Abstract
We evaluated the performance characteristics of a new, real-time nucleic acid sequence-based amplification (NASBA) assay that incorporates molecular beacon technology for detection of human immunodeficiency virus type 1 (HIV-1). The quantitative results were comparable to those obtained with three leading commercially available assays. The analytical sensitivity was 37 IU/ml. The NASBA assay detected clinically relevant recombinant viruses and all group M HIV-1 subtypes.
Current FDA-approved human immunodeficiency virus type 1 (HIV-1) viral load assays are labor intensive and rely on older detection technologies that limit the throughput capabilities of the assays (4). Most assays use a separate detection step, which results in additional (hands-on) time. For example, the AMPLICOR HIV-1 Monitor test uses an initial reverse transcription-PCR amplification reaction and a subsequent colorimetric step performed in a microwell plate for manual detection. With the COBAS AMPLICOR tests, the detection step is automated on the COBAS instrument.
The NucliSens EasyQ HIV-1 assay (bioMérieux, Boxtel, The Netherlands) is a quantitative, next-generation amplification assay designed to overcome the labor and throughput obstacles in the earlier assays. The EasyQ HIV-1 assay uses nucleic acid sequence-based amplification (NASBA) and provides real-time detection by incorporating molecular beacons in the NASBA reaction (2, 3, 6, 7). Like the AMPLICOR assay, the NucliSens EasyQ test targets the gag region of the HIV-1 genome. Combining the amplification and detection steps facilitates high-throughput capabilities and provides greater user convenience. In this study, we evaluated the performance characteristics of the NucliSens EasyQ HIV-1 assay for its interassay precision, sensitivity and dynamic range, and ability to detect HIV-1 group M subtypes and clinically relevant recombinant viruses and for the degree of concordance of the results of the assay with those of three other marketed HIV-1 assay systems.
We used the NucliSens extractor for nucleic acid isolation and the NucliSens EasyQ HIV-1 assay (version 1.1) according to the manufacturer's protocols. An internal calibration standard (synthetic HIV-1 RNA) was added to the sample before nucleic acid extraction and isolation by a silica bead procedure (1). NASBA of the sample RNA together with the calibrator RNA was performed by using primers specific for wild-type HIV-1 RNA and calibrator RNA.
For comparison, assays were also performed by using one or more of the following HIV-1 assay systems approved for market in the United States: NucliSens HIV-1 QT (bioMérieux), AMPLICOR UltraSensitive v1.0 (Roche Molecular Systems, Pleasanton, Calif.), and COBAS AMPLICOR v1.5 (Roche). In each case the samples were extracted and the assays were performed by using the protocols provided by the manufacturer.
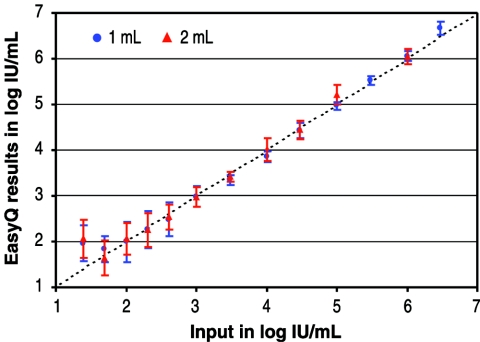
To evaluate the precision of the NucliSens EasyQ HIV-1 assay, we analyzed a panel of serial dilutions of an HIV-1 RNA reference strain calibrated against a WHO standard HIV-1 RNA (NIBSC code 97/656). The viral concentrations in the panel ranged from 25 to 3 × 106 copies/ml, and 8 to 12 replicates from a total of six runs were tested at each concentration. Response curves were generated from the serial dilutions. Although the assay precision was higher at concentrations >1,000 IU/ml, the assay was linear over a range of 50 IU/ml to 3 × 106 IU/ml, with a mean standard deviation of 0.22 log10 (Fig. 1). Probit analysis of the sensitivity of the assay showed probabilities of detection of 50% and 95% at 37 IU/ml and 200 IU/ml, respectively, with a 1-ml input volume.
FIG. 1.
Assessment of the NucliSens EasyQ HIV-1 assay against a panel of serial dilutions of an HIV-1 RNA reference strain.
For assessment of clinical reactivity of the assay, we used repository plasma samples from clinical studies of antiretroviral therapy sponsored by GlaxoSmithKline (Research Triangle Park, N.C.). All plasma samples used were from institutional review board-approved studies in which subjects had provided written informed consent approving the use of plasma samples for research purposes. Repository plasma samples from subjects participating in North American clinical trials were used as a source of HIV-1 subtype B for comparison of the NucliSens EasyQ HIV-1 assay with the NucliSens HIV-1 QT assay and the Roche AMPLICOR UltraSensitive v1.0 assay. All repository plasma samples used for this purpose had been verified earlier to be infected with subtype B. For evaluation of virus subtypes A, C, D, E F, G, and H and clinically relevant recombinant viruses (5), we used repository plasma samples from antiretroviral therapy-naive subjects from different demographic regions who were participating in clinical trials in North and South America. The HIV-1 subtypes in these clinical samples had already been determined by DNA sequencing of the reverse transcriptase-coding region (ViroSeq HIV-1 Genotyping System v2; Celera Diagnostics, Alameda, Calif.) and phylogenetic analysis of >600 nucleotides of the reverse transcriptase-coding region by using the NCBI Retroviruses HIV subtyping tool (NIH, Bethesda, MD).
