Abstract
We describe a case of an adult organ recipient patient with a pulmonary cavitary lesion due to Neisseria lactamica, a harmless commensal organism that rarely causes human infection. To our knowledge, this is the first report of pulmonary disease caused by this organism and the second case of N. lactamica infection in an adult patient.
CASE REPORT
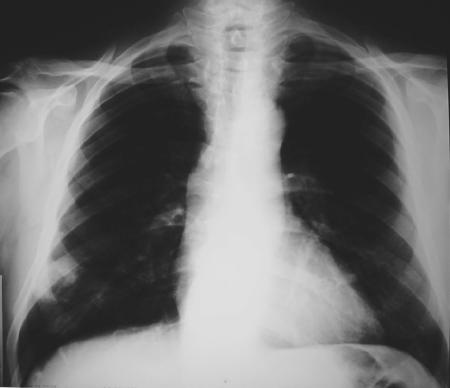
A 64-year-old man who had received a cadaver kidney allograft 2 years previously consulted with a primary-care physician 6 months before his hospital admission. He complained of high fever and productive cough with purulent sputum. Maintenance immunosuppressive therapy was composed of cyclosporine at 125 mg twice a day, mycofenolate mofetil at 1,000 mg twice a day, and prednisone at 10 mg daily. A chest X-ray image showed right lower lobe opacity (Fig. 1), and the patient was diagnosed with a mild community-acquired pneumonia and treated with oral levofloxacin at 500 mg/daily for 14 days, with complete resolution of the symptoms. No other chest X-ray examination was performed after this treatment.
FIG. 1.
Chest X-ray image showing a right lower lobe opacity.
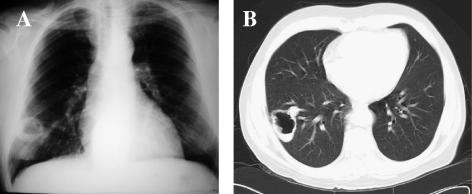
At admission he presented with 3-day nonproductive mild cough. No other symptoms were present. A new chest X-ray exam showed a cavitary lesion in the right lower lobe (Fig. 2A), and he was admitted to the hospital for further investigation. The patient was in good health status with normal body weight and had normal vital signs. The physical examination was unremarkable.
FIG. 2.
Cavitary lesion at the anterior and lateral basal segments transition of the right lower lung lobe on chest X-ray (A) and computed tomographic (B) images.
A chest computed tomogram showed an irregular cavity, measuring 5.4 cm by 3.6 cm in its major transversal axis, with a moderately thickened wall (0.7 cm) and a small air-fluid level at the anterior and lateral basal segments transition of the right lower lung lobe (Fig. 2B) There was no evidence of other alterations in the lung parenchyma or mediastinum. The erythrocyte and leukocyte counts were normal. The erythrocyte sedimentation rate was 24 mm/h. The serum creatinine level was 1.4 mg/dl. Several blood cultures were negative. The tuberculin skin test was nonreactive.
A transthoracic pulmonary fine-needle aspiration was performed, and approximately 1 ml of nonfetid purulent secretion was obtained. The Gram stain showed intracellular, gram-negative diplococci and many polymorphonuclear leukocytes. The sample was inoculated onto chocolate agar and 5% sheep blood agar (bioMérieux, Brazil). The agar media were incubated at 36°C for 48 h. Opaque, oxidase-positive colonies that looked like gram-negative cocci were observed. The panel HNID (Dade Behring, Inc., West Sacramento, CA) was used according to the manufacturer's protocol. The following tests were performed: indoxyl-phosphatase, nitrate, nitrite, glucose, sucrose, maltose, fructose, lactose, urea, ornithine, indole, l-prolyl-β-naphtylamide, N-γ-l-glutamil-β-naphtylamide, N-α-benzoyl-dl-arginine, β-naphtylamide, p-nitrophenil-α-d-glucoside, starch, and o-nitrophenil-β-d-galactoside. The tests with a positive result included glucose, maltose, lactose, o-nitrophenil-β-d-galactoside, and l-prolyl-β-naphtylamide. The organism was identified as Neisseria lactamica with 99.9% probability. A chromogenic cephalosporin test, with nitrocefin (Becton Dickinson, Sparks, MD), was performed to check the organism's ability to produce β-lactamase, and the result was negative.
