Abstract
High-resolution images of the chlorophyll fluorescence parameter Fq′/Fm′ from attached leaves of commelina (Commelina communis) and tradescantia (Tradescantia albiflora) were used to compare the responses of photosynthetic electron transport in stomatal guard cell chloroplasts and underlying mesophyll cells to key environmental variables. Fq′/Fm′ estimates the quantum efficiency of photosystem II photochemistry and provides a relative measure of the quantum efficiency of non-cyclic photosynthetic electron transport. Over a range of light intensities, values of Fq′/Fm′ were 20% to 30% lower in guard cell chloroplasts than in mesophyll cells, and there was a close linear relationship between the values for the two cell types. The responses of Fq′/Fm′ of guard and mesophyll cells to changes of CO2 and O2 concentration were very similar. There were similar reductions of Fq′/Fm′ of guard and mesophyll cells over a wide range of CO2 concentrations when the ambient oxygen concentration was decreased from 21% to 2%, suggesting that both cell types have similar proportions of photosynthetic electron transport used by Rubisco activity. When stomata closed after a pulse of dry air, Fq′/Fm′ of both guard cell and mesophyll showed the same response; with a marked decline when ambient CO2 was low, but no change when ambient CO2 was high. This indicates that photosynthetic electron transport in guard cell chloroplasts responds to internal, not ambient, CO2 concentration.
Stomata are the main routes for leaf gas exchange, controlling CO2 uptake and transpiration. Stomatal movements are regulated by both internal and external factors. Opening of stomata is stimulated by low CO2 concentrations, blue light, and other photosynthetically active wavelengths, whereas stomatal closure occurs in response to a number of environmental cues, most notably darkness, low air humidity, and high temperature (for reviews, see Assmann, 1993; Willmer and Fricker, 1996). Stomatal movements are brought about through changes in turgor within guard cells and accessory cells. This involves the active (energy requiring) net accumulation or loss of K+ ions and the parallel loss or accumulation of organic solutes, such as malate and Suc (e.g. Outlaw, 1996; Willmer and Fricker, 1996; Asai et al., 2000).
Chloroplasts are a characteristic feature of guard cells and are present in most (but not all) species, and they show photosynthetic electron transport (Willmer and Fricker, 1996; Tsionsky et al., 1997). Although guard cell chloroplasts are generally smaller, less numerous, and have fewer grana than mesophyll chloroplasts (Sack, 1987; Willmer and Fricker, 1996), photophosphorylation, on a chlorophyll basis, has been reported to be as high as 80% of that in the mesophyll cells (Shimazaki and Zeiger, 1985). Although guard cells have much lower chlorophyll contents than mesophyll cells (25- to 100-fold lower), they are also considerably smaller (approximately 10-fold smaller; Table III.1 in Willmer and Fricker, 1996), so their chloroplasts could represent a significant energy source for guard cell ion transport and other processes (Wu and Assman, 1993). The role of guard cell chloroplasts in CO2 fixation is still a matter of debate (e.g. Lee and Bowling, 1995; Willmer and Fricker, 1996; Lu et al., 1997). Although some researchers have concluded that guard cell chloroplasts do not reduce CO2 through the Calvin cycle at appreciable rates (Outlaw, 1989; Reckmann et al., 1990), they could function as part of an environmental sensing and signaling system (Outlaw, 1989; Goh et al., 1999). For example, Melis and Zeiger (1982) found that photosynthetic electron transport in guard cells of Chlorophytum comosum and Aspidistra eliator responded to changes in ambient CO2 concentration (Ca), whereas Cardon and Berry (1992) observed modulation of the chlorophyll fluorescence signal from guard cell chloroplasts in response to changes in Ca and ambient O2 concentration, confirming that Rubisco was acting as a significant sink for ATP and NADPH. In these experiments, changes in fluorescence with ambient O2 were only observed at low Ca (< 130 μmol mol−1), indicating that photorespiration was the main sink for ATP and NADPH at low Ca and that this was inhibited at higher Ca.
Previous measurements of chlorophyll fluorescence from guard cells have been largely restricted to epidermal peels (Melis and Zeiger, 1982; Ogawa et al., 1982) or guard cell protoplasts (Goh et al., 1999). Cardon and Berry (1992) made the important advance of studying intact tissue; however, they were only able to compare changes in the steady-state fluorescence signal (F′) from guard cell chloroplasts located within white regions of variegated leaves of tradescantia (Tradescantia albiflora) with those from mesophyll chloroplasts within green regions of the same leaves. Although guard cells in the white regions of leaves are capable of opening, they are obviously not subject to the same influence of mesophyll cell activity as they are in green areas (e.g. Scarth and Shaw, 1951).
It is well established that the “Genty factor” fluorescence parameter (Genty et al., 1989) can provide a good estimate of the quantum efficiency of photosystem (PS)II photochemistry, and consequently can be used to examine changes in photosynthetic electron transport. Images of the Genty factor (defined by Fq′/Fm′ in the terminology used in this paper) from guard cells and mesophyll cells of green leaves have been produced previously (Oxborough and Baker, 1997a; Baker et al., 2001). In this study, the high resolution imaging system described previously by Oxborough and Baker (1997a) has been combined with an infrared gas analyzer system to compare the responses of photosynthetic electron transport in chloroplasts of guard and mesophyll cells in attached variegated and green leaves of tradescantia and commelina (Commelina communis) to changes in photosynthetically active photon flux density (PPFD), Ca, and leaf-air vapor pressure difference (VPD).