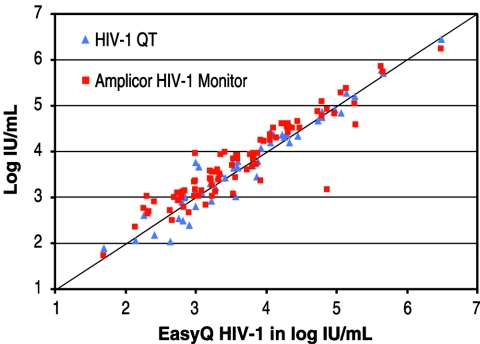
For comparisons of the NucliSens EasyQ HIV-1 assay with the NucliSens HIV-1 QT assay and with the Roche AMPLICOR UltraSensitive v1.0 assay, 55 and 91 samples, respectively, were extracted and tested in parallel. Overall, the concordance of the results of both assays was very good, with slightly better concordance observed between the results of the two NucliSens assays (Fig. 2). Eighty-three of 91 samples were positive by both the Roche AMPLICOR UltraSensitive v1.0 and the NucliSens EasyQ HIV-1 assays. Six samples had viral loads below the lower detection limits of both assays. One sample was negative by the Roche AMPLICOR UltraSensitive assay and positive by the NucliSens EasyQ HIV-1 assay, and another was negative by the EasyQ assay and positive by the AMPLICOR assay.
FIG. 2.
Comparisons of the NucliSens EasyQ HIV-1 assay with the NucliSens HIV-1 QT assay (55 samples) and with the Roche AMPLICOR Ultrasensitive v1.0 assay (91 samples).
Concordance with the HIV-1 QT assay gave a correlation coefficient (R) of 0.97, whereas with the AMPLICOR UltraSensitive assay had an R value of 0.93. The log10 biases were 0.04 and 0.13 for the comparisons with the NucliSens HIV-1 QT and the Roche AMPLICOR UltraSensitive assays, respectively.
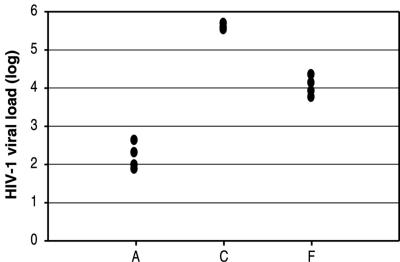
To assess assay reproducibility with clinical samples infected with non-subtype B HIV-1 isolates, three plasma samples infected with HIV-1 subtypes A, C, and F, respectively, were each independently extracted and tested four times by the NucliSens EasyQ HIV-1 assay. The EasyQ HIV-1 assay quantitated each of the three HIV-1 subtypes reproducibly, with standard deviations of 0.35 for subtype A, 0.08 for subtype C, and 0.24 for subtype F (Fig. 3).
FIG. 3.
Assessment of assay reproducibility with clinical samples infected with non-subtype B HIV-1 isolates.
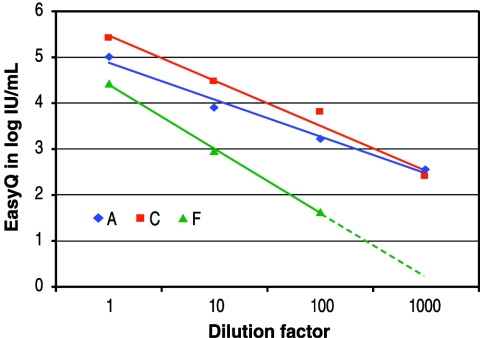
Subtype linearity in quantitation was assessed by performing the NucliSens EasyQ HIV-1 assay by using a 10-fold serial dilution of each of the three HIV-1 subtype-infected samples over a dilution range from 1 to 103. Excellent linearity was exhibited for all three subtypes (Fig. 4), although the slope for subtype F seemed to deviate somewhat compared to those for the other subtypes. This might be attributed to the lower viral loads and subsequent higher variability obtained with subtype F.
FIG. 4.
Assessment of assay linearity with non-subtype B isolates by using 10-fold serial dilutions.
