Abstract
We report a case of acute cholecystitis accompanied by acute pancreatitis and caused by Dolosigranulum pigrum in a 76-year-old male with gallstones. D. pigrum was isolated from a blood culture and confirmed by biochemistry tests and 16S rRNA sequencing. The isolate was susceptible to the β-lactams ampicillin, penicillin, cephalothin, ceftriaxone, ceftazidime, chloramphenicol, and vancomycin but was intermediate to erythromycin and clindamycin. The patient recovered without sequelae after treatment with appropriate antibiotics for two weeks.
CASE REPORT
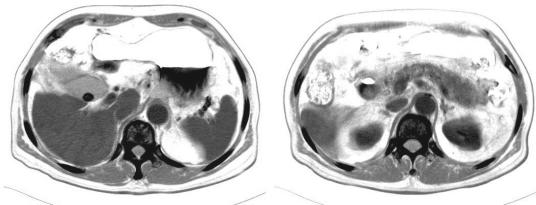
A 76-year-old male with dementia living in a day care center suffered from nausea, vomiting, chills, and fever for 1 day. He was admitted to Tri-Service General Hospital on 11 August 2005 with a diagnosis of gallstones, with acute cholecystitis and acute pancreatitis. He had no underlying diseases, such as diabetes, hypertension, hematological disorders, or malignant solid organs. On admission, physical examination was remarkable, with an oral temperature of 38°C, drowsy consciousness with icteric sclera, and tenderness over the right upper quadrant without rebound pain. Laboratory tests showed a white blood cell count of 9.9 × 109/liter, with 86% neutrophils, 128 U/liter aspartate aminotransferase (normal, <37 U/liter), 65 U/liter alanine aminotransferase (normal, <41 U/liter), 10.7 mg/dl total bilirubin (normal, <1 mg/dl), 0.8 mg/dl creatine, 9.21 mg/dl C-reactive protein (normal, <0.5 mg/dl), 761 U/liter amylase (normal, 28 to 100 U/liter), and 818 U/liter lipase (normal, 13 to 60 U/liter). Abdominal computed tomography showed a 1.4-cm-diameter stone within the gallbladder, with minimal fluid accumulation over the fossa and swelling of pancreatic tissue (Fig. 1). Empirical parenteral administration of 1.0 g of ampicillin and 500 mg of sulbactam every 8 h was begun on the first day of hospitalization. On the third day of hospitalization, a blood culture showed gram-positive cocci in small grayish-white alpha-hemolytic colonies on a blood agar plate. Biochemistry results were negative for catalase, positive for l-pyrrolidonyl-β-naphthylamide, and positive with bile esculin medium. Biochemical tests for Enterococcus, such as those using mannitol, arginine, methyl-a-d-glucopyranoside, arabinose, sorbose, and pyruvate, were negative. Serologic tests for streptococcus groups A, B, C, D, F, and G were all negative. Sequencing of 16S rRNA identified the presence of Dolosigranulum pigrum (7, 11). The MICs of nine antimicrobial agents were determined by Etest using interpretive standards based on CLSI recommendations (4). The results demonstrated that the D. pigrum isolate was susceptible to the β-lactams ampicillin, penicillin, cephalothin, ceftriaxone, ceftazidime, chloramphenicol, and vancomycin but was intermediate to erythromycin and clindamycin, with MICs of 0.75 μg/ml and 0.5 μg/ml, respectively. The patient's body temperature, abdominal pain, nausea, and vomiting improved after antibiotic administration. The amylase, lipase, and C-reactive protein concentrations decreased to within normal ranges. The patient was discharged on the seventh day of hospitalization with a prescription for oral cephalosporin antibiotics.
FIG. 1.
Abdominal computed tomography. The image shows a stone within the gallbladder, with minimal fluid accumulation over the fossa and swelling of pancreatic tissue.
Discussion.
