Abstract
Antibiotic disks with and without clavulanic acid, 3-aminophenylboronic acid, or EDTA were tested with a set of 55 Klebsiella pneumoniae and Escherichia coli strains producing well-characterized extended-spectrum, AmpC, or carbapenem-hydrolyzing β-lactamases. A relatively simple scheme was devised for distinguishing β-lactamase types in clinical isolates with or without intact outer membrane porins.
The ever increasing variety of β-lactamases that have been reported in Escherichia coli, Klebsiella pneumoniae, Klebsiella oxytoca, and other members of the family Enterobacteriaceae constitute a diagnostic challenge for the clinical microbiology laboratory (18, 22, 31). Many methods for the detection of extended-spectrum β-lactamases (ESBLs), plasmid-mediated AmpC β-lactamases, and carbapenemases have been proposed; but some procedures are technically demanding and time-consuming, others are hard to interpret, and still others require specialized reagents and reagents that are difficult to obtain (6).
The CLSI has published guidelines for ESBL detection that involve an initial screening with standard cefpodoxime, ceftazidime, aztreonam, cefotaxime, or ceftriaxone disks, followed by a confirmatory test with ceftazidime and cefotaxime disks alone and in combination with clavulanic acid (11). Augmentation of the zone of inhibition by ≥5 mm is considered a positive test result. Resistance to a cephamycin is very suggestive of the presence of an AmpC-type enzyme but can be mimicked by porin loss in K. pneumoniae (14, 24). Recently, a test for AmpC-type β-lactamases that involves augmentation of the inhibition zone around ceftazidime and cefotaxime disks by a boronic acid compound has been proposed by Yagi et al. (46). Other inhibitors have also been proposed to aid with the detection of metallo-β-lactamases (2, 21). Testing is complicated by the fact that the pattern of resistance may be altered by porin loss (24).
The aim of the present study was to evaluate the performance of such simple disk tests with a sample of clinical isolates and E. coli transconjugants making a variety of well-characterized β-lactamases with the hope of proposing a relatively simple disk scheme that could be used by laboratories without sophisticated equipment to distinguish the main classes of β-lactamases.
MATERIALS AND METHODS
Bacterial strains, chemicals, and antibiotics.
Table 1 lists the bacterial strains used in this study and the β-lactamases that they produce. The enzymes produced by previously unpublished strains were identified by isoelectric focusing, PCR amplification, cloning, and sequencing, as described previously (1, 19, 28, 36). Plasmids were transferred to porin-deficient K. pneumoniae strain C2 (24) by conjugation (16). Lithium clavulanate was purchased from the U.S. Pharmacopeia (www.usp.org), while 3-aminophenylboronic acid (APB) and EDTA disodium salt (EDTA) were obtained from Sigma (St. Louis, MO). Mueller-Hinton agar and antibiotic disks were purchased from Becton Dickinson and Company (Sparks, MD). The aztreonam, cefepime, cefotaxime, cefoxitin, ceftazidime, and ceftriaxone disks each contained 30 μg of antibiotic, while the cefpodoxime and imipenem disks each contained 10 μg of antibiotic.
TABLE 1.
Test strains and β-lactamases
| Strain | Designation | β-Lactamase(s) | Reference |
|---|---|---|---|
| K. pneumoniae | 3689 | TEM-3 | 19 |
| E. coli | J53 pUD16 | TEM-4 | 32 |
| K. pneumoniae | CF504 | TEM-5 | 33 |
| E. coli | J53 pIF100 | TEM-7 | 13 |
| K. pneumoniae | 2633E | TEM-9 | 41 |
| E. coli | 14714 | TEM-10 | 17 |
| K. pneumoniae | G11 | TEM-11 | 43 |
| K. pneumoniae | 5934 | TEM-12 | 39 |
| E. coli | MCV36 | TEM-12 | Unpublished |
| E. coli | J53 pMG274 | TEM-15 | 29 |
| K. pneumoniae | CF1304 | TEM-16 | 10 |
| E. coli | UAB5 | TEM-19 | Unpublished |
| E. coli | TEM-20 | TEM-20 | 5 |
| E. coli | TEM-21 | TEM-21 | 5 |
| K. pneumoniae | CF1104 | TEM-24 | 10 |
| E. coli | CF1609 | TEM-25 | 35 |
