. 4. Cluster V′ is missing. In general, strains found in one cluster in Fig. 3 are found in the same cluster in Fig. 4, with exceptions. Here again, B. halodurans and B. subtilis form specific branches. All three B. anthracis strains are grouped together as cluster I′, which now appears as a subgroup of cluster IV′. Clusters VI′ and VIII′ contain only subsets of B. thuringiensis serovars. All other clusters comprise strains from at least two, and sometimes three, species of the B. cereus sensu lato group. Here also, B. thuringiensis serovars from the same H serotypes are often found clustered together, with exceptions.
DISCUSSION
Flagellins from B. thuringiensis.
The B. thuringiensis H antigen classification is highly diverse, with at least 69 H serotypes and 82 serological varieties, serovars, reported as of 1999 (7). Flagellin, the bacterial filament's protein, encoded by the hag gene, is responsible for eliciting the immunological reaction. Presumably, different H serotypes, and different serovars, harbor different hag alleles encoding different flagellin amino acid sequences. Although useful as a classification method, H serotyping suffers from some limitations. It is limited to a small number of laboratories in possession of all antisera. Several B. thuringiensis strains are not amenable to H serotyping because either they are nonmotile or they autoagglutinate. Also, certainly as important, no genetic relatednesses among B. thuringiensis H serotypes can be established. In our work, cloning and sequencing of the flagellin alleles has revealed levels of flagellin protein homology among B. thuringiensis serovars and allowed the grouping of serovars. In most cases, different serovars had different flagellin amino acid sequences, with exceptions. Likewise, highly related serovars from the same H serotype were grouped together, with exceptions. Indeed, and rather unexpectedly, some different B. thuringiensis serovars shared identical flagellin amino acid sequences. This was the cases for B. thuringiensis serovar rongseni and B. thuringiensis serovar azorensis, for B. thuringiensis subsp. wuhanensis and B. thuringiensis serovar galleriae, and for B. thuringiensis serovar argentinensis, B. thuringiensis serovar asturiensis, and B. thuringiensis serovar indiana. Additional work is needed to determine why they all belong to different serovars while sharing identical flagellin amino acid sequences. In addition, and perhaps surprisingly, some different serovars from the same H serotype carry flagellins with sufficiently different amino acid sequences as to be located in distant clusters. This was the case for serovars in the H3 serotype, with B. thuringiensis serovar kurstaki, B. thuringiensis serovar alesti, and B. thuringiensis serovar fukuokaensis all grouped into cluster II, whereas B. thuringiensis serovar sumiyoshiensis was ungrouped, and for the two serovars in the H24 serotype, with B. thuringiensis serovar novosibirsk in cluster VI but B. thuringiensis serovar neoleonensis in cluster VIII. Here also, additional work is needed to determine why serovars from the same H serotype may be located in distant clusters. As mentioned above, most of the 96 bacterial strains assayed yielded a single amplicon. A small number of strains yielded two or more amplicons. The number of hag alleles per strain has not been determined. Certainly, should more than one hag allele be present in some strains, silent or expressed, the positioning of the bacterial strains on either phylogenetic tree would be allele dependent, whereas proper identification of the H serotype is dependent on the expressed allele. We are following up on this by studying the copy number of the hag alleles, and possible sequence diversity, at the intrastrain level for selected strains.
Two unserotypeable B. thuringiensis strains, B. thuringiensis subsp. wuhanensis and B. thuringiensis M15, deserve additional comments. B. thuringiensis subsp. wuhanensis is nonmotile and possesses no flagellum (5) and, hence, is not amenable to serotyping but was shown here to harbor a hag gene. Surprisingly enough, its sequence is identical to the one of B. thuringiensis serovar galleriae. Additional work is needed to determine why B. thuringiensis subsp. wuhanensis harbors no flagellum. This finding also opens the door to further comparisons between B. thuringiensis subsp. wuhanensis and B. thuringiensis serovar galleriae using markers other than flagellin to determine the exact relationship between both strains. Conversely, B. thuringiensis M15 possesses flagella. However, because it is an autoagglutinable strain, it is not amenable to serotyping. We have shown here that this strain harbors a hag gene whose protein shares extensive amino acid sequence identities with the one of B. thuringiensis serovar konkukian, which belongs to serotype H34. It would be interesting to conduct immunological assays of B. thuringiensis M15, perhaps with purified filaments, to determine its H serotype.
Flagellins from the B. cereus sensu lato group.
