Abstract
Agar-based antifungal susceptibility testing is an attractive alternative to the microdilution method. We examined the correlation between the microdilution, E-test, and disk diffusion methods for posaconazole against Candida spp. A total of 270 bloodstream isolates of Candida spp. with a broad range of posaconazole MICs were tested using the CLSI M27-A2 method for microdilution, as well as the M-44A method and E-test methods for agar-based testing on Mueller-Hinton agar supplemented with 2% glucose and 0.5 μg of methylene blue. MICs and inhibitory zone diameters at the prominent growth reduction endpoint were recorded at 24 and 48 h. The Candida isolates included Candida albicans (n = 124), C. parapsilosis (n = 44), C. tropicalis (n = 41), C. glabrata (n = 36), C. krusei (n = 20), C. lusitaniae (n = 3), and C. dubliniensis (n = 2). The overall concordance (i.e., the percentage of isolates within two dilutions) between the E-test and microdilution was 64.8% at 24 h and 82.6% at 48 h. When we considered an arbitrary breakpoint of ≤1 μg/ml, the agreement between the E-test and microdilution methods was 87.8% at 24 h and 93.0% at 48 h. The correlation of MICs with disk diffusion zone diameters was better for the E-test than the microdilution method. Zone correlation for diameters produced by the disks of two manufacturers was high, with a Pearson test value of 0.941 at 24 h. The E-test and microdilution MICs show good concordance and interpretative agreement. The disk diffusion zone diameters are highly reproducible and correlate well with both the E-test and the microdilution method, making agar-based methods a viable alternative to microdilution for posaconazole susceptibility testing.
Standardization of antifungal susceptibility testing is an area of active research since the availability of reference methods for the testing of yeasts (Clinical and Laboratory Standards Institute [CLSI] M27-A2 method) and molds (CLSI M38-A method) (17). The emergence of fluconazole-resistant Candida albicans and selection for inherently fluconazole-resistant Candida spp. has prompted the use of alternative agents for the treatment of invasive Candida infections (7, 8). The alternatives include the echinocandins and the newer azoles, voriconazole, ravuconazole, and posaconazole. The azoles are inhibitors of the sterol 14-alpha-demethylase enzyme, blocking the production of the ergosterol component of the fungal cell membrane. Posaconazole, a triazole agent currently in clinical trials, is a more potent inhibitor of this enzyme than itraconazole and voriconazole in Aspergillus species and retains activity against the mutated enzyme responsible for resistance to fluconazole, itraconazole, and voriconazole in Candida (11). It has shown activity superior to fluconazole and itraconazole against Candida spp. in previous in vitro surveys using the broth microdilution (MD) technique according to the CLSI method (15). There has been much research interest in agar-based antifungal susceptibility via E-test (ET) and disk diffusion (DD) methods due to their relative ease and the lack of need for specialized equipment (6, 17). The MD susceptibility results for posaconazole have been compared to E-test results with good correlation (16); however, no correlation has been made with disk diffusion methods for posaconazole.
In the present study, we compared the MD, ET, and DD methods for the determination of susceptibilities to posaconazole against 270 Candida spp. isolates. The ET and DD methods are well studied with respect to fluconazole and voriconazole (2, 9, 13), and the present study expands that knowledge base to include posaconazole.
MATERIALS AND METHODS
Isolates.
A total of 270 bloodstream isolates of Candida spp. identified with the API 20C AUX system (Biomerieux Vitek, Hazelwood, MO) were randomly selected from a collection from the United States for testing. The isolates included 124 isolates of Candida albicans (46.3%), 44 isolates of C. parapsilosis (15.9%), 41 isolates of C. tropicalis (15.2%), 36 isolates of C. glabrata (13.3%), 20 isolates of C. krusei (7.4%), 3 isolates of C. lusitaniae (1.1%), and 2 isolates of C. dubliniensis (0.8%). The isolates were stored in distilled water at room temperature or in 30% glycerin at −80°C. Each isolate was subcultured at least twice on Sabouraud dextrose agar and incubated at 35°C to ensure purity and optimal growth. All isolates were subcultured again 24 h prior to testing. Quality control isolates included C. albicans ATCC 90028, C. parapsilosis ATCC 22019, and C. krusei ATCC 6258.
Inoculum suspensions.
Yeast inoculum suspensions were prepared as described in CLSI M27-A2 (5). Using spectrophotometry at 530 nm, turbidity was measured and adjusted to match a 0.5 McFarland density standard resulting in an inoculum containing 1 × 106 to 5 × 106 yeast cells/ml. This suspension was used to directly inoculate agar plates for the ET and DD procedures or was diluted as directed by CLSI M27-A2 for the MD procedure.
