Abstract
The clinical course of Chagas' disease varies widely among different patients and geographic regions. For reasons that are not completely understood but involve host and parasite factors, some patients never develop the disease while others present cardiac and/or gastrointestinal symptoms. Many studies have been conducted in order to correlate the genetic variability of the parasites with the clinical forms of the disease, but no conclusive data have been obtained. Our research aims at characterizing the genetic profiles of Trypanosoma cruzi isolates recently obtained from 70 chagasic patients who either showed pathological lesions with symptoms of various intensities or were asymptomatic. All patients came from an area where Chagas' disease is endemic in southeast Brazil where vectorial transmission has been controlled and different clinical forms of the disease can be found. The molecular characterization of parasites evaluated the polymorphisms of the 3′ region of the 24Sα rRNA gene and the variability of kinetoplast DNA (kDNA) minicircles of T. cruzi populations by low-stringency single specific primer PCR. Data presented here provide a strong correlation between T. cruzi II and human infection in this region. However, a high degree of variability was observed within T. cruzi II, as demonstrated by intense kDNA polymorphism among all clinical forms and also within each of them, irrespective of the intensity of pathological processes.
Chagas' disease is characterized by a short and frequently nonsymptomatic acute phase, followed by a chronic phase with a variable clinical course and different manifestations. Most patients have the indeterminate form of infection and never develop the disease, while others may exhibit cardiac, digestive, cardiodigestive, or neurological symptoms or even acute exacerbations. The prevalence and geographical distribution of the clinical forms of Chagas' disease vary among countries and even among different areas where the disease is endemic within the same country (6). The exact causes of the pleomorphism of Chagas' disease remain unknown, and even a major role of the parasite in determining the pathogenesis of the disease has already been challenged (19). However, immunohistochemical techniques (12) and PCR (13, 14, 36) have shown a strict correlation between the presence of the parasite and tissue lesions (33).
Trypanosoma cruzi populations show a high degree of intraspecific variability detected by biological, biochemical, immunological, and genetic markers (20). Nevertheless, until now such polymorphism could not be properly correlated with the clinical manifestations of the disease. Early studies based on enzyme electrophoresis classified T. cruzi populations into three major zymodeme groups, named Z1, Z2, and Z3 (22), but when using more enzyme markers a greater heterogeneity was disclosed (34). T. cruzi populations are now generally clustered into two major phylogenetic groups based on miniexon and rRNA gene polymorphism analyses (9, 32). These major lineages, named T. cruzi I and T. cruzi II (2a), are associated with sylvatic (Z1) and domestic (Z2) transmission cycles, respectively. Further evidence suggested that T. cruzi II populations could be more complex than originally thought, being subdivided into five groups (IIa to IIe) (4, 5, 17). Recent results, however, have demonstrated that T. cruzi populations showing distinct and/or hybrid characteristics, including those belonging to Z3, can be clustered into a third major lineage (3, 30).
Findings related to the genetic variability of T. cruzi kinetoplast DNA (kDNA) minicircles suggest that the degree of similarity displayed by the profiles of two strains reflects, at least in part, the genetic distance between them (37). An association between tissue tropism and parasite-specific clones has been demonstrated in humans and mice, with possible implications for the clinical forms of Chagas' disease (2, 36).
New clinical and epidemiological perspectives could be generated with the genetic characterization of T. cruzi strains recently isolated from chagasic patients resident in an area where the disease is endemic that presents several clinical forms of the disease. In this context, we have characterized the rRNA gene and kDNA sequences of T. cruzi populations isolated from patients whose clinical presentations of Chagas' disease were carefully analyzed in order to investigate a possible association between genetic parameters and pathogenic potential.
MATERIALS AND METHODS
Patients.