RESULTS
Fluorescence Parameters
The calculation of useful fluorescence parameters, irrespective of whether fluorescence measurements are made with conventional modulated fluorimeters or imaging systems, requires that the fluorescence signal is recorded while the photosynthetic system is in well-defined states. With dark-adapted material, the Fo level of fluorescence is recorded at very low PPFD (less than 1 μmol m−2 s−1), which leaves virtually all PSII centers in the “open” state (capable of photochemistry), whereas the Fm level of fluorescence is recorded during a short light pulse of very high PPFD (typically less than 1 s at several thousand μmol m−2 s−1), which transiently drives a very high proportion of PSII centers into the “closed” state (making the capacity for photochemistry close to zero). With light-adapted material, the equivalent terms are Fo′ and Fm′. At any point between Fo′ and Fm′ (where a variable proportion of PSII centers are in the open state), the fluorescence signal is termed F′. The difference between Fm and Fo is termed Fv and the difference between Fm′ and Fo′ is termed Fv′. The term Fq′ has recently been introduced to denote the difference between Fm′ and F′ measured immediately before application of the saturating pulse used to measure Fm′ (Oxborough and Baker, 2000; Oxborough et al., 2000; Baker et al., 2001). In theory (Baker et al., 2001), the fluorescence parameter Fq′/Fm′ provides a good estimate of the quantum efficiency of PSII photochemistry at the point of measurement (hereafter referred to as PSII operating efficiency). Fq′/Fm′ is actually the product of two other useful fluorescence parameters, Fv′/Fm′ and Fq′/Fv′:
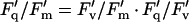 |
1 |
Fv′/Fm′ provides an estimate of the maximum quantum efficiency of PSII photochemistry (PSII maximum efficiency), i.e. the PSII operating efficiency when all PSII centers are in the open state at the point of measurement. Its value is largely determined by down-regulation, which appears to involve the operation of one or more processes that increase the rate constant for non-radiative decay within the PSII pigment matrix, and which is responsible for non-photochemical quenching of chlorophyll fluorescence. The parameter Fq′/Fv′ is a factor (the PSII efficiency factor) that relates the PSII maximum efficiency to the PSII operating efficiency. Its value is non-linearly related to the proportion of PSII centers in the open state, which determines the level of photochemical quenching of chlorophyll fluorescence (Baker et al., 2001). The value of Fo′ is required for the calculation of both Fv′/Fm′ and Fq′/Fv′ (since Fv′ = Fm′ − Fo′). Images of Fo′ cannot be taken with the imaging system used here (or with any other imaging system that we are aware of). However, Oxborough and Baker (1997b) demonstrated that Fo′ can be calculated from the following equation:
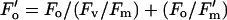 |
2 |
Recently it has been suggested that this method of estimating Fo′ cannot be applied in situations where plants are stressed and significant amounts of photoinhibition may occur (Maxwell and Johnson, 2000). This is not the case. The only requirements for the calculation of Fo′ from the above equation are that (a) all the PSII centers are open at the point at which Fo is measured, (b) there is no reversal of down-regulation between the measurement of Fo and Fm, and (c) there is no reversal of photoinhibition between the measurement of Fm′ and Fm. In reality, given the inherent difficulty of accurately measuring Fo′, it can be reasonably argued that the calculation of Fo′ from the above equation gives a more accurate estimate of the parameter than does direct measurement.
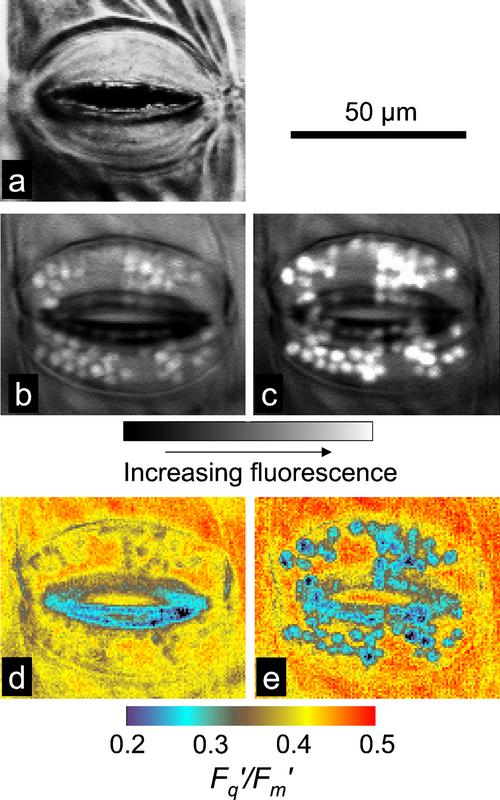
Images of F′ from stomatal guard cell chloroplasts and the underlying mesophyll in a tradescantia leaf are shown in Figure 1, b and c. Chlorophyll fluorescence from individual chloroplasts in the guard cells can be clearly identified (Fig. 1c) and isolated using the FluorImager software (see “Materials and Methods”; Oxborough and Baker, 1997a). Consequently, images can be generated for Fq′/Fm′ of isolated chloroplasts in the guard cells and adjacent mesophyll tissue (Fig. 1e; Oxborough and Baker, 1997a). Stomatal aperture (maximum pore width) can be measured from the reflected light image (Fig. 1a).
Figure 1.
Images of reflected light (a) and fluorescence parameters (b–e) from stomatal guard cells and surrounding mesophyll in a tradescantia leaf. Images of F′ taken using a 695-nm longpass filter (b) or a 680-nm bandpass filter (c); images of Fq′/Fm′ taken using a 695-nm longpass filter (d) or a 680-nm bandpass filter (e). Values of Fq′/Fm′ for guard cell chloroplasts can be determined from images d and e by isolating the chloroplasts using the FluorImager software (see “Materials and Methods”; Oxborough and Baker, 1997).
The main aim of this study was to compare changes in Fq′/Fm′ from guard cell chloroplasts and mesophyll cells in response to changes in PPFD, Ca, and VPD. While conducting these experiments, it was clear that lower values of Fq′/Fm′ for both guard and mesophyll cells were obtained with closed compared with open stomata, which we attribute to the restricted CO2 diffusion into the leaf at any given external CO2 concentration. In addition, the differences between values of Fq′/Fm′ for guard and mesophyll cells were usually larger for tradescantia than for commelina, which is probably because tradescantia has fewer stomata per area and only on the lower side, and therefore has more restricted CO2 exchange compared with commelina, which is amphistomatous.
Imaging of Fluorescence Using 680-nm Bandpass and 695-nm Longpass Filters
Although the editing tools built into the imaging software (FluorImager) allow for the isolation of guard cell chloroplasts from within an image, they cannot remove fluorescence that might be transmitted through guard cell chloroplasts from underlying mesophyll cells. Chlorophyll fluorescence of shorter wavelengths (between approximately 675 and 685 nm) is strongly reabsorbed, because these wavelengths are close to the long wavelength maximum of the chlorophyll a absorption spectrum. Consequently, use of a 680-nm bandpass filter (Fig. 1c), rather than a 695-nm longpass filter (Fig. 1b), gives much greater weight to fluorescence from chloroplasts that are close to the imaged surface (including the chloroplasts within guard cells). With the imaging system used here, the incident PPFD is predominantly at wavelengths that are absorbed well by chlorophylls a and b. Consequently, there is an expectation that chloroplasts in cells at the surface will exhibit lower values of Fq′/Fm′ than those in cells deep within the leaf, simply because they are absorbing more photons. Using a 680-nm bandpass filter, rather than a 695-nm longpass filter, when imaging also decreases the contribution of PSI fluorescence to the overall fluorescence signal. The yield of fluorescence from PSI is reasonably constant and independent of PPFD (Dau, 1994; Pfündel, 1998). A consequence of this is that PSI fluorescence makes a proportionally larger contribution to the fluorescence signal at F′ than at Fm′. Removing most of the PSI signal with the 680-nm bandpass filter has the effect of increasing the measured value of Fq′/Fm′ (compare mesophyll regions in Fig. 1d with Fig. 1e).