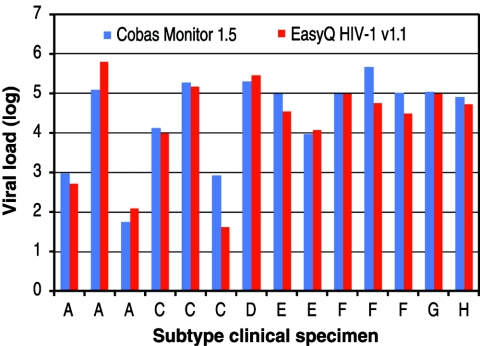
We assessed the HIV-1 subtype reactivity of the NucliSens EasyQ HIV-1 assay in parallel with the Roche AMPLICOR COBAS v1.5 assay against a panel of clinical specimens with different HIV-1 subtypes (subtype A, three samples; subtype C, three samples; subtype D, one sample; subtype E, two samples; subtype F, three samples; subtype G, one sample; subtype H, one sample). The concordance of the results of the two assays was very good, with an R value of 0.92 (Fig. 5).
FIG. 5.
Comparison of the NucliSens EasyQ HIV-1 assay and the Roche AMPLICOR Ultrasensitive v1.0 assay for assessment of HIV-1 subtype reactivity.
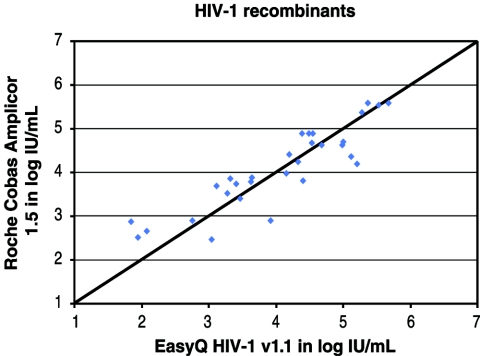
To assess assay reactivity against recombinant clinical strains, we analyzed 30 plasma samples selected from antiretroviral therapy-naive subjects of multiple demographic backgrounds in North and South America. Viruses from these 30 subjects were typed as B/C (n = 11), B/F1 (n = 7), B/D (n = 4), B/F1/D (n = 3), C (n = 1), B/H/F1 (n = 1), B/C/D (n = 1), C/H (n = 1), and F1 (n = 1). Samples were extracted and assayed in parallel by the NucliSens EasyQ HIV-1 assay and the Roche COBAS AMPLICOR v1.5 assay. Both assays detected all recombinants. The concordance of the results of the two assays was good, with an R value of 0.86 (Fig. 6).
FIG. 6.
Comparison of the NucliSens EasyQ HIV-1 and the Roche COBAS AMPLICOR v1.5 assays for reactivities against recombinant clinical strains.
In summary, the NucliSens EasyQ HIV-1 assay gave quantitative results comparable to those of the Roche AMPLICOR UltraSensitive v1.5 assay and showed clinical sensitivity equivalent to that of the Roche AMPLICOR UltraSensitive v1.5 assay. The assay was effective in detecting all group M subtypes and clinically relevant recombinant viruses. By incorporating molecular beacon technology in the detection step, the NucliSens EasyQ HIV-1 assay provides real-time detection and permits a single test format to be used for samples from a wide variety of patient populations with variable viral loads.
REFERENCES
- 1.Boom, R., C. J. Sol, M. M. Salimans, C. L. Jansen, P. M. Wertheim-van Dillen, and J. van der Noordaa. 1990. Rapid and simple method for purification of nucleic acids. J. Clin. Microbiol. 28:495-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deiman, B., P. van Aarle, and P. Sillekens. 2002. Characteristics and applications of nucleic acid sequence based amplification (NASBA). Mol. Biotechnol. 20:163-179. [DOI] [PubMed] [Google Scholar]
- 3.de Mendoza, C., M. Koppelman, B. Montes, V. Ferre, V. Soriano, H. Cuypers, M. Segondy, and T. Oosterlaken. 2005. Multicenter evaluation of the NucliSens EasyQ HIV-1 v1.1 assay for the quantitative detection of HIV-1 RNA in plasma. J. Virol. Methods 127:54-59. [DOI] [PubMed] [Google Scholar]
- 4.Murphy, D. G., L. Cote, M. Fauve, P. Rene, and J. Vincelette. 2000. Multicenter comparison of Roche COBAS AMPLICOR MONITOR version 1.5, Organon Teknika NucliSens QT with Extractor, and Bayer Quantiplex version 3.0 for quantification of human immunodeficiency virus type 1 RNA in plasma. J. Clin. Microbiol. 38:4034-4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spira, S., M. A. Wainberg, H. Loemba, D. Turner, and B. G. Brenner. 2003. Impact of clade diversity on HIV-1 virulence, antiretroviral drug sensitivity and drug resistance. J. Antimicrob. Chemother. 51:229-240. [DOI] [PubMed] [Google Scholar]
- 6.Stevens, W., T. Wiggill, P. Horsfield, L. Coetzee, and L. E. Scott. 2005. Evaluation of the NucliSens EasyQ assay in HIV-1-infected individuals in South Africa. J. Virol. Methods 124:105-110. [DOI] [PubMed] [Google Scholar]
- 7.Yao, J., Z. Liu, L.-S. Ko, G. Pan, and Y. Jiang. 2005. Quantitative detection of HIV-1 RNA using NucliSens EasyQ HIV-1 assay. J. Virol. Methods 129:40-46. [DOI] [PubMed] [Google Scholar]