Our sample was composed of 132 chagasic patients referred for clinical and parasitological evaluation at the Universidade Federal do Triângulo Mineiro (UFTM; Uberaba, Minas Gerais, Brazil). Most of the patients (127/132) come from a region of southeast Brazil (Minas Gerais) where Chagas' disease is endemic and where vectorial transmission has been interrupted. In this region, there are extensive variations in the prevalence of Chagas' disease and its distinct clinical manifestations can be observed. Three types of serological tests confirmed the chagasic etiology of patients: indirect immunofluorescence, indirect hemagglutination, and enzyme-linked immunosorbent assay. The clinical classification of the patients was based on the results of esophagrams, electrocardiograms, barium enemas, and/or the number of days of constipation. Of the 132 patients studied, 12.9% had the indeterminate (I) form of Chagas' disease, 33.3% had the cardiac (C) form, 4.5% had megacolon (MC), 11.4% had megaesophagus (ME), and 37.9% had mixed forms (3.8% MC-C, 22.7% ME-C, and 11.4% ME-MC-C). T. cruzi strains were isolated from 70 autochthonous patients with positive blood cultures and different clinical forms of Chagas' disease. This work and all procedures were carried out with the informed consent of the participants and were approved by the Medical Research Ethics Committee of UFTM. Experiments were performed in a double-blinded fashion, and patients were numbered chronologically.
Parasite isolation and DNA extraction.
Parasite isolation was performed by blood culture as previously described (16). Immediately after collection, 30 ml of venous blood was centrifuged at 4°C and 1,000 × g for 10 min in order to remove the plasma. The packed blood cells were washed by centrifugation at 4°C in 10 ml of liver infusion tryptose (LIT) medium, resuspended in 6 ml of LIT medium, and uniformly distributed among six plastic tubes. Cultures were maintained at 28°C, homogenized weekly, and examined monthly for 90 days. Microscopic examination was carried out in 10-μl aliquots of each preparation under a 22-mm2 coverslip at a magnification of 150×.
In order to minimize parasite selection, positive blood cultures in LIT medium were maintained in individual tubes in the laboratory for a short period of time without passage. LIT medium was added every 10 to 15 days for a maximum of 8 weeks. Culture samples were then collected and mixed with equal volumes of a 6 M guanidine hydrochloride-0.2 M EDTA solution and stored at 4°C for DNA extraction. Positive blood culture samples collected in guanidine-EDTA were boiled for 15 min before DNA extraction, which was carried out in duplicate with 200 μl of guanidine-EDTA-blood as previously described (11).
LSSP-PCR.
Genetic characterization of T. cruzi kDNA was performed by low-stringency single specific primer PCR (LSSP-PCR), a technique which allows the generation of specific gene signatures and which has been shown to be reproducible and sensitive (37). The technique is based on a two-step procedure. The first one consists of the specific PCR amplification of a 330-bp fragment corresponding to the four variable regions of T. cruzi kDNA with primers 121 (5′-AAATAATGTACGGG(T/G)GAGATGCATGA-3′) and 122 (5′-GGTTCGATTGGGGTTGGTGTAATATA-3′) (Operon Technology Inc., Alameda, CA) and 1.0 U of Taq DNA polymerase enzyme (Promega, Madison, WI). The PCR-amplified products were submitted to electrophoresis in a 1.5% agarose gel (1.0% agarose, 0.5% low-melting-point agarose) and ethidium bromide stained. The 330-bp fragments of individual amplifications, corresponding to approximately 150 ng of DNA, were excised from the gel, melted, diluted 10-fold in double-distilled water, and used as the template for a second step of amplification with a single 121 modified primer (5′-AAATAATGTACGGGGGAGATG-3′). The LSSP-PCR products were then visualized by 7.5% polyacrylamide gel electrophoresis after silver staining. In order to demonstrate the stability of the amplification, each DNA sample was analyzed in duplicate. The genetic profiles obtained were analyzed by the DNA-POP software, which compares the number of DNA bands shared between strains (27).
PCR amplification of the D7 domain of the 24Sα rRNA gene.
