Abstract
We compared the robustness and discriminatory power of the enterobacterial repetitive intergenic consensus (ERIC) and random amplified polymorphic DNA (RAPD) fingerprinting methods for detecting cases of mixed Helicobacter pylori infection in Peruvian shantytown residents. H. pylori isolates from 63 participants were cultured, and five single colonies and a pool of additional colonies from each participant were analyzed by ERIC-PCR and by RAPD tests with four 10-nucleotide primers (one primer per reaction). There was 94% agreement between the ERIC and RAPD profiles in classifying sets of isolates as uniform versus closely related but not identical versus probably unrelated, indicating a high kappa statistic of 0.8942. Subtle differences in related ERIC or RAPD patterns likely reflect gene transfer between strains, recombination, and/or mutation, whereas markedly different patterns reflect infection by unrelated strains. At least half of infected shantytown residents seemed to carry more than one H. pylori strain, although in 19 of 31 persons, the strains were closely related. Three RAPD tests, each with a different primer, were needed to achieve the sensitivity of one ERIC test. ERIC-PCR constitutes a resource- and time-efficient method for H. pylori strain differentiation.
Helicobacter pylori is a genetically diverse gastric pathogen that chronically infects billions of people worldwide, typically beginning in infancy and lasting for decades. It is a major cause of peptic ulcers and an early risk factor for gastric cancer, one of the most frequently lethal malignancies globally (1, 2, 12, 13). Also important are H. pylori contributions to growth faltering in infants, susceptibility to diarrheal disease, and the development of iron deficiency anemia (5, 14, 17).
The random amplified polymorphic DNA (RAPD), or arbitrarily primed PCR, method has been valuable for studies of H. pylori genetic diversity and transmission (2, 3, 11, 16). This method entails PCR amplification with one or more oligonucleotide primers (one primer per amplification reaction) that do not strongly match particular sites in target bacterial genomes and the use of low-stringency conditions for primer-template annealing (21, 23). Most current RAPD protocols use primers that are just 10 nucleotides (nt) long, a legacy of the early 1990s when oligonucleotide synthesis was very costly and large oligonucleotide libraries were being screened to find primers that gave “good” RAPD profiles (2, 4, 7, 10, 21, 22, 23). Approximately 3 to 10 discrete DNA fragments are typically obtained per H. pylori strain with any such good primer (2), and each independent H. pylori isolate typically yields a fragment pattern that is reproducibly different from those of other isolates. Important to this method's success are (i) low- stringency conditions to allow priming of DNA synthesis wherever just a few bases at the primer's 3′ end match sequences in target DNA; (ii) amplification when pairs of such fortuitous binding sites are near each other (<∼3 kb apart) in genomic DNA; (iii) efficient continued amplification of products generated in the first rounds; and (iv) differences among strains in the distribution of such sites and, hence, of the arrays of products amplified from them. The arrays of PCR products obtained vary with the strain tested and thus distinguish individual strains from one another. Several longer primers that were first designed for gene-specific PCR from other species also gave robust and informative RAPD profiles in early studies (2, 21, 22). They have not been used much since then, however, in part because useful data had been obtained with 10-nt primers.
A cDNA fingerprinting strategy, developed initially for enteric species such as Escherichia coli and Salmonella enterica serovar Typhimurium, was based on the discovery of a family of repetitive sequences, two or more copies of which occur between many pairs of neighboring genes (8, 20). PCR with outward-facing 22-nt-long primers specific for these enterobacterial repetitive intergenic consensus (ERIC) sequences yielded arrays of products that also differed among strains, nominally because of differences in the underlying distribution and spacing of ERIC sequences (20). The conditions used for ERIC fingerprinting are only moderately stringent: annealing at 45 or 49°C in recent H. pylori studies (9, 18), whereas the calculated melting temperature for ERIC primers is ∼61°C. The low stringency used for H. pylori probably allows priming of DNA synthesis from fortuitously matched (non-ERIC) sites in genomic DNAs, much as in RAPD-PCR with longer primers.
