Abstract
Mixed Plasmodium malariae and P. vivax infections in humans are reported very infrequently. The case of a 27-year-old male who sustained malaria quartana/tertiana caused by an unbalanced mixed P. malariae-P. vivax infection is reported here. Conventional tests and serology for malarial parasites were uniformly negative. Identification and quantification of the parasites were accomplished by examining bone-marrow specimens using specific real-time TaqMan PCR.
CASE REPORT
A 27-year-old development aid volunteer, deployed as a construction engineer in Sudan (East Africa) for 6 months, sustained several fever attacks and occasional bouts of mild diarrhea during his stay there. He treated the symptoms himself using local drugs from unknown sources. Prior to his travel to Sudan, he was vaccinated against yellow fever virus and received malaria prophylaxis with mefloquine, which was incomplete because of noncompliance after 3 months. Five months after his return from Africa, he complained of fatigue with a sudden onset of flu-like symptoms and sustained recurrent severe fever attacks of unknown etiology with temperatures of 39.5 through 40.5°C, rigor, and myalgia every 2 to 4 days. Despite the unusually long duration of the asymptomatic period, both domestically acquired and tropical infections were suspected. Detailed examination of the patient and abdominal sonograms revealed a mild lymphadenopathy (axillary, inguinal, and mesenteric) and pronounced hepatosplenomegaly. Furthermore, a thrombocytopenia was diagnosed with reduced thrombocyte levels (37,000/μl). These symptoms strongly suggested a relapsing or recrudescent form of malaria. Examinations for malaria using thick and thin blood films, as well as the NOW malaria test (Binax, Portland, OR) were, however, negative in four consecutive blood samples collected over 2 days. Even the serodiagnosis for malaria was negative. The patient was empirically placed on a regimen of intravenous doxycycline (seven consecutive doses of 200 mg daily for 1 week) and ampicillin-sulbactam (seven consecutive doses of 1.11 g daily for 1 week). Serological examination for Bartonella henselae, Borrelia burgdorferi, Brucella spp., Coxiella burnetii, Echinococcus spp., Entamoeba histolytica, Leishmania spp., Leptospira spp., Microfilaria spp., Rickettsia spp., Schistosoma spp., and Treponema pallidum was negative. In addition, the stool samples were tested negative for Campylobacter spp., E. histolytica, Salmonella spp., Shigella spp., and Yersinia enterocolitica. Both aerobic and anaerobic blood cultures were culture negative, and examination of the respiratory and the urogenital tract revealed no evidence of an infection. The patient was tested negative for active hepatitis A, B, and C virus, cytomegalovirus, and Epstein-Barr virus infections. Six days after starting the treatment with doxycycline and ampicillin-sulbactam the patient suffered a renewed bout of fever. A Plasmodium-specific real-time TaqMan PCR analysis (19) using a total DNA extract of EDTA-blood obtained during this bout of fever was also negative.
An alternative line of examination based on hematological and oncological causes underlying the symptoms was also followed. Whole-body computed tomography was performed, but no signs indicating Hodgkin or non-Hodgkin lymphoma or other localized oncological processes were detected. To exclude leukemia, a bone-marrow puncture was conducted and a bone-marrow smear was examined by an experienced hematologist. Microscopic examination revealed weak pale red dots in erythrocytes with suspected similarity to Schüffner dots, which normally are typical signs of malaria tertiana. However, no malaria parasites were detected. We therefore extracted total DNA from 200 μl of the bone-marrow puncture and performed Plasmodium-specific real-time quantitative TaqMan PCR (19). We detected malaria as the underlying source of the symptoms caused by an uncommon mixed-species infection comprising both P. malariae and P. vivax. We calculated the relative loads to be ∼1,000 and ∼10 copies per reaction for P. malariae and P. vivax, respectively (ratio of 100:1). The patient was subsequently put on a therapy regimen (day 1, a first dose of 600 mg and a second dose after 6 h of 300 mg; days 2 and 3, 300 mg of each) with chloroquine (Resochin; Bayer HealthCare, Leverkusen, Germany) and, after exclusion of glucose-6-phosphate dehydrogenase deficiency, with Primaquine (Sanofi-Aventis, Bridgewater, NJ) at 14 consecutive doses of 15 mg daily for 2 weeks, after which the patient recovered uneventfully.
