Abstract
Pro dehydrogenase (PDH) catalyzes the first and rate-limiting step in the Pro catabolic pathway. In Arabidopsis, this enzyme is encoded by At-PDH. To investigate the role of Pro catabolism in plants, we generated transgenic Arabidopsis plants with altered levels of PDH by sense (PDH-S plants) and antisense (PDH-AS plants) strategies. Free Pro levels were reduced by up to 50% in PDH-S plants under stress and recovery conditions and enhanced by a maximum of 25% in PDH-AS plants, despite large modifications of the At-PDH transcript and At-PDH protein levels. A similar trend in free Pro levels was observed in the PDH-S and PDH-AS seeds without visible effects on germination or growth. Under stress conditions, PDH transgenic plants showed no signs of change in osmotolerance. However, addition of exogenous Pro increased survival rates of salt-stressed PDH-S plants by 30%. Isotope-labeling studies showed that the conversion of [14C]Pro to Glu was reduced in PDH-AS plants and increased in PDH-S plants, especially under stress conditions. Furthermore, PDH-AS plants were hypersensitive to exogenous Pro, whereas PDH-S plants were sensitive to Pro analogs. These findings demonstrate that altered At-PDH levels lead to weakly modified free Pro accumulation with a limited impact on plant development and growth, suggesting a tight control of Pro homeostasis and/or gene redundancy.
Pro accumulation is a well-known response to osmotic stress in plants (Singh et al., 1972; Rhodes, 1987). This amino acid is a compatible solute and has been proposed to stabilize subcellular structures and to scavenge free radicals (Smirnoff and Cumbes, 1989). In plants, Pro accumulates through an increase in its synthesis concomitantly with inhibition of its catabolism (Delauney and Verma, 1993; Yoshiba et al., 1997). Pro catabolism is capable of a high-energy output and has been proposed to donate electrons to the respiratory electron transport chain (Hare and Cress, 1997). Each molecule of Pro, when oxidized, can yield 30 ATP equivalents (Atkinson, 1977) and has been considered a primary fuel in energy-intensive processes, such as insect flight (Gade, 1992) or pollen germination (Zhang et al., 1982; Skubatz et al., 1989). In plants, Pro catabolism may provide amino nitrogen and reducing power to cells that recover from stress (Peng et al., 1996; Verbruggen et al., 1996). Pro is oxidized to Glu in the mitochondria (Boggess and Koeppe, 1978; Huang and Cavalieri, 1979; Elthon and Stewart, 1981) by the sequential action of Pro dehydrogenase (PDH) and pyrroline-5-carboxylate (P5C) dehydrogenase.
The first plant cDNA coding for PDH was isolated in Arabidopsis (At-PDH; Kiyosue et al., 1996; Peng et al., 1996; Verbruggen et al., 1996). Although At-PDH has been referred to as Pro oxidase, its classification as a dehydrogenase is more appropriate (N. Verbruggen, unpublished results). At-PDH expression is down-regulated during osmotic stress, whereas Pro accumulates and is rapidly induced upon recovery (Verbruggen et al., 1996). On the other hand, exogenous Pro, in the absence of stress, is a good inducer of At-PDH expression (Kiyosue et al., 1996; Verbruggen et al., 1996). Furthermore, in the absence of stress, At-PDH expression seems to be correlated with free Pro content (Verbruggen et al., 1996). Recent work on the At-PDH promoter has confirmed its negative regulation during dehydration and up-regulation during rehydration (Nakashima et al., 1998). In addition, the At-PDH promoter is up-regulated by Pro and is developmentally regulated, being active especially in reproductive tissues such as pollen and pistils, which are rich in free Pro.
The role of Pro catabolism has been highlighted in the field of nitrogen fixation. Plants such as soybean (Glycine max) synthesize high levels of Pro in the host cells of nodules, and bacteroids are capable of high Pro-oxidizing activity, suggesting that free Pro entering the bacteroids from host cells might be an important energy source for nitrogen fixation (Kohl et al., 1988). Nodulation tests with a bacteroid PDH-deficient mutant revealed that PDH activity is essential for nodulation efficiency. Thus, increased oxidative flux of Pro in bacteroids might provide an agronomically significant yield advantage (Straub et al., 1997).
We have investigated the function of At-PDH in plants by analysis of transgenic Arabidopsis plants with altered At-PDH transcript levels under normal and stress conditions. Our results are analyzed and discussed in view of the results on At-PDH antisense (anti-PRODH) plants published by Nanjo et al (1999a).
RESULTS
To engineer Arabidopsis plants with altered levels of At-PDH, the At-PDH cDNA was overexpressed in sense or antisense orientation under the control of the 35S promoter.
Selection of PDH-S Transgenic Lines with Increased At-PDH Levels
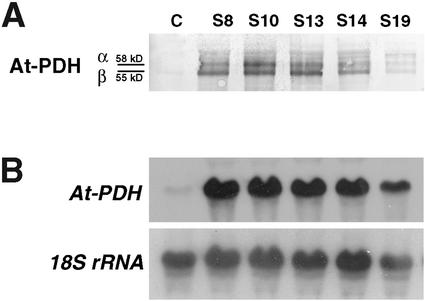
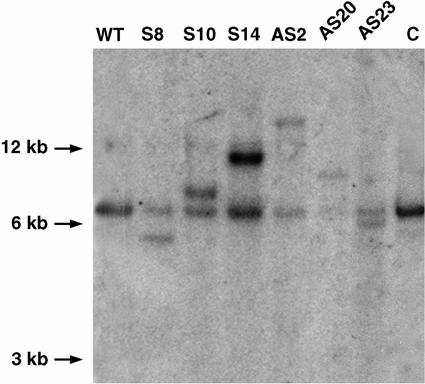
Thirty transgenic lines transformed with the At-PDH cDNA in sense orientation (referred to as PDH-S plants) were analyzed. Screening by western analysis of PDH on total protein extracts showed a marked increase in the At-PDH protein levels in approximately 60% of the transgenic plants. Five positive lines were selected to perform a western analysis on mitochondrial extracts (Fig. 1A). A clear overproduction of the At-PDH protein was observed that confirmed mitochondrial targeting. At least two distinct bands at 58 kD and 55 kD (designated α and β) were detected that were probably the premature and mature forms of At-PDH. Northern analysis revealed an increase in At-PDH transcript levels (Fig. 1B). Quantification of At-PDH transcript levels showed an increase of up to 15-fold, whereas the protein level was increased by 5- to 10-fold. Three lines (S8, S10, and S14) were chosen for subsequent analysis. Southern analysis of these three lines showed a single integration event (Fig. 2) that correlated with the 3:1 segregation analysis on kanamycin-containing medium.