The use of ERIC fingerprinting to distinguish unrelated Swedish H. pylori strains from one another (18) and for phylogenetic analyses of DNAs from several sources (9) led us to compare the robustness and discriminatory power of ERIC versus RAPD tests for detecting mixed infections with strains that are closely related genetically. We found that three RAPD tests, each with a different 10-nt primer, were needed to achieve the discriminatory power of one ERIC test and that at least half of infected shantytown residents carried more than one H. pylori strain.
MATERIALS AND METHODS
Bacterial strains, bacterial growth, and DNA extraction.
H. pylori strains were cultured from residents of Lima, Peru, area pueblo jovenes (shantytowns), either from string test samples (15, 19) (23 of 63 participants) or from gastric biopsy samples in cases of patients who had undergone upper gastrointestinal endoscopy (40 of 63 participants). All protocols related to the collection and analysis of these patient samples were approved by local human studies committees at each hospital, and all samples were collected with informed consent. Standard methods (3) were used for initial H. pylori isolation and subsequent routine culture on Difco brain heart infusion agar with 10% horse blood plus Dent Supplement (Oxoid, Lexena, KS) (10 mg of vancomycin, 5 mg of trimethoprim, 5 mg of cefsulodin, and 5 mg of amphotericin B per liter) to suppress growth of other gastric bacterial species in a microaerobic atmosphere (5% O2, 10% CO2, and 85% N2) at 37°C. H. pylori isolates were identified by their characteristic colony morphology, the appearance of the cells after Gram staining, and positive urease test results (16). Chromosomal DNA was extracted by using the QIAamp tissue kit (QIAGEN, Chatsworth, CA) and quantified and preserved according to methods described elsewhere (3). For each positive plate, DNA was extracted from five individual colonies and from a pool comprised of at least six additional colonies from the plate.
ERIC-PCR fingerprinting.
ERIC-PCR fingerprinting was carried out essentially as recommended previously (9, 18, 20) in a PTC-100 instrument (MJ Research, Inc.). The 20-μl reaction volumes contained 1× PCR buffer (Invitrogen), 2 mM MgCl2 (Invitrogen), 80 μM deoxynucleoside triphosphates (Invitrogen), 0.5 μM concentrations each of the forward and reverse primers, 2 U of Taq DNA polymerase (Invitrogen), 0.1 μg of bovine serum albumin (pH 7.0; ICN Biomedicals)/μl, the ERIC primers 5′-ATGTAAGCTCCTGGGGATTCAC-3′ and 5′-AAGTAAGTGACTGGGGTGAGCG-3′, and 5 ng of genomic DNA per reaction (after initial test results showed identical banding patterns with 5, 9, and 20 ng of DNA per reaction). The following cycling conditions were used: 5 min at 94°C for initial denaturation; 35 cycles of 30 s at 90°C, 1 min at 49°C, and 5 min at 70°C; and then a final extension for 10 min at 70°C. After PCR, 3 μl of gel loading buffer (0.1% bromophenol blue, 50% glycerol) was mixed with each amplified product, and 15 μl of the mixture was loaded onto a 1% agarose gel containing 0.5 μg ethidium bromide/μl in 1× TAE buffer (40 mM Tris-acetate, 1 mM EDTA [pH 8.0]). A 1-kb DNA ladder (Gibco-BRL, Gaithersburg, MD) was used as a size marker. The gels were photographed under UV light after electrophoresis to record results and visually compare isolates and thus test for diversity within each given patient. We did not rigorously compare isolates from different patients, which generally are not related to one another (2). Reproducibility was monitored when warranted by comparing the results of ERICs carried out with the same samples on different days.
RAPD fingerprinting.