Divergent domain D7 of the 24Sα rRNA gene was PCR amplified with D71 (5′-AAGGTGCGTCGACAGTGTGG-3′) and D72 (5′-TTTTCAGAATGGCCGAACAGT-3′) (Operon Technology Inc., Alameda, CA) as described previously (32). PCR products were visualized by 6.0% polyacrylamide gel electrophoresis after silver staining. The amplification of a fragment of 110 or 125 bp identified the T. cruzi population as belonging to T. cruzi I or T. cruzi II, respectively (2a).
RESULTS
Clinical and parasitological characteristics of patients.
Blood culture positivity was 53.03% (70/132), and we did not observe differences in the distribution of clinical forms between patients with positive blood cultures and patients with negative blood cultures. Of the patients with positive parasitological evaluations, 11.43% had the I form and 88.6% had clinical manifestations of Chagas' disease; 27.14% had the C form, 14.3% were diagnosed with ME, and 2.85% were diagnosed with MC. Other patients had associated clinical forms; 4.28% had MC-C, 25.7% had ME-C, and 14.3% had ME-MC-C (Table 1). The intensities of the pathological alterations in the C form varied from I to III, and the degrees of ME varied from II to IV (21, 26, 29).
TABLE 1.
DNA-POP analysis of LSSP-PCR profiles obtained from T. cruzi strains isolated from blood cultures of chagasic patients
| Clinical form(s)a | % of patients (no. of patients/total) | Avg no. of bands/ lane ± SD | Avg no. of shared bands by pairs ± SD | % of shared bands |
|---|---|---|---|---|
| I | 11.43 (8/70) | 11.4 ± 1.5 | 3.9 ± 1.3 | 34 |
| C | 27.14 (19/70) | 9.8 ± 1.0 | 2.6 ± 1.3 | 27 |
| ME | 14.3 (10/70) | 11.0 ± 0.8 | 4.3 ± 1.6 | 39 |
| ME-C | 25.7 (18/70) | 10.1 ± 1.8 | 2.6 ± 1.6 | 26 |
| ME-MC-C | 14.3 (10/70) | 12.1 ± 2.0 | 5.0 ± 1.9 | 41 |
| MC | 2.85 (2/70) | 9.5 ± 0.5 | 5.5 ± 0.5 | 53 |
| MC-C | 4.28 (3/70) | 11.0 ± 1.4 | 6.1 ± 1.6 | 55 |
| All groups | NAb | 11.8 ± 1.8 | 2.9 ± 1.6 | 27 |
Results are presented for all detected clinical forms of Chagas' disease. For group definitions, see the text. All groups, simultaneous analysis of 21 blood culture samples representative of all clinical forms of Chagas' disease analyzed in the same polyacrylamide gel.
NA, not applicable.
Molecular characterization of T. cruzi genotypes.
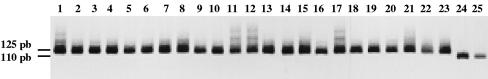
T. cruzi strains isolated from chagasic patients were initially characterized by PCR amplification of the 3′region of the 24Sα rRNA gene (Fig. 1). All of the parasite strains isolated presented the amplification of a 125-bp fragment, which demonstrates that they belong to T. cruzi II, which is associated with the domestic transmission cycle of Chagas' disease. Control samples of the T. cruzi I and T. cruzi II lineages were analyzed, providing fragments of the expected sizes.
FIG. 1.
Characterization of T. cruzi strains. Representative polyacrylamide gel showing amplification of the 3′ regions of 24Sα rRNA gene sequences from T. cruzi strains isolated from patients with different clinical forms of Chagas' disease. Lanes 1 to 5, isolates from patients with the cardiac form. Lanes 6 to 10, isolates from patients with megaesophagus. Lanes 11 to 14, isolates from patients with the indeterminate form. Lanes 15 to 21, isolates from patients with mixed clinical forms of Chagas' disease. Lanes 22 to 23, control DNA samples from T. cruzi II (125 bp). Lanes 24 to 25, control DNA samples from T. cruzi I (110 bp).