D. pigrum, described by Aguirre et al. in 1993 (1), is a gram-positive coccus arranged in pairs, tetrads, and clusters (13). D. pigrum is an infrequent clinical isolate found as an opportunistic organism of infection in immunocompromised hosts. It is a part of the normal floras of the oral cavity, the respiratory and alimentary tracts, and the skin (1). Articles about disease caused by D. pigrum are rare (9, 12). LaClaire et al. reported an analysis of clinical demographic data and antimicrobial susceptibilities of infections due to 27 different strains of D. pigrum (12). They found the age of patients with D. pigrum infection ranged from 2 months to more than 80 years. Most of them were either very young (under 2 years old) or very old (over 65 years old). There were equal percentages of male and female patients. The most common isolation sites were from blood (44%, 12/27), eyes (22%, 6/27), and the nasopharyngeal region (15%, 4/27). D. pigrum caused diseases in clinics, including sepsis, blepharitis, pneumonia, sinusitis, and synovitis (9, 12). There are no reports of D. pigrum organisms causing acute cholecystitis or acute pancreatitis. Although the organism was not directly isolated from the patient's biliary tract or pancreatic bed, both inflammations subsided after the patient was treated with appropriate antibiotics.
Laboratory identification of D. pigrum is based on a determination of colony morphology on a blood agar plate, Gram staining, and biochemistry tests, including those for catalase, esculin hydrolysis, vancomycin sensitivity, l-pyrrolidonyl-β-naphthylamide hydrolysis, leucine aminopeptidase hydrolysis, and growth on 6.5% NaCl broth, to differentiate it from other unusual alpha-hemolytic gram-positive cocci (1, 5, 15). When results of reliable biochemistry tests for clinically relevant bacteria cannot be adequately documented, molecular identification is an important and essential routine for microbiology laboratories. The technique of 16S rRNA gene sequencing and analysis can identify unusual strains, such as D. pigrum strains (6, 7, 14). In this case, the 16S rRNA sequence of D. pigrum was confirmed to be the same as that of the GenBank reference number AF193886. The 16S rRNA sequencing method is a robust alternative method for rapid identification of pathogens, especially those which are rare or difficult to identify by conventional methods.
The antimicrobial patterns of our strain are similar to those of the LaClaire et al. study. LaClaire et al. demonstrated that all 27 D. pigrum isolates were sensitive to 11 tested drugs (including clindamycin), but the majority (15/27) of their strains were resistant to erythromycin (12) and 10 were intermediate. Our strain was also sensitive to seven tested drugs but had intermediate resistance to erythromycin and clindamycin.
Acute pancreatitis is a disease with a wide variety of clinical presentations (16). About 80% to 90% of pancreatitis presents as a mild edematous inflammation of the pancreas with low morbidity and mortality rates (<1%). Necrotizing pancreatitis represents a severe form of the disease that develops in about 15% of patients and has a reported mortality rate as high as 50% (2, 3, 8, 16). The most common pathogens in acute pancreatitis are the gram-negative aerobic bacteria Escherichia coli, Pseudomonas spp., Proteus spp., and Klebsiella spp. (10, 16). Anaerobes and gram-positive bacteria of Staphylococcus aureus and Enterococcus faecalis have also been isolated from patients with this condition (16).
In summary, D. pigrum infection remains rare but could become a potential pathogen, especially in infants and the elderly. Sequencing of 16S rRNA is a reliable method for rapid identification of this rare and difficult-to-identify pathogen.
Acknowledgments
This study was supported by grants TSGH-C93-32 and TSGH-C94-83 from the Tri-Service General Hospital, Taiwan, Republic of China.