| K. pneumoniae | 5657 | TEM-26 | 39 |
| K. pneumoniae | LIJ64974 | TEM-26 | Unpublished |
| E. coli | J53 pMG276 | TEM-52 | 29 |
| K. pneumoniae | G19 | TEM-61 | 43 |
| K. pneumoniae | 3635 | TEM-71 | 36 |
| K. pneumoniae | K28 | TEM-88 | 29 |
| K. pneumoniae | NEDH-1 | SHV-2 | 24 |
| E. coli | J53 pUD18 | SHV-3 | 27 |
| E. coli | J53 pUD21 | SHV-4 | 9 |
| K. pneumoniae | 147460 | SHV-5 | Unpublished |
| K. pneumoniae | WCMC12 | SHV-5 | Unpublished |
| K. pneumoniae | Mary12 | SHV-5 | Unpublished |
| K. pneumoniae | JD227 | SHV-5 | Unpublished |
| E. coli | J53 pSLH47 | SHV-6 | 3 |
| K. pneumoniae | WCMC9 | SHV-7 | Unpublished |
| K. pneumoniae | 48188 | SHV-12 | Unpublished |
| K. pneumoniae | 49760 | SHV-12 | Unpublished |
| K. pneumoniae | MCV41a | SHV-18 | 37 |
| E. coli | GC4209 | CTX-M-5 | 8 |
| E. coli | J53 p4-3 | CTX-M-9 | 44 |
| E. coli | J53 pMG267 | CTX-M-14 | 28 |
| K. pneumoniae | India17 | CTX-M-15 | 20 |
| E. coli | 4202 | CTX-M-15 | Unpublished |
| K. pneumoniae | SLK54 | ACC-1 | 26 |
| K. pneumoniae | BL18 | ACT-1 | 1 |
| E. coli | UMMC29 | CMY-2 | 1 |
| K. pneumoniae | UCLA14 | DHA-1 | 1 |
| K. pneumoniae | BA32 | FOX-1 | 12 |
| E. coli | J53 p1734 | FOX-3 | 23 |
| E. coli | GCE | FOX-4 | 7 |
| K. pneumoniae | UAB1 | FOX-5 | 1 |
| E. coli | XL1Blue pHP15 | LAT-1 | 42 |
| K. pneumoniae | 96D | MIR-1 | 30 |
| E. coli | J53 pRMOX1 | MOX-1 | 15 |
| E. coli | J53 pKOL | MOX-2 | 38 |
| K. pneumoniae | 6206b | KPC-3 | Unpublished |
| E. coli | DH10B pNOR-2001 | VIM-2 | 34 |
| K. pneumoniae | 77845 | ACT-1 and TEM-10 | Unpublished |
| K. pneumoniae | JD225 | FOX-5 and SHV-5 | Unpublished |
Identical in source and phenotype to ATCC 700603, a reference strain for ESBL testing.
Isolated at New York University Medical Center in 2003.
Susceptibility testing.
Disk susceptibility testing followed the recommendations of the CLSI by using unsupplemented Mueller-Hinton agar and incubation at 37°C for 16 to 20 h (11). E. coli ATCC 25922 was used for quality control. Inhibitory disks were made by adding 10 μg clavulanate (11), 300 μg APB (46), or 200 μg neutralized EDTA to antibiotic disks.
RESULTS
Oxyimino-β-lactam resistance.
The following isolates were tested: 27 clinical isolates of K. pneumoniae and E. coli and 12 E. coli transconjugants producing TEM-, SHV-, and CTX-M-type ESBLs; 8 clinical isolates and 4 E. coli transconjugants making AmpC type β-lactamases; 2 strains producing carbapenemases; and 2 clinical isolates making both plasmid-mediated extended-spectrum and AmpC β-lactamases. The enzymes in all strains were fully characterized. About 30% of the ESBL-producing strains and 50% of the AmpC-producing strains also made a pI 5.4 enzyme consistent with the TEM-1 β-lactamase, and most of the K. pneumoniae clinical isolates had a pI 7.6 enzyme consistent with SHV-1; but except for the two strains indicated in Table 1, none of the strains made more than a single ESBL, AmpC, or carbapenem-hydrolyzing β-lactamase.
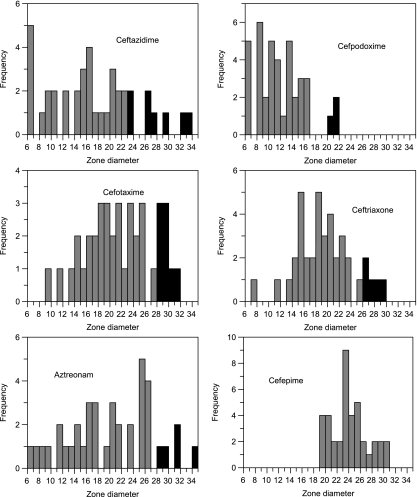
For the ESBL-producing strains, the distributions of the zone diameters obtained on disks containing ceftazidime, cefotaxime, aztreonam, cefpodoxime, ceftriaxone, or cefepime are shown in Fig. 1, where the darker bars represent isolates that failed to meet the currently recommended CLSI breakpoints for ESBL screening by the disk test. The particular β-lactamases produced by such strains are listed in Table 2. No disk criteria recognized every ESBL-producing strain. Screening with a cefpodoxime disk provided the fewest false-negative results, but nonetheless, three ESBL-producing strains would have been overlooked. The number of strains overlooked increased to five with an aztreonam disk and to eight with single ceftazidime or cefotaxime disks. Screening with two disks increased the rate of ESBL detection, but the combination of ceftazidime and cefotaxime still missed two strains producing the SHV-5 and SHV-7 ESBLs.