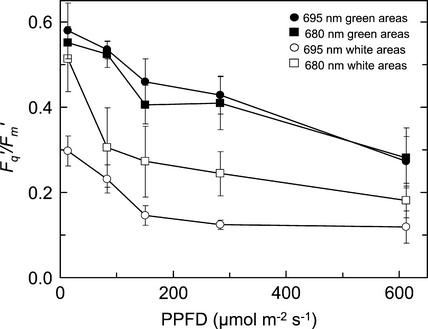
Changes in Fq′/Fm′ with increasing PPFD for guard cell chloroplasts of a variegated tradescantia leaf are shown in Figure 2. These data are derived from images of guard cell chloroplasts that were isolated from images of white and green areas of a tradescantia leaf. These images were taken using either the 680-nm bandpass filter or the 695-nm longpass filter, and the values for Fq′/Fm′ for white region guard cell chloroplasts were significantly lower than those for green region guard cell chloroplasts (P < 0.001). As expected, use of the 680-nm bandpass filter resulted in higher values for Fq′/Fm′ from the white areas (P = 0.10), due to removal of most of the PSI signal. Over the green areas, the two filters produced very similar values of Fq′/Fm′. These data suggest that the increase in Fq′/Fm′ that results from removing most of the PSI fluorescence with the 680-nm bandpass filter was offset by a decrease in Fq′/Fm′ because of the decrease in the contribution of fluorescence from cells located at depth within the leaf.
Figure 2.
Responses of Fq′/Fm′ to increasing PPFD of chloroplasts of guard cells of a tradescantia leaf. Data are derived from images of F′ and Fm′ taken from white areas (○, □) or green areas (●, ▪). Chlorophyll fluorescence was measured using a 695-nm longpass filter (○, ●) or 680-nm bandpass filter (□, ▪). Measurements were made at 25°C and a Ca of 356 μmol mol−1. Data are the means of six replicates ± se.
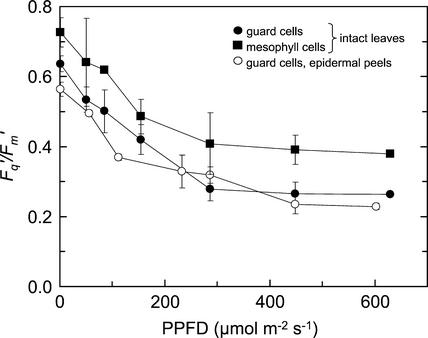
Although use of the 680-nm bandpass filter clearly reduces contamination of the fluorescence signal from guard cell chloroplasts by fluorescence from the underlying mesophyll cells, it is inevitable that some contamination will result. The extent of this contamination is difficult to quantify precisely, although a number of observations suggest that it is not large. First, if there was a significant level of contamination of the guard cell chloroplasts fluorescence with fluorescence from underlying mesophyll cells, Fq′/Fm′ values from guard cell chloroplasts in intact leaves would be higher than those from the guard cell chloroplasts in epidermal peels. Figure 3 shows that Fq′/Fm′ values from guard cell chloroplasts of intact leaves were indistinguishable those from peels but significantly different from those of mesophyll cells within the same leaves (P = 0.02; Fig. 3). Further evidence for a low level of contamination came from comparison of the fluorescence signal (F′) from guard cell chloroplasts of green and white areas of tradescantia leaves. The values of F′ from the guard cell chloroplasts in the green, compared with the white, areas were indistinguishable (96.6 ± 33.7 compared with 91.2 ± 4.28 at PPFD of 93 μmol m−2 s−1 and 154.2 ± 50.9 compared with 145.4 ± 57.4 at 256 μmol m−2 s−1), which would not be the case if there had been significant contamination from the mesophyll cells.
Figure 3.
Responses of Fq′/Fm′ to increasing PPFD for mesophyll (▪) and guard cells (●) of a commelina leaf, and for guard cells in a peel from a commelina leaf (○). Measurements were made at 25°C and a Ca of 360 μmol mol−1. Data are the means of four replicates ± se.
Responses to Changing PPFD
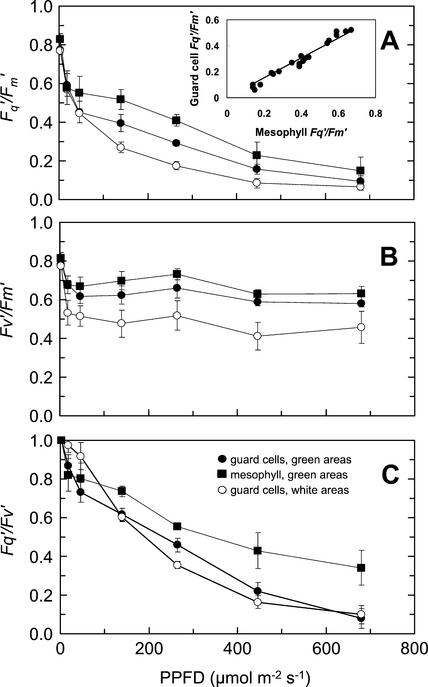
Images from green and white areas of an intact tradescantia leaf were taken over a range of PPFDs between 0 and 680 μmol m−2 s−1 with the leaf at steady-state photosynthesis. The changes in Fq′/Fm′, Fv′/Fm′, and Fq′/Fv′ with increasing PPFD, calculated from the images, were qualitatively similar for both guard and mesophyll cells (Fig. 4). There were significantly lower values of Fq′/Fm′ from guard cell chloroplasts within both green and white areas of leaves, compared with mesophyll cells, at PPFDs above 20 μmol m−2 s−1 (Fig. 4A; P = 0.031 and P = 0.005, for green and white areas respectively). Values of Fq′/Fm′ from guard cell chloroplasts in the white area were noticeably lower than from those in the green area at PPFDs between 46 μmol m−2 s−1 and 446 μmol m−2 s−1 (P = 0.13). The lower values of Fq′/Fm′ from guard cell chloroplasts in the white area resulted from lower values of Fv′/Fm′ (P = 0.14), suggesting a higher level of down-regulation in these cells. The differences in Fq′/Fm′ between the mesophyll and guard cell chloroplasts in green areas at PPFDs above 20 μmol m−2 s−1 were due to higher values of Fq′/Fv′ within the mesophyll cells (P = 0.013; Fig. 4C), as there were no statistically significant differences in Fv′/Fm′. There was a close linear relationship between Fq′/Fm′ for guard and mesophyll cells over the PPFD range (see inset in Fig. 4A), suggesting that the response of the photosynthetic apparatus to changing PPFD was similar in both cell types.
Figure 4.
Responses of the fluorescence parameters Fq′/Fm′ (A), Fv′/Fm′ (B), and Fq′/Fv′ (C) of a variegated tradescantia leaf to increasing PPFD. Data were obtained from guard cells in white (○) areas of the leaf and from mesophyll (▪) and guard cells (●) in green areas. Measurements were made at 25°C and a Ca of 360 μmol mol−1. Data are the means of six replicates ± se. The inset in A shows the relationship between Fq′/Fm′ for mesophyll and guard cells in green areas of the leaf over the range of PPFDs (the linear relationship is defined by y = 0.76x; r2 = 0.92).