Figure 1.
Western (A) and northern (B) analysis of 2-week-old PDH-S plants. Western analysis was done on crude mitochondrial extracts. α and β represent hypothetical premature and mature forms of the At-PDH protein. C, Control plants (transformed with the pBIN19 binary vector without insert); S, PDH-S lines; 18S rRNA, control for loading.
Figure 2.
Southern analysis of At-PDH transgenic plants. Five micrograms of genomic DNA was digested with XbaI, blotted to nylon membrane (Hybond N; Amersham Biosciences), and probed with the At-PDH cDNA. Hybridization was done overnight at 65°C as described by Verbruggen et al. (1996). WT, Wild type; S, PDH-S lines; AS, PDH-AS lines; C, control plants.
Selection of PDH-AS Transgenic Lines with Decreased At-PDH Levels
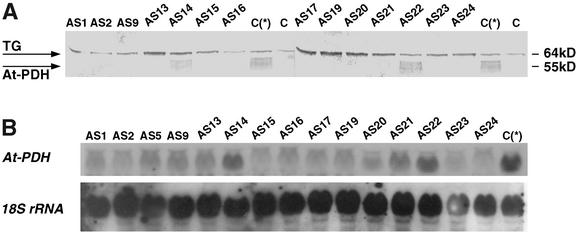
Twenty-seven transgenic lines transformed with the At-PDH cDNA in antisense orientation (referred as PDH-AS plants) were analyzed. Given that At-PDH protein level is very low under normal growth conditions (Fig. 1A), screening of the transgenic lines was performed 12 h after treatment with 100 mm Pro. Exogenous Pro is known to be a good inducer of At-PDH (Verbruggen et al., 1996; Nakashima et al., 1998). Under induced conditions, the levels of the At-PDH protein were undetectable in most of the transgenic lines (Fig. 3A). Northern analysis under these conditions showed reduced levels of the At-PDH transcripts in all the transgenic lines when compared with the control plants (Fig. 3B). Three best lines (AS2, AS20, and AS23) were selected. Southern analysis of the selected PDH-AS transgenic lines showed a single integration of T-DNA transgene (Fig. 2) that correlated with the 3:1 segregation pattern on kanamycin.
Figure 3.
Western (A) and northern (B) analysis of 2-week-old PDH-AS plants treated with 100 mm l-Pro for 12 h. Western analysis was done on crude protein extracts. C, Control plants; C(*), Pro-induced control plants; TG, thioglucosidase; AS, PDH-AS plants; 18S rRNA, control for loading.
PDH Transgenic Plants Accumulate Altered Free Pro Levels in Seeds during Drought and Recovery from Stress
Previous studies on free Pro levels in Arabidopsis during development showed a significant increase in reproductive tissues (floret and seeds), whereas lower levels were found in vegetative tissues (Chiang and Dandekar, 1995; Savouré et al., 1995). Free Pro contents in control, PDH-S, and PDH-AS seeds were 504 ± 130.8, 266 ± 20.6, and 820.5 ± 196.3 μg g−1 fresh weight, respectively (sd for 3–5 replicates). Altered Pro levels in PDH transgenic seeds had no impact on germination or further growth. During development, a 2-fold increase in PDH activity was measured in floral extracts of PDH-S plants, whereas PDH-AS plants showed a decrease of 25% in PDH activity (data not shown). Furthermore, Pro also accumulated in Arabidopsis in response to osmotic stress (Delauney and Verma, 1993). A maximal 20-fold increase in free Pro levels was recorded in Arabidopsis by applying 20% polyethylene glycol (PEG) 6000 for 24 h (Chiang and Dandekar, 1995; Verbruggen et al., 1996).
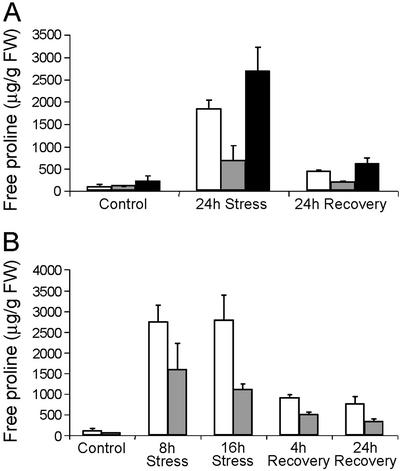
We analyzed free Pro levels on 2-week-old plants exposed for 24 h to PEG-induced drought or 24 h after recovery from stress. Under unstressed conditions, no significant differences were observed in free Pro levels of control and PDH transgenic plants (Fig. 4A). After 24 h of stress, free Pro accumulated up to 20-fold in control and PDH-AS plants and only up to 10-fold in PDH-S plants. After 24 h of recovery from stress, the free Pro levels were still 50% reduced in PDH-S plants and weakly increased in PDH-AS plants (Fig. 4A). Statistical analysis showed significant differences in free Pro levels between the control and PDH transgenic plants (Table I). Each PDH-AS line accumulated significantly more free Pro than the control. Similarly, each PDH-S line accumulated significantly less free Pro than the control. A comparable trend of reduced Pro accumulation was observed in PDH-S plants when subjected to hyperosmotic stress induced by mannitol (500 mm), and this reduced level was maintained under recovery conditions (Fig. 4B). Despite significant changes in free Pro, no phenotype was observed in PDH transgenic plants upon stress or recovery as a consequence of reduced free Pro levels during stress (data not shown).