Intraspecific diversity of T. cruzi strains.
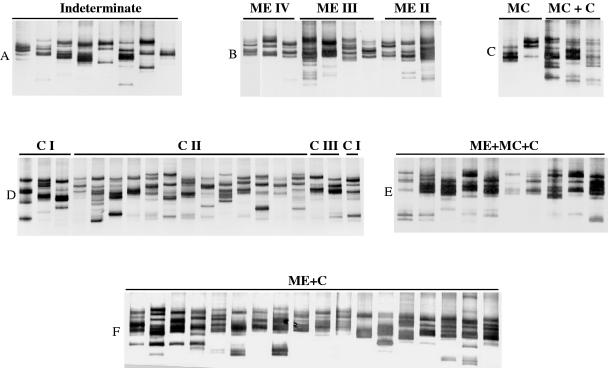
In order to conduct an extensive investigation concerning the occurrence of putative genetic profiles associated with each of the clinical forms of Chagas' disease, we carried out comparative LSSP-PCR analyses among parasites isolated from patients with the same clinical forms of the disease. Analysis of the genetic profiles obtained from the variable region of T. cruzi kDNA minicircles showed a high degree of intraspecific variability. A unique T. cruzi LSSP-PCR gene signature was obtained for each patient (Fig. 2). A high degree of genetic polymorphism was observed in the kDNA sequences of T. cruzi strains isolated from patients with the same clinical form of the disease, irrespective of the severity of the lesions, showing the following percentages of shared bands: MC-C, 55%; MC, 53%; ME-MC-C, 41%; ME, 39%; I, 34%; C, 27%; ME-C, 26%. The highest percentage of similarity was detected within T. cruzi isolates from patients who presented complex clinical manifestations associated with MC (Table 1); however, the sample size is not enough to draw any conclusions. The simultaneous comparison of 21 representative samples of all distinct clinical forms of Chagas' disease also revealed an intense kDNA polymorphism, with an average of 27% of the bands shared by pairs (Table 1).
FIG. 2.
kDNA signatures of T. cruzi obtained by LSSP-PCR. Amplified DNA fragments of parasites isolated by blood cultures from patients with different clinical forms of Chagas' disease were resolved in 7.5% polyacrylamide gels and silver stained. A, indeterminate form; B, megaesophagus (ME) with degrees of dilation II, III, and IV; C, megacolon (MC) and megacolon associated with the cardiac form (MC + C); D, cardiac form with degrees of lesions CI, CII, and CIII; E, cardiac form associated with megaesophagus and megacolon (ME+MC+C); F, cardiac form associated with megaesophagus (ME+C).
DISCUSSION
In this study, most of the patients examined had typical clinical symptoms of Chagas' disease (88.6%), with a high frequency of mixed forms (44.3%) and few intermediate-form cases, probably because they were selected in a hospital unit. T. cruzi II was detected in all of the patients, including those without clinical alterations, and was also found to be associated with different symptoms and various degrees of pathological processes in all clinical forms of the disease, although T. cruzi I is present in the sylvatic transmission cycle in the region of Triângulo Mineiro (28).
Our findings are in agreement with previously published data from other Brazilian areas where the disease is endemic and where T. cruzi II is mainly distributed in the domestic cycle and is associated with severe human infections (8, 38). Furthermore, the presence of T. cruzi II in cardiac and megaesophagus lesions of distinct chagasic patients was recently demonstrated (10). Moreover, high incidences of human chagasic cardiac lesions in Argentina (23) and digestive forms in Brazil (15) have also been correlated with the Z12 and Z2 zymodemes, respectively, both associated with T. cruzi II. These and other results have been used as evidences that humans could act as a biological filter selecting more adaptable T. cruzi strains (17). When clinical-genetic approaches are adopted, it is important to take into account the facts that some patients may harbor more than one T. cruzi population (25, 35) and that tissue and blood samples or isolates may not represent all of the clones of a strain. As a result of low parasitemia, different parasite populations with distinct tissue tropism may not always be detected and it is also possible that some clones are selected by the available isolation techniques.