Responses to Changes in Ambient CO2 and O2 Concentration
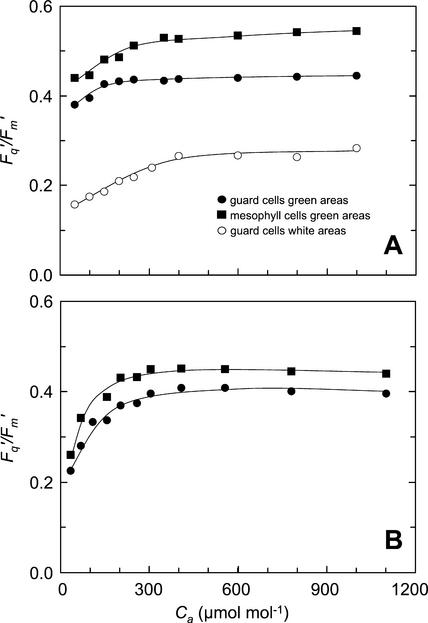
To further examine whether there may be differences in photosynthetic metabolism between the two cell types, the responses of the cells to changes in ambient CO2 and O2 concentration were studied. The changes in Fq′/Fm′ within guard cell chloroplasts and mesophyll cells of attached leaves of tradescantia and commelina, in response to increasing Ca, are shown in Figure 5. Fq′/Fm′ increased with Ca up to approximately 350 μmol mol−1, after which the response curves flattened out. Values of Fq′/Fm′ of guard cell chloroplasts in the green regions of tradescantia and in commelina leaves were lower than those from the adjacent mesophyll cells over the Ca range. In tradescantia (Fig. 5A), stomata started fully open in the white area, with apertures of approximately 30 μm, and partially open in the green area (with apertures of approximately 18 μm compared with 30 μm for fully open stomata). Values of Fq′/Fm′ of guard cell chloroplasts within the white area of tradescantia were very much lower than for mesophyll cells (by 50%–60%) or guard cell chloroplasts in green areas (by 45%–50%). The stomata in the commelina leaves (Fig. 5b) started fully open with apertures of approximately 35 μm, therefore the difference between Fq′/Fm′ values of guard cell chloroplasts and mesophyll was not so large; however, the response to CO2 in the two cell types was the same and similar to that of tradescantia.
Figure 5.
Response of Fq′/Fm′ of tradescantia (A) and commelina leaves (B) to increasing Ca. A, Data were obtained from guard cells in white (○) areas of the tradescantia leaf and from mesophyll (▪) and guard cells (●) in green areas. B, Data are from mesophyll (▪) and guard cells (●) of the commelina leaf. Measurements were made at an ambient temperature of 25°C and at PPFDs of 215 and 265 μmol m−2 s−1 for tradescantia and commelina leaves, respectively.
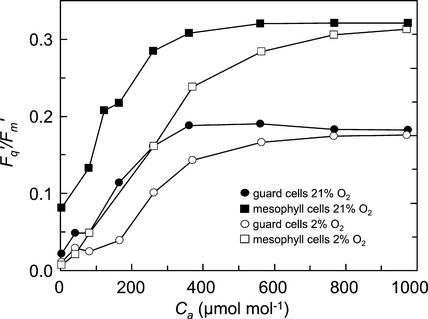
The responses of Fq′/Fm′ of guard and mesophyll cells of a green area of a tradescantia leaf to changes in Ca at 2% and 21% O2 are shown in Figure 6. Fq′/Fm′ of both cell types was lower at 2% O2, compared with 21% O2, over a Ca range of 0 to 600 μmol mol−1 (Fig. 6). As Ca increased, the difference in Fq′/Fm′ at the two O2 concentrations decreased, and became close to zero at 974 μmol mol−1. As would be expected, the values of Fq′/Fm′ in 21% O2 when the stomata were closed (Fig. 6) were much lower at any given Ca than was the case when the stomata were open (Fig. 5A), because of restricted CO2 diffusion.
Figure 6.
Response of Fq′/Fm′ of mesophyll (□, ▪) and guard cells (○, ●) to increasing Ca in the green areas of a tradescantia leaf in an atmosphere containing 2% (○, □) or 21% O2 (●, ▪). Measurements were made at a PPFD of 215 μmol m−2 s−1 and an ambient temperature of 25°C.
The responses of photosynthetic electron transport, as monitored by Fq′/Fm′, in guard and mesophyll cells in response to changes in ambient CO2 and O2 were qualitatively similar (Figs. 5 and 6) suggesting that photosynthetic metabolism in the two cell types is similar.
Responses to Changes in VPD
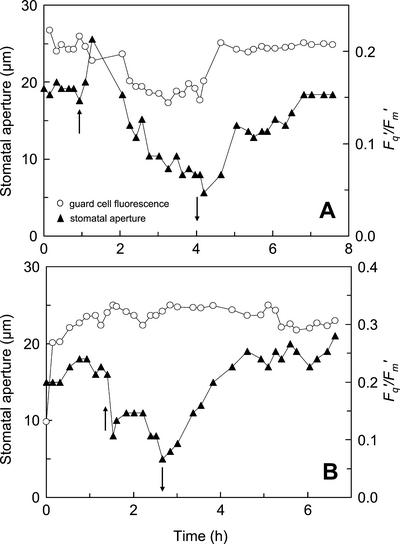
The effects of changes in VPD on stomatal aperture and Fq′/Fm′ of guard cells in white areas of a tradescantia leaf at a Ca of 80 or 220 μmol mol−1 are shown in Figure 7. Stomatal closure was stimulated by rapidly decreasing the humidity in the leaf cuvette so that VPD increased from approximately 1.0 to 1.5 to 3.0 to 3.5 kPa. At low Ca (80 μmol mol−1), the initial effect of the increase in VPD was a rapid hydropassive opening of the stomata over approximately 5 min (Fig. 7A), because of changes in the balance between epidermal and guard cell turgor pressures (Ståfelt, 1955; Kappen et al., 1987; Willmer and Fricker, 1996). The initial increase in stomatal aperture was followed by a much slower decrease (over 2–3 h), which was accompanied by a decrease in Fq′/Fm′. When VPD was decreased to the original level, both stomatal aperture and Fq′/Fm′ increased to within a few percent of their original values (over a period of approximately 1 h). At a higher Ca of 200 μmol mol−1, the same increase in the VPD induced a much more rapid closure of the stoma with little effect on Fq′/Fm′ (Fig. 7B). Returning the VPD to original levels at this higher Ca resulted in a slow increase in stomatal aperture over approximately 2 h (Fig. 7B).
Figure 7.
The effects of changes in VPD on stomatal aperture (▴) and Fq′/Fm′ (○) from guard cells in white areas of a tradescantia leaf at a Ca of 80 μmol mol−1 (A) or 220 μmol mol−1 (B). Stomatal aperture was determined from reflected light images. Measurements were made at a PPFD of 215 μmol m−2 s−1 and an ambient temperature of 25°C. ↑ indicate the time at when VPD was increased from 1.0 to 1.5 kPa to 3.0 to 3.5 kPa; ↓ indicate the time when VPD was decreased back to 1.0 to 1.5 kPa.