Figure 4.
Free Pro levels of 3-week-old plants treated with PEG 6000 and upon recovery on Murashige and Skoog medium in the absence of stress (A) and with 500 mm of mannitol (B). Control plants (white bars), PDH-S plants (gray bars), and PDH-AS plants (black bars). Data represent average of three replicates.
Table I.
Analysis of variance of free Pro levels between control plants (C), PDH-S lines (S8, S10, S14), and PDH-AS lines (AS2, AS20, AS23) in three treatments (control, 24 h of stress, and 24 h of recovery from stress)
| Source of Variation | df | MS | F |
|---|---|---|---|
| Sense lines | |||
| Treatment | 2 | 2,385,448 | 102.08a |
| Sense line | 3 | 512,952 | 21.95a |
| Interaction T × SL | 6 | 341,645 | 14.62a |
| Error | 21 | 23,367 | |
| Antisense lines | |||
| Treatment | 2 | 40,027,700 | 111.37a |
| Antisense line | 3 | 7,263,856 | 20.21a |
| Interaction T × ASL | 6 | 6,269,294 | 17.44a |
| Error | 16 | 359,426 |
df, Degree of freedom; MS, mean of square.
P < 0.001.
Osmotolerance and Pro Catabolism in Control, PDH-S, and PDH-AS Plants
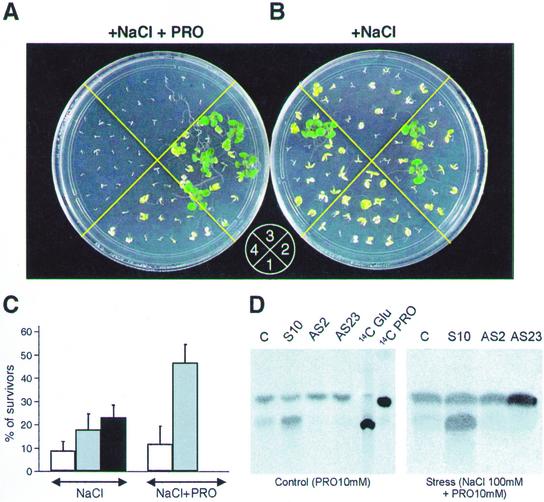
Tolerance to hyperosmotic stress was investigated in control and PDH transgenic plants by monitoring germination rates and subsequent growth in the presence of minimal mineral medium devoid of sugar and supplemented with NaCl. The addition of 100 mm NaCl or higher concentrations affected growth of control and PDH transgenic plants dramatically, based on survival rates after 2 weeks (Fig. 5). On 100 mm NaCl, Arabidopsis plants could adapt and complete their life cycle. When analyzed under these conditions, no significant differences in growth were observed in PDH transgenic plants when compared with the control plants. However, addition of exogenous Pro under these conditions showed dramatic effects on the growth of PDH transgenic plants. Scoring the germination efficiency of control and PDH transgenic plants after 2 weeks of sowing in the presence of NaCl (100 mm) supplemented with Pro (10 mm) revealed a hypersensitive response of PDH-AS plants (Fig. 5A). On the other hand, under these conditions PDH-S plants had up to 30% better survival rates than control plants (Fig. 5B). Interestingly, addition of Pro did not alleviate stress in control plants.
Figure 5.
In vitro grown At-PDH transgenic plants in the presence of NaCl. A, Growth on Murashige and Skoog minimal medium containing 100 mm NaCl + 10 mm Pro (+NaCl+PRO); B, 100 mm NaCl (+NaCl). 1, control plants; 2, PDH-S line S10; 3 and 4, PDH-AS lines AS2 and AS23, respectively. Photographs were taken 2 weeks after germination. C, Percentage of survivors. Data represent average of five replicates with total of 75 to 100 seeds for control and 225 to 300 seeds for PDH transgenic plants. Transgenic control (white bars), PDH-S (gray bars; mean of lines S8, S10, and S14), and PDH-AS (black bars; mean of lines AS2, AS20, and AS23). D, Analysis of 2-week-old PDH transgenic plants by thin-layer chromatography. Experiments were done as described in “Materials and Methods”. Incubation was performed for 4 h under normal and stress conditions.
The efficiency of Pro catabolism in PDH transgenic plants was estimated by [14C]Pro-labeling experiments, which showed reduced [14C]Glu formation in PDH-AS plants (Fig. 5D). Under stress (100 mm NaCl), reduced Pro oxidation was observed in the control plants, whereas enhanced conversion to Glu was observed in PDH-S plants (Fig. 5D). Furthermore, Pro uptake rates were similar in PDH-S and control plants (data not shown). Thus, reduced or increased [14C]Glu formation reflects the different oxidation rates of Pro in transgenic plants.