FIG. 1.
Distribution of diameters of zones of inhibition obtained with various antibiotic disks and ESBL-producing strains. The darker shaded columns lie outside the current CLSI screening criteria for ESBL detection. No criteria have been established for cefepime.
TABLE 2.
ESBL-producing strains failing to meet CLSI criteria
| Test | Enzymea |
|---|---|
| Screening test | |
| Ceftazidime zone size >22mm | TEM-20, TEM-25, SHV-3, SHV-5, SHV-7, CTX-M-5, CTX-M-14, CTX-M-15 |
| Cefotaxime zone size >27 mm | TEM-7, TEM-10, TEM-11, TEM-12, TEM-26, SHV-5, SHV-6, SHV-7 |
| Aztreonam zone size >27 mm | TEM-7, TEM-20, TEM-25, SHV-3, SHV-6 |
| Cefpodoxime zone size >17 mm | TEM-11, TEM-12, SHV-6 |
| Ceftriaxone zone size >25 mm | TEM-7, TEM-10, TEM-11, SHV-6, SHV-18 |
| Ceftazidime and cefotaximeb | SHV-5, SHV-7 |
| Ceftazidime and aztreonam | TEM-20, TEM-25, SHV-3 |
| Ceftazidime and cefpodoxime | None |
| Ceftazidime and ceftriaxone | None |
| Confirmatory test | |
| Ceftazidime | TEM-88, SHV-2, CTX-M-14, CTX-M-15 |
| Cefotaxime | TEM-5, TEM-7, TEM-10, TEM-11, TEM-12, TEM-26, TEM-61, SHV-5, SHV-6 |
| Aztreonam | TEM-10, TEM-12, TEM-25, SHV-6, CTX-M-15 |
| Cefpodoxime | Many |
| Ceftriaxone | Many |
| Ceftazidime and cefotaximec | None |
| Ceftazidime and aztreonam | CTX-M-15 |
The enzyme in strains failing the CLSI screening criteria (screening test) or the enzyme in strains failing the CLSI clavulanate inhibition test (zone enhancement, <5 mm).
Screening test failure for both agents.
Confirmatory test failure for both agents.
Strains making KPC-3, VIM-2, or 12 different AmpC-type β-lactamases were also positive with each antibiotic disk by use of the criteria for the ESBL screening test, with two exceptions: a transconjugant making MOX-1, which would not have been identified as a resistance suspect with a ceftazidime or aztreonam disk, and an E. coli transconjugant making VIM-2, which had a 40-mm zone diameter with aztreonam, an antibiotic known to be unaffected by VIM-2 (34).
Clavulanic acid enhancement.
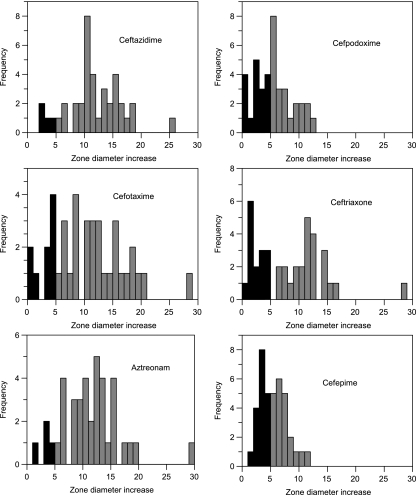
The distributions of the zone diameter increases with the test strains and the various antibiotic disks on addition of clavulanic acid are shown in Fig. 2, while the particular enzymes present in strains that failed this confirmatory test are indicated in Table 2. It is evident that many strains failed to show zone diameters of 5 mm or more by clavulanic acid enhancement of the cefpodoxime or ceftriaxone disks, whose use, appropriately, is not recommended as part of the CLSI ESBL detection criteria. Ceftazidime plus clavulanic acid was the best single disk combination, but 4 of the 39 ESBL-producing strains failed the confirmatory test. By combined testing with ceftazidime and cefotaxime, all the ESBL-producing strains had zone diameters of ≥5 mm with clavulanic acid enhancement. Fittingly, the CLSI confirmatory test requires the use of both ceftazidime and cefotaxime alone and in combination with clavulanic acid (11).
FIG. 2.
Distribution of zone diameter increases with addition of 10 μg clavulanic acid to antibiotic disks for ESBL-producing strains. The darker shaded columns represent strains with <5 mm of zone diameter enhancement. The ESBL confirmatory test recommended by the CLSI uses clavulanic acid only with ceftazidime and cefotaxime.
