Abstract
Many pathogenic yeast species are asexual and therefore not involved in intra- or interspecies mating. However, high-frequency transfer of plasmid DNA was observed when pathogenic and food-borne yeasts were grown together. This property could play a crucial role in the spread of virulence and drug resistance factors among yeasts.
For millennia, yeasts belonging to the genus Saccharomyces have been widely used in preparation of food and beverages. Historically, natural isolates of Saccharomyces species have been considered to be harmless, nonpathogenic saprophytes. However, in the past few decades these yeasts have been found to be causative agents of human infections (18, 19). How these originally inoffensive yeasts evolved into pathogenic forms is not understood. Paradoxically, one of the reasons behind the emergence of novel human fungal pathogens is the success of modern medical care, which leads to the survival of immunocompromised patients (23).
Candida glabrata, formerly classified as Torulopsis glabrata, can be found as a commensal yeast in healthy individuals, but it is also a human opportunistic pathogen (9). C. glabrata is a close relative of Saccharomyces species, and recently it was added together with other Saccharomyces yeasts to the joint Saccharomyces clade (13). C. glabrata diverged from the common ancestor of Saccharomyces sensu stricto species after whole-genome duplication occurred, and the C. glabrata genome subsequently underwent reductive evolution, possibly associated with the emergence of this yeast as a human pathogen (6, 26). The widespread use of immunosuppressive therapy, chemo- and radiotherapy for cancer, and broad-spectrum antimycotic therapy has increased the frequency of both systemic and mucosal infections caused by C. glabrata (7). Candida species are some of the most common bloodstream pathogens in the United States, and a substantial shift in the epidemiology of hematogenous candidiasis to non-Candida albicans species, such as C. glabrata, has been observed recently. Fluconazole treatment may have played a role in this shift (1). A nationwide survey of candidemia from 1991 to 2000 in Swiss tertiary care hospitals showed that C. glabrata was the major non-C. albicans cause of candidiasis, with an incidence of 15% (14). C. glabrata was the second most frequently occurring fungus and the dominant non-C. albicans species causing candidal vulvovaginitis in pregnant women in China (25). In the Flemish population in Belgium the incidence of infection placed C. glabrata third among the organisms identified in symptomatic vulvovaginal candidiasis patients after fluconazole treatment (5). Vulvovaginal candidiasis is an example of a common mucosal infection that also occurs in healthy, immunocompetent women.
Could intra- and interspecies exchange of genetic material be responsible, at least in part, for the spread of virulence factors from pathogenic species to nonpathogenic species? It has been proven previously that in experimental populations Saccharomyces species can form hybrids and thereby create novel combinations of genetic material (15). These results suggest that Saccharomyces yeasts have the potential to exchange genetic material in nature, and this view is supported by the fact that several natural isolates are indeed hybrids between different species (8, 16). It may well be that some Saccharomyces isolates that are human pathogens are naturally occurring interspecific hybrids (4, 17). However, mating per se has not been observed yet for C. glabrata (12), and therefore it is not thought that C. glabrata can be involved in interspecies mating. To evaluate the possibility of gene transfer between pathogenic and food-borne yeasts species, we studied possible plasmid transfer between C. glabrata and the Saccharomyces species S. cerevisiae and S. bayanus.
Plasmid transfer from S. cerevisiae to C. glabrata.