Exogenous Pro Induces Hypersensitivity in PDH-AS Plants
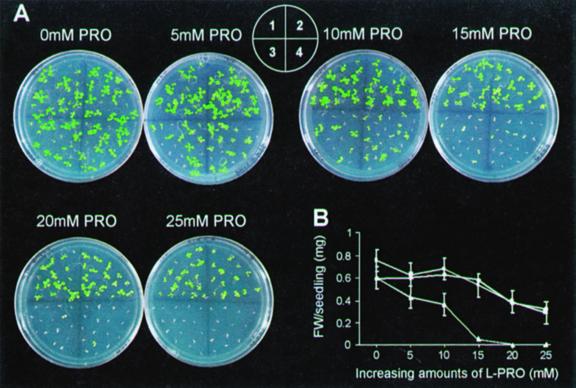
Pro toxicity was further investigated by sowing control and PDH transgenic seeds on minimal mineral medium devoid of sugar containing increased doses of exogenous l-Pro. PDH-AS plants were clearly hypersensitive to Pro and the phenotype severity was directly correlated with the concentration of exogenous Pro added to the medium (Fig. 6). Germination rates recorded after 1 week of growth on Pro were not significantly different in PDH transgenic and control plants. However, significant morphological changes were visible in PDH-AS plants after the emergence of cotyledons and further development was severely retarded. Microscopic analysis of these Pro-hypersensitive plants with Evans blue staining showed very few dead cells (data not shown). Almost all of the PDH-AS plants lost chlorophyll and continued to remain dormant for weeks. On the contrary, PDH-S plants did not exhibit a significant phenotype on Pro compared with the control plants. Estimation of free Pro levels in 1-week-old control, PDH-S, and PDH-AS plants after 22-h treatment with exogenous Pro (10 mm) were 410 ± 19.51, 265.26 ± 20.84, and 1,068.5 ± 154 μg g−1 fresh weight, respectively. Enhanced free Pro levels in PDH-AS plants were a consequence of reduced Pro catabolism.
Figure 6.
Hypersensitivity of PDH-AS plants in the presence of exogenous Pro. A, Growth on Murashige and Skoog minimal medium containing increasing amounts of exogenous Pro (0 mm PRO, 5 mm PRO, 10 mm PRO, 15 mm PRO, 20 mm PRO, and 25 mm PRO). 1, control plants; 2, PDH-S line S10; 3 and 4, PDH-AS lines AS2 and AS23, respectively. B, Fresh weights. Control plants (solid circle; mean of line S10), PDH-S (solid square; mean of line S10), PDH-AS (solid diamond; mean of lines AS2 and AS23). Data represent average of three replicates with an average of five seedlings for each line. Photographs were taken 2 weeks after germination.
Pro Analogs Induce Early Sensitivity in PDH-S Plants
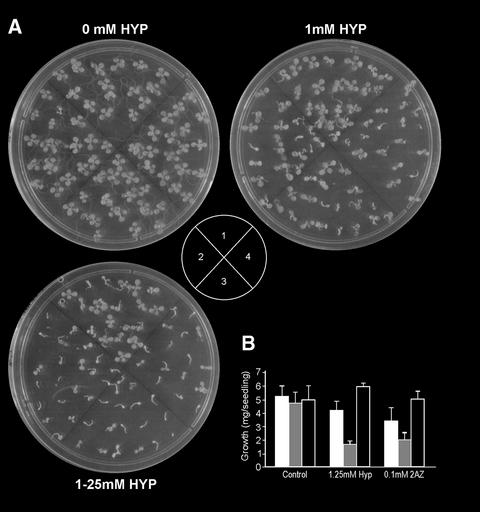
Pro analogs, such as azetidine-2-carboxylic acid (2AZ) and trans-4-Hyp (HYP), resemble Pro with respect to Mr, steric conformation, and charge. We tested the effect of HYP and 2AZ on the growth of PDH transgenic plants. PDH-S plants were more sensitive than the control plants (Fig. 7A). One week after germination on Pro analogs, the PDH-S and PDH-AS plants had lower and higher survival rates than control plants, respectively (data not shown). Further quantification of the phenotypes showed a reduction in fresh weight of up to 50% by PDH-S plants, whereas PDH-AS plants had 20% increased fresh weight when compared with the control plants grown under similar conditions (Fig. 7). After 6 weeks of growth on 1 mm HYP, PDH-AS plants flowered and completed their life cycle, whereas the control plants were dead (data not shown).
Figure 7.
Sensitivity of PDH-S transgenic plants in the presence of Pro analogs. A, Growth on HYP. 1, control plants; 2, 3, and 4, PDH-S lines S8, S10, and S14, respectively. B, Fresh weights. Transgenic control (white bars), PDH-S (gray bars; mean of lines S8, S10, and S14), and PDH-AS (black bars; mean of lines AS2, AS20, and AS23). Data represent average of 15 seedlings for each line. Photographs were taken 1 week after germination.
DISCUSSION
Physiological Significance of Altered Pro Catabolism
To investigate the role of Pro catabolism in plants, transgenic Arabidopsis plants were engineered that overexpressed At-PDH in sense and antisense orientation. Significantly modified free Pro levels were observed in PDH transgenics under conditions in which free Pro accumulates naturally.
Previous studies have shown that during plant development, significant proportions of different amino acids accumulate in different tissues. The most notable changes were found for Pro, which constitutes 17% to 26% of the total free amino acid concentration in reproductive tissues (floret and seed) but only 1% to 3% of the total free amino acid concentration in vegetative tissues (rosette leaf and root; Chiang and Dandekar, 1995). Pro catabolic rates were investigated through enzymatic measurements of PDH activity in different organs of Arabidopsis wild-type plants. Results showed that the PDH activity could be easily measured in reproductive tissues, such as flowers and siliques, but was very low in vegetative tissues (N. Verbruggen, unpublished results). Comparison of PDH activity in floral extracts of control and PDH transgenic plants showed an increase of up to 2-fold in PDH-S plants, whereas PDH-AS flowers exhibited a 25% decrease (data not shown). We quantified the free Pro content in PDH transgenic seeds in which the levels were reduced by 50% and increased by 60% in PDH-S and PDH-AS lines, respectively. These changes in free Pro levels of seeds did not alter germination or further development of PDH transgenic plants when compared with the control plants. Furthermore, isotope-labeling studies with [14C]Pro reflected changes in Pro catabolism in PDH-S and PDH-AS plants that correlated with the altered free Pro levels observed under stress conditions (Figs. 4 and 5). Interestingly, thin-layer chromatography analysis showed an absence of radiolabeled P5C in control and PDH-S plants. In accordance, previous studies by Wang (1968) and Stewart et al. (1977) have shown that Glu was the only visible compound to be radioactive in [14C]Pro feeding experiments with wilted and turgid barley leaves. Similarly, study of the Pro-biosynthetic pathway with [14C]Glu in tobacco (Nicotiana tabacum) plants showed the absence of radiolabeled P5C accumulation (Kishor et al., 1995).
