Abstract
We report here the first Portuguese case of acute fatal granulomatous encephalitis attributed to Balamuthia mandrillaris, initially thought to be a brain tumor, which had a progressive and fatal outcome. Balamuthia mandrillaris is a free-living amoeba recognized as an uncommon agent of granulomatous encephalitis. Infections have been identified in immunocompromised hosts and in immunocompetent pediatric patients. Balamuthia infections are very rare, with only two reported cases in Europe. The case presented here occurred in a previously healthy boy who died 5 weeks after the onset of the symptoms. No evidence of immunological deficiency was noted, and testing for human immunodeficiency virus antibodies was negative. The symptoms were initially thought to be the result of a tumor, but histopathologic examination showed evidence of amoebic infection. Immunofluorescence staining of brain tissue identified B. mandrillaris as the infectious agent. The diagnosis was confirmed with PCR by detecting Balamuthia DNA in formalin-fixed brain tissue sections. Despite initiation of empirical antimicrobial therapy for balamuthiasis, the patient died 3 weeks after being admitted to the hospital. No source of infection was readily apparent.
CASE REPORT
An 8-year-old male was transferred to our hospital because of signs of intracranial hypertension. His past medical history was unremarkable except for an episode of varicella infection a year before. Prior to his illness, he practiced swimming in several swimming pools but had not traveled outside Portugal. Symptoms with sporadic headaches started a few months earlier but had become daily for the 2 weeks prior to admission. His parents noted medial esotropia 2 days before admission, and diplopia developed 1 day before. His headaches were more intense in the morning, he had frequent episodes of vomiting and lethargy, and papilledema was noted upon physical examination.
Upon admission he appeared well, with a good general status, and his speech was age appropriate. After unilateral eye occlusion, he had no visual defects. Mycobacterium tuberculosis infection was ruled out from urine and sputum samples, and the tuberculin test (2U, SC) was negative. Human immunodeficiency virus type 1 (HIV-1) and HIV-2 serum antibodies and immunologic studies all showed no changes.
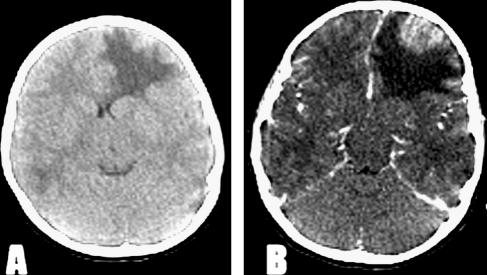
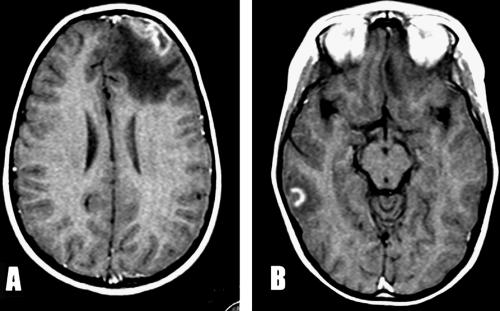
Fundoscopy revealed bilateral papilledema and right retinal hemorrhages. Neither alteration of consciousness nor neurological focal deficits were noted. There was no fever. Noncontrast computerized tomography (CT) scanning revealed an expansive hypodense lesion in the right frontal lobe, causing distortion of the middle line and ventricular thinning. With contrast enhancement, a heterogeneous cortical lesion surrounded by a huge area of edema was noted for the first time in the right temporal lobe (Fig. 1A and B). A brain tumor was suspected, and dexamethasone was started because of the clinical symptoms. Upon preoperative magnetic resonance imaging (MRI), the frontal lesion was heterogeneous, with hypodense inner and hyperintense outer areas on T1-weighted imaging. Another heterogeneous image, with a thick ring of gadolinium enhancement in the right temporal lobe, was noted for the first time (Fig. 2A and B). Frontal craniotomy with resection of a cortical lesion was performed. Postoperative recovery was uneventful, and the child was asymptomatic on the second day after surgery.
FIG. 1.
(A) CT scan showing expansive hypodense lesion in the right frontal lobe. (B) CT scan with contrast enhancement reveals a heterogeneous cortical lesion surrounded by a huge area of edema.
FIG. 2.
(A) MRI with T1-weighted imaging shows a heterogeneous frontal lesion with hypodense inner and hyperintense outer areas. (B) MRI with gadolinium enhancement shows a heterogeneous image with a thick ring in the temporal lobe.