Increased global tourism and traveling have contributed to the dramatic emergence and reemergence of infectious diseases (17). Approximately 25 to 30 million international travelers from nontropical regions annually visit countries where malaria is endemic. Thus, malaria is the most common cause of fever in returning travelers and a major imported disease of developed nations (9). From 1991 through 2001, a total of 13,900 cases were reported in the United States (5). For Germany, the number was 9,148 cases reported between 1993 and 2003 (21). Although microscopic examination of blood smears remains the “gold standard” for diagnosis, this method suffers from poor sensitivity and requires considerable expertise and experience. Molecular diagnosis using real-time PCR offers improved and standardized diagnosis, enabling not only detection but also quantification of the parasites for the monitoring of patients receiving antimalarial therapy (19).
Chromosomal DNAs of P. falciparum ATCC 30074, P. malariae MRA-344G, P. vivax ATCC 30060, and P. ovale MRA-600G (American Type Culture Collection [ATCC], MR4, Malaria Research and Reference Reagent Resource Center) were used as positive controls. Since only limited amounts of chromosomal DNAs (<1 μg) were available, the whole genomes of these Plasmodium species were amplified by using the GenomiPhi DNA amplification kit (GE Healthcare, Freiburg, Germany) to obtain sufficient DNA of these positive controls for Plasmodium species-specific real-time PCR assays. This novel method is not based on PCR but rather relies on the highly processive and high-fidelity phage Phi29 DNA polymerase to replicate genomic DNA by multiple strand displacement amplification following random priming of hexamer primers. This method permits virtually unlimited numbers of DNA tests to be performed from a small number of cells or from limited amounts of precious samples and requires as little as 1 ng of genomic DNA template. There is no difference in the rate of efficiency of amplification among various DNA templates regardless of the GC/AT content. The material is suitable for unrestricted downstream genetic analysis such as gene-specific PCR, direct sequencing, microsatellite marker analysis, and single nucleotide polymorphism allelic discrimination using real-time PCR (2, 8).
Detection of Plasmodium species was performed with P. falciparum, P. vivax, P. ovale, and P. malariae species-specific monoplex real-time PCR assays using oligonucleotides, TaqMan probes, and PCR conditions described previously (19) with the exception that all of the TaqMan probes used were labeled with 5′FAM and 3′TAMRA since each species-specific PCR was run as an individual and independent reaction. All of the Plasmodium species amplified with the GenomiPhi DNA amplification kit and subjected to Plasmodium genus- and species-specific monoplex real-time PCR assays were determined to be positive using the Plasmodium genus-specific primers and probe (Fig. 1; Table 1), and the individual primer sets allowed specific detection of P. falciparum, P. vivax, P. ovale, and P. malariae, respectively. No difference was observed when the original Plasmodium DNA samples (data not shown) was used. The specificities of the genus- and species-specific Plasmodium probes were assessed on DNAs from other 18S-possessing eukaryotes as described previously (19) and could be confirmed.
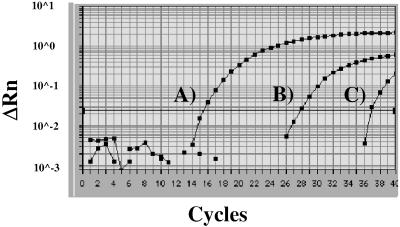
FIG. 1.
Quantitative real-time PCR assays to detect Plasmodium genus- and species-specific DNA from P. falciparum, P. vivax, P. ovale, and P. malariae in EDTA-blood and bone-marrow punctures. The data from the individual and independent real-time PCR assays have been combined and are presented in one figure. (A) Positive control (GenomiPhi amplified), consisting of a mixture of P. falciparum, P. vivax, P. ovale, and P. malariae (∼108 copies/reaction), detected by the Plasmodium genus-specific real-time PCR. (B and C) Specific detection of P. malariae (∼1,000 copies/reaction) (B) and P. vivax (∼10 copies/reaction) (C) by the P. malariae and P. vivax species-specific real-time PCR assays. Both P. malariae and P. vivax species were only detected in the bone-marrow puncture sample. The EDTA-blood was negative (data not visible).
TABLE 1.