To develop a potential acceptor of plasmids, C. glabrata type strain Y475 was mutagenized with ethyl methanesulfonate as described previously (15). Auxotrophic ura3 mutants were selected on plates containing 5-fluoroorotic acid (5-FOA) at a concentration of 1.00 mg/ml. 5-FOA-resistant mutants were tested for growth on a minimal medium with uracil, on a minimal medium without uracil, and on a synthetic complex medium without uracil, and they were checked for the ability to revert using standard yeast techniques. Only nonreverting strains were used in experiments. Plasmids to be used in the plasmid transfer experiment (P158, P159, and P199) (Table 1) were first transformed into C. glabrata ura3 mutants (Table 2). Only the C. glabrata mutant strains (Y718 and Y719) which were able to grow under selective conditions when they were transformed by plasmids were used as plasmid recipients in the plasmid transfer experiments. S. cerevisiae strain Y391 was used as a potential donor of genetic material in the plasmid transfer experiment and was transformed for this purpose with the P158, P159, and P199 plasmids. Overnight cultures of C. glabrata ura3 mutant strains Y718 and Y719 were mixed with the same volume (300 μl) of overnight cultures of S. cerevisiae plasmid donor strains Y745, Y746, and Y747. On YPD plates 5-μl portions of mixed cultures were spotted and replica plated onto the selective minimal medium the following day. Neither the S. cerevisiae plasmid donor nor the C. glabrata plasmid acceptor could grow on minimal medium plates without uracil (Fig. 1), but after a few days small colonies appeared in the “mixed” spots. The frequencies of plasmid transfer between plasmid donor strains and plasmid recipient strains were calculated by determining the fractions of the plasmid recipient strain clones possessing the plasmids based on all of the plasmid recipient strain colonies plated. In the case of P199 the frequency was 1.2 × 10−4. Several colonies (referred to below as “resulting strains”) (Table 2) were checked for their karyotype patterns, the presence of exchanged plasmid, and the ability to lose the acquired plasmid. Chromosomes were prepared as described by Petersen et al. (20) and were separated by pulsed-field gel electrophoresis using a five-step program, as follows: step 1, 240-s pulse for 6 h; step 2, 160-s pulse for 13 h; step 3, 120-s pulse for 10 h; step 4, 90-s pulse for 10 h; and step 5, 60-s pulse for 3 h. The angle was 60°, and the potential was 150 V (4.5 V/cm). All but one of the resulting strains exhibited the same karyotype pattern as the C. glabrata plasmid recipient (Fig. 2A). Thus, C. glabrata cells acquired the plasmid from the donor S. cerevisiae strain. The karyotype of Y732 displayed both S. cerevisiae and C. glabrata sets of chromosome bands, but this strain was later shown to consist of cells of both parents. The presence of a plasmid in C. glabrata recipient strains Y718 and Y719 was confirmed by PCR with total DNA isolated from strains resulting from plasmid transfer (designated strains Y727 to Y732) using primers Ampr12.01 (5′-CAA ATA TGT ATC CGC TCA TGA GAC A-3′) and Ampr12.02 (5′-GTA AAC TTG GTC TGA CAG TTA CC-3′). For amplification of the plasmid ampicillin resistance-encoding gene, the following conditions were used: 94°C of initial denaturation for 3 min and then 35 cycles of 30 s at 94°C, 1 min at 55°C, and 1.5 min at 72°C, followed by 72°C for 3 min. Total DNA from Y718 and Y719 served as a negative control. The possibility of plasmid loss from strains resulting from plasmid transfer was tested by growing these strains under nonselective conditions. The resulting strains were grown overnight in liquid YPD medium, and then the cells were spread on solid YPD medium. After 3 days colonies were replica plated onto minimal medium without uracil. Only the colonies which had not lost the plasmid could grow under selective conditions. The percentage of the P199 plasmid lost from C. glabrata strain Y729 was 84%. In conclusion, C. glabrata indeed acquired the plasmid at a relatively high frequency when it was grown in the presence of S. cerevisiae cells.
TABLE 1.
Shuttle plasmids (E. coli-yeast) used in the experiments and their characteristics
TABLE 2.
Strains used in this study and their characteristics
| Strain | Species | Genotype or origin | Reference or commenta |
|---|---|---|---|
| Y244 | S. bayanus | MATα ura3 | 15 |
| Y391 | S. cerevisiae | MATα ura3 trp1 gal2 | M1-2B from T. Nilsson-Tillgren |
| Y475 | C. glabrata | Asexual prototroph | Type strain NRRL Y-65 |
| Y718 | C. glabrata | ura3 | EMS mutagenesis of Y475 |
| Y719 | C. glabrata | ura3 | EMS mutagenesis of Y475 |
| Y727 | C. glabrata | Y718 with P158 obtained by plasmid transfer (from Y745) | |
| Y728 | C. glabrata | Y718 with P159 obtained by plasmid transfer (from Y746) | |
| Y729 | C. glabrata | Y718 with P199 obtained by plasmid transfer (from Y747) | |
| Y730 | C. glabrata | Y719 with P158 obtained by plasmid transfer (from Y745) | |
| Y731 | C. glabrata | Y719 with P159 obtained by plasmid transfer (from Y746) | |
| Y732 | C. glabrata | Y719 with P199 obtained by plasmid transfer (from Y747) | |
| Y745 | S. cerevisiae | Y391 transformed with P158 | Plasmid donor |
| Y746 | S. cerevisiae | Y391 transformed with P159 | Plasmid donor |
| Y747 | S. cerevisiae | Y391 transformed with P199 | Plasmid donor |
| Y760 | C. glabrata | his3 | From type strain BG98, from B. Cormack |
| Y765 | C. glabrata | his3 ura3 | EMS mutagenesis of Y760 |
| Y781 | C. glabrata | Y765 transformed with P159 | Plasmid donor |
| Y782 | C. glabrata | Y765 transformed with P159 | Plasmid donor |
| Y784 | C. glabrata | Y765 transformed with P157 | Plasmid donor |
| Y832 | S. bayanus | Y244 with P159 obtained by plasmid transfer (from Y782) | |
| Y833 | S. bayanus | Y244 with P159 obtained by plasmid transfer (from Y782) | |
| Y834 | S. bayanus | Y244 with P159 obtained by plasmid transfer (from Y782) | |
| Y835 | S. bayanus | Y244 with P159 obtained by plasmid transfer (from Y782) | |
| Y836 | S. bayanus | Y244 with P159 obtained by plasmid transfer (from Y782) | |
| Y837 | S. bayanus | Y244 with P159 obtained by plasmid transfer (from Y781) | |
| Y840 | S. bayanus | Y244 with P157 obtained by plasmid transfer (from Y784) | |
| Y841 | S. bayanus | Y244 with P157 obtained by plasmid transfer (from Y784) |
EMS, ethyl methanesulfonate.