However, large modifications in transcript levels were observed despite less dramatic changes in free Pro levels. This suggests the existence of a second PDH gene or posttranslational modifications, which are known to occur in prokaryotes (Ostrovsky and Maloy, 1995). Interestingly, the Arabidopsis genome contains an open reading frame that shares 84% identity with At-PDH at the protein level (gi 15240986, chromosome locus At5g38710; data not shown). Another interpretation for the moderate change in free Pro may be linked to compartmentalization. Free Pro accumulates mostly in the cytosol, which accounts for 5% of the cell size, whereas the At-PDH enzyme is localized in the mitochondria. Thus, availability of Pro for catabolism probably depends on transporters, which may be highly regulated.
Cause and Effect Relationship of Exogenous Pro and Pro Analogs
Despite the low modification of free Pro levels under normal conditions, PDH transgenics showed hypersensitive responses to Pro or Pro analogs (Figs. 6 and 7). It is interesting that the PDH-AS phenotype on exogenous Pro resembles that of the reduced sugar response (rsr) and Pro hypersensitive (Pro HS) mutants (Hellmann et al., 2000). However, the molecular mechanisms involved in the Pro-hypersensitive response might be different in rsr mutants and PDH-AS plants. Our data show that Pro catabolism is decreased in PDH-AS plants, whereas the rsr mutants exhibit a higher At-PDH expression, suggesting a higher Pro catabolism. Furthermore, unlike in rsr mutants, Pro hypersensitivity cannot be rescued by the addition of NaCl in PDH-AS plants (Fig. 5). Unfortunately, the targets of Pro toxicity are unknown. Previous studies on amino acid inhibition have shown that high doses of all amino acids, except Gln, lead to toxicity resulting from imbalances in specific pathways or alteration in feedback mechanisms of other related amino acids (Bonner et al., 1996). Because Pro breakdown is reduced or suppressed in PDH-AS plants, a toxic response is expected earlier than in the wild-type or control plants (Fig. 6).
In PDH-S transgenic plants, addition of low concentrations of Pro analogs caused hypersensitivity in PDH-S plants earlier than in control plants (Fig. 7). Moreover, Pro analogs seemed to induce At-PDH expression in control plants (data not shown), which could emphasize Pro catabolism, thus the ratio of Pro analogs/Pro in PDH-S plants.
Pro Accumulation during Stress: Its Relevance to Osmotolerance in Arabidopsis
No significant change in osmotolerance was observed in PDH transgenic plants (Fig. 5). Similar studies have been reported on carrot (Daucus carota) cell lines, which had no increased osmotolerance despite a 6-fold increase in free Pro levels (Maggio et al., 1997). In contrast, other Arabidopsis transgenic PDH antisense lines, referred to as anti-ProDH plants by Nanjo et al. (1999a), exhibited higher osmotolerance under very restricted conditions. Comparative studies of PDH-AS and anti-ProDH plants showed a similar proportion of free Pro accumulation under normal and stress conditions. During recovery after stress, one of the anti-ProDH lines accumulated approximately 6-fold more free Pro than the recovering control plants and further exhibited osmotolerance when treated with 600 mm NaCl for 30 min (Nanjo et al., 1999a). We performed similar experiments on 2-week-old control and PDH transgenic plants treated with 500 mm NaCl for 30 min up to 12 h and recovery was monitored for several weeks. We did not observe any significant alteration in osmotolerance in PDH-AS plants when compared with the control plants (data not shown).
Addition of exogenous Pro during osmotic stress resulted in a marked increase in survival rates of PDH-S plants, but it did not ameliorate the osmotolerance of wild-type or control plants. Because Pro catabolism is suggested to be linked to respiration and ATP synthesis, we evaluated the respiratory rates of PDH-S plants under stress and in the presence of exogenous Pro by measuring O2 consumption using a Clark electrode. No significant increase was recorded (data not shown). On the other hand, root respiration is a rather crude measurement as other stress responses can interfere and may involve O2 consumption or O2 production processes. Previous studies on Pro catabolism in rice (Oryza sativa) showed that increased amounts of P5C could induce osmoresponsive genes (Iyer and Caplan, 1998). P5C levels may increase when P5C dehydrogenase is rate limiting. However, P5C accumulation does not seem to occur in PDH-S plants given that P5C accumulation is toxic (Hellmann et al., 2000) and that the PDH-S plants look healthy. In addition, thin-layer chromatography analysis showed no P5C accumulation (Fig. 5).