Quantitative real-time TaqMan PCR to detect Plasmodium genus- and species-specific DNA
| Species | Remarks | PCR | CT value | No. of copies/reaction |
|---|---|---|---|---|
| Plasmodium spp. | Positive control | + | 15.5 | ∼108 |
| P. malaria | Specific detection | + | 27.8 | ∼103 |
| P. vivax | Specific detection | + | 36.9 | ∼101 |
| P. falciparum | Specific detection | − | ||
| P. ovale | Specific detection | − |
In order to detect Plasmodium species in patient samples, total DNA from 200 μl each of fresh EDTA-blood and bone-marrow-derived material was extracted by using commercially available genomic DNA isolation kits according to the vendor's instructions (Invisorb Spin Blood Minikit and Invisorb Spin Tissue Minikit; Invitek, Berlin, Germany) and directly used for all further template-based amplification studies. The GenomiPhi DNA amplification kit was not used to amplify the whole-genome DNA of the clinical samples. Initially, both samples of the EDTA-blood and the bone marrow were analyzed using the Plasmodium genus-specific real-time PCR, and only the bone-marrow-derived material gave a positive signal. The EDTA-blood sample turned out to be negative. Further differentiation of the bone-marrow sample using Plasmodium species-specific real-time PCR assays revealed mixed Plasmodium infection comprising P. malariae and P. vivax (Fig. 1; Table 1). The identification of the two Plasmodium species was independently confirmed by sequencing the respective PCR products. Negative extraction controls (healthy individual) were tested for each species, and negative amplification controls (no template), as well as inhibition amplification controls (a spiked sample with a known amount of positive control DNA), were included for each sample. The parasitic burdens for P. malariae (∼1,000 copies/reaction) and for P. vivax (∼10 copies/reaction) were calculated by using the respective standard curves, taking into consideration the relative efficiencies of the real-time PCRs (19). The amount of P. vivax was 100-fold lower than the amount of P. malariae and marginally above the detection limit, which was approximately one to five parasites/reaction (19).
Potential explanations for testing the EDTA-blood negatively include the absence of parasites or that the parasitic burden in the peripheral blood was below the detection limit of the real-time PCR. Poor detection can be attributed to the quality of the DNA extraction kit used, since the method used to extract DNA from blood samples can influence the sensitivity of malaria detection by PCR (7, 19). However, prior treatment with doxycycline for 1 week could have resulted in a clearance of the parasites from peripheral blood. Doxycycline is a member of a group of antibacterial drugs with antimalarial activity and has been exploited for the prevention and treatment of malaria, in particular in the treatment of chloroquine-resistant P. falciparum malaria (4, 18). Clearance in peripheral blood can occur within 5 to 6 days. Here, both the EDTA-blood sample and the bone-marrow puncture for real-time PCR analysis were extracted 7 days after the start of doxycycline treatment. Although the penetration of doxycycline into tissues and bone marrow is not retarded, it is known that the activity in bone marrow is significantly reduced because it forms complexes with calcium (20). Therefore, the lowered activity of the drug enabled the detection and quantification of the remaining P. malariae and P. vivax microorganisms. The detection of Plasmodium species in bone marrow has previously been found to be effective in demonstrating that the diagnostic efficacy of bone marrow for evidence of malaria was very useful in febrile individuals for whom the diagnosis was otherwise unknown (14).
This case report extends the number of rare reports of mixed P. malariae and P. vivax infections in humans and demonstrates the power of real-time quantitative TaqMan PCR in the diagnosis of malaria (24). The incidence of mixed Plasmodium species infections in humans is nearly always underestimated, in particular for imported malaria, and requires substantially more attention. The estimated prevalence of P. malariae-P. vivax mixed infections is clearly less than 3% (3, 6). However, it is known that simultaneous infection with different Plasmodium species can result in the suppression of one of the species, e.g., P. vivax can suppress P. falciparum, resulting in a reduced severity of the disease (6, 11, 13, 23, 24). Hence, the correct diagnosis of a mixed-species infection using microscopic blood film examination is complicated and often results in underestimation since one species dominates over the other one (23, 24). The quantitative real-time TaqMan PCR assays used demonstrated a P. malariae/P. vivax ratio of 100:1. The patient stayed 6 months in a sub-Saharan area of Africa where P. malariae is endemic, whereas P. vivax has been found infrequently (23). It is acknowledged that environmental factors, nutrition, and the genetic predispositions of the human hosts, as well as the biology of the transmitting mosquito vector populations, influence the prevalence of mixed Plasmodium species infections in humans (23). Susceptible hosts are possibly infected by the bites of different mosquitoes carrying different single Plasmodium species or by one type of mosquito carrying more than one Plasmodium species (1, 10, 12).