The addition of B. cereus sensu lato group species to the B. thuringiensis H serotypes shows the relationships between flagellin proteins among these different related species. All five B. cereus strains used in this study did not cluster together but, rather, were scattered throughout both phylograms and were located next to B. thuringiensis strains. This is perhaps better exemplified by the close proximity of B. cereus BGSC 6A17, B. thuringiensis serovar dakota, and B. thuringiensis serovar colmeri in cluster VII or by B. cereus E33L and B. thuringiensis serovar cameroun.
It has been known for at least a decade that B. thuringiensis and B. cereus share an extremely high frequency of common flagellar antigens (9, 18). Whether these B. cereus strains were derived from their respective neighboring B. thuringiensis strains, or conversely, or perhaps from another related strain not included here, or a more ancient one, or whether they have always been B. cereus strains is unknown and a challenging question worthy of further investigation. Our work also indicates that because they share extensive flagellin amino acid sequence identities, some B. thuringiensis, B. weihenstephanensis, and B. mycoides strains may also share a high frequency of common flagellar antigens. Immunological assays are yet to confirm this. The story with B. anthracis is a different one. First, all three B. anthracis strains studied here share identical flagellin amino acid sequences with no apparent point mutation in the corresponding, potentially functional, hag alleles, yet B. anthracis is a nonmotile species in the B. cereus sensu lato group. It has previously been reported that four essential proteins in the flagellar gene cluster contain point mutations and subsequent frameshifts, rendering the flagellum nonfunctional (12, 13). Second, all three B. anthracis strains were grouped together in either phylogram to form a distinct cluster, somehow suggesting that the B. anthracis species may be distinct from other species in the B. cereus sensu lato group. The present work on B. anthracis, however, was limited by the number of sequences publicly available from the GenBank database. It would be interesting to add more flagellin sequences from other B. anthracis strains as they become available to determine whether they all group within cluster I (or I′), hence establishing the flagellin sequence as a B. anthracis-discriminating criterion, or whether additional sequences may be scattered throughout either phylogram.
H serotyping is still the method of choice for the classification of B. thuringiensis strains today. The approach presented here benefits from several major improvements over classical H serotyping: it is not limited by the possession of all, or any, antisera, and phylogenetic relationships can be revealed among H serotypes and also among bacteria that either are not amenable to H serotyping or have not yet been serotyped. It is now possible to determine the nucleotide sequence of the hag gene from a novel bacterial strain and position it on the phylogenetic tree, giving an indication as to its possible H serotype. It is also possible to build on this study by designing primer pairs for the screening of either specific H serotypes or specific clusters revealed here among B. thuringiensis strain collections.
Supplementary Material
Acknowledgments
We thank Monika Ehling-Schulz from the Microbial Ecology Group, Technical University of Munich, Freising, Germany, for providing us with B. weihenstephanensis DNA. We also thank Huguette de Barjac, Marguerite M. Lecadet, Emmanuel Frachon, and Jean-François Charles, formerly from the International Entomopathogenic Bacillus Centre, Institut Pasteur, Paris, France; Daniel R. Zeigler from the Bacillus Genetic Stock Center, The Ohio State University, Columbus, OH; and Zdena Pacova from the Czech Collection of Microorganisms, Masaryk University, Brno, Czech Republic, for providing us with bacterial strains. We also thank Brahim Soufiane for isolating total DNA from some Bacillus strains.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Birnboim, H. C., and J. Doly. 1979. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 7:1513-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Claus, D., and R. C. W. Berkeley. 1986. The genus Bacillus, p. 1105-1139. In P. H. A. Sneath, N. S. Mair, M. E. Sharpe, and J. G. Holt (ed.), Bergey's manual of systematic bacteriology, vol. 2. Williams & Wilkins, Baltimore, Md. [Google Scholar]
- 3.de Barjac, H., and A. Bonnefoi. 1962. Essai de classification biochimique et sérologique de 24 souches de Bacillus du type B. thuringiensis. Entomophaga 7:5-31. [Google Scholar]
- 4.Entomogenous Organism Research Group. 1976. Bacillus thuringiensis “140,” a new variety without flagellum. Acta Microbiol. Sin. 16:12-16. [Google Scholar]
- 5.Glare, T. R., and M. O'Callaghan. 2000. Bacillus thuringiensis: biology, ecology and safety. John Wiley & Sons, Ltd., Toronto, Canada.