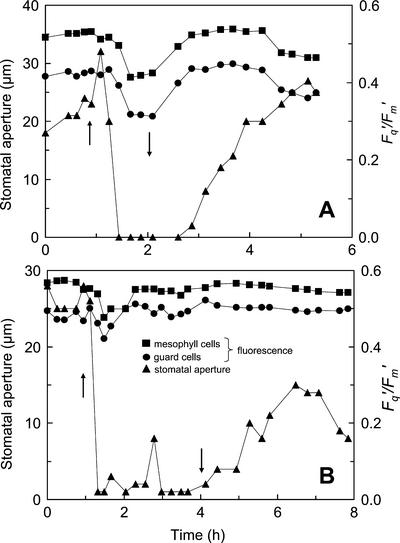
Similar experiments were conducted on a commelina leaf, but using higher Ca values (180 and 330 μmol mol−1). At the Ca of 180 μmol mol−1, the effect of increasing the VPD on stomatal aperture was qualitatively the same (Fig. 8A) as was observed for the white areas of the tradescantia leaf (Fig. 7A). However, the response was much more rapid and more extreme; a large, transient hydropassive movement being followed by almost complete closure within 10 min. Decreases in Fq′/Fm′ within both guard cell chloroplasts and mesophyll cells occurred almost as rapidly within 15 min (Fig. 8A). Before the increase in VPD, Fq′/Fm′ of guard cells was approximately 10% lower than from the mesophyll cells. After the increase in VPD, this difference increased to approximately 15%. When the VPD was decreased to the original level, stomatal aperture and Fq′/Fm′ of both cell types also increased to the pretreatment levels. The recovery of Fq′/Fm′ in both guard and mesophyll cells of commelina from the VPD-induced decrease occurred considerably before the stomata were fully open. This could reflect patchy stomatal opening on the lower (imaged) surface or a more rapid opening of stomata on the upper surface of the leaf. Commelina leaves are amphistomatous, unlike tradescantia, with the ratio of stomatal density between upper and lower surface in our plant material being approximately 1:3. At the higher Ca of 330 μmol mol−1, a similar stomatal closing response to VPD was observed, with Fq′/Fm′ in both the guard and mesophyll cells showing a small initial decrease, which returned to its initial value within about 30 min, well before any increase in stomatal aperture (Fig. 8B).
Figure 8.
The effects of changes in VPD on stomatal aperture (▴) and Fq′/Fm′ from mesophyll (▪) and guard cells (●) in a commelina leaf at a Ca of 180 μmol mol−1 (A) or 330 μmol mol−1 (B). Stomatal aperture was determined from reflected light images. Measurements were made at a PPFD of 265 μmol m−2 s−1 and an ambient temperature of 25°C. ↑ indicate the time at when VPD was increased from 1.0 to 1.5 kPa to 3.0 to 3.5 kPa; ↓ indicate the time when VPD was decreased back to 1.0 to 1.5 kPa.
DISCUSSION
This is the first study, to our knowledge, to show the effect of changing environmental conditions on PSII photochemical efficiency (measured by Fq′/Fm′) within guard and mesophyll cells of intact, green leaves. Although there have been a number of chlorophyll fluorescence studies on stomata in white areas of variegated leaves (Zeiger et al., 1981; Cardon and Berry, 1992), the PPFD response curves in Figure 4A show Fq′/Fm′ of guard cells to be substantially lower in the white than in the green areas of variegated tradescantia leaves. We agree with Scarth and Shaw (1951) that the responses of the guard cells in white areas of leaves cannot be considered to be indicative of the responses in green tissues. Figure 7 also confirms that guard cells from albino portions of leaves are slower to move than those in the green areas (Scarth 1932).
We found (Figs. 3 and 4A) that there was no difference in the dark adapted Fv/Fm of guard and mesophyll cells, whereas in the light Fq′/Fm′ of guard cell chloroplasts was lower than that of mesophyll cells, which confirms for intact, photosynthesizing leaves the results of Goh et al. (1999) with Vicia faba protoplasts. A marked decrease in Fv′/Fm′ upon illumination was observed for both mesophyll and guard cell chloroplasts (Fig. 4B), which is consistent with the substantial light-induced, non-photochemical quenching observed previously in V. faba guard cell protoplasts (Goh et al., 1999). The above results agree with other studies on guard cells of both V. faba and Arabidopsis, which indicated that some zeaxanthin was maintained in the dark and that additional zeaxanthin was produced on exposure to low light levels (Frechilla et al., 1999). The accumulation of zeaxanthin would explain the observed rapid drop in Fv′/Fm′ at low PPFD levels, because this could result in an increase in non-radiative decay of excitation energy. Our data for both intact leaves (Figs. 2 and 4–8) and epidermal peels (Fig. 3) showing high PSII operating efficiencies support the suggestion from a number of earlier studies that guard cell chloroplasts are capable of photophosphorylation (Melis and Zeiger, 1982; Shimazaki and Zeiger, 1985; Mawson and Zeiger, 1991; Goh et al., 1999). The fact that Fq′/Fm′ is only 20% to 30% lower in the guard cells compared with the mesophyll cells (Figs. 3 and 4) would imply that the photosynthetic electron transport rate in the guard cell chloroplasts is likely to be 70% to 80% of that in chloroplasts of mesophyll cells, if light absorption was similar, which seems a reasonable assumption. However, we have not attempted to estimate electron transport rates from Fq′/Fm′, because there are uncertainties in the exact light absorption and contribution of PSI fluorescence for guard and mesophyll chloroplasts.
Modulation of the steady-state fluorescence signal (F′) from guard cells within white areas of Tradescantia leaves by ambient O2 concentration was observed by Cardon and Berry (1992), and was taken as evidence for photorespiration (and hence Rubisco oxygenase activity) within these cells. A major difficulty with the interpretation of these data was that F′ is affected by both photochemistry (photochemical quenching) and down-regulation (non-photochemical quenching). Consequently, decreases in F′ induced by increases in O2 concentration, although consistent with an increase in photochemical quenching of chlorophyll fluorescence (because of increased photorespiration producing an increased rate of electron transport), could also be attributable to increases in non-photochemical quenching (down-regulation). However, we have demonstrated a very clear codependence of the more definitive parameter Fq′/Fm′ in guard cell chloroplasts on CO2 and O2 concentration (Fig. 6). Decreasing the concentration of ambient O2 at low CO2 markedly decreased the PSII operating efficiency. As Cardon and Berry (1992) did, we interpret this O2-CO2 interaction as strong evidence for Rubisco-mediated carbon assimilation as a sink for the products of PSII electron transport in guard cell chloroplasts. Furthermore, as the fluorescence in guard cells showed very similar O2-CO2 sensitivity to that of the mesophyll this must indicate that the proportion of electron transport being used by Rubisco is similar in the two cell types. This Calvin cycle activity may have an important role in producing signal metabolites (Outlaw, 1989; Reckmann et al., 1990; Goh et al., 1999). Alternatively, if the above logic on the actual rates of photosynthetic electron transport is accepted, the similarity of the responses of mesophyll and guard cell chloroplasts suggests that Rubisco activity in guard cell chloroplasts is broadly comparable with that of the mesophyll cells. When extrapolating to the whole cell, the photosynthetic activity in guard cells would be much lower compared with the underlying spongy mesophyll (our results are from the abaxial surface) due to the 20- to 50-fold lower chlorophyll content per cell (Table III.1 in Willmer and Fricker, 1996). However, the guard cell volume is approximately a tenth that of the spongy mesophyll cells, so the possible contribution of such CO2 assimilation to the guard cell carbon balance might be about one-third to one-tenth that of mesophyll cells. We can offer no explanation for the difference between this fluorescence-derived, in vivo estimate and the biochemical assays of Outlaw and colleagues (Outlaw et al., 1979; Outlaw, 1989) and Reckmann et al. (1990), which have suggested minimal Rubisco activity and Calvin cycle contribution (in broad bean [V. faba] and pea [Pisum sativum], respectively).