To sum up, our results demonstrated clearly that bone marrow is a useful source material to diagnose malaria, in particular in a patient sustaining recurrent severe fever attacks of unknown origin and under treatment with doxycycline. Therefore, bone marrow may constitute an ecological niche for the survival and periodic dissemination of P. malariae and P. vivax causing recurrent fever attacks, even after for months postinfection. For P. vivax it is known that this parasite preferentially infects reticulocytes in bone marrow via the Duffy antigen receptor (16, 22). Despite limited evidence from clinical trials indicating that doxycycline is an effective drug in the treatment of uncomplicated or severe malaria, its antimalarial activity is acknowledged. The tetracyclines are consistently active against all species of Plasmodium, and doxycycline is the most widely used agent both for prophylaxis and for treatment of malaria (23). It has been even suggested that the daily dose of doxycycline be increased for the treatment of malaria in combination with antimalarial drugs (15). Given the increasing number of cases of imported malaria annually and that microscopic examination of blood smears relies on considerable expertise and experienced examiners, it is vital to standardize and to improve diagnostic techniques so that species-specific parasitemia can be determined more efficiently and with greater precision. The absence of serological correlates, as described here, makes it important that alternative methods for diagnosis are also available. The specific real-time TaqMan PCR assays used enabled the clear identification of an unbalanced and rare mixed Plasmodium species infection and even the quantification of the parasites. Our own experience with five more malaria patients (data not shown) and the results of Rougemont et al. (19) showed that the values estimated by microscopy had a significant correlation with the values calculated by real-time PCR. Thus, real-time PCR reliably facilitates the calculation of the ratio of a mixed Plasmodium species infection and the monitoring of patients receiving antimalarial treatment.
Acknowledgments
We thank Martina Leyerer and Kirsten-Susann Bommersheim for excellent technical assistance and Rolf Horstmann (Bernhard-Nocht-Institute for Tropical Medicine, Hamburg, Germany), as well as Holger Repp (Rudolf-Buchheim-Institute for Pharmacology, Giessen, Germany) for helpful discussions.
This study was supported by grants from the Bundesministerium fuer Bildung und Forschung (Germany) within the framework of the National Genome Research Network (contract no. 01GS0401).
REFERENCES
- 1.Arez, A. P., K. Palsson, J. Pinto, A. S. Franco, J. Dinis, T. G. Jaenson, G. Snounou, and V. E. doRosario. 1997. Transmission of mixed malaria species and strains by mosquitoes, as detected by PCR, in a study area in Guinea-Bissau. Parassitologia 39:65-70. [PubMed] [Google Scholar]
- 2.Chakraborty, T., G. Bein, and E. Domann. 2003. Whole-genome amplification and downstream analysis from limited amounts of DNA from human blood samples using GenomiPhi. Abstract. International Symposium on Functional Genomics of Infectious Diseases and Inflammation, Tübingen, Germany.
- 3.Eliades, M. J., S. Shah, P. Nguyen-Dinh, R. D. Newman, A. M. Barber, J. M. Roberts, S. Mali, M. E. Parise, and R. Steketee. 2003. Malaria surveillance-United States. Morb. Mortal. Wkly. Rep. Surveill. Summ. 54:25-40. [PubMed] [Google Scholar]
- 4.Elkheir, H. K., E. F. Elkarim, I. B. Eltayeb, A. E. Elkadaru, H. A. Babiker, and A. M. Ibrahim. 2001. Efficacy of sulphadoxine and pyrimethamine, doxycycline and their combination in the treatment of chloroquine resistant falciparum malaria. Saudi Med. J. 22:690-693. [PubMed] [Google Scholar]
- 5.Filler, S., L. M. Causer, R. D. Newman, A. M. Barber, J. M. Roberts, J. MacArthur, M. E. Parise, and R. W. Steketee. 2001. Malaria surveillance-United States. Morb. Mortal. Wkly. Rep. Survell. Summ. 52:1-14. [PubMed] [Google Scholar]
- 6.Foca, A., G. S. Barreca, V. Barbieri, G. Matera, M. C. Liberto, and M. DeRosa. 2004. Fourteen-year experience with imported malaria. Infez. Med. 12:186-192. [PubMed] [Google Scholar]