- 6.Höfte, H., and H. R. Whiteley. 1989. Insecticidal crystal proteins of Bacillus thuringiensis. Microbiol. Rev. 53:242-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lecadet, M.-M., E. Frachon, V. C. Dumanoir, H. Ripouteau, S. Hamon, P. Laurent, and I. Thiéry. 1999. Updating the H-antigen classification of Bacillus thuringiensis. J. Appl. Microbiol. 86:660-672. [DOI] [PubMed] [Google Scholar]
- 8.Macnab, R. M. 1992. Genetics and biogenesis of bacterial flagella. Annu. Rev. Genet. 26:131-158. [DOI] [PubMed] [Google Scholar]
- 9.Murakami, T., K. Hiraoka, T. Mikami, T. Matsumoto, S. Katagiri, K. Shinagawa, and M. Suzuki. 1993. Analysis of common antigen of flagella in Bacillus cereus and Bacillus thuringiensis. FEMS Microbiol. Lett. 107:179-184. [DOI] [PubMed] [Google Scholar]
- 10.Page, R. D. M. 1996. TREEVIEW: an application to display phylogenetic trees on personal computers. Comp. Appl. Biosci. 12:357-358. [DOI] [PubMed] [Google Scholar]
- 11.Page, R. D. M. 2000. TreeView—tree drawing software for Apple Macintosh and Windows. http://taxonomy.zoology.gla.ac.uk/rod/treeview.html.
- 12.Rasko, D. A., J. Ravel, O. A. Økstad, E. Helgason, R. Z. Cer, L. Jiang, K. A. Shores, D. E. Fouts, N. J. Tourasse, S. V. Angiuoli, J. Kolonay, W. C. Nelson, A.-B. Kolstø, C. M. Fraser, and T. D. Read. 2004. The genome sequence of Bacillus cereus ATCC 10987 reveals metabolic adaptations and a large plasmid related to Bacillus anthracis pXO1. Nucleic Acids Res. 32:977-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Read, T. D., S. N. Peterson., N. Tourasse, L. W. Baillie, I. T. Paulsen, K. E. Nelson, H. Tettelin, D. E. Fouts, J. A. Eisen, S. R. Gill, E. K. Holtzapple, O. A. Økstad, E. Helgason, J. Rilstone, M. Wu, J. F. Kolonay, M. J. Beanan, R. J. Dodson, L. M. Brinkac, M. Gwinn, R. T. DeBoy, R. Madpu, S. C. Daugherty, A. S. Durkin, D. H. Haft, W. C. Nelson, J. D. Peterson, M. Pop, H. M. Khouri, D. Radune, J. L. Benton, Y. Mahamoud, L. Jiang, I. R. Hance, J. F. Weidman, K. J. Berry, R. D. Plaut, A. M. Wolf, K. L. Watkins, W. C. Nierman, A. Hazen, R. Cline, C. Redmond, J. E. Thwaite, O. White, S. L. Salzberg, B. Thomason, A. M. Friedlander, T. M. Koehler, P. C. Hanna, A. B. Kolstø, and C. M. Fraser. 2003. The genome sequence of Bacillus anthracis Ames and comparison to closely related bacteria. Nature 423:81-86. [DOI] [PubMed] [Google Scholar]
- 14.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 15.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 16.Sanchis, V., J. Chafaux, and D. Lereclus. 1996. Amélioration biotechnologique de Bacillus thuringiensis: les enjeux et les risques. Ann. Inst. Pasteur Actual. 7:271-284. [Google Scholar]
- 17.Sanger, F., S. Nicklen, and A. R. Coulson. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 74:5463-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shisa, N., N. Wasano, A. Ohgushi, D.-H. Lee, and M. Ohba. 2002. Extremely high frequency of common flagellar antigens between Bacillus thuringiensis and Bacillus cereus. FEMS Microbiol. Lett. 213:93-96. [DOI] [PubMed] [Google Scholar]
- 19.Stephen, D., C. Jones, and J. P. Schofield. 1990. A rapid method for isolating high quality plasmid DNA suitable for DNA sequencing. Nucleic Acids Res. 18:7463-7464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. Clustal W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu, D., and J.-C. Côté. 2003. Phylogenetic relationships between Bacillus species and related genera inferred from comparison of 3′ end 16S rDNA and 5′ end 16S-23S ITS nucleotide sequences. Int. J. Syst. Evol. Microbiol. 53:695-704. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.