RAPD fingerprinting was carried out essentially as recommended elsewhere (2) in a PTC-100 instrument. Each 25-μl volume contained 1× PCR buffer (Invitrogen), 4 mM MgCl2 (Invitrogen), 0.25 mM deoxynucleoside triphosphates (Invitrogen), 0.1 μg of bovine serum albumin/μl (pH 7.0; ICN Biomedicals), 1.2 U Taq DNA polymerase (Invitrogen), an 0.8 μM concentration of one of four RAPD primers, i.e., 1254 (5′-CCGCAGCCAA-3′), 1281 (5′-AACGCGCAAC-3′), 1283 (5′-GCGATCCCCA-3′), or 1290 (5′-GTGGATGCGA-3′), and 9 ng of genomic DNA (after control tests showed that the patterns obtained did not depend on DNA concentration in the range of 5 to 20 ng per 25-μl reaction). The following conditions were used for 45 cycles: 1 min at 94°C, 1 min at 36°C, and then 1 min at 72°C. Products were electrophoresed and photographed under UV light, as for the ERIC analyses.
Gel analysis and ERIC-RAPD comparison.
The uniqueness of each patient's fingerprint was assessed by comparing the banding patterns generated with DNAs from single colonies or pools of colonies and noting differences in band number and size. Pools and single colonies were scored as the “same” if they yielded identical profiles and scored as “similar or related” if just one band differed among profiles (see Fig. 1). Isolates with two or more band differences per seven bands scored on average were considered “different or unrelated.” ERIC designations were based on the single ERIC primer pair used. RAPD designations were based on four assays. Blind RAPD and ERIC comparisons were performed separately in order to ensure that there was no analyst (subjectivity) bias. The correlation of ERIC and RAPD data was analyzed by using a kappa statistic (SPSS, Chicago, IL).
FIG. 1.
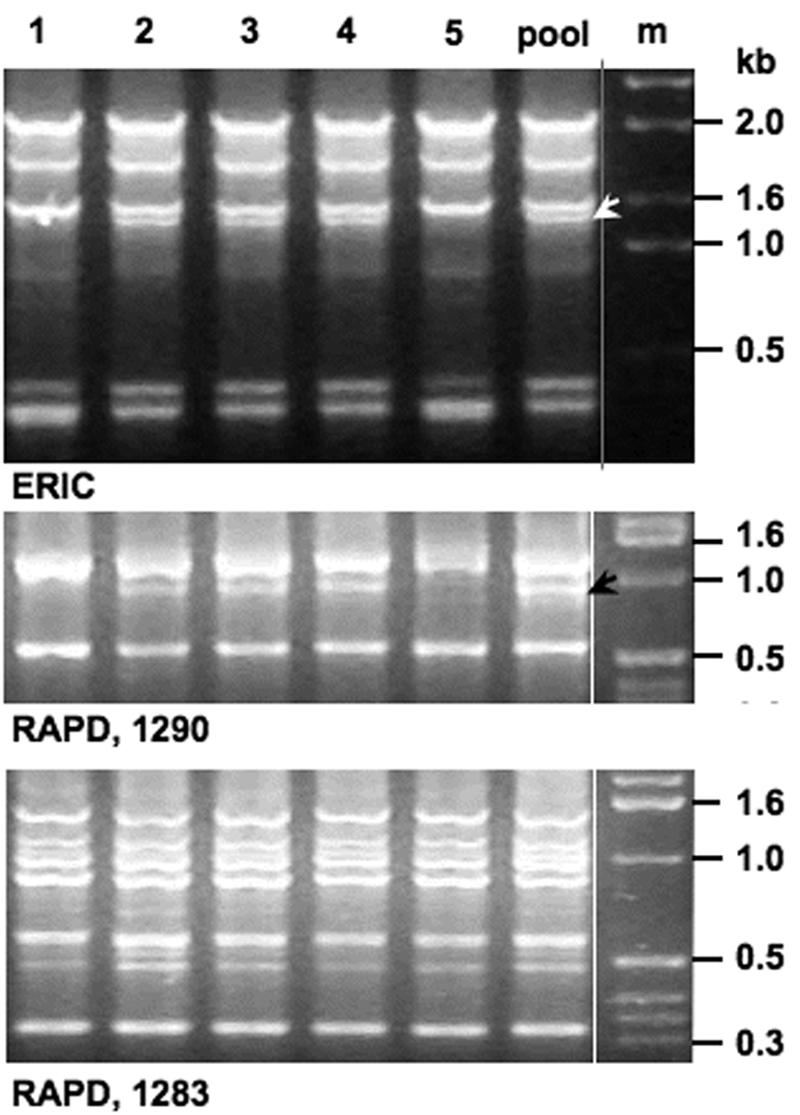
ERIC and two RAPD profiles representative of isolates from one person's mixed infection by closely related but nonidentical H. pylori strains (lanes 1 and 5 versus other lanes). As indicated by the arrows in ERIC and RAPD 1290 fingerprints, no more than one of the multiple bands observed in a given profile varied among these isolates; sometimes no difference was detectable (RAPD primer 1283).