Our consistent observation was that Fq′/Fm′ for guard cell chloroplasts was lower than for mesophyll cells. The difference between the two cell types varied considerably depending upon the species and to a smaller extent on stomatal aperture, which ranged from approximately 5% lower in commelina leaves with fully open stomata (Fig. 5B) to more than 40% lower in green areas of tradescantia with closed stomata (Fig. 6). Small differences may be simply attributable to differences in the amount of light absorbed by the two cell types. In addition, in green areas of tradescantia and commelina with reduced stomatal aperture, the lower Fq′/Fm′ values from guard cells compared with mesophyll cells could reflect reduced availability of CO2 to the guard cells. However, guard cells are obviously closer to the external atmosphere, and have numerous mitochondria (Willmer and Fricker, 1996), so this would be surprising. Alternatively, differences in the response of the two cell types to environmental conditions could produce differences in organization and/or poising of the photosynthetic systems, which may result in differences in the PSII operating efficiency.
The time courses of Fq′/Fm′ and stomatal aperture changes shown in Figures 7 and 8 indicate that Fq′/Fm′ of both the mesophyll and guard cells is strongly affected by CO2 supply through the stomatal aperture. Similarly, when the internal CO2 concentration was changed by manipulation of Ca changes in Fq′/Fm′ were observed (Fig. 5). The simplest conclusion is that the change in guard cell Fq′/Fm′ as aperture changed was not due to any change in guard cell metabolism but solely due to the change in CO2 diffusion through the stomatal pore, because there was no change in Fq′/Fm′ at high Ca even though aperture changes were as large as those observed at low Ca (Figs. 7A and 8B). During preliminary studies it was observed that illumination of a large area of the adaxial surface of the leaf was necessary to determine reliable and reproducible responses of Fq′/Fm′and stomatal aperture to CO2, O2, and humidity. When only a small area on the abaxial leaf surface was illuminated through the microscope lens, then the effects of high internal CO2concentrations (presumably resulting from respiration in surrounding dark areas of the leaf) overrode any influences of changes in Ca.
The highly sensitive response of Fq′/Fm′ to CO2 supply mediated via the guard cell pore emphasizes that stomatal aperture must to be taken into account in assessments of photosynthetic activity using fluorimeters (Maxwell and Johnson, 2000). It also reveals another key aspect of the sensitivity of stomatal metabolism to CO2. Bulk stomatal conductance derived from gas exchange measurements with attached leaves has been shown to respond to changes Ci, not Ca (Mott, 1988). The data presented in Figures 7 and 8 indicate that Ci must be sensed by a guard cell metabolic process directly. If stomata responded to Ca (or a combination of Ca and Ci) there would be either no (or a reduced) response of Fq′/Fm′ to changes in stomatal aperture, and hence Ci. However, the changes in guard cell Fq′/Fm′ matched those of the adjacent mesophyll cells in all cases, indicating that the CO2 supply route and sensitivity are the same for both guard and mesophyll cells.
The responses of guard cell Fq′/Fm′ to changing Ca (Figs. 5 and 6) indicate that the largest changes occur below a Ca of 400 μmol mol−1, and closely mirror the previously observed response of stomatal conductance to changing Ca (e.g. Morison, 1998). Many of the physiological studies seeking to elucidate the mechanism behind the CO2sensitivity of stomata have focused on large changes and comparisons between Ca levels of 350, 700, or 1,000 μmol mol−1 and CO2-free air, whereas leaf conductance measurements show high sensitivity over changes of 50 μmol mol−1 or less (see reviews by Morison, 1998; Assmann, 1999). Our measurements on guard cells in intact leaves reveal, for the first time, large changes in Fq′/Fm′ in response to changing Ca, and demonstrate that small changes in Ca produce large changes in photosynthetic electron transport rate in guard cells. However, whereas stomatal conductance declines with increasing Ca, PSII operating efficiency increases. Farquhar and Wong (1984) and Jarvis and Davies (1998) have proposed that a carbon metabolite pool in the guard cells may be the link between increased carbon assimilation and reduced stomatal conductance. Our results showing CO2 and O2 modulation of photosynthetic electron transport and, by inference, Calvin cycle activity in the guard cells are compatible with this hypothesis. In addition, our evidence for Calvin cycle activities in both guard and mesophyll cells provides an explanation for the parallel acclimation of CO2 assimilation rate and stomatal conductance sometimes observed when plants are grown at high Ca (Morison, 1998; Assmann, 1999; Lodge et al., 2001).
CONCLUSIONS
This study has shown that guard cell chloroplasts have a 20% to 30% lower quantum efficiency of photosynthetic electron transport, as estimated from Fq′/Fm′, than mesophyll cells. This measure of photosynthetic efficiency exhibited similar responses in these two cell types to changes in light-, CO2-, O2-, and humidity-induced changes in stomatal aperture. We infer that photosynthetic electron transport rates (on a per chlorophyll basis) in guard cell chloroplasts are of similar magnitude to those in mesophyll cells, and that the Calvin cycle and Rubisco are active in guard cell chloroplasts of the two species examined. Photosynthetic electron transport in guard cell chloroplasts was found to respond to intercellular CO2 concentration (Ci), not Ca, and the sensitivity to changes in Ci was similar to that of the response of stomatal conductance.
MATERIALS AND METHODS
Plant Material
Seeds of commelina (Commelina communis) were sown in a peat- and loam-based compost (F2, Levington, Horticulture Ltd, Ipswich, UK) in a controlled environment chamber (SGC066, Fitotron, Sanyo Gallenkamp, Leicester, UK). After 3 weeks, seedlings were potted up into 100-mm pots and used 6 to 7 weeks after sowing. Cuttings of the variegated plant tradescantia (Tradescantia albiflora) were grown in the same compost and environment chamber. The chamber air temperature was maintained at 18°C at night, and 22°C through the day. Light was provided by halogen quartz iodide lamps (Powerstar HQI-TS 250 W/NDL, Osram, Munich) from 6 am to 10 pm, at a constant PPFD of 530 μmol m−2 s−1. Relative humidity was maintained at 70% through the day and 65% at night. Plants were kept well watered using capillary matting.