A high intraspecific variability among the T. cruzi II kDNAs was demonstrated by the diversity of LSSP-PCR gene signatures, which did not correlate with clinical manifestations. Although most of the isolates had the same geographical origin, the parasite genetic profiles were unique and specific for each patient, irrespective of the clinical features and stages of the disease. The intense polymorphism of kDNA may result from the presence of different classes of minicircle sequences in each parasite characterized, the high mutation rates in their hypervariable regions (31), reversible changes in kDNA minicircle sequences (1), or the low sequence identity among them (7). However, we cannot rule out the possibility that the hypervariability of kDNA gene signatures is a result of several years of an intimate host-parasite interaction in specific tissues with active pressure from the immune system. These factors may explain the difficulty in establishing genetic profiles associated with specific clinical manifestations. Therefore, studies using DNA fingerprinting (18) and restriction fragment length polymorphism of kDNA minicircles (24) have failed to correlate specific variability markers with the clinical prognosis of Chagas' disease (17).
The correct choice or development of other genetic markers based on both nuclear and kDNA variability could define the role of T. cruzi in determining the clinical forms of Chagas' disease. One important question to be answered is whether the stability of a clinical form would correlate with changes in parasite genetic profiles of a chagasic patient over a few years. Several factors should be further investigated in carefully controlled clinical and epidemiological studies in different regions where the disease is endemic in order to better address this issue.
Acknowledgments
This study was supported by grants from the Financiadora de Estudos e Projetos (41/96/0894/00; FINEP/PRONEX), the Fundação de Amparo a Pesquisa de Minas Gerais (FAPEMIG), the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) Edital Universal 2001, the UNDP/World Bank/WHO Special Program for Research and Training in Tropical Disease (TDR), and the Fundação de Ensino e Pesquisa de Uberaba da Universidade Federal do Triângulo Mineiro (FUNEPU/UFTM).
REFERENCES
- 1.Alves, A. M., D. F. De Almeida, and W. M. von Kruger. 1994. Changes in Trypanosoma cruzi kinetoplast DNA minicircles induced by environmental conditions and subcloning. J. Eukaryot. Microbiol. 41:415-419. [DOI] [PubMed] [Google Scholar]
- 2.Andrade, L. O., C. R. Machado, E. Chiari, S. D. Pena, and A. M. Macedo. 1999. Differential tissue distribution of diverse clones of Trypanosoma cruzi in infected mice. Mol. Biochem. Parasitol. 100:163-172. [DOI] [PubMed] [Google Scholar]
- 2a.Anonymous. 1999. Recommendations from a satellite meeting. Mem. Inst. Oswaldo Cruz 94:429-432. [DOI] [PubMed] [Google Scholar]