Antifungal agents.
Posaconazole research powder was obtained from Schering-Plough (Kenilworth, NJ) and was stored at −20°C until reconstituted for the MD procedure. Posaconazole and fluconazole E-test strips were obtained from AB Biodisk (Solna, Sweden) with concentration ranges of 0.002 to 32 μg/ml and 0.016 to 256 μg/ml, respectively. Two varieties of paper disks containing posaconazole at 5 μg were obtained from Oxoid, Ltd. (Basingstoke, England), and BD (Maryland).
Susceptibility testing methods.
Broth MD testing was performed as described in the CLSI M27-A2 using RPMI 1640 broth buffered with morpholinepropanesulfonic acid (Sigma Chemical Co., St. Louis, MO), and the pH was adjusted to 7.0. Posaconazole was serially diluted (twofold) in media, and 100-μl aliquots were dispensed into microdilution plates for a final drug concentration range of 0.03125 to 16 μg/ml. Then, 100 μl of yeast inoculum containing 1 × 103 to 5 × 103 yeast cells/ml was added to each well. The plates were incubated at 35°C and read at 24 and 48 h by visual inspection and spectrophotometry at 490 nm. The MIC was defined as the lowest drug concentration that reduced growth by 50% compared to drug-free controls.
The ET and DD testing methods were performed according to the CLSI M44-A (4) method on Mueller-Hinton agar plates (10 cm, with 60 ml of agar) supplemented with 2% glucose and 0.5 μg of methylene blue (Hardy Diagnostics, Santa Maria, CA). For each isolate, duplicate plates were inoculated by dipping two sterile cotton swabs into the inoculum and evenly streaking the entire surface of the plates in three directions. After drying for 15 min, an E-test strip was applied to one plate, and two disks were applied to the other plate. The plates were incubated at 35°C. In addition to the posaconazole tests, quality control specimens were tested with a fluconazole E-test. The plates were read at 24 and 48 h. The MIC for the E-test and the inhibitory zone diameter for the disks were measured at the transition point where growth abruptly decreased as determined by a marked reduction in colony size, number, and density.
RESULTS
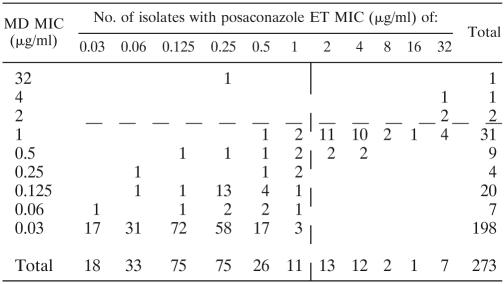
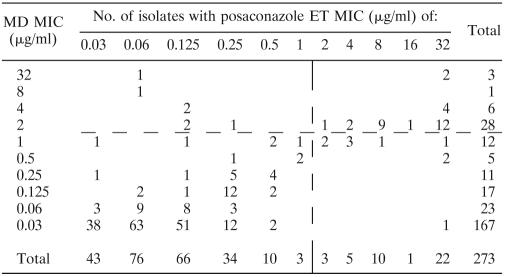
Table 1 summarizes the drug MICs measured by the MD and ET methods after 24 and 48 h of incubation. Table 2 summarizes the concordance between the ET and MD methods measured at 24 and 48 h. Values were considered concordant if the measured MICs by each method were within two or fewer dilutions of each other. Overall, concordance was 64.8% at 24 h and 82.6% at 48 h. Table 2 also summarizes the interpretive agreement for the two methods based on an arbitrary breakpoint of ≤1 μg/ml (14) for determining a susceptible isolate. Overall, the agreement between MD and ET was 87.8% at 24 h and 93.0% at 48 h. Tables 3 and 4 demonstrate the correlation between MD and ET with Spearman's rho values of 0.635 at 24 h and 0.648 at 48 h. The reading time specified for MD by CLSI 27-A2 and ET by the manufacturer is 48 h.
TABLE 1.