Microscopy of the resected tissue showed that a dense infiltrate of mononuclear inflammatory cells enlarged the subarachnoid space where it involved the wall of some vessels. Chronic inflammation with lymphocytes, plasma cells, histiocytes, and occasional multinucleated giant cells also obliterated the normal architecture of the cerebral cortex in many areas. The best-preserved cortical tissue was in the most superficial layers, and it was here that the majority of the amoebic trophozoites (Fig. 3A) were found, although a few could be identified in the subarachnoid space. The trophozoites were generally round to oval, with a well-defined cell border and a central nucleus in which a single nucleolus could sometimes be identified. Small cytoplasmic granules were visible in some trophozoites. Occasionally, trophozoites formed a collar around a vessel. Tissue necrosis and polymorphonuclear infiltration occurred in some superficial areas where trophozoites were particularly numerous. In deeper areas, a massive proliferation of astrocytes accompanied the inflammation. In these areas, isolated cyst forms were encountered, with a clear space surrounding their shrunken cytoplasm and prominent nucleus.
FIG. 3.
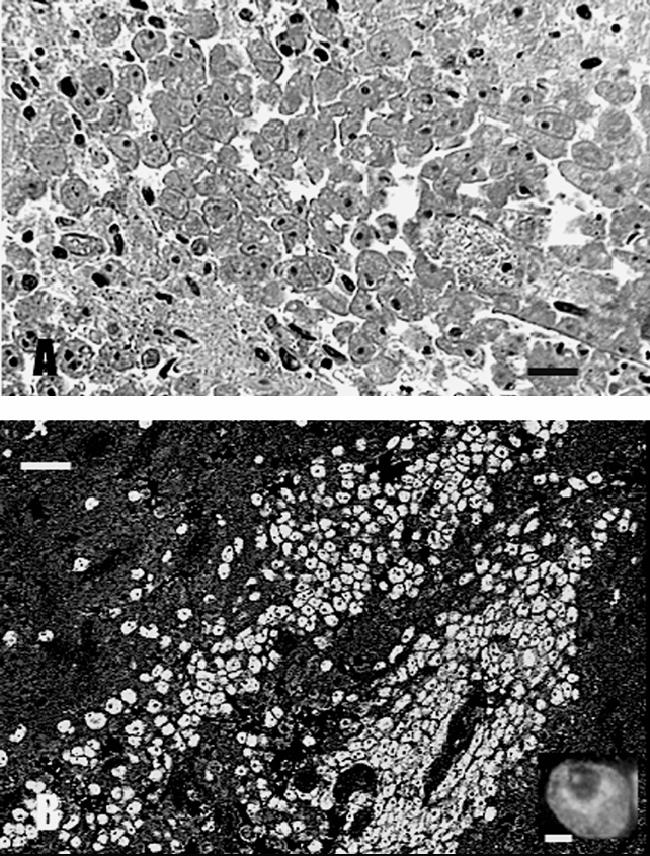
(A) H&E-stained CNS section demonstrating a huge cluster of amoebae. The amoebae were round to oval, measured 25 to 30 μm, and contained a single nucleus with a dense nucleolus. (B) The amoebae in the brain sections reacted only with the anti-B. mandrillaris serum and produced bright apple green fluorescence. Inset, a higher magnification of a single amoeba.
Combined treatment was started with fluconazole (10 mg/kg), trimethoprim-sulfamethoxazole (15 mg/kg), and rifampin (10 mg/kg) because of the indication of amoebic infection. Nevertheless, on hospital day 15, the child suffered clinical deterioration with increasing headaches, vomiting, and reduced consciousness. Emergent MRI examination revealed disseminated heterogeneous hypointense lesions on T1-weighted images distributed to the bilateral hemispheres and cerebellum. On T2-weighted images, cerebral edema was widespread. The patient started irregular breathing and bradycardia that required admission to the pediatric intensive care unit 36 h later. Hemodynamic and ventilatory supports were provided, and intracranial pressure was monitored. Brain edema was refractory to all instituted measures, and progressive brain damage led to death on hospital day 21. A necropsy was not done.
Slides of biopsied brain tissue were subjected to indirect immunofluorescence staining using rabbit polyclonal anti-Acanthamoeba and -Balamuthia sera, and the results were positive for Balamuthia mandrillaris (Fig. 3B) and negative for Acanthamoeba (26, 27). For confirmation, the PCR was done on brain tissue sections scraped from slides following deparaffinization and DNA extraction for amplification and sequencing (28). Two primer sets were used: one for Balamuthia mandrillaris and a second one for Acanthamoeba spp. The primer set 5′Balspec16S (5′-CGCATGTATGAAAGAAGACCA-3′) and Balspec16Sr610 (5′-CCCCTTTTTAACTCTAGTCATATAGT-3′), producing an amplicon of 230 bp, was used to test for the presence of Balamuthia mandrillaris DNA (6). The primer set including Aca16Sf1010 (5′-TTATATTGACTTGTACAGGTGCT-3′) and Aca16Sr1180 (5′-CATAATGATTTGACTTCTTCTCCT-3′), producing an amplicon of 161 bp, was used for the demonstration of Acanthamoeba DNA (6). The resultant bands were matched to control Balamuthia or Acanthamoeba DNAs (Fig. 4). DNA extracted from brain tissue produced a band that corresponded to control Balamuthia DNA (Fig. 4), confirming the identity of the infectious agent. The PCR products were directly sequenced, and the 16S rRNA gene sequence of B. mandrillaris, available under GenBank accession number DQ508945, matched those of other Balamuthia sequences deposited in GenBank. No band was produced with the Acanthamoeba primer set.