FIG. 1.
C. glabrata auxotrophic ura3 strain Y718 was grown together with S. cerevisiae strains Y745, Y746, and Y747 containing different plasmids carrying the URA3 gene on YPD plates. The colonies were replica plated onto the selective minimal medium the following day, and the images show the growth on the minimal medium after 1 week. Four controls (parental strains Y745, Y746, Y747, and Y718) were also grown on the minimal medium.
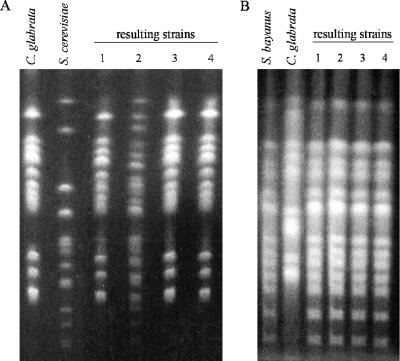
FIG. 2.
(A) Karyotypes of C. glabrata recipient strains Y718 and Y719, S. cerevisiae donor strains Y745, Y746, and Y747, and the four resulting strains (lane 1, Y729; lane 2, Y732; lane 3, Y719/Y606 no. 3; lane 4, Y719/Y748 no. 4). All but one strain had the same karyotype pattern as the C. glabrata plasmid recipient. The karyotype of resulting strain Y732 (lane 2) had both S. cerevisiae and C. glabrata chromosome bands. (B) Karyotypes of S. bayanus recipient strain Y244, C. glabrata donor strains Y781, Y782, and Y784, and the four resulting prototrophs (lane 1, Y837; lane 2, Y832; lane 3, Y840; lane 4, Y841). All four resulting strains had the same karyotype pattern as the recipient strain.
Plasmid transfer from C. glabrata to S. bayanus.
C. glabrata strain Y760 was mutagenized with ethyl methanesulfonate and selected with 5-FOA. Double mutant Y765 was transformed with URA3 gene-containing plasmid P157 (Y784) or P159 (Y781 and Y782) and was used as a C. glabrata donor of genetic material (Table 2). A food-borne yeast species belonging to the Saccharomyces sensu stricto group, S. bayanus (Y244), was chosen as the potential plasmid acceptor. Plasmid transfer experiments were carried out as described above, and prototrophic strains were isolated. Plasmid transfer frequencies were calculated; for plasmid P157 the transfer frequency was 2.2 × 10−4, and for plasmid P159 the transfer frequency was 1.6 × 10−4. For several strains chromosomes were separated by pulsed-field gel electrophoresis using the program described above, and all of the strains tested exhibited the S. bayanus chromosomal pattern (Fig. 2B). Thus, S. bayanus cells acquired the plasmid from the C. glabrata donor strain. The presence of the plasmid in S. bayanus plasmid recipient strain Y244 was also confirmed by PCR when plasmids were first isolated from the strains and rescued in Escherichia coli strain XL-1 Blue (laboratory designation, P311; Stratagene) (24), and then the plasmid tetracycline resistance-encoding gene was amplified with gene-specific primers Tetr11.27 (5′-AGT GCC ACC TGA CGT CTA AGA-3′) and Tetr11.28 (5′-GTT TGC GCA TTC ACA GTT CTC C-3′). The following conditions were used for the PCR: 94°C of initial denaturation for 4 min and then 35 cycles of 40 s at 94°C, 45 s at 54°C, and 2.5 min at 72°C, followed by 72°C for 5 min. In conclusion, S. bayanus also acquired the plasmid when it was grown in the presence of C. glabrata cells.