Increased osmotolerance in spite of reduced free Pro has been reported previously in Arabidopsis mutants with photoautotrophic salt tolerance1 (pst1; Tsugane et al., 1999) and reduced salt sensitivity (rss; Werner and Finkelstein, 1995). Under salt stress, 20% of the pst1 mutants show osmotolerance and accumulate 50% less free Pro under stress conditions (Tsugane et al., 1999). Thus, Pro does not seem to play an important role in pst1 or rss osmoregulation. Similarly, exogenous Pro does not seem to be a good osmolyte in wild-type Arabidopsis and does not alleviate stress injury (Fig. 5; Hellmann et al., 2000). Under these circumstances, the role of free Pro in Arabidopsis development and stress resistance is questionable. Previous reports on the role of free Pro in osmotic adjustment of Pro-overproducing transgenic tobacco plants that accumulated free Pro up to 20-fold more than control plants has been subject of controversy (Blum et al., 1996; Murthy and Tester, 1996; Sharp et al., 1996). In fact, recent evidence favors the role of Pro as a protein constituent rather than an osmolyte in Arabidopsis. Pro-deficient Arabidopsis transgenic (TF3) plants, engineered to overexpress At-P5CS1 (encoding the rate-limiting enzyme in the Pro-biosynthetic pathway) in antisense orientation were morphologically affected and hypersensitive to osmotic stress (Nanjo et al., 1999b). Deficiency in Pro synthesis also resulted in lower levels of Pro and HYP-rich proteins, which are important constituents of structural cell wall proteins and are probable candidates for mutated phenotypes in TF3 plants (Nanjo et al., 1999b). These phenotypes were suppressed by addition of l-Pro, which suggests a function for Pro in morphogenesis as a major constituent of cell wall structural proteins in plants (Nanjo et al., 1999b). Despite restoration of growth, the internal free Pro pool of TF3 plants was only 7% of that in control plants, thus questioning the role of free Pro accumulation in Arabidopsis wild-type/control plants.
Free Pro is suggested to play an important role in plant freezing tolerance, as revealed in eskimo (esk) mutants (Xin and Browse, 1998). Arabidopsis esk1 mutants accumulate large amounts of free Pro (up to 30-fold), which account for 2% of total dry matter and are constitutively freezing tolerant. This increase in free Pro in esk1 mutants was due to its increased synthesis (Xin and Browse, 1998). Furthermore, study of the expression pattern of genes involved in Pro metabolism support a role in development and osmoregulation (Hare and Cress, 1997). In contrast to what has been proposed, the osmolyte function of Pro seems to be controversial in Arabidopsis. In addition to the work in this study, further modification of Pro catabolism should take account of the new predicted PDH isoform.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Arabidopsis ecotype Columbia was used in this study. Wild-type and transgenic seeds were grown in vitro on Murashige and Skoog mineral medium (Valvekens et al., 1988) or on minimal medium containing Murashige and Skoog salt, 0.5 g L−1 MES and 8 g L−1 plant tissue culture agar (LabM, Lancashire, UK) and grown in a growth chamber as described by Verbruggen et al. (1996). Plants were also grown in soil and incubated under illumination of approximately 100 μmol m−2 s−1 with a 16-h light/8-h dark photoperiod and 60% relative humidity at 22°C.
Construction of Recombinant Binary Vectors and Arabidopsis Transformation
The At-PDH cDNA, previously named At-POX (Verbruggen et al., 1996), cloned at the EcoRI site of pBluescript KS plasmid (Stratagene, La Jolla, CA), was partially digested with ApoI. The 1.5-kb fragment obtained containing the full coding sequence was fused in sense and antisense orientation to the EcoRI site of the expression vector pBin19 (Bevan, 1984). The expression of the At-PDH cDNA was driven by the cauliflower mosaic virus 35S promoter. Transgenic lines were generated by Agrobacterium tumefaciens-mediated transfer of the constructs into the Arabidopsis Columbia ecotype as described previously (Valvekens et al., 1988). Control plants with the pBIN19 vector devoid of insert were also generated. The T1 transgenic seeds obtained were selected on medium supplemented with 50 mg L−1 kanamycin and checked for segregation. The kanamycin-resistant plants with a single copy were self-pollinated and the homozygous seeds obtained from the T2 and T3 generations were used for subsequent experiments.
Northern Analysis
Total RNA was prepared as described (Verwoerd et al., 1989) and analyzed by northern blotting. Twenty micrograms of total RNA was fractionated by electrophoresis on 1% agarose gel containing formaldehyde and blotted onto nylon filter (Hybond N; Amersham Biosciences, Little Chalfont, UK). The At-PDH cDNA riboprobe was labeled with digoxigenin-11-dUTP (Roche Diagnostics, Brussels) and hybridization was done according to the manufacturer's instructions. Northern blots were rehybridized with 18S rRNA as a control for loading. Signals were quantified with a phosphoimager (Amersham Biosciences).
14C-Feeding Experiments and Thin-Layer Chromatography Analysis
Two-week-old plants were incubated for 3, 4, or 20 h in 0.5 mL of Murashige and Skoog medium containing a mixture of cold Pro (10 mm) and 1 μCi [14C]Pro. For analysis by thin-layer chromatography, incubated samples were washed three times with water containing 5 mm Pro, rinsed, and briefly dried on tissue paper before being weighed, frozen in liquid N2, and stored at −20°C or extracted immediately in methanol-chloroform-water (12:5:3, v/v) as described (Boggess et al., 1976). Ascending thin-layer chromatography was done on MN300 cellulose sheets with butanol-pyridine-water (1:1:1, v/v) as solvent. [14C]Pro (Amersham Biosciences) and [14C]Glu (Amersham Biosciences) were used as standards. Plates were dried in a fume-hood and analyzed on a phosphoimager (Amersham Biosciences).
Preparation of Mitochondria and Immunoblot Analysis
Crude mitochondria were prepared from 4-week-old plants grown under greenhouse conditions as described (Klein et al., 1999). Protein (10 μg) was separated by 10% SDS-PAGE and transferred onto a polyvinylidene difluoride membrane (Immobilon; Millipore, Bedford, MA) in 25 mm Tris, 192 mm Gly, and 20% (v/v) methanol. For immunological detection, rabbit anti-At-PDH antibody (dilution 1/2500) was used, followed by incubation with anti-rabbit IgG alkaline phosphatase (Roche Diagnostics). The immunoreactive protein was visualized by using nitro blue tetrazolium (Duchefa, Haarlem, The Netherlands) and 5-bromo-4-chloro-3-indolyl-phosphate (Sigma-Aldrich, St. Louis) as substrates. Western analysis on total extracts revealed a cross-reaction of anti-At-PDH antibody with a glycoprotein thioglucosidase at 64 kD (N. Verbruggen, unpublished data) that was absent in mitochondrial extracts.