Cephalosporin (acetyl cefuroxime) treatment was then started, with immediate resolution of the symptoms and with partial regression of the cavity after 1 month of treatment. Oral ciprofloxacin replaced acetyl cefuroxime after 3 months. A 5-month follow-up revealed an almost complete resolution of the lesion, and ciprofloxacin therapy was then stopped.
We describe here a case of N. lactamica infection that likely began as a partially treated pneumonia and then asymptomatically evolved over 6 months to a cavitary lung lesion. To our knowledge, only one other case of N. lactamica infection in an adult patient has been reported (3). It occurred in a 46-year-old woman who developed meningitis caused by a nasal cerebral spinal fluid leak after skull trauma (3). Although another case in an 18-year-old female patient with vaginal discharge has been reported, the role of N. lactamica as an etiologic agent of the vaginitis was not certain (12). The present case is the first report of N. lactamica causing pulmonary infection and the second report of an N. lactamica infection in an adult patient.
N. lactamica is a harmless human commensal organism closely related to N. meningitidis and N. gonorrhoeae, both pathogens of global significance (1). Infection by this organism is an extremely rare entity, although a few cases have been reported, most of them in infants and newborns (2, 3, 5, 6, 8, 10-12, 14) (Table 1). This may be explained by the fact that N. lactamica carriage throughout the world is high in infants and young children and declines with age (4).
TABLE 1.
Infections by Neisseria lactamica reported in the literature
| Reference | Age, sex | Clinical presentation | Potential predisposing condition | Treatmenta | Outcome |
|---|---|---|---|---|---|
| 8 | 7 mo, F | Meningitis | None | i.v. ampicillin followed by i.v. aqueous penicillin G | Cured |
| 14 | 23 mo, F | Otitis and bacteremia | Previous cardiac arrest; patent ductus arteriosus with pulmonary hypertension and gastrointestinal malrotation | i.v. ampicillin | Cured |
| 5 | 6 mo, F | Meningitis | None | i.v. ampicillin | Cured |
| 6 | 6 yr, M | Meningitis | None | Cured | |
| 11 | 7 yr, F | Bacteremia | Chemotherapy-induced neutropenia/acute lymphocytic leukemia | i.v. penicillin followed by oral penicillin | Cured |
| 2 | 15 wk, M | Bacteremia | Cerebral infarcts | i.v. benzyl-penicillin | Cured |
| 10 | 28 mo, M | Otitis | NDb | ND | |
| 12 | 18 yr, F | Vaginitis? Vaginal colonization? | None | Oral cotrimoxazole | Cured |
| 2 | 46 yr, F | Meningitis | Cerebrospinal fluid leak following skull trauma | i.v. ceftizoxime and i.v. penicillin | Cured |
i.v., intravenous.
ND, not described.
Bacteremia is considered a key step in the pathophysiology of meningococcal disease and precedes any form of invasive infection (13). It has either been confirmed or probably occurred in most of the cases of N. lactamica infection (2, 5, 8, 11, 14). However, in the previous case in an adult patient, the mode of entry of the organism into the cranium was via a cerebrospinal fluid leak through the cribriform plate (3). In the present case, it was not possible to determine whether lung infection followed hematogenic dissemination or aspiration of nasopharyngeal secretions. It is possible that the immunosuppressive state of the patient may have contributed to the carriage state, the invasiveness of the N. lactamica, and further development of lung tissue damage.
Treatments of previous cases of N. lactamica infections were usually short, and all patients were cured (Table 1). Our patient was treated for a longer time, since the length of the therapy was based on the radiological resolution of the cavitary lesion, although the infection had possibly been resolved earlier.
Neisseria spp. may be differentiated by their patterns of acid production from glucose, maltose, lactose, sucrose, and fructose. The most prominent biochemical feature of the species is the production of acid from lactose, which distinguishes N. lactamica from all other species of the genus (1). The HNID panel is a microdilution tray system that uses chromogenic enzyme substrates and modified conventional tests for the identification of Neisseria spp., Haemophilus spp., and Moraxella spp. These tests provide a reaction profile that allows immediate differentiation among the groups of Neisseria spp. (7). Although in vitro susceptibility testing is recommended for any gram-negative, oxidase-positive diplococcus isolated from clinically relevant specimens (7), the Clinical and Laboratory Standards Institute does not provide interpretive criteria for testing members of the genus Neisseria other than N. gonorrhoeae and N. meningitidis. Thus, we performed the direct nitrocefin-based β-lactamase test, which detects β-lactamase-mediated penicillin resistance (7).