- 3.Augusto-Pinto, L., S. M. Teixeira, S. D. Pena, and C. R. Machado. 2003. Single-nucleotide polymorphisms of the Trypanosoma cruzi MSH2 gene support the existence of three phylogenetic lineages presenting differences in mismatch-repair efficiency. Genetics 164:117-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brisse, S., J. C. Dujardin, and M. Tibayrenc. 2000. Identification of six Trypanosoma cruzi lineages by sequence-characterised amplified region markers. Mol. Biochem. Parasitol. 111:95-105. [DOI] [PubMed] [Google Scholar]
- 5.Brisse, S., J. Henriksson, C. Barnabe, E. J. Douzery, D. Berkvens, M. Serrano, M. R. De Carvalho, G. A. Buck, J. C. Dujardin, and M. Tibayrenc. 2003. Evidence for genetic exchange and hybridization in Trypanosoma cruzi based on nucleotide sequences and molecular karyotype. Infect. Genet. Evol. 2:173-183. [DOI] [PubMed] [Google Scholar]
- 6.Coura, J. R., N. Anunziato, and H. P. Willcox. 1983. Chagas' disease morbidity. I. Study of cases originating in various states of Brazil, observed in Rio de Janeiro. Mem. Inst. Oswaldo Cruz 78:363-372. [DOI] [PubMed] [Google Scholar]
- 7.Degrave, W., S. P. Fragoso, C. Britto, H. van Heuverswyn, G. Z. Kidane, M. A. Cardoso, R. U. Mueller, L. Simpson, and C. M. Morel. 1988. Peculiar sequence organization of kinetoplast DNA minicircles from Trypanosoma cruzi. Mol. Biochem. Parasitol. 27:63-70. [DOI] [PubMed] [Google Scholar]
- 8.Fernandes, O., S. Santos, A. Junqueira, A. Jansen, E. Cupolillo, D. Campbell, B. Zingales, and J. R. Coura. 1999. Populational heterogeneity of Brazilian Trypanosoma cruzi isolates revealed by the mini-exon and ribosomal spacers. Mem. Inst. Oswaldo Cruz 94(Suppl. 1):195-197. [DOI] [PubMed] [Google Scholar]
- 9.Fernandes, O., R. P. Souto, J. A. Castro, J. B. Pereira, N. C. Fernandes, A. C. Junqueira, R. D. Naiff, T. V. Barrett, W. Degrave, B. Zingales, D. A. Campbell, and J. R. Coura. 1998. Brazilian isolates of Trypanosoma cruzi from humans and triatomines classified into two lineages using mini-exon and ribosomal RNA sequences. Am. J. Trop. Med. Hyg. 58:807-811. [DOI] [PubMed] [Google Scholar]
- 10.Freitas, J. M., E. Lages-Silva, E. Crema, S. D. Pena, and A. M. Macedo. 2005. Real time PCR strategy for the identification of major lineages of Trypanosoma cruzi directly in chronically infected human tissues. Int. J. Parasitol. 35:411-417. [DOI] [PubMed] [Google Scholar]
- 11.Gomes, M. L., A. M. Macedo, A. R. Vago, S. D. Pena, L. M. Galvao, and E. Chiari. 1998. Trypanosoma cruzi: optimization of polymerase chain reaction for detection in human blood. Exp. Parasitol. 88:28-33. [DOI] [PubMed] [Google Scholar]
- 12.Higuchi, M. L., P. S. Gutierrez, V. D. Aiello, S. Palomino, E. Bocchi, J. Kalil, G. Bellotti, and F. Pileggi. 1993. Immunohistochemical characterization of infiltrating cells in human chronic chagasic myocarditis: comparison with myocardial rejection process. Virchows Arch. A Pathol. Anat. Histopathol. 423:157-160. [DOI] [PubMed] [Google Scholar]
- 13.Jones, E. M., D. G. Colley, S. Tostes, E. R. Lopes, C. L. Vnencak-Jones, and T. L. McCurley. 1993. Amplification of a Trypanosoma cruzi DNA sequence from inflammatory lesions in human chagasic cardiomyopathy. Am. J. Trop. Med. Hyg. 48:348-357. [DOI] [PubMed] [Google Scholar]
- 14.Lages-Silva, E., E. Crema, L. E. Ramírez, A. M. Macedo, S. D. Pena, and E. Chiari. 2001. Relationship between Trypanosoma cruzi and human chagasic megaesophagus: blood and tissue parasitism. Am. J. Trop. Med. Hyg. 65:435-441. [DOI] [PubMed] [Google Scholar]