- 7.Henning, L., I. Felger, and H. P. Beck. 1999. Rapid DNA extraction for molecular epidemiological studies of malaria. Acta Trop. 72:149-155. [DOI] [PubMed] [Google Scholar]
- 8.Holbrook, J. F., D. Stabley, and K. Sol-Church. 2005. Exploring whole genome amplification as a DNA recovery tool for molecular genetic studies. J. Biomol. Tech. 16:125-133. [PMC free article] [PubMed] [Google Scholar]
- 9.Leder, K., J. Black, D. O'Brien, Z. Greenwood, K. C. Kain, E. Schwartz, G. Brown, and J. Torresi. 2004. Malaria in travelers: a review of the GeoSentinel surveillance network. Clin. Infect. Dis. 39:1104-1112. [DOI] [PubMed] [Google Scholar]
- 10.Marques, P. X., F. Saute, V. V. Pinto, S. Cardoso, J. Pinto, P. L. Alonso, V. E. doRosario, and A. P. Arez. 2005. Plasmodium species mixed infections in two areas of Manhica District, Mozambique. Int. J. Biol. Sci. 1:96-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.May, J., F. P. Mockenhaupt, O. G. Ademowo, A. G. Falusi, P. E. Olumese, U. Bienzle, and C. G. Meyer. 1999. High rate of mixed and subpatent malarial infections in southwest Nigeria. Am. J. Trop. Med. Hyg. 61:339-343. [DOI] [PubMed] [Google Scholar]
- 12.McKenzie, F. E., and W. H. Bossert. 1997. Mixed-species Plasmodium infections of Anopheles (Diptera:Culicidae). J. Med. Entomol. 34:417-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehlotra, R. K., K. Lorry, W. Kastens, S. M. Miller, M. P. Alpers, M. Bockarie, J. W. Kazura, and P. A. Zimmerman. 2000. Random distribution of mixed species malaria infections in Papua New Guinea. Am. J. Trop. Med. Hyg. 62:225-231. [DOI] [PubMed] [Google Scholar]
- 14.Mirdha, B. R., J. C. Samantray, B. Mishra, and I. Xess. 1999. Bone-marrow examination for identifying malaria in fever of unknown origin. J. Assoc. Physicians India 47:177-179. [PubMed] [Google Scholar]
- 15.Newton, P. A., J. F. Chaulet, A. Brockman, W. Chierakul, A. Dondrop, R. Ruangveerayuth, S. Looareesuwan, C. Mounier, and N. J. White. 2005. Pharmacokinetics of oral doxycycline during combination treatment of severe falciparum malaria. Antimicrob. Agents Chemother. 49:1622-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ocampo, M., R. Vera, L. Eduardo Rodriguez, H. Curtidor, M. Urquiza, J. Suarez, J. Garcia, A. Puentes, R. Lopez, M. Trujillo, E. Torres, and M. E. Patarroyo. 2002. Plasmodium vivax Duffy binding protein peptides specifically bind to reticulocytes. Peptides 23:13-22. [DOI] [PubMed] [Google Scholar]
- 17.Ostroff, S. M., and P. Kozarsky. 1998. Emerging infectious diseases and travel medicine. Infect. Dis. Clin. N. Am. 12:231-241. [DOI] [PubMed] [Google Scholar]
- 18.Pukrittayakamee, S., R. Clemens, A. Chantra, A. Nontprasert, T. Luknam, S. Looareesuwan, and N. J. White. 2001. Therapeutic responses to antibacterial drugs in vivax malaria. Trans. R. Soc. Trop. Med. Hyg. 95:524-528. [DOI] [PubMed] [Google Scholar]
- 19.Rougemont, M., M. Van Saanen, R. Sahli, H. P. Hinrikson, J. Bille, and K. Jaton. 2004. Detection of four Plasmodium species in blood from humans by 18S rRNA gene subunit-based and species-specific real-time PCR assays. J. Clin. Microbiol. 42:5636-5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sande, M. E., and G. L. Mandell. 1990. Antimicrobial agents: tetracyclines, chloramphenicol, erythromycin, and miscellaneous antibacterial agents, p. 1117-1145. In A. G. Gilman, Th. W. Rall, A. S. Nies, and P. Taylor (ed.), Goodman and Gilman's the pharmacological basis of therapeutics, 8th ed. Pergamon Press, New York, N.Y.
- 21.Schoneberg, I., K. Stark, D. Altmann, and G. Krause. 2005. Malaria in Germany 1993 to 2003: data from the Robert Koch Institute on affected groups of people, countries traveled to and treatment. Dtsch. Med. Wochenschr. 130:937-941. [DOI] [PubMed] [Google Scholar]
- 22.Schuster, F. L. 2002. Cultivation of Plasmodium spp. Clin. Microbiol. Rev. 15:355-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.White, N. J. 2003. Malaria, p. 1205-1295. In G. C. Cook and A. I. Zumla (ed.), Manson's tropical diseases: protozoan infections. Elsevier Science, Ltd., London, United Kingdom.
- 24.Zimmerman, P. A., R. K. Mehlotra, L. J. Kasehagen, and J. W. Kazura. 2004. Why do we need to know more about mixed Plasmodium species infections in humans? Trends Parasitol. 20:440-447. [DOI] [PMC free article] [PubMed] [Google Scholar]