There were a few false-positive results with the clavulanic acid enhancement test. A clinical isolate making the AmpC enzyme ACT-1 had a 5-mm enhancement of the zone of inhibition around a ceftazidime disk, while a transconjugant making the AmpC enzyme MOX-2 showed a 5-mm zone enhancement with clavulanic acid and an aztreonam disk. A clinical isolate making the carbapenemase KPC-3 also had a 5-mm enhancement with clavulanic acid of the zone around a cefotaxime disk and a 6-mm enhancement of the zone around an aztreonam disk, a result that was not unexpected since this class A enzyme is known to be susceptible to clavulanic acid inhibition (45). Other resistance properties of the ACT-1- and MOX-2-producing strains, such as their resistance to cefoxitin, would, however, correct any confusion that they produced ESBLs.
Cefoxitin resistance.
The 12 AmpC β-lactamase-producing strains had zone diameters with a cefoxitin disk of 11 mm or less, with the exception of a clinical isolate making ACC-1, which had an 18-mm cefoxitin zone and which, consequently, would be considered cefoxitin susceptible, a known peculiarity of the enzyme (4). The strain producing the VIM-2 carbapenemase was also cefoxitin resistant, but the strain making the KPC-3 carbapenemase and all the ESBL-producing strains were cefoxitin susceptible, except for the special case of strain NEDH-1.
Strain NEDH-1 carries a plasmid encoding the ESBL SHV-2 and lacks both the OmpK35 and the OmpK36 porins (24). Cefoxitin resistance is due to porin loss since it persists if the plasmid is eliminated, while the plasmid itself does not express cefoxitin resistance if it is transferred to a new host. Consequently, porin loss or the presence of a metallo-β-lactamase such as VIM-2 needs to be ruled out before cefoxitin resistance can be considered a reliable indicator of the presence of an AmpC β-lactamase.
APB enhancement.
Yagi et al. (46) have proposed augmentation of ceftazidime or cefotaxime resistance with APB as diagnostic of the presence of an AmpC-type β-lactamase. By adoption of a ≥5-mm enlargement of the zone of inhibition as a positive test result (46), APB inhibited ceftazidime and cefotaxime resistance in most AmpC-producing strains; the exceptions were ACT-1- and MOX-2-producing strains, which failed with ceftazidime plus APB, and DHA-1- and FOX-1-producing strains, which failed with cefotaxime plus APB. There were, however, no false-positive results with any ESBL-producing strain or with strains making VIM-2 or KPC-3. With cefoxitin and cefoxitin-APB disks, all AmpC-producing strains were positive except for the strain making ACC-1, which was unaffected (because the enzyme has hardly any activity against cefoxitin) (4). Also, no change in cefoxitin resistance when APB was used with cefoxitin was seen when plasmids encoding the AmpC enzymes ACT-1 and DHA-1 were introduced into porin-deficient strain C2 because strain C2 itself had no zone of inhibition around the cefoxitin disk. The strain C2 derivatives, however, still showed positive responses with ceftazidime and APB.
Imipenem resistance.
Only two carbapenemase-producing strains were available for testing. Both KPC-3 and VIM-2 caused a decrease in imipenem susceptibility (zone diameters, 16 and 20 mm, respectively) in strains of E. coli and K. pneumoniae, but the zones were still within the susceptible range (≥16 mm). For the strain producing KPC-3, clavulanic acid increased the imipenem zone diameter, but only by 3 mm.
AmpC-type enzymes can also produce carbapenem resistance in porin-deficient K. pneumoniae strains (24). K. pneumoniae strain C2 was derived from NEDH-1 by elimination of the plasmid encoding SHV-2. On introduction of AmpC-encoding plasmids into strain C2, imipenem zone diameters of 10 mm (plasmid pMG251 making ACT-1) or 18 mm (plasmid pMG247 making DHA-1) were observed, indicating that the degree of imipenem resistance depends on the particular AmpC β-lactamase involved. In both strains, resistance was still affected by APB, with zone diameter increases of 9 and 5 mm, respectively. Strain C2 carrying pNOR-2001 and producing VIM-2 was fully resistant, with no zone of inhibition around the 6-mm imipenem disk.
EDTA enhancement.
EDTA chelates the metal required for class B β-lactamase activity and has been used in screening tests for metallo-β-lactamase production (2). Addition of 200 μg EDTA to imipenem disks produced a 12-mm zone diameter enhancement with an E. coli strain producing VIM-2 and a 15-mm zone diameter enhancement with the VIM-2-encoding plasmid in porin-deficient strain C2. EDTA had no effect on the imipenem susceptibilities of strains making the AmpC enzyme ACT-1 or DHA-1 or class A carbapenemase KPC-3 whether it was in a strain with normal or deficient porins.
Dual resistance.