- 15.Luquetti, A. O., M. A. Miles, A. Rassi, J. M. de Rezende, A. A. de Souza, M. M. Povoa, and I. Rodrigues. 1986. Trypanosoma cruzi: zymodemes associated with acute and chronic Chagas' disease in central Brazil. Trans. R. Soc. Trop. Med. Hyg. 80:462-470. [DOI] [PubMed] [Google Scholar]
- 16.Luz, Z. M., M. G. Coutinho, J. R. Cancado, and A. U. Krettli. 1994. Hemoculture: sensitive technique in the detection of Trypanosoma cruzi in chagasic patients in the chronic phase of Chagas disease. Rev. Soc. Bras. Med. Trop. 27:143-148. [DOI] [PubMed] [Google Scholar]
- 17.Macedo, A. M., C. R. Machado, R. P. Oliveira, and S. D. Pena. 2004. Trypanosoma cruzi: genetic structure of populations and relevance of genetic variability to the pathogenesis of Chagas disease. Mem. Inst. Oswaldo Cruz 99:1-12. [DOI] [PubMed] [Google Scholar]
- 18.Macedo, A. M., M. S. Martins, E. Chiari, and S. D. Pena. 1992. DNA fingerprinting of Trypanosoma cruzi: a new tool for characterization of strains and clones. Mol. Biochem. Parasitol. 55:147-153. [DOI] [PubMed] [Google Scholar]
- 19.Macedo, A. M., and S. D. Pena. 1998. Genetic variability of Trypanosoma cruzi: implications for pathogenesis of Chagas disease. Parasitol. Today 14:119-123. [DOI] [PubMed] [Google Scholar]
- 20.Macedo, A. M., J. R. Pimenta, R. S. Aguiar, A. I. Melo, E. Chiari, B. Zingales, S. D. Pena, and R. P. Oliveira. 2001. Usefulness of microsatellite typing in population genetic studies of Trypanosoma cruzi. Mem. Inst. Oswaldo Cruz 96:407-413. [DOI] [PubMed] [Google Scholar]
- 21.Macedo, V. O. 1976. Influência da exposição à reinfecção na evolução da doença de Chagas (estudo longitudinal de 5 años). Rev. Patol. Trop. 5:33-115. [Google Scholar]
- 22.Miles, M. A., A. Souza, M. Povoa, J. J. Shaw, R. Lainson, and P. J. Toye. 1978. Isozymic heterogeneity of Trypanosoma cruzi in the first autochthonous patients with Chagas' disease in Amazonian Brazil. Nature 272:819-821. [DOI] [PubMed] [Google Scholar]
- 23.Montamat, E. E., G. M. De Luca D'Oro, R. H. Gallerano, R. Sosa, and A. Blanco. 1996. Characterization of Trypanosoma cruzi populations by zymodemes: correlation with clinical picture. Am. J. Trop. Med. Hyg. 55:625-628. [DOI] [PubMed] [Google Scholar]
- 24.Morel, C. M., E. Chiari, E. Plessman Camargo, D. M. Mattei, A. J. Romanha, and L. Simpson. 1980. Strains and clones of Trypanosoma cruzi can be characterized by pattern of restriction endonuclease products of kinetoplast DNA minicircles. Proc. Natl. Acad. Sci. USA 77:6810-6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oliveira, R. P., N. E. Broude, A. M. Macedo, C. R. Cantor, C. L. Smith, and S. D. Pena. 1998. Probing the genetic population structure of Trypanosoma cruzi with polymorphic microsatellites. Proc. Natl. Acad. Sci. USA 95:3776-3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Organização Mundial de Saúde/Organização Panamericana de Saúde (OMS/OPAS). 1974. Aspectos clínicos de la enfermidad de Chagas. Informe de una reunión conjunta OMS/OPS de investigadores. Bol. Of. Sanit. Panam. 76:141-158. [Google Scholar]