Summary of MIC50 and MIC90 values determined by the MD and ET methods at 24 and 48 h
| Species (no. of isolates) | MIC (μg/ml)
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MD method
|
ET method
|
|||||||||||
| 24 h
|
48 h
|
24 h
|
48 h
|
|||||||||
| MIC50 | MIC90 | Range | MIC50 | MIC90 | Range | MIC50 | MIC90 | Range | MIC50 | MIC90 | Range | |
| C. albicans (124) | 0.03 | 0.03 | 0.03-32 | 0.03 | 0.03 | 0.03-32 | 0.125 | 0.25 | 0.03-32 | 0.06 | 0.25 | 0.03-32 |
| C. dubliniensis (2) | 0.03 | 0.03 | 0.03-0.03 | 0.03 | 0.03 | 0.03-0.03 | 0.06 | 0.125 | 0.03-0.125 | 0.03 | 0.03 | 0.03-0.03 |
| C. glabrata (36) | 1.0 | 1.0 | 0.03-2.0 | 2.0 | 2.0 | 0.03-32 | 2.0 | 16 | 0.03-32 | 8.0 | 32 | 0.03-32 |
| C. krusei (20) | 0.125 | 0.125 | 0.03-0.25 | 0.25 | 0.25 | 0.03-1.0 | 0.25 | 0.50 | 0.06-0.50 | 0.25 | 0.50 | 0.06-1.0 |
| C. lusitaniae (3) | 0.03 | 0.03 | 0.03-0.03 | 0.03 | 0.03 | 0.03-0.03 | 0.03 | 0.03 | 0.03-0.03 | 0.03 | 0.03 | 0.03-0.03 |
| C. parapsilosis (44) | 0.03 | 0.125 | 0.03-4.0 | 0.03 | 0.125 | 0.03-32 | 0.125 | 1.0 | 0.03-32 | 0.06 | 0.5 | 0.03-32 |
| C. tropicalis (41) | 0.03 | 0.06 | 0.03-1.0 | 0.06 | 2.0 | 0.03-4.0 | 0.25 | 0.50 | 0.03-2.0 | 0.125 | 0.25 | 0.03-16 |
| Total (278) | 0.03 | 1.0 | 0.03-32 | 0.03 | 2.0 | 0.03-32 | 0.25 | 2.0 | 0.03-32 | 0.125 | 8.0 | 0.03-32 |
TABLE 2.
Concordance and agreement of MIC data measured by the MD and ET methods at 24 and 48 h
| Species | % Concordance
|
% Agreement
|
||
|---|---|---|---|---|
| 24 h | 48 h | 24 h | 48 h | |
| C. albicans | 56.8 | 87.2 | 96.8 | 97.6 |
| C. dubliniensis | 100 | 100 | 100 | 100 |
| C. glabrata | 72.2 | 55.6 | 25.0 | 77.8 |
| C. krusei | 100 | 100 | 100 | 100 |
| C. lusitaniae | 100 | 100 | 100 | 100 |
| C. parapsilosis | 86.0 | 93.0 | 97.7 | 95.3 |
| C. tropicalis | 39.0 | 70.7 | 97.6 | 85.4 |
| Total | 64.8 | 82.6 | 87.8 | 93.0 |
TABLE 3.
Plot of posaconazole ET MIC versus MD MIC at 24 ha
The vertical and horizontal dashed lines represent an arbitrary breakpoint MIC (14). Spearman's rho = 0.635.
TABLE 4.
Plot of posaconazole ET MIC versus MD MIC at 48 ha
The vertical and horizontal dashed lines represent an arbitrary breakpoint MIC (14). Spearman's rho = 0.648.
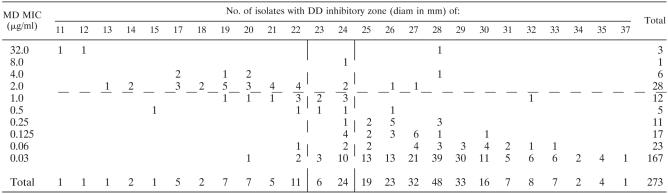
Comparison of MD MIC with DD inhibitory zone exhibited good correlation, as evidenced by a Spearman's rho values of −0.580 and −0.605 for the two disks at 24 h and Spearman's rho values of −0.551 and −0.571 at 48 h. Table 5 demonstrates the correlation between the MICs obtained by MD with the DD inhibitory zone diameters (for the BD disk) compared at the reading times specified by CLSI M27-A2 and CLSI M44-A. This comparison showed slightly lower correlation with a Spearman's rho value of −0.470. The results were similar for the Oxoid disk, and zone diameters correlated well between the two disk types, as shown by Pearson test values of 0.941 at 24 h and 0.960 at 48 h.
TABLE 5.
Plot of 24-h posaconazole disk inhibition zone diameter versus 48-h MD MICa
Comparison of the ET MIC with the DD inhibitory zone exhibited even better correlation, as evidenced by Spearman's rho values of −0.793 and −0.791 for the two disks at 24 h and by Spearman's rho values of −0.827 and −0.839 at 48 h.