Two clinical isolates produced both an AmpC enzyme and an ESBL (either TEM-10 or SHV-12). Testing showed that the strains met the screening criteria for ESBL production with any of the recommended antibiotic disks and were also cefoxitin resistant. By the use of conventional susceptibility criteria, they would have been labeled as ceftazidime, aztreonam, and cefpodoxime resistant but cefotaxime and ceftriaxone intermediate. Both strains gave dual responses to inhibitors, with zone diameter increases of ≥5 mm on disk supplementation with either clavulanic acid or APB, indicating that each of the resistance genes could be independently recognized in these two strains.
Provisional interpretation.
So many individual enzymes are known that full characterization of the β-lactamase responsible for resistance requires the use of molecular techniques such as bla gene amplification by PCR and DNA sequencing, but some empirical rules can be gleaned from simple disk zone diameters. For example, the ratio of the zone diameter obtained with cefotaxime to that obtained with ceftazidime was 0.6 or less for the three strains with CTX-M-type enzymes but was more than 0.6 for all 28 of the TEM and SHV ESBL producers. The same cefotaxime zone diameter/ceftazidime zone diameter ratio was 1.8 or less for 9 of 9 strains producing ESBLs in the SHV family but for only 9 of 19 TEM ESBL producers. More strains will need to be tested to see if such a ratio is consistently discriminating.
DISCUSSION
Based on the response to disk tests with and without inhibitors, a relatively simple scheme for distinguishing β-lactamases is described in Table 3. Initial screening uses ceftazidime and cefotaxime disks and the screening criteria proposed for ESBL detection by the CLSI: positive responses are zones of inhibition of ≤22 mm for ceftazidime and ≤27 mm for cefotaxime. AmpC- and carbapenem-producing strains will also be screen test positive. Positive strains should be retested with ceftazidime and cefotaxime disks containing clavulanic acid and with cefoxitin and imipenem disks. ESBL-producing strains will show a ≥5-mm zone enhancement with clavulanic acid (Fig. 2 and Table 2). Strains with one of the currently uncommon class A carbapenemases, such as a plasmid-mediated KPC enzyme, give similar responses to clavulanic acid but may demonstrate reduced susceptibility rather than resistance to imipenem. Strains producing AmpC β-lactamases or class B carbapenemases will not respond to clavulanic acid and will generally be cefoxitin resistant. They should be retested with disks containing APB. If resistance to ceftazidime, cefotaxime, or cefoxitin is reduced by APB, an AmpC β-lactamase is present. If it is not, testing should be repeated with disks containing EDTA; a positive response indicates the presence of a class B carbapenemase. The testing can be done in a single batch to speed detection, especially if strains with enzymes other than ESBLs are prevalent, but at the expense of using additional resources.
TABLE 3.
Summary of diagnostic criteriaa
| β-Lactamase | Porin lossb | CAZ or CTXc | Clavulanic acid response | FOXd | APB response | IPMd | EDTA response |
|---|---|---|---|---|---|---|---|
| ESBL | − | P | Yese | S | No | S | No |
| + | P | Yes | R | No | S | No | |
| AmpC | − | P | No | R | Yes | S | No |
| + | P | No | R | Nof | R/↓S | No | |
| Carbapenemase class A | − | P | Yes | S | No | ↓S | No |
| + | P | Yes | R | No | ↓S | No | |
| Carbapenemase class B | − | P | No | R | No | ↓S | Yes |
| + | P | No | R | No | R | Yes |
CAZ, ceftazidime; CTX, cefotaxime; FOX, cefoxitin; IPM, imipenem.
Loss of porins OmpK35 and OmpK36 in K. pneumoniae.
P, positive CLSI screening test result.
R, resistant; S, susceptible; ↓S, decreased susceptibility by CLSI disk interpretive criteria.
The enhancement of the zone of inhibition was ≥5 mm.
For AmpC, an APB response was seen with CAZ or CTX but not FOX.
The scheme is not perfect. An occasional ESBL-producing strain may be overlooked in an initial screen with ceftazidime and cefotaxime (Fig. 1 and Table 2). A strain producing a class A carbapenemase will have a positive response to clavulanic acid and may be falsely categorized as producing an ESBL if a reduction in imipenem susceptibility is not evident, a detection problem previously noted by Smith Moland et al. (40). A positive response to APB indicates the production of an AmpC β-lactamase but not necessarily a plasmid-mediated enzyme since in E. coli strains this phenotype may result as well from overexpression of the chromosomal ampC gene (25). More strains producing CTX-M-type ESBLs and carbapenemases also need to be evaluated to improve the criteria for their detection.
Acknowledgments
This study was supported in part by a grant from Merck & Co., Inc.