- 27.Pena, S. D. J., and A. Nunes. 1990. DNA-POP and PATER two simple computer programs for population studies and paternity analyses with DNA fingerprintings. Fingerprinting News 2:7-8. [Google Scholar]
- 28.Ramírez, L. E., E. Lages-Silva, F. Alvarenga-Franco, A. Matos, N. Vargas, O. Fernandes, and B. Zingales. 2002. High prevalence of Trypanosoma rangeli and Trypanosoma cruzi in opossums and triatomids in a formerly-endemic area of Chagas disease in southeast Brazil. Acta Trop. 84:189-198. [DOI] [PubMed] [Google Scholar]
- 29.Rezende, J. M., K. M. Lauar, and A. de Oliveira. 1960. Clinical and radiological aspects of aperistalsis of the esophagus. Rev. Bras. Gastroenterol. 12:247-262. [PubMed] [Google Scholar]
- 30.Santos, S. S., E. Cupolillo, A. Junqueira, J. R. Coura, A. Jansen, N. R. Sturm, D. A. Campbell, and O. Fernandes. 2002. The genetic diversity of Brazilian Trypanosoma cruzi isolates and the phylogenetic positioning of zymodeme 3, based on the internal transcribed spacer of the ribosomal gene. Ann. Trop. Med. Parasitol. 96:755-764. [DOI] [PubMed] [Google Scholar]
- 31.Shapiro, T. A., and P. T. Englund. 1995. The structure and replication of kinetoplast DNA. Annu. Rev. Microbiol. 49:117-143. [DOI] [PubMed] [Google Scholar]
- 32.Souto, R. P., O. Fernandes, A. M. Macedo, D. A. Campbell, and B. Zingales. 1996. DNA markers define two major phylogenetic lineages of Trypanosoma cruzi. Mol. Biochem. Parasitol. 83:141-152. [DOI] [PubMed] [Google Scholar]
- 33.Tarleton, R. L. 2001. Parasite persistence in the aetiology of Chagas disease. Int. J. Parasitol. 31:550-554. [DOI] [PubMed] [Google Scholar]
- 34.Tibayrenc, M., and F. Ayala. 1988. Isoenzyme variability in Trypanosoma cruzi, the agent of Chagas' disease: genetical, taxonomic and epidemiological significance. Evolution 42:277-292. [DOI] [PubMed] [Google Scholar]
- 35.Vago, A. R., L. O. Andrade, A. A. Leite, D. d'Avila Reis, A. M. Macedo, S. J. Adad, S. Tostes, Jr., M. C. Moreira, G. B. Filho, and S. D. Pena. 2000. Genetic characterization of Trypanosoma cruzi directly from tissues of patients with chronic Chagas disease: differential distribution of genetic types into diverse organs. Am. J. Pathol. 156:1805-1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vago, A. R., A. M. Macedo, S. J. Adad, D. D. Reis, and R. Correa-Oliveira. 1996. PCR detection of Trypanosoma cruzi DNA in oesophageal tissues of patients with chronic digestive Chagas' disease. Lancet 348:891-892. [DOI] [PubMed] [Google Scholar]
- 37.Vago, A. R., A. M. Macedo, R. P. Oliveira, L. O. Andrade, E. Chiari, L. M. Galvao, D. Reis, M. E. Pereira, A. J. Simpson, S. Tostes, and S. D. Pena. 1996. Kinetoplast DNA signatures of Trypanosoma cruzi strains obtained directly from infected tissues. Am. J. Pathol. 149:2153-2159. [PMC free article] [PubMed] [Google Scholar]
- 38.Zingales, B., R. P. Souto, R. H. Mangia, C. V. Lisboa, D. A. Campbell, J. R. Coura, A. Jansen, and O. Fernandes. 1998. Molecular epidemiology of American trypanosomiasis in Brazil based on dimorphisms of rRNA and mini-exon gene sequences. Int. J. Parasitol. 28:105-112. [DOI] [PubMed] [Google Scholar]