Stress Treatment for Free Pro Analysis
Stress treatments with PEG and NaCl were done as previously described (Verbruggen et al., 1996). Free Pro content was measured as described by Bates et al. (1973) using l-Pro as a standard.
Statistical Analysis
Statistical analysis on free Pro between control and PDH transgenic plants subjected to stress (PEG 6000) and recovery conditions was compared by ANOVA. Similar statistical analysis was done on fresh weight between control and PDH transgenic plants grown under normal and in presence of Pro analogs. Two-factor with replication ANOVA test was performed with a statistical program (Statistica; StatSoft, Inc., Tulsa, OK). The analysis represented control and three independent PDH-S (S8, S10, and S14) and PDH-AS (AS2, AS20, and AS20) lines.
ACKNOWLEDGMENTS
The authors wish to thank Catherine Bernard for help with the statistical analysis, and Martine De Cock, Rebecca Verbanck, and Karel Spruyt for help preparing the manuscript.
Footnotes
This work was supported by the European Union Training and Mobility (grant no. FMRX–CT96–0007). S.M. is indebted to the “Bijzondere Onderzoekfonds” of the Ghent University for a predoctoral fellowship (011NEU199).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010572.
LITERATURE CITED
- Atkinson DE. Cellular Energy Metabolism and its Regulation. New York: Academic Press; 1977. [Google Scholar]
- Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39:205–207. [Google Scholar]
- Bevan M. Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res. 1984;12:8711–8721. doi: 10.1093/nar/12.22.8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum A, Munns R, Passioura JB, Turner NC. Genetically engineered plants resistant to soil drying and salt stress: how to interpret osmotic relations? Plant Physiol. 1996;110:1051. doi: 10.1104/pp.110.4.1051. (Letter) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggess SF, Koeppe DE. Oxidation of proline by plant mitochondria. Plant Physiol. 1978;62:22–25. doi: 10.1104/pp.62.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggess SF, Stewart CR, Aspinall D, Paleg LG. Effect of water stress on proline synthesis from radioactive precursors. Plant Physiol. 1976;58:398–401. doi: 10.1104/pp.58.3.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner CA, Williams DS, Aldrich HC, Jensen RA. Antagonism by l-glutamine toxicity and growth inhibition caused by other amino acids in suspension cultures of Nicotiana silvestris. Plant Sci. 1996;113:43–58. [Google Scholar]
- Chiang HH, Dandekar AM. Regulation of proline accumulation in Arabidopsis thaliana (L.) Heynh during development and in response to desiccation. Plant Cell Environ. 1995;18:1280–1290. [Google Scholar]
- Delauney AJ, Verma DPS. Proline biosynthesis and osmoregulation in plants. Plant J. 1993;4:215–223. [Google Scholar]
- Elthon TE, Stewart CR. Submitochondrial location and electron transport characteristics of enzymes involved in proline oxidation. Plant Physiol. 1981;67:780–784. doi: 10.1104/pp.67.4.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gade G. The hormonal integration of insect flight metabolism. Zool Jahrb Abt Allg Zool Physiol Tiere. 1992;96:211–225. [Google Scholar]
- Hare PD, Cress WA. Metabolic implications of stress-induced proline accumulation in plants. Plant Growth Regul. 1997;21:79–102. [Google Scholar]
- Hellmann H, Funck D, Rentsch D, Frommer WB. Hypersensitivity of an Arabidopsis sugar signaling mutant toward exogenous proline application. Plant Physiol. 2000;123:779–790. doi: 10.1104/pp.123.2.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang AHC, Cavalieri AJ. Proline oxidase and water stress-induced proline accumulation in spinach leaves. Plant Physiol. 1979;63:531–535. doi: 10.1104/pp.63.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer S, Caplan A. Products of proline catabolism can induce osmotically regulated genes in rice. Plant Physiol. 1998;116:203–211. [Google Scholar]
- Kishor PBK, Hong Z, Miao G-H, Hu C-AA, Verma DPS. Overexpression of Δ1-pyrroline-5-carboxylate synthetase increases proline production and confers osmotolerance in transgenic plants. Plant Physiol. 1995;108:1387–1394. doi: 10.1104/pp.108.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyosue T, Yoshiba Y, Yamaguchi-Shinozaki K, Shinozaki K. A nuclear gene encoding mitochondrial proline dehydrogenase, an enzyme involved in proline metabolism, is upregulated by proline but downregulated by dehydration in Arabidopsis. Plant Cell. 1996;8:1323–1335. doi: 10.1105/tpc.8.8.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M, Binder S, Brennicke A. Purification of mitochondria from Arabidopsis. In: Martínez-Zapater JM, Salinas J, editors. Arabidopsis Protocols, Methods in Molecular Biology. Vol. 82. Totowa: Humana Press; 1999. pp. 49–53. [DOI] [PubMed] [Google Scholar]
- Kohl DH, Schubert KR, Carter MB, Hagedorn CH, Shearer G. Proline metabolism in N2-fixing root nodules: energy transfer and regulation of purine synthesis. Proc Natl Acad Sci USA. 1988;85:2036–2040. doi: 10.1073/pnas.85.7.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio A, Bressan RA, Hasegawa PM, Locy RD. Moderately increased constitutive proline does not alter osmotic stress tolerance. Physiol Plant. 1997;101:240–246. [Google Scholar]
- Murthy M, Tester M. Compatible solutes and salt tolerance: misuse of transgenic tobacco. Trends Plant Sci. 1996;1:294–295. [Google Scholar]