The Imaging System
The optical part of the instrument used in these experiments is essentially the same as that described previously (Oxborough and Baker, 1997a). One change has been modification of the lower light source (which is used to illuminate the opposite side of the leaf to the one being imaged) so that a much larger area of leaf (a circle of 1.5 cm in diameter) is illuminated. This was required because only a small area is illuminated by the upper light source (through the lens), and this left the Ci dependent on diffusion within the leaf from the surrounding (non-illuminated) tissue, rather than on changes in stomatal aperture. With most leaves, very little of the light from the lower light source is transmitted through to the upper surface. However, the system has been designed in such a way that the upper and lower light sources can be controlled independently. This allows the change in light output from the upper and lower light sources during a saturating pulse to be matched or for the lower light source to be shuttered out completely while imaging is taking place. Chlorophyll fluorescence was defined by either a 680-nm bandpass filter (Coherent, Watford, UK) or an RG 695 longpass filter (Schott, Mainz, Germany). These filters were located within a filter wheel, located between the camera and microscope, along with the 630-nm shortpass filter used for reflected light images (Oxborough and Baker, 1997a).
A purpose-designed microscope cuvette attached to a portable photosynthesis system (CIRAS2, PP Systems, Hitchin, Hertsfordshire, UK) was used to control Ca and VPD. Cuvette and leaf temperatures were maintained at between 25°C and 28°C for all experiments. The stirred chamber was sealed to a modified objective lens using a condom with the end cut off, providing an uninterrupted light path between the lens and leaf.
All images were taken from the abaxial surface of leaves using a 40× objective, which provides images of 310 × 205 μm with a pixel size of (534 nm)2. This area covers an average of one to two stomatal complexes on the surface of a tradescantia leaf or two to three stomatal complexes on the surface of a commelina leaf. A reflected visible light image was used to measure the maximum width of the stomatal pore as an indicator of aperture. Chloroplasts within guard cell pairs were isolated from images using the ends-in search and other editing tools described in Oxborough and Baker (1997a) and Oxborough et al. (2000). Figures 6 through 8 showing the effect of CO2 and humidity on stomatal and mesophyll responses use one representative example out of three experiments, because of the considerable variability between plants. This variability is most likely the result of varying internal CO2 concentrations because of heterogeneity in stomatal aperture (not only those guard cells being measured but also those in the surrounding area) and photosynthetic activity.
Images of Fv′/Fm′ and Fq′/Fv′ were generated from images of Fo, Fm, and Fm′. This was achieved through production of a virtual image of Fo′ (in computer memory), calculated as described previously (Oxborough and Baker, 1997b). In the specific case of chloroplasts within guard cells, stomatal movements between the dark and light-adapted states often make it very difficult to match up the locations of chloroplasts within all three required images. Although this can prevent the generation of images of Fv′/Fm′ and Fq′/Fv′, it is still possible to generate mean values of Fo, Fm, and Fm′ for the guard cell chloroplasts within each image, which can then be used in the calculation of mean values of Fv′/Fm′ and Fq′/Fv′.
The fluorescence imaging system was controlled by a computer program called FluorImager (Technologia Ltd., Colehester, UK), which was written in house using Microsoft Visual C++. FluorImager was run on a dual Pentium PC under the Windows NT 4.0 operating system (Microsoft, Redmond, WA).
Epidermal Peels
Epidermal peels were removed from commelina leaves using the method of Weyers and Johansen (1985). The lower epidermis was removed from intact leaves of 6-week-old commelina plants and floated on an incubation buffer (50 mm KCl in 10 mm PIPES at pH 6.8; Weyers, 1994) in 50-mm diameter Petri dishes. The Petri dishes were placed on a water bath, to maintain a temperature of 25°C, under fluorescent lights, which provided an incident PPFD of 200 μmol m−2 s−1. A small syringe needle attached to a pump via tubing was inserted into the lid of the Petri dish to maintain a constant air supply to the peels. The bubbles produced by the air pumping also ensured a constant mixing of the buffer solution.
Statistical Analysis
Data shown in Figures 2 to 4 are means (±1 se) from three to five replicates (stomata on different leaves). The differences between cell type (Figs. 2 to 4) or filters (Fig. 2) was analyzed using ANOVA, with a repeated measures design for the different PPFD levels, except for the comparison of epidermal peel with intact leaf guard cells (Fig. 3) where different PPFD values were used, and therefore regression comparison was carried out.
ACKNOWLEDGMENTS
We are grateful to Drs. Jörg Leipner and Ian McKee for helpful discussions.
Footnotes
This research was supported by the UK Biotechnology and Biological Sciences Research Council (grant no. 84/P10409).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010317.