RESULTS
Preliminary ERIC tests of 20 independent H. pylori isolates generated approximately 4 to 17 well-defined bands per isolate (9 bands on average), with each isolate yielding a unique profile of products. The set of four RAPD tests carried out on these same isolates yielded approximately 4 to 15 bands per isolate per reaction (7 bands on average); again, each isolate exhibited a unique profile. This ability to readily distinguish independent H. pylori isolates by ERIC or RAPD fingerprinting is in accord with previous results (2, 3, 9, 16, 18).
To assess genetic diversity among isolates recovered from a given patient and the power of the ERIC versus RAPD methods to distinguish closely related strains, we cultured H. pylori from 63 Peruvian residents of a Lima region shantytown and fingerprinted five representative single colony isolates and also pools of at least six additional colonies from each study participant. In 35 of 63 cases (56%), the ERIC profiles from each single colony isolate and the pool were identical. In 16 cases (25%), one or more colonies differed reproducibly from the others by one band, as illustrated in Fig. 1 (top frame). This suggested carriage of a mixture of related but not identical strains. In the remaining 12 cases (19%), ERIC profiles from at least one colony differed from others by at least two bands, suggesting distinct strains.
Similar conclusions emerged from RAPD tests of the same DNAs: no differences among the five single colony isolates or the pool were found with 33 of the 63 participants (52%), slight differences were found with one or more RAPD primers with 18 participants (29%), and quite different profiles were obtained with 12 participants (19%). There was 94% agreement between classifications based on results from one ERIC test versus four RAPD tests (each with a different primer) of sets of isolates as uniform, versus closely related but not identical, versus probably unrelated, indicating a high kappa statistic of 0.8942.
The sensitivity of an individual RAPD test varied, however, with the RAPD primer used. For example, of the 30 samples interpreted as not identical based on data from tests with all four RAPD primers (one primer per test), 22 (73%) and 21 (70%) were thus identified with the primers 1254 or 1281, respectively, whereas only 14 (47%) and 11 (37%) were thus identified with the primers 1283 and 1290, respectively. Combinations of the best two and three primers gave 26/30 (87%) and 30/30 (100%) agreement, respectively, with interpretations based on all four primers. The apparent complexity of a subject's H. pylori population was not related to whether the bacteria had been cultured from biopsy versus from string test samples.
DISCUSSION
The results of RAPD and ERIC fingerprinting of H. pylori from 63 infected residents of a shantytown in Lima, Peru, indicated that approximately one-half of the people carried more than one distinct strain. This is probably a minimum estimate: strains that comprised <∼20% of a person's H. pylori population would often have been missed, since we analyzed just five single colonies and a pool of colonies from each participant. About one-third of mixed infections (12 of 31) involved strains that differed in multiple bands and thus were likely unrelated and acquired from different sources. This abundance fits with data from an eradication trial (16), indicating that the risk of new infection in this community is >15% per person per year, far higher than the <1% per person per year estimated for adults in North America or western Europe. The other mixed infections involved strains that seemed closely related, in that no more than one of the multiple bands observed in a given profile varied among isolates. Many such polymorphic bands may stem from plasmid transfer or chromosomal recombination between unrelated and potentially transient strains, which generally involves the transfer of a few kilobases or less from donor to recipient, and selection for recombinants with improved fitness (6, 11); recombination between duplicate and divergent sequences within the genome and mutation without recombination can also underlie certain individual cases (4). These alternatives can be distinguished by DNA sequencing, focusing initially on individual polymorphic bands (11).