REFERENCES
- 1.Alvarez, M., J. H. Tran, N. Chow, and G. A. Jacoby. 2004. Epidemiology of conjugative plasmid-mediated AmpC β-lactamases in the United States. Antimicrob. Agents Chemother. 48:533-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arakawa, Y., N. Shibata, K. Shibayama, H. Kurokawa, T. Yagi, H. Fujiwara, and M. Goto. 2000. Convenient test for screening metallo-β-lactamase-producing gram-negative bacteria by using thiol compounds. J. Clin. Microbiol. 38:40-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arlet, G., M. Rouveau, D. Bengoufa, M. H. Nicolas, and A. Philippon. 1991. Novel transferable extended-spectrum β-lactamase (SHV-6) from Klebsiella pneumoniae conferring selective resistance to ceftazidime. FEMS Microbiol. Lett. 81:57-62. [DOI] [PubMed] [Google Scholar]
- 4.Bauernfeind, A., I. Schneider, R. Jungwirth, H. Sahly, and U. Ullmann. 1999. A novel type of AmpC β-lactamase, ACC-1, produced by a Klebsiella pneumoniae strain causing nosocomial pneumonia. Antimicrob. Agents Chemother. 43:1924-1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ben Redjeb, S., G. Fournier, C. Mabilat, A. Ben Hassen, and A. Philippon. 1990. Two novel transferable extended-spectrum β-lactamases from Klebsiella pneumoniae in Tunisia. FEMS Microbiol. Lett. 67:33-38. [DOI] [PubMed] [Google Scholar]
- 6.Black, J. A., K. S. Thomson, J. D. Buynak, and J. D. Pitout. 2005. Evaluation of β-lactamase inhibitors in disk tests for detection of plasmid-mediated AmpC β-lactamases in well-characterized clinical strains of Klebsiella spp. J. Clin. Microbiol. 43:4168-4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bou, G., A. Oliver, M. Ojeda, C. Monzón, and J. Martínez-Beltran. 2000. Molecular characterization of FOX-4, a new AmpC-type plasmid-mediated β-lactamase from an Escherichia coli strain isolated in Spain. Antimicrob. Agents Chemother. 44:2549-2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradford, P. A., Y. Yang, D. Sahm, I. Grope, D. Gardovska, and G. Storch. 1998. CTX-M-5, a novel cefotaxime-hydrolyzing β-lactamase from an outbreak of Salmonella typhimurium in Latvia. Antimicrob. Agents Chemother. 42:1980-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buré, A., P. Legrand, G. Arlet, V. Jarlier, G. Paul, and A. Philippon. 1988. Dissemination in five French hospitals of Klebsiella pneumoniae serotype K25 harboring a new transferable enzymatic resistance to third generation cephalosporins and aztreonam. Eur. J. Clin. Microbiol. 7:780-782. [DOI] [PubMed] [Google Scholar]
- 10.Chanal, C. M., D. L. Sirot, A. Petit, R. Labia, A. Morand, J. L. Sirot, and R. A. Cluzel. 1989. Multiplicity of TEM-derived β-lactamases from Klebsiella pneumoniae strains isolated at the same hospital and relationships between the responsible plasmids. Antimicrob. Agents Chemother. 33:1915-1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.CLSI. 2003. Performance standards for antimicrobial susceptibility testing; fifteenth informational supplement. Clinical and Laboratory Standards Institute, Wayne, Pa.
- 12.Gonzalez Leiza, M., J. C. Perez-Diaz, J. Ayala, J. M. Casellas, J. Martinez-Beltran, K. Bush, and F. Baquero. 1994. Gene sequence and biochemical characterization of FOX-1 from Klebsiella pneumoniae, a new AmpC-type plasmid-mediated β-lactamase with two molecular variants. Antimicrob. Agents Chemother. 38:2150-2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gutmann, L., M. D. Kitzis, D. Billot-Klein, F. Goldstein, G. Tran Van Nhieu, T. Lu, J. Carlet, E. Collatz, and R. Williamson. 1988. Plasmid-mediated β-lactamase (TEM-7) involved in resistance to ceftazidime and aztreonam. Rev. Infect. Dis. 10:860-866. [DOI] [PubMed] [Google Scholar]
- 14.Hernández-Allés, S., M. Conejo, A. Pascual, J. M. Tomás, V. J. Benedí, and L. Martínez-Martínez. 2000. Relationship between outer membrane alterations and susceptibility to antimicrobial agents in isogenic strains of Klebsiella pneumoniae. J. Antimicrob. Chemother. 46:273-277. [DOI] [PubMed] [Google Scholar]