FIG. 4.
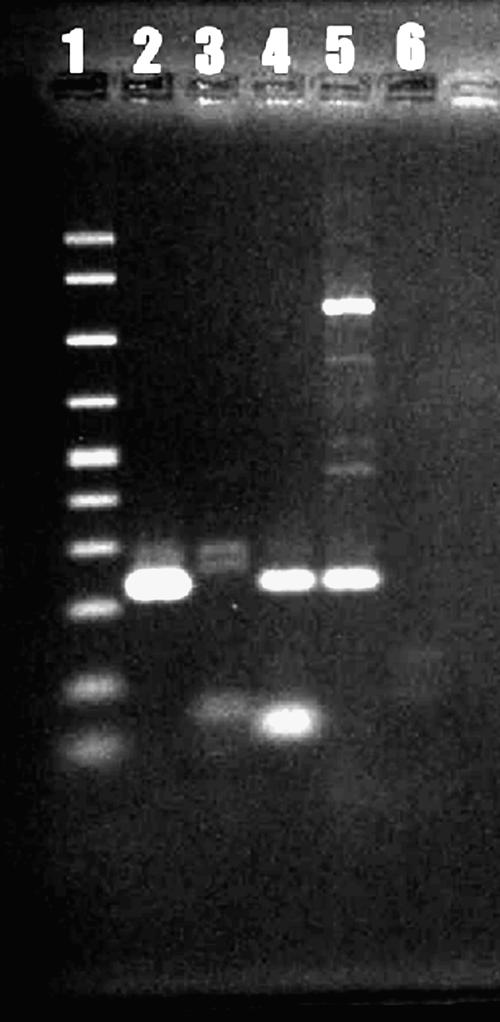
PCR amplification of B. mandrillaris DNA. Acanthamoeba DNA was not amplified. Lane 1, molecular size ladder (100 to 1,000 bp); lane 2, tissue sample from Portugal (note band at about 230 bp); lane 3, Acanthamoeba DNA; lane 4, positive control (Balamuthia DNA); lane 5, same as lane 4 but different dilution; lane 6, Sigma water.
Discussion.
Balamuthia mandrillaris, a free-living amoeba, has caused granulomatous encephalitis in humans, nonhuman primates, a sheep, a horse, and a dog (14, 15, 24). Human infection with Balamuthia is rare, with many cases associated with HIV infection and other types of immunodepression (1, 27), although the disease affects both immunocompetent and immunocompromised hosts (15). Infections in immunocompetent patients, especially children, are now more frequently reported (18). The application of immunofluorescence staining and molecular detection techniques, as well as greater awareness of the etiologic agent, has led to increased reports of the amoeba as an infectious agent. The infection is usually fatal, with a clinical picture that is slow and insidious, and often simulates a cerebral tumor or other granulomatous diseases. In the absence of early detection, prognosis is poor (2).
This is the first reported case of balamuthiasis in Portugal and only the third case in Europe (12, 29). Cases have occurred in the United States, Canada, Mexico, Australia, South America (including Argentina, Brazil, Mexico, and Peru), Thailand, Japan, the Czech Republic, and England. A significant number of the published cases from the United States have occurred in persons of Hispanic ethnicity (22).
Potential portals of entry for the amoeba are the nasal mucosa, skin, and lungs, with hematogenous dissemination to the central nervous system (CNS). The infection may have several modes of appearance: a cutaneous lesion, nasopharyngeal or sinus infection, or a disseminated infection (including encephalitis).
Balamuthia mandrillaris is found in soil and possibly water (5). In most cases, as in the present one, there is no readily apparent source of infection. In one case, infection was associated with an indolent cutaneous lesion that developed through injury while digging in soil (3). Gardening and soil were implicated as a source of infection in another case (11). In still another case, Balamuthia was isolated from flowerpot soil, which the patient had contact with over an extended time period (20). The patient in the case reported here had a history of swimming in several swimming pools and occasional outdoor plant-collecting activity, both possible sources of infection. However, no cutaneous lesions were evident on the child during his hospital stay.