Concluding remarks.
Although sexual activity (zygote, G1 arrest) between C. glabrata and S. cerevisiae cells has not been observed previously, we observed a relatively high frequency of transfer of genetic material between these yeast species. The mechanism(s) for this transfer is not clear. Previously, DNA transfer between bacteria (E. coli) and yeasts (S. cerevisiae and S. kluyveri) was reported as transkingdom conjugation (10, 11). In our experiments recipient species could have picked up plasmid molecules released by the donor strains through cell lysis, or the plasmid transfer could have resulted from cytoduction. In addition, despite the “asexual” nature of C. glabrata, a potential cryptic sexual life cycle (27) could promote rare interspecies mating. The transfer observed could allow the spread of virulence factors and resistance to medical drugs even between distantly related yeast species and could probably help in the “transformation” of harmless saprophytes into potential causative agents of human infections.
Acknowledgments
We thank Jørgen Stenderup for his interest in this work.
REFERENCES
- 1.Abi-Said, D., E. Anaissie, O. Uzun, I. Raad, H. Pinzcowski, and S. Vartivarian. 1997. The epidemiology of hematogenous candidiasis caused by different Candida species. Clin. Infect. Dis. 24:1122-1128. [DOI] [PubMed] [Google Scholar]
- 2.Botstein, D., S. C. Falco, S. E. Stewart, M. Brennan, S. Scherer, D. T. Stinchcomb, K. Struhl, and R. W. Davis. 1979. Sterile host yeasts (SHY): a eukaryotic system of biological containment for recombinant DNA experiments. Gene 8:17-24. [DOI] [PubMed] [Google Scholar]
- 3.Botstein, D., and R. W. Davis. 1982. Principles and practice of recombinant DNA research with yeast, p. 607-636. In J. N. Strathan, E. W. Jones, and J. R. Broach (ed.), The molecular biology of the yeast Saccharomyces. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 4.Clemons, K. V., P. Park, J. H. McCusker, M. J. McCullough, R. W. Davis, and D. A. Stevens. 1997. Application of DNA typing methods and genetic analysis to epidemiology and taxonomy of Saccharomyces isolates. J. Clin. Microbiol. 35:1822-1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Vos, M. M., M. Cuenca-Estrella, T. Boekhout, B. Theelen, N. Matthijs, T. Bauters, H. Nailis, M. A. Dhont, J. L. Rodriguez-Tudela, and H. J. Nelis. 2005. Vulvovaginal candidiasis in a Flemish patient population. Clin. Microbiol. Infect. 11:1005-1011. [DOI] [PubMed] [Google Scholar]
- 6.Dujon, B., D. Sherman, G. Fischer, P. Durrens, S. Casaregola, I. Lafontaine, J. De Montigny, C. Marck, C. Neuveglise, E. Talla, N. Goffard, L. Frangeul, M. Aigle, V. Anthouard, A. Babour, V. Barbe, S. Barnay, S. Blanchin, J. M. Beckerich, E. Beyne, C. Bleykasten, A. Boisrame, J. Boyer, L. Cattolico, F. Confanioleri, A. De Daruvar, L. Despons, E. Fabre, C. Fairhead, H. Ferry-Dumazet, A. Groppi, F. Hantraye, C. Hennequin, N. Jauniaux, P. Joyet, R. Kachouri, A. Kerrest, R. Koszul, M. Lemaire, I. Lesur, L. Ma, H. Muller, J. M. Nicaud, M. Nikolski, S. Oztas, O. Ozier-Kalogeropoulos, S. Pellenz, S. Potier, G. F. Richard, M. L. Straub, A. Suleau, D. Swennen, F. Tekaia, M. Wesolowski-Louvel, E. Westhof, B. Wirth, M. Zeniou-Meyer, I. Zivanovic, M. Bolotin-Fukuhara, A. Thierry, C. Bouchier, B. Caudron, C. Scarpelli, C. Gaillardin, J. Weissenbach, P. Wincker, and J. L. Souciet. 2004. Genome evolution in yeasts. Nature 430:35-44.15229592 [Google Scholar]
- 7.Fidel, P. L., Jr., J. A. Vazquez, and J. D. Sobel. 1999. Candida glabrata: review of epidemiology, pathogenesis, and clinical disease with comparison to C. albicans. Clin. Microbiol. Rev. 12:80-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Groth, C., J. Hansen, and J. Piskur. 1999. A natural chimeric yeast containing genetic material from three species. Int. J. Syst. Bacteriol. 49:1933-1938. [DOI] [PubMed] [Google Scholar]