The high quality and discriminatory ability of ERIC profiling encourage the use of this method for analyses of H. pylori diversity, transmission, persistence, and virulence. Indeed, we imagine that with H. pylori, ERIC and standard RAPD fingerprinting are equivalent in terms of underlying target specificity. It is instructive in this context that we found no significant homology between H. pylori genome sequences and either canonical E. coli ERIC sequences or the 22-nt-long primers used for ERIC profiling (8, 20). This is in accord with the very different placements of E. coli and H. pylori in the proteobacteria (gamma versus epsilon phylogenetic groups, respectively). The robustness of ERIC fingerprinting may reflect the length of the primers used (22 nt), which allows high-efficiency priming of further amplification from products of any earlier ERIC amplification cycle; well-chosen long RAPD primers (2) might offer equivalent robustness and sensitivity. We conclude that one ERIC amplification is equivalent to several RAPD tests in reliability and sensitivity and, accordingly, that use of the ERIC protocol allows significant labor and financial savings without a loss in sensitivity or reliability when many samples are to be analyzed.
Acknowledgments
This study was supported by grants T35 AI07646, RO1 AI38166, P01 AI 52976-01, RO1 DK063041, and P30 DK52574 from the U.S. National Institutes of Health.
REFERENCES
- 1.Achtman, M., T. Azuma, D. E. Berg, Y. Ito, G. Morelli, Z. J. Pan, S. Suerbaum, S. A. Thompson, A. van der Ende, and L. J. van Doorn. 1999. Recombination and clonal groupings within Helicobacter pylori from different geographical regions. Mol. Microbiol. 32:459-470. [DOI] [PubMed] [Google Scholar]
- 2.Akopyanz, N., N. O. Bukanov, T. U. Westblom, S. Kresovich, and D. E. Berg. 1992. DNA diversity among clinical isolates of Helicobacter pylori detected by PCR-based RAPD fingerprinting. Nucleic Acids Res. 20:5137-5142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berg, D. E., R. H. Gilman, J. Lelwala-Guruge, Y. Valdez, J. Watanabe, J. Miyagi, N. S. Akopyants, K. Srivastava, A. Ramirez-Ramos, T. H. Yoshiwara, S. Recavarren, and R. Leon Barua. 1997. Helicobacter pylori populations in individual Peruvian patients. Clin. Infect. Dis. 25:996-1002. [DOI] [PubMed] [Google Scholar]
- 4.Brikun, I., K. Suziedelis, and D. E. Berg. 1994. DNA sequence divergence among derivatives of Escherichia coli K-12 detected by arbitrary primer PCR (random amplified polymorphic DNA) fingerprinting. J. Bacteriol. 176:1673-1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cardenas, V. M., Z. D. Mulla, M. Ortiz, and D. Y. Graham. 2006. Iron deficiency and Helicobacter pylori infection in the United States. Am. J. Epidemiol. 163:127-134. [DOI] [PubMed] [Google Scholar]
- 6.Falush, D., C. Kraft, N. S. Taylor, P. Correa, J. G. Fox, M. Achtman, and S. Suerbaum. 2001. Recombination and mutation during long-term gastric colonization by Helicobacter pylori: estimates of clock rates, recombination size, and minimal age. Proc. Natl. Acad. Sci. USA 98:15056-15061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grattapaglia, D., and R. Sederoff. 1994. Genetic linkage maps of Eucalyptus grandis and Eucalyptus urophylla using a pseudo-testcross: mapping strategy and RAPD markers. Genetics 137:1121-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hulton, C. S., C. F. Higgins, and P. M. Sharp. 1991. ERIC sequences: a novel family of repetitive elements in the genomes of Escherichia coli, Salmonella typhimurium and other enterobacteria. Mol. Microbiol. 5:825-834. [DOI] [PubMed] [Google Scholar]