- 15.Horii, T., Y. Arakawa, M. Ohta, S. Ichiyama, R. Wacharotayankun, and N. Kato. 1993. Plasmid-mediated AmpC-type β-lactamase isolated from Klebsiella pneumoniae confers resistance to broad-spectrum β-lactams, including moxalactam. Antimicrob. Agents Chemother. 37:984-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacoby, G. A., and P. Han. 1996. Detection of extended-spectrum β-lactamases in clinical isolates of Klebsiella pneumoniae and Escherichia coli. J. Clin. Microbiol. 34:908-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacoby, G. A., A. A. Medeiros, T. F. O'Brien, M. E. Pinto, and H. Jiang. 1988. Broad-spectrum, transmissible β-lactamases. N. Engl. J. Med. 319:723-724. [DOI] [PubMed] [Google Scholar]
- 18.Jacoby, G. A., and L. S. Munoz-Price. 2005. The new β-lactamases. N. Engl. J. Med. 352:380-391. [DOI] [PubMed] [Google Scholar]
- 19.Jacoby, G. A., and R. Vacheva-Dobrevsky. 2003. Epidemiology of extended-spectrum β-lactmases in Sofia, Bulgaria. Eur. J. Clin. Microbiol. Infect. Dis. 22:385-388. [DOI] [PubMed] [Google Scholar]
- 20.Jacoby, G. A., K. E. Walsh, D. M. Mills, V. J. Walker, H. Oh, A. Robicsek, and D. C. Hooper. 2006. qnrB, another plasmid-mediated gene for quinolone resistance. Antimicrob. Agents Chemother. 50:1178-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee, K., Y. S. Lim, D. Yong, J. H. Yum, and Y. Chong. 2003. Evaluation of the Hodge test and the imipenem-EDTA double-disk synergy test for differentiating metallo-β-lactamase-producing isolates of Pseudomonas spp. and Acinetobacter spp. J. Clin. Microbiol. 41:4623-4629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Livermore, D. M. 1995. β-Lactamases in laboratory and clinical resistance. Clin. Microbiol. Rev. 8:557-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marchese, A., G. Arlet, G. C. Schito, P. H. Lagrange, and A. Philippon. 1998. Characterization of FOX-3, an AmpC-type plasmid-mediated β-lactamase from an Italian isolate of Klebsiella oxytoca. Antimicrob. Agents Chemother. 42:464-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martínez-Martínez, L., A. Pascual, S. Hernández-Allés, D. Alvarez-Díaz, A. I. Suárez, J. Tran, V. J. Benedí, and G. A. Jacoby. 1999. Roles of β-lactamases and porins in activities of carbapenems and cephalosporins against Klebsiella pneumoniae. Antimicrob. Agents Chemother. 43:1669-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mulvey, M. R., E. Bryce, D. A. Boyd, M. Ofner-Agostini, A. M. Land, A. E. Simor, and S. Paton. 2005. Molecular characterization of cefoxitin-resistant Escherichia coli from Canadian hospitals. Antimicrob. Agents Chemother. 49:358-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nadjar, D., M. Rouveau, C. Verdet, J. Donay, J. Herrmann, P. H. Lagrange, A. Philippon, and G. Arlet. 2000. Outbreak of Klebsiella pneumoniae producing transferable AmpC-type β-lactamase (ACC-1) originating from Hafnia alvei. FEMS Microbiol. Lett. 187:35-40. [DOI] [PubMed] [Google Scholar]
- 27.Nicolas, M. H., V. Jarlier, N. Honore, A. Philippon, and S. T. Cole. 1989. Molecular characterization of the gene encoding SHV-3 β-lactamase responsible for transferable cefotaxime resistance in clinical isolates of Klebsiella pneumoniae. Antimicrob. Agents Chemother. 33:2096-2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pai, H., E. H. Choi, H. J. Lee, J. Y. Hong, and G. A. Jacoby. 2001. Identification of CTX-M-14 extended-spectrum β-lactamase in clinical isolates of Shigella sonnei, Escherichia coli, and Klebsiella pneumoniae in Korea. J. Clin. Microbiol. 39:3747-3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pai, H., H. J. Lee, E. H. Choi, J. Kim, and G. A. Jacoby. 2001. Evolution of TEM-related extended-spectrum β-lactamases in Korea. Antimicrob. Agents Chemother. 45:3651-3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Papanicolaou, G. A., A. A. Medeiros, and G. A. Jacoby. 1990. Novel plasmid-mediated β-lactamase (MIR-1) conferring resistance to oxyimino- and alpha-methoxy β-lactams in clinical isolates of Klebsiella pneumoniae. Antimicrob. Agents Chemother. 34:2200-2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paterson, D. L., and R. A. Bonomo. 2005. Extended-spectrum β-lactamases: a clinical update. Clin. Microbiol. Rev. 18:657-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paul, G. C., G. Gerbaud, A. Bure, A. M. Philippon, B. Pangon, and P. Courvalin. 1989. TEM-4, a new plasmid-mediated β-lactamase that hydrolyzes broad-spectrum cephalosporins in a clinical isolate of Escherichia coli. Antimicrob. Agents Chemother. 33:1958-1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petit, A., D. L. Sirot, C. M. Chanal, J. L. Sirot, R. Labia, G. Gerbaud, and R. A. Cluzel. 