- 9.Haley, L. D. 1961. Yeasts of medical importance. Am. J. Clin. Pathol. 36:227-234. [DOI] [PubMed] [Google Scholar]
- 10.Heinemann, J. A., and G. F. Sprague, Jr. 1989. Bacterial conjugative plasmids mobilize DNA transfer between bacteria and yeast. Nature 340:205-209. [DOI] [PubMed] [Google Scholar]
- 11.Inomata, K., M. Nishikawa, and K. Yoshida. 1994. The yeast Saccharomyces kluyveri as a recipient eukaryote in transkingdom conjugation: behavior of transmitted plasmids in transconjugants. J. Bacteriol. 176:4770-4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaur, R., R. Domergue, M. L. Zupancic, and B. P. Cormack. 2005. A yeast by any other name: Candida glabrata and its interaction with the host. Curr. Opin. Microbiol. 8:378-384. [DOI] [PubMed] [Google Scholar]
- 13.Kurtzman, C. P., and C. J. Robnett. 1998. Identification and phylogeny of ascomycetous yeasts from analysis of nuclear large subunit (26S) ribosomal DNA partial sequences. Antonie Leeuwenhoek 73:331-371. [DOI] [PubMed] [Google Scholar]
- 14.Marchetti, O., J. Bille, U. Fluckiger, P. Eggimann, C. Ruef, J. Garbino, T. Calandra, M. P. Glauser, M. G. Tauber, and D. Pittet. 2004. Epidemiology of candidemia in Swiss tertiary care hospitals: secular trends, 1991-2000. Clin. Infect. Dis. 38:311-320. [DOI] [PubMed] [Google Scholar]
- 15.Marinoni, G., M. Manuel, R. F. Petersen, J. Hvidtfeldt, P. Sulo, and J. Piskur. 1999. Horizontal transfer of genetic material among Saccharomyces yeasts. J. Bacteriol. 181:6488-6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masneuf, I., J. Hansen, C. Groth, J. Piskur, and D. Dubourdieu. 1998. New hybrids between Saccharomyces sensu stricto yeast species found among wine and cider production strains. Appl. Environ. Microbiol. 64:3887-3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCullough, M. J., K. V. Clemons, J. H. McCusker, and D. A. Stevens. 1998. Intergenic transcribed spacer PCR ribotyping for differentiation of Saccharomyces species and interspecific hybrids. J. Clin. Microbiol. 36:1035-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCusker, J. H., K. V. Clemons, D. A. Stevens, and R. W. Davis. 1994. Genetic characterization of pathogenic Saccharomyces cerevisiae isolates. Genetics 136:1261-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nielsen, H., J. Stenderup, and B. Bruun. 1990. Fungemia with Saccharomycetaceae. Report of four cases and review of the literature. Scand. J. Infect. Dis. 22:581-584. [DOI] [PubMed] [Google Scholar]
- 20.Petersen, R. F., T. Nilsson-Tillgren, and J. Piskur. 1999. Karyotypes of Saccharomyces sensu lato species. Int. J. Syst. Bacteriol. 49:1925-1931. [DOI] [PubMed] [Google Scholar]
- 21.Rose, M. D., P. Novick, J. H. Thomas, D. Botstein, and G. R. Fink. 1987. A Saccharomyces cerevisiae genomic plasmid bank based on a centromere-containing shuttle vector. Gene 60:237-243. [DOI] [PubMed] [Google Scholar]
- 22.Sikorski, R. S., and P. Hieter. 1989. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Burik, J. A., and P. T. Magee. 2001. Aspects of fungal pathogenesis in humans. Annu. Rev. Microbiol. 55:743-772. [DOI] [PubMed] [Google Scholar]
- 24.Ward, A. C. 1990. Single-step purification of shuttle vectors from yeast for high frequency back-transformation into E. coli. Nucleic Acids Res. 18:5319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wenjin, Q., and S. Yifu. 2006. Epidemiological study on vaginal Candida glabrata isolated from pregnant women. Scand. J. Infect. Dis. 38:49-54. [DOI] [PubMed] [Google Scholar]
- 26.Wolfe, K. 2004. Evolutionary genomics: yeasts accelerate beyond BLAST. Curr. Biol. 14:R392-R394. [DOI] [PubMed] [Google Scholar]
- 27.Wong, S., M. A. Fares, W. Zimmermann, G. Butler, and K. H. Wolfe. 2003. Evidence from comparative genomics for a complete sexual cycle in the ‘asexual’ pathogenic yeast Candida glabrata. Genome. Biol. 4:R10-R19. [DOI] [PMC free article] [PubMed] [Google Scholar]