- Nakashima K, Satoh R, Kiyosue T, Yamaguchi-Shinozaki K, Shinozaki K. A gene encoding proline dehydrogenase is not only induced by proline and hypoosmolarity, but is also developmentally regulated in the reproductive organs of Arabidopsis. Plant Physiol. 1998;118:1233–1241. doi: 10.1104/pp.118.4.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanjo T, Kobayashi M, Yoshiba Y, Kakubari Y, Yamaguchi-Shinozaki K, Shinozaki K. Antisense suppression of proline degradation improves tolerance to freezing and salinity in Arabidopsis thaliana. FEBS Lett. 1999a;461:205–210. doi: 10.1016/s0014-5793(99)01451-9. [DOI] [PubMed] [Google Scholar]
- Nanjo T, Kobayashi M, Yoshiba Y, Sanada Y, Wada K, Tsukaya H, Kakubari Y, Yamaguchi-Shinozaki K, Shinozaki K. Biological functions of proline in morphogenesis and osmotolerance revealed in antisense transgenic Arabidopsis thaliana. Plant J. 1999b;18:185–193. doi: 10.1046/j.1365-313x.1999.00438.x. [DOI] [PubMed] [Google Scholar]
- Ostrovsky PC, Maloy S. Protein phosphorylation on serine, threonine, and tyrosine residues modulates membrane-protein interactions and transcriptional regulation in Salmonella typhimurium. Genes Dev. 1995;9:2034–2041. doi: 10.1101/gad.9.16.2034. [DOI] [PubMed] [Google Scholar]
- Peng Z, Lu Q, Verma DPS. Reciprocal regulation of Δ1-pyrroline-5-carboxylate synthetase and proline dehydrogenase genes controls proline levels during and after osmotic stress in plants. Mol Gen Genet. 1996;253:334–341. doi: 10.1007/pl00008600. [DOI] [PubMed] [Google Scholar]
- Rhodes D. Metabolic responses to stress. In: Davies DD, editor. Physiology of Metabolism, The Biochemistry of Plants: A Comprehensive Treatise. Vol. 12. New York: Academic Press; 1987. pp. 201–241. [Google Scholar]
- Savouré A, Jaoua S, Hua X-J, Ardiles W, Van Montagu M, Verbruggen N. Isolation, characterization, and chromosomal location of a gene encoding the Δ1-pyrroline-5-carboxylate synthetase in Arabidopsis thaliana. FEBS Lett. 1995;372:13–19. doi: 10.1016/0014-5793(95)00935-3. [DOI] [PubMed] [Google Scholar]
- Singh TN, Aspinall D, Paleg LG. Proline accumulation and varietal adaptability to drought in barley: a potential metabolic measure of drought resistance. Nature. 1972;236:188–190. doi: 10.1038/newbio236188a0. [DOI] [PubMed] [Google Scholar]
- Sharp RE, Boyer JS, Nguyen HT, Hsiao TC. Genetically engineered plants resistant to soil drying and salt stress: how to interpret osmotic relations? Plant Physiol. 1996;110:1051–1052. doi: 10.1104/pp.110.4.1051. (Letter) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skubatz H, Meeuse BJD, Bendich AJ. Oxidation of proline and glutamate by mitochondria of the inflorescence of voodoo lily (Sauromatum guttatum) Plant Physiol. 1989;91:530–535. doi: 10.1104/pp.91.2.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnoff N, Cumbes QJ. Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry. 1989;28:1057–1060. [Google Scholar]
- Stewart CR, Boggess SF, Aspinall D, Paleg LG. Inhibition of proline oxidation by water stress. Plant Physiol. 1977;59:930–932. doi: 10.1104/pp.59.5.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub PF, Shearer G, Reynolds PHS, Sawyer SA, Kohl DH. Effect of disabling bacteroid proline catabolism on the response of soybeans to repeated drought stress. J Exp Bot. 1997;48:1299–1307. [Google Scholar]
- Tsugane K, Kobayashi K, Niwa Y, Ohba Y, Wada K, Kobayashi H. A recessive Arabidopsis mutant that grows photoautotrophically under salt stress shows enhanced active oxygen detoxification. Plant Cell. 1999;11:1195–1206. doi: 10.1105/tpc.11.7.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valvekens D, Van Montagu M, Van Lijsebettens M. Agrobacterium tumefaciens-mediated transformation of Arabidopsis thaliana root explants by using kanamycin selection. Proc Natl Acad Sci USA. 1988;85:5536–5540. doi: 10.1073/pnas.85.15.5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen N, Hua X-J, May M, Van Montagu M. Environmental and developmental signals modulate proline homeostasis: evidence for a negative transcriptional regulator. Proc Natl Acad Sci USA. 1996;93:8787–8791. doi: 10.1073/pnas.93.16.8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verwoerd TC, Dekker BMM, Hoekema A. A small-scale procedure for the rapid isolation of plant RNAs. Nucleic Acids Res. 1989;17:2362. doi: 10.1093/nar/17.6.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D. Metabolism of 14C-labeled proline in higher plants. Contrib Boyce Thompson Inst. 1968;24:117–122. [Google Scholar]
- Werner JE, Finkelstein RR. Arabidopsis mutants with reduced response to NaCl and osmotic stress. Physiol Plant. 1995;93:659–666. [Google Scholar]
- Xin Z, Browse J. eskimo1 mutants of Arabidopsis are constitutively freezing-tolerant. Proc Natl Acad Sci USA. 1998;95:7799–7804. doi: 10.1073/pnas.95.13.7799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiba Y, Kiyosue T, Nakashima K, Yamaguchi-Shinozaki K, Shinozaki K. Regulation of levels of proline as an osmolyte in plants under water stress. Plant Cell Physiol. 1997;38:1095–1102. doi: 10.1093/oxfordjournals.pcp.a029093. [DOI] [PubMed] [Google Scholar]
- Zhang H-q, Croes AF, Linskens HF. Protein synthesis in germinating pollen of Petunia: role of proline. Planta. 1982;154:199–203. doi: 10.1007/BF00387864. [DOI] [PubMed] [Google Scholar]