DISCUSSION
MD and ET MICs show good concordance and interpretive agreement. The ET MIC was generally higher than the MIC, as determined by the MD method, at both 24 and 48 h. The ET MIC was generally one dilution higher at 24 h than at 48 h, in contrast to the MD method in which the 24-h MIC was usually lower. This phenomenon is explained by the fact that, for most Candida spp., there was little or no distinction between the ET zone of markedly decreased growth and the zone of no growth at 24 h. At 48 h and further growth, the distinction between the zones of markedly reduced growth and no growth became more prominent. In addition, the differences may relate to the use of glucose-supplemented Mueller-Hinton agar for ET as opposed to the manufacturer-recommended glucose-supplemented RPMI agar or the CLSI M27-A-recommended media used for MD. This medium was selected since it is specifically recommended by CLSI M44-A for use in routine agar-based antifungal susceptibility testing. This phenomenon was not seen in the more resistant species (C. glabrata and krusei), resulting in similar results using the ET and MD methods.
Concordance was higher at 48 h for all species (except C. glabrata) and was >85% for C. albicans, C. dubliniensis, C. krusei, C. lusitaniae, and C. parapsilosis. C. albicans disconcordance was mainly due to the phenomenon discussed above. C. tropicalis disconcordance was due to the well-described trailing growth phenomenon (1, 12). C. glabrata isolates showed the lowest rates of concordance with a much higher MIC measured by the ET than the MD method. The isolates with high MIC determined by ET method were the same isolates with high MICs as determined by the MD method; however, the ET measurements were several dilutions higher.
There are no established clinical breakpoints for posaconazole, so it is unclear whether these higher MICs determined by ET would change the “resistant” or “susceptible” interpretive category of these isolates compared to the MICs measured by MD method. However, when an arbitrary MIC breakpoint of ≤1 μg/ml was used to identify potentially susceptible isolates (14), all species except C. glabrata showed an agreement of >96% at 24 h. At 48 h, the MD and ET agreement for C. glabrata increased to >75% and increased for all species except C. tropicalis.
DD inhibitory zone diameters are highly reproducible and correlate well with ET and MD MICs. Correlation may have been decreased by the fact that MD MICs of <0.03 μg/ml were grouped and by trailing growth at 48 h.
Using the data in Tables 3 to 5, comparisons of the MD method with ET and DD using the error-rate-bounded method (10) and the arbitrary MIC breakpoint of ≤1 μg/ml are shown in Table 6. The numbers of major errors and very major errors are similar to the acceptable error rates recommended by the CLSI M23-A2 (3) for this method of analysis. Of the four isolates causing very major errors, three are C. tropicalis.
TABLE 6.
Comparison of MD, ET, and DD categorical agreement and error rates
| Comparison | % Categorical agreement | No. of errors/total no. of samples tested (%)
|
||
|---|---|---|---|---|
| Major errors | Very major errors | Indeterminate | ||
| MD vs ET at 24 h | 87.8 | 32/273 (11.7) | 1/273 (0.3) | |
| MD vs ET at 48 h | 93.0 | 10/273 (3.6) | 7/273 (2.5) | |
| MD at 48 h vs DD at 24 h | 12/273 (4) | 4/273 (0.7) | 30/273 (11.0) | |
The results of the present study are similar to those of previous studies comparing the MD, ET, and DD methods of susceptibility testing for other antifungal drugs (2, 9). The agar-based methods compare well to the reference method, especially for the more susceptible species of Candida. In the present study, C. glabrata accounted for most of the outlying results, although previous work with other antifungal drugs has shown better concordance between the MD and ET methods for this species (13). Agar-based methods are a valid alternative to MD methods for posaconazole susceptibility testing in Candida spp., although more clinical experience is needed prior to true breakpoint determination.
REFERENCES
- 1.Arthington-Skaggs, B. A., W. Lee-Yang, M. A. Ciblak, J. P. Frade, M. E. Brandt, R. A. Hajjeh, L. H. Harrison, A. N. Sofair, and D. W. Warnock. 2002. Comparison of visual and spectrophotometric methods of broth microdilution MIC endpoint determination and evaluation of a sterol quantitation method for in vitro susceptibility testing of fluconazole and itraconazole against trailing and nontrailing Candida isolates. Antimicrob. Agents Chemother. 46:2477-2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barry, A. L., and S. D. Brown. 1996. Fluconazole disk diffusion procedure for determining susceptibility of Candida species. J. Clin. Microbiol. 34:2154-2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clinical Laboratory Standards Institute. 1999. Development of in vitro susceptibility testing criteria and quality control parameters. Approved guideline, 2nd ed, vol. M23-A2. CLSI, Wayne, Pa.
- 4.Clinical Laboratory Standards Institute. 2002. Method for antifungal disk diffusion susceptibility testing in yeasts. Approved guideline, vol. M44-A. CLSI, Wayne, Pa.
- 5.Clinical Laboratory Standards Institute. 2002. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard, vol. M27-A2. CLSI, Wayne, Pa.
- 6.Espinel-Ingroff, A., M. Pfaller, S. A. Messer, C. C. Knapp, N. Holliday, and S. B. Killian. 2004. Multicenter comparison of the Sensititre YeastOne colorimetric antifungal panel with the NCCLS M27-A2 reference method for testing new antifungal agents against clinical isolates of Candida spp. J. Clin. Microbiol. 42:718-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gleason, T. G., A. K. May, D. Caparelli, B. M. Farr, and R. G. Sawyer. 1997. Emerging evidence of selection of fluconazole-tolerant fungi in surgical intensive care units. Arch. Surg. 132:1197-1202. [DOI] [PubMed] [Google Scholar]
- 8.Krcmery, V., and A. J. Barnes. 2002. Non-albicans Candida spp. causing fungaemia: pathogenicity and antifungal resistance. J. Hosp. Infect. 50:243-260. [DOI] [PubMed] [Google Scholar]
- 9.Matar, M. J., L. Ostrosky-Zeichner, V. L. Paetznick, J. R. Rodriguez, E. Chen, and J. H. Rex. 2003. Correlation between E-test, disk diffusion, and microdilution methods for antifungal susceptibility testing of fluconazole and voriconazole. Antimicrob. Agents Chemother. 47:1647-1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Metzler, C. M., and R. M. DeHaan. 1974. Susceptibility tests of anaerobic bacteria: statistical and clinical considerations. J. Infect. Dis. 130:588-594. [DOI] [PubMed] [Google Scholar]
- 11.Munayyer, H. K., P. A. Mann, A. S. Chau, T. Yarosh-Tomaine, J. R. Greene, R. S. Hare, L. Heimark, R. E. Palermo, D. Loebenberg, and P. M. McNicholas. 2004. Posaconazole is a potent inhibitor of sterol 14α-demethylation in yeasts and molds. Antimicrob. Agents Chemother. 48:3690-3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ostrosky-Zeichner, L., J. H. Rex, P. G. Pappas, R. J. Hamill, R. A. Larsen, H. W. Horowitz, W. G. Powderly, N. Hyslop, C. A. Kauffman, J. Cleary, J. E. Mangino, and J. Lee. 2003. Antifungal susceptibility survey of 2,000 bloodstream Candida isolates in the United States. Antimicrob. Agents Chemother. 47:3149-3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pfaller, M. A., D. J. Diekema, L. Boyken, S. A. Messer, S. Tendolkar, and R. J. Hollis. 2003. Evaluation of the Etest and disk diffusion methods for determining susceptibilities of 235 bloodstream isolates of Candida glabrata to fluconazole and voriconazole. J. Clin. Microbiol. 41:1875-1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pfaller, M. A., S. A. Messer, L. Boyken, R. J. Hollis, C. Rice, S. Tendolkar, and D. J. Diekema. 2004. In vitro activities of voriconazole, posaconazole, and fluconazole against 4,169 clinical isolates of Candida spp. and Cryptococcus neoformans collected during 2001 and 2002 in the ARTEMIS global antifungal surveillance program. Diagn. Microbiol. Infect. Dis. 48:201-205. [DOI] [PubMed] [Google Scholar]
- 15.Pfaller, M. A., S. A. Messer, R. J. Hollis, and R. N. Jones. 2001. In vitro activities of posaconazole (Sch 56592) compared with those of itraconazole and fluconazole against 3,685 clinical isolates of Candida spp. and Cryptococcus neoformans. Antimicrob. Agents Chemother. 45:2862-2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfaller, M. A., S. A. Messer, K. Mills, A. Bolmstrom, and R. N. Jones. 2001. Evaluation of Etest method for determining posaconazole MICs for 314 clinical isolates of Candida species. J. Clin. Microbiol. 39:3952-3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rex, J. H., M. A. Pfaller, T. J. Walsh, V. Chaturvedi, A. Espinel-Ingroff, M. A. Ghannoum, L. L. Gosey, F. C. Odds, M. G. Rinaldi, D. J. Sheehan, and D. W. Warnock. 2001. Antifungal susceptibility testing: practical aspects and current challenges. Clin. Microbiol. Rev. 14:643-658. [DOI] [PMC free article] [PubMed] [Google Scholar]