REFERENCES
- 1.Aguirre, M., D. Morrison, B. D. Cookson, F. W. Gay, and M. D. Collins. 1993. Phenotypic and phylogenetic characterization of some Gemella-like organisms from human infections: description of Dolosigranulum pigrum gen. nov., sp. nov. J. Appl. Bacteriol. 75:608-612. [DOI] [PubMed] [Google Scholar]
- 2.Beger, H. G., B. Rau, J. Mayer, and U. Pralle. 1997. Natural course of acute pancreatitis. World J. Surg. 21:130-135. [DOI] [PubMed] [Google Scholar]
- 3.Buchler, M. W., B. Gloor, C. A. Muller, H. Friess, C. A. Seiler, and W. Uhl. 2000. Acute necrotizing pancreatitis: treatment strategy according to the status of infection. Ann. Surg. 232:619-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clinical and Laboratory Standards Institute/NCCLS. 2005. Performance standards for antimicrobial susceptibility testing: fifteenth informational supplement. CLSI/NCCLS document M100-S15. Clinical and Laboratory Standards Institute, Wayne, Pa.
- 5.Collins, M. D., R. Higgins, S. Messier, M. Fortin, R. A. Hutson, P. A. Lawson, and E. Falsen. 2003. Allofustis seminis gen. nov., sp. nov., a novel Gram-positive, catalase-negative, rod-shaped bacterium from pig semen. Int. J. Syst. Evol. Microbiol. 53:811-814. [DOI] [PubMed] [Google Scholar]
- 6.Ferris, M. J., A. Masztal, and D. H. Martin. 2004. Use of species-directed 16S rRNA gene PCR primers for detection of Atopobium vaginae in patients with bacterial vaginosis. J. Clin. Microbiol. 42:5892-5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fontana, C., M. Favaro, M. Pelliccioni, E. S. Pistoia, and C. Favalli. 2005. Use of the MicroSeq 500 16S rRNA gene-based sequencing for identification of bacterial isolates that commercial automated systems failed to identify correctly. J. Clin. Microbiol. 43:615-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerzof, S. G., P. A. Banks, A. H. Robbins, W. C. Johnson, S. J. Spechler, S. M. Wetzner, J. M. Snider, R. E. Langevin, and M. E. Jay. 1987. Early diagnosis of pancreatic infection by computed tomography-guided aspiration. Gastroenterology 93:1315-1320. [DOI] [PubMed] [Google Scholar]
- 9.Hall, G. S., S. Gordon, S. Schroeder, K. Smith, K. Anthony, and G. W. Procop. 2001. Case of synovitis potentially caused by Dolosigranulum pigrum. J. Clin. Microbiol. 39:1202-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartwig, W., J. Werner, W. Uhl, and M. W. Buchler. 2002. Management of infection in acute pancreatitis. J. Hepatobiliary Pancreat. Surg. 9:423-428. [DOI] [PubMed] [Google Scholar]
- 11.Kolbert, C. P., and D. H. Persing. 1999. Ribosomal DNA sequencing as a tool for identification of bacterial pathogens. Curr. Opin. Microbiol. 2:299-305. [DOI] [PubMed] [Google Scholar]
- 12.LaClaire, L., and R. Facklam. 2000. Antimicrobial susceptibility and clinical sources of Dolosigranulum pigrum cultures. Antimicrob. Agents Chemother. 44:2001-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LaClaire, L. L., and R. R. Facklam. 2000. Comparison of three commercial rapid identification systems for the unusual gram-positive cocci Dolosigranulum pigrum, Ignavigranum ruoffiae, and Facklamia species. J. Clin. Microbiol. 38:2037-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mikosza, A. S., M. A. Munshi, and D. J. Hampson. 2004. Analysis of genetic variation in Brachyspira aalborgi and related spirochaetes determined by partial sequencing of the 16S rRNA and NADH oxidase genes. J. Med. Microbiol. 53:333-339. [DOI] [PubMed] [Google Scholar]
- 15.Miller, P. H., R. R. Facklam, and J. M. Miller. 1996. Atmospheric growth requirements for Alloiococcus species and related gram-positive cocci. J. Clin. Microbiol. 34:1027-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmid, S. W., W. Uhl, H. Friess, P. Malfertheiner, and M. W. Buchler. 1999. The role of infection in acute pancreatitis. Gut 45:311-316. [DOI] [PMC free article] [PubMed] [Google Scholar]