- 9.Hussain, M. A., F. Kauser, A. A. Khan, S. Tiwari, C. M. Habibullah, and N. Ahmed. 2004. Implications of molecular genotyping of Helicobacter pylori isolates from different human populations by genomic fingerprinting of enterobacterial repetitive intergenic consensus regions for strain identification and geographic evolution. J. Clin. Microbiol. 42:2372-2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kersulyte, D., J. P. Woods, E. J. Kreath, W. E. Goldman, and D. E. Berg. 1992. Diversity among clinical isolates of Histoplasma capsulatum detected by polymerase chain reaction with arbitrary primers. J. Bacteriol. 174:7075-7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kersulyte, D., H. Chalkauskas, and D. E. Berg. 1999. Emergence of recombinant strains of Helicobacter pylori during human infection. Mol. Microbiol. 31:31-43. [DOI] [PubMed] [Google Scholar]
- 12.Marshall, B. J., and H. M. Windsor. 2005. The relation of Helicobacter pylori to gastric adenocarcinoma and lymphoma: pathophysiology, epidemiology, screening, clinical presentation, treatment, and prevention. Med. Clin. N. Am. 89:313-344. [DOI] [PubMed] [Google Scholar]
- 13.Parsonnet, J., G. D. Friedman, D. P. Vandersteen, Y. Chang, J. H. Vogelman, N. Orentreich, and R. K. Sibley. 1991. Helicobacter pylori infection and the risk of gastric carcinoma. N. Engl. J. Med. 325:1127-1131. [DOI] [PubMed] [Google Scholar]
- 14.Passaro, D. J., D. N. Taylor, R. Meza, L. Cabrera, R. H. Gilman, and J. Parsonnet. 2001. Acute Helicobacter pylori infection is followed by an increase in diarrheal disease among Peruvian children. Pediatrics 108:E87. [DOI] [PubMed] [Google Scholar]
- 15.Samuels, A. L., H. M. Windsor, G. Y. Ho, L. D. Goodwin, and B. J. Marshall. 2000. Culture of Helicobacter pylori from a gastric string may be an alternative to endoscopic biopsy. J. Clin. Microbiol. 38:2438-2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soto, G., C. T. Bautista, D. E. Roth, R. H. Gilman, B. Velapatino, M. Ogura, G. Dailide, M. Razuri, R. Meza, U. Katz, T. P. Monath, D. E. Berg, D. N. Taylor, et al. 2003. Helicobacter pylori reinfection is common in Peruvian adults after antibiotic eradication therapy. J. Infect. Dis. 188:1263-1275. [DOI] [PubMed] [Google Scholar]
- 17.Thomas, J. E., A. Dale, J. E. Bunn, M. Harding, W. A. Coward, T. J. Cole, and L. T. Weaver. 2004. Early Helicobacter pylori colonization: the association with growth faltering in The Gambia. Arch. Dis. Child 89:1149-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thoreson, A. C., N. Hosseini, A. M. Svennerholm, and I. Bolin. 2000. Different Helicobacter pylori strains colonize the antral and duodenal mucosa of duodenal ulcer patients. Helicobacter 5:69-78. [DOI] [PubMed] [Google Scholar]
- 19.Velapatino, B., J. Balqui, R. H. Gilman, A. Bussalleu, W. Quino, S. A. Finger, L. Santivanez, P. Herrera, A. Piscoya, J. Valdivia, J. Cok, and D. E. Berg. 2006. Validation of string for diagnosis of Helicobacter pylori infections. J. Clin. Microbiol. 44:976-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Versalovic, J., T. Koeuth, and J. R. Lupski. 1991. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 19:6823-6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Welsh, J., and M. McClelland. 1990. Fingerprinting genomes using PCR with arbitrary primers. Nucleic Acids Res. 18:7213-7218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Welsh, J., and M. McClelland. 1991. Genomic fingerprinting using arbitrarily primed PCR and a matrix of pairwise combinations of primers. Nucleic Acids Res. 19:5275-5279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams, J. G., A. R. Kubelik, K. J. Livak, J. A. Rafalsky, and S. V. Tingey. 1990. DNA polymorphisms amplified by arbitrary primers are useful genetic markers. Nucleic Acids Res. 18:6531-6535. [DOI] [PMC free article] [PubMed] [Google Scholar]