Clinical presentation varies. Most patients present with focal neurologic findings, mostly cranial nerve palsies (third to sixth) (2); some are febrile and have signs of meningeal irritation. In the majority of cases, there is a subacute clinical course leading to death in weeks to months, even as long as 1 to 2 years (17, 25). Otitis media has preceded infection in several juveniles (2, 4, 12). In some cases, cerebrospinal fluid (CSF) examination shows a discrete pleocytosis with lymphocytic predominance and significantly increased protein levels (2). Glucose levels are usually depressed, which, in association with granulomatous lesions found on imaging studies, leads to an exhaustive search for tuberculosis, even in immunocompetent patients. In other studies, examination of the CSF has been normal (7). In addition to tuberculosis, the differential diagnoses for balamuthiasis include neurocysticercosis, brain abscess, tumor, acute disseminated encephalomyelitis, and viral encephalitis.
The amoeba has been isolated from brain tissue (2, 26, 27) and, in a single case, from CSF obtained at autopsy (10). Neither the CSF nor unfixed brain tissue was available for attempting culture isolation of the amoeba. Neuroimaging studies usually show unilateral lesions surrounded by edema, and hydrocephalus or cystic lesions upon admission are not rare, resembling acute events like stroke (4, 8, 19). MRI examination reveals some typical lesions, as was previously shown (3, 8). Ring-enhancing lesions with heterogeneous intensity on T1-weighted images are suggestive of Balamuthia infection.
Symptoms of balamuthiasis are nonspecific and, without a high index of suspicion on the part of the physician, a correct diagnosis may be challenging. Examination of hematoxylin and eosin (H&E)-stained tissue sections of the brain reveals the presence of amoebae, usually surrounded by extensive inflammatory cells. Trophozoites can be seen in the perivascular spaces or throughout the brain parenchyma, as was found in the present case. Granulomas may be present, although granulomatous lesions may not be found, particularly in the immunocompromised host. The present case had definitive granulomas as well as necrotic and hemorrhagic foci dispersed through the brain specimen. These features are commonly seen in both Acanthamoeba and Balamuthia infections.
The current gold standard for diagnosis of balamuthiasis is indirect immunofluorescent staining of brain tissue sections (27). Immunofluorescent-antibody (IFA) staining of serum may also be useful. IFA is a rapid and noninvasive means of detecting Balamuthia antibodies and has been used in a few cases (3, 11, 19, 27). However, although detectable Balamuthia antibodies may be present in humans, possibly as a result of contact with amoebae in the environment (9, 21), it is not known whether these antibodies provide protection against amoebic infection and disease. Unfortunately, serum samples from this case were not available for antibody testing. The PCR technique can also demonstrate Balamuthia DNA in CSF of patients (28). Thus, to be a reliable indicator of balamuthiasis, IFA has to be coupled with H&E and indirect immunofluorescent staining of tissue sections and/or PCR of CSF.
Features of balamuthiasis are also commonly seen in acanthamoebiasis, an infection caused by Acanthamoeba spp., another free-living amoeba that causes granulomatous encephalitis (14). The two organisms have a similar morphological appearance in histopathologic specimens and a similar disease course. The resemblance of the two amoebae has resulted in the past in Balamuthia being misidentified as Acanthamoeba (27). A case that was initially diagnosed as neurocysticercosis was, upon autopsy, reported as acanthamoebiasis and later confirmed as balamuthiasis (16; G. S. Visvesvara, unpublished data). Other diagnoses include Wegener's granulomatosis (7), chronic skin lesions (17), and brain stem glioma (13).
At this time, only three survivors have been reported worldwide (3, 11). The majority of patients had a fatal outcome even when a premortem diagnosis was made. Amebicidal drugs have been identified in laboratory trials, but their in vivo efficacy can only be conjectured because of potential toxicity in proven therapeutic doses and their uncertain effect on dormant cystic forms of Balamuthia in the CNS (23). It is not known if Balamuthia cysts that develop and remain in the brain and other tissues may later activate in the absence of antimicrobials to cause a flare-up of the amoebic infection.
Survivors were started on a combination of macrolide antibiotic (azithromycin or clarithromycin), flucytosine, sulfadiazine, and phenothiazine compounds, since these drugs have shown some amebicidal activity in vitro (3, 11). Patients undergoing treatment still exhibit serologic evidence of infection, albeit with reduced Balamuthia antibody titers (3).
In the present case, empirical antimicrobial therapy was started to treat what was initially thought to be Acanthamoeba encephalitis but was subsequently identified as balamuthiasis by immunofluorescent staining and PCR. Despite combined drug administration (sulfadiazine, a macrolide antibiotic, rifampin, and fluconazole), the patient died 3 weeks after admission, with progressive deterioration after the start of antimicrobial therapy.
Although there is high mortality from free-living amoebic infections, early diagnosis and initiation of treatment can be important to a successful recovery (3, 11). Balamuthiasis should be considered in the differential diagnosis of multifocal lesions in