Thus far, the clinical relevance of N. lactamica has been limited to the fact that, like other Neisseria species, this organism can transfer antibiotic resistance markers into the closely related species N. meningitidis (9). However, although still extremely rare, N. lactamica may be important as a human pathogen, particularly in immunocompromised patients. A better understanding of a possible relationship between immunosuppression, nasopharyngeal colonization, and invasive disease may determine the real importance of N. lactamica as an adult human pathogen.
REFERENCES
- 1.Alber, D., M. Oberkötter, S. Suerbaum, H. Claus, M. Frosch, and U. Vogel. 2001. Genetic diversity of Neisseria lactamica strains from epidemiologically defined carriers. J. Clin. Microbiol. 39:1710-1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown, N. M., N. K. Ragge, and D. C. Speller. 1987. Septicaemia due to Neisseria lactamica: initial confusion with Neisseria meningitidis. J. Infect. 15:243-245. [DOI] [PubMed] [Google Scholar]
- 3.Denning, D. W., and S. S. Gill. 1991. Neisseria lactamica meningitis following skull trauma. Rev. Infect. Dis. 13:216-218. [DOI] [PubMed] [Google Scholar]
- 4.Gold, R., I. Goldschneider, M. L. Lepow, T. F. Draper, and M. Randolph. 1978. Carriage of Neisseria meningitidis and Neisseria lactamica in infants and children. J. Infect. Dis. 137:112-121. [DOI] [PubMed] [Google Scholar]
- 5.Greenberg, L. W., and E. Kleinerman. 1978. Neisseria lactamica meningitis. J. Pediatr. 93:1061-1062. [DOI] [PubMed] [Google Scholar]
- 6.Hansman, D. 1978. Meningitis caused by Neisseria lactamica. N. Engl. J. Med. 299:491. [PubMed] [Google Scholar]
- 7.Janda, W. M., and J. S. Knapp. 2003. Neisseria and Moraxella catarrhalis, p. 609-622. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. A. Pfaller, and R. H. Yolken (ed.), Manual of clinical microbiology, 8th ed. ASM Press, Washington, D.C.
- 8.Lauer, B. A., and C. E. Fisher. 1976. Neisseria lactamica meningitis. Am. J. Dis. Child. 130:198-199. [DOI] [PubMed] [Google Scholar]
- 9.Maiden, M. C. J. 1998. Horizontal genetic exchange, evolution, and spread of antibiotic resistance in bacteria. Clin. Infect. Dis. 27(Suppl. 1):12-20. [DOI] [PubMed] [Google Scholar]
- 10.Orden, B., and M. A. Amerigo. 1991. Acute otitis media caused by Neisseria lactamica. Eur. J. Clin. Microbiol. Infect. Dis. 10:986-987. [DOI] [PubMed] [Google Scholar]
- 11.Schifman, R. B., and K. J. Ryan. 1983. Neisseria lactamica septicemia in an immunocompromised patient. J. Clin. Microbiol. 17:934-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Telfer Brunton, W. A., H. Young, and D. R. Fraser. 1980. Isolation of Neisseria lactamica from the female genital tract: a case report. Br. J. Vener. Dis. 56:325-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vienne, P., M. Ducos-Galand, A. Guiyoule, R. Pires, D. Giorgini, M. K. Taha, and J. M. Alonso. 2003. The role of particular strains of Neisseria meningitidis in meningococcal arthritis, pericarditis, and pneumonia. Clin. Infect. Dis. 37:1639-1642. [DOI] [PubMed] [Google Scholar]
- 14.Wilson, H. D., and T. L. Overman. 1976. Septicemia due to Neisseria lactamica. J. Clin. Microbiol. 4:214-215. [DOI] [PMC free article] [PubMed] [Google Scholar]