1988. Novel plasmid-mediated β-lactamase in clinical isolates of Klebsiella pneumoniae more resistant to ceftazidime than to other broad-spectrum cephalosporins. Antimicrob. Agents Chemother. 32:626-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poirel, L., T. Naas, D. Nicolas, L. Collet, S. Bellais, J. D. Cavallo, and P. Nordmann. 2000. Characterization of VIM-2, a carbapenem-hydrolyzing metallo-β-lactamase and its plasmid- and integron-borne gene from a Pseudomonas aeruginosa clinical isolate in France. Antimicrob. Agents Chemother. 44:891-897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poupart, M. C., C. Chanal, D. Sirot, R. Labia, and J. Sirot. 1991. Identification of CTX-2, a novel cefotaximase from a Salmonella mbandaka isolate. Antimicrob. Agents Chemother. 35:1498-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rasheed, J. K., G. J. Anderson, A. M. Queenan, J. W. Biddle, A. Oliver, G. A. Jacoby, K. Bush, and F. C. Tenover. 2002. TEM-71, a novel plasmid-encoded, extended-spectrum β-lactamase produced by a clinical isolate of Klebsiella pneumoniae. Antimicrob. Agents Chemother. 46:2000-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rasheed, J. K., G. J. Anderson, H. Yigit, A. M. Queenan, A. Doménech-Sánchez, J. M. Swenson, J. W. Biddle, M. J. Ferraro, G. A. Jacoby, and F. C. Tenover. 2000. Characterization of the extended-spectrum β-lactamase reference strain, Klebsiella pneumoniae K6 (ATCC 700603), which produces the novel enzyme SHV-18. Antimicrob. Agents Chemother. 44:2382-2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raskine, L., I. Borrel, G. Barnaud, S. Boyer, B. Hanau-Bercot, J. Gravisse, R. Labia, G. Arlet, and M. J. Sanson-Le-Pors. 2002. Novel plasmid-encoded class C β-lactamase (MOX-2) in Klebsiella pneumoniae from Greece. Antimicrob. Agents Chemother. 46:2262-2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rice, L. B., S. H. Willey, G. A. Papanicolaou, A. A. Medeiros, G. M. Eliopoulos, R. C. Moellering, Jr., and G. A. Jacoby. 1990. Outbreak of ceftazidime resistance caused by extended-spectrum β-lactamases at a Massachusetts chronic-care facility. Antimicrob. Agents Chemother. 34:2193-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith Moland, E., N. D. Hanson, V. L. Herrera, J. A. Black, T. J. Lockhart, A. Hossain, J. A. Johnson, R. V. Goering, and K. S. Thomson. 2003. Plasmid-mediated, carbapenem-hydrolysing β-lactamase, KPC-2, in Klebsiella pneumoniae isolates. J. Antimicrob. Chemother. 51:711-714. [DOI] [PubMed] [Google Scholar]
- 41.Spencer, R. C., P. F. Wheat, T. G. Winstanley, D. M. Cox, and S. J. Plested. 1987. Novel β-lactamase in a clinical isolate of Klebsiella pneumoniae conferring unusual resistance to β-lactam antibiotics. J. Antimicrob. Chemother. 20:919-921. [DOI] [PubMed] [Google Scholar]
- 42.Tzouvelekis, L. S., E. Tzelepi, A. F. Mentis, and A. Tsakris. 1993. Identification of a novel plasmid-mediated β-lactamase with chromosomal cephalosporinase characteristics from Klebsiella pneumoniae. J. Antimicrob. Chemother. 31:645-654. [DOI] [PubMed] [Google Scholar]
- 43.Vuye, A., G. Verschraegen, and G. Claeys. 1989. Plasmid-mediated β-lactamases in clinical isolates of Klebsiella pneumoniae and Escherichia coli resistant to ceftazidime. Antimicrob. Agents Chemother. 33:757-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang, M., J. H. Tran, G. A. Jacoby, Y. Zhang, F. Wang, and D. C. Hooper. 2003. Plasmid-mediated quinolone resistance in clinical isolates of Escherichia coli from Shanghai, China. Antimicrob. Agents Chemother. 47:2242-2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Woodford, N., P. M. Tierno, Jr., K. Young, L. Tysall, M. F. Palepou, E. Ward, R. E. Painter, D. F. Suber, D. Shungu, L. L. Silver, K. Inglima, J. Kornblum, and D. M. Livermore. 2004. Outbreak of Klebsiella pneumoniae producing a new carbapenem-hydrolyzing class A β-lactamase, KPC-3, in a New York medical center. Antimicrob. Agents Chemother. 48:4793-4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yagi, T., J. Wachino, H. Kurokawa, S. Suzuki, K. Yamane, Y. Doi, N. Shibata, H. Kato, K. Shibayama, and Y. Arakawa. 2005. Practical methods using boronic acid compounds for identification of class C β-lactamase-producing Klebsiella pneumoniae and Escherichia coli. J. Clin. Microbiol. 43:2551-2558. [DOI] [PMC free article] [PubMed] [Google Scholar]