LITERATURE CITED
- Asai N, Nakajima N, Tamaoki M, Kamada H, Kondo N. Role of malate synthesis mediated by phosphoenolpyruvate carboxylase in guard cells in the regulation of stomatal movements. Plant Cell Physiol. 2000;41:10–15. doi: 10.1093/pcp/41.1.10. [DOI] [PubMed] [Google Scholar]
- Assmann SM. Signal transduction in stomatal guard cells. Annu Rev Cell Biol. 1993;9:345–375. doi: 10.1146/annurev.cb.09.110193.002021. [DOI] [PubMed] [Google Scholar]
- Assmann SM. The cellular basis of guard cell sensing of rising CO2. Plant Cell Environ. 1999;22:629–637. [Google Scholar]
- Baker NR, Oxborough K, Lawson T, Morison JIL. High resolution imaging of photosynthetic activities of tissues, cells and chloroplasts in leaves. J Exp Bot. 2001;52:615–621. [PubMed] [Google Scholar]
- Cardon ZG, Berry J. Effects of O2 and CO2 concentration on the steady-state fluorescence yield of single guard cell pairs in intact leaf discs of Tradescantia albiflora. Plant Physiol. 1992;99:1238–1244. doi: 10.1104/pp.99.3.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dau H. Molecular mechanisms and quantitative models of variable photosystem II fluorescence. Photochem Photobiol. 1994;60:1–23. [Google Scholar]
- Farquhar GD, Wong SC. An empirical model of stomatal conductance. Aust J Plant Physiol. 1984;11:191–210. [Google Scholar]
- Frechilla S, Zhu J, Talbott LD, Zeiger E. Stomata from npq1, a zeaxanthin-less Arabidopsis mutant, lack a specific response to blue light. Plant Cell Physiol. 1999;40:949–954. doi: 10.1093/oxfordjournals.pcp.a029627. [DOI] [PubMed] [Google Scholar]
- Genty B, Briantais JM, Baker NR. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta. 1989;990:87–92. [Google Scholar]
- Goh C-H, Schreiber U, Hedrich R. New approach of monitoring changes in chlorophyll a fluorescence of single guard cells and protoplasts in response to physiological stimuli. Plant Cell Environ. 1999;22:1057–1070. [Google Scholar]
- Jarvis AJ, Davies WJ. The coupled response of stomatal conductance to photosynthesis and transpiration. J Exp Bot. 1998;49:399–406. [Google Scholar]
- Kappen L, Andresen G, Lösch R. In situ observations of stomatal movements. J Exp Bot. 1987;38:126–141. [Google Scholar]
- Lee J, Bowling DJF. Influence of the mesophyll on stomatal opening. Aust J Plant Physiol. 1995;22:357–363. [Google Scholar]
- Lodge RJ, Dijkstra P, Drake BG, Morison JIL. Stomatal acclimation to increased CO2 concentration in a Florida scrub oak species Quercus myrtifolia Willd. Plant Cell Environ. 2001;24:77–88. [Google Scholar]
- Lu P, Outlaw HW, Jr, Smith BG, Freed GA. A new mechanism for the regulation of stomatal aperture size in intact leaves: accumulation of mesophyll-derived sucrose in the guard-cell wall of Vicia faba L. Plant Physiol. 1997;114:109–114. doi: 10.1104/pp.114.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawson BT, Zeiger E. Blue light-modulation of chlorophyll a fluorescence transients in guard cell chloroplasts. Plant Physiol. 1991;96:642–647. doi: 10.1104/pp.96.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell K, Johnson GN. Chlorophyll fluorescence: a practical guide. J Exp Bot. 2000;51:659–668. doi: 10.1093/jxb/51.345.659. [DOI] [PubMed] [Google Scholar]
- Melis A, Zeiger E. Chlorophyll a fluorescence transients in mesophyll and guard cells. Plant Physiol. 1982;69:642–647. doi: 10.1104/pp.69.3.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morison JIL. Stomatal response to increased CO2 concentration. J Exp Bot. 1998;49:443–453. [Google Scholar]
- Mott KA. Do stomata respond to CO2 concentrations other than intercellular? Plant Physiol. 1988;86:200–203. doi: 10.1104/pp.86.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T, Grantz D, Boyer J, Govindjee Effects of cations and abscisic acid on chlorophyll a fluorescence in guard cells of Vicia faba. Plant Physiol. 1982;69:1140–1144. doi: 10.1104/pp.69.5.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outlaw WH., Jr Critical examination of the quantitative evidence for and against photosynthetic CO2 fixation by guard cells. Physiol Plant. 1989;77:275–281. [Google Scholar]
- Outlaw WH., Jr . Stomata: biophysical and biochemical aspects. In: Baker NR, editor. Photosynthesis and the Environment. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1996. pp. 241–259. [Google Scholar]
- Outlaw WH, Jr, Manchester J, Di Camelli CA, Randell DD, Rapp B, Veith GM. Photosynthetic carbon reduction pathway is absent in chloroplasts of Vicia faba guard cells. Proc Natl Acad Sci USA. 1979;76:6371–6375. doi: 10.1073/pnas.76.12.6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxborough K, Baker NR. An instrument capable of imaging chlorophyll a fluorescence from intact leaves at very low irradiance and at cellular and subcellular levels of organization. Plant Cell Environ. 1997a;20:1473–1483. [Google Scholar]
- Oxborough K, Baker NR. Resolving chlorophyll a fluorescence images of photosynthetic efficiency into photochemical and non-photochemical components: calculation of qP and Fv′/Fm′ without measuring Fo′. Photosynth Res. 1997b;54:135–142. [Google Scholar]
- Oxborough K, Baker NR. An evaluation of the potential triggers of photoinactivation of photosystem II in the context of a Stern-Volmer model for down-regulation and the reversible radical pair equilibrium model. Philos Trans R Soc Lond B. 2000;355:1489–1498. doi: 10.1098/rstb.2000.0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxborough K, Hanlon ARM, Underwood GJC, Baker NR. In vivo estimation of the photosystem II photochemical efficiency of individual microphytobenthic cells using high-resolution imaging of chlorophyll a fluorescence. Limnol Oceanogr. 2000;45:1420–1425. [Google Scholar]
- Pfündel E. Estimating the contribution of photosystem I to total leaf chlorophyll fluorescence. Photosynth Res. 1998;56:185–195. [Google Scholar]
- Reckmann U, Scheibe R, Raschke K. Rubisco activity in guard cells compared with the solute requirement for stomatal opening. Plant Physiol. 1990;92:246–253. doi: 10.1104/pp.92.1.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack FD. The development and structure of stomata. In: Zeiger E, Farquhar GD, Cowan IR, editors. Stomatal Function. Stanford, CA: Stanford University Press; 1987. pp. 59–90. [Google Scholar]
- Scarth GW. Mechanism of the action of light and other factors on stomatal movement. Plant Physiol. 1932;7:481–504. doi: 10.1104/pp.7.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarth GW, Shaw M. Stomatal movement and photosynthesis in Pelargonium: I. Effect of light and carbon dioxide. Plant Physiol. 1951;26:207–225. doi: 10.1104/pp.26.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazaki K-I, Zeiger E. Cyclic and non-cyclic photophosphorylation in isolated guard cell chloroplasts from Vicia faba L. Plant Physiol. 1985;78:211–214. doi: 10.1104/pp.78.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ståfelt MG. The stomata as a hydrophotic regulator of the water deficit of the plant. Physiol Plant. 1955;8:572–593. [Google Scholar]
- Tsionsky M, Cardon ZG, Bard AJ, Jackson RB. Photosynthetic electron transport in single guard cells as measured by scanning electrochemical microscopy. Plant Physiol. 1997;113:895–901. doi: 10.1104/pp.113.3.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyers JDB. Investigating stomatal physiology with epidermal strips from Commelina communis L. (Dayflower) J Biol Educ. 1994;28:255–259. [Google Scholar]
- Weyers JDB, Johansen LG. Accurate estimation of stomatal aperture from silicone rubber impressions. New Phytol. 1985;101:1–7. doi: 10.1111/j.1469-8137.1985.tb02820.x. [DOI] [PubMed] [Google Scholar]
- Willmer C, Fricker M. Stomata. Ed 2. London, UK: Chapman & Hall; 1996. [Google Scholar]
- Wu W, Assman SM. Photosynthesis by guard cell chloroplasts of Vicia faba L.: effects of factors associated with stomatal movement. Plant Cell Physiol. 1993;34:1015–1022. [Google Scholar]
- Zeiger E, Armond P, Melis A. Fluorescence properties of guard cell chloroplasts: evidence for linear electron transport and light-harvesting pigments of photosystems I and II. Plant Physiol. 1981;67:17–20. doi: 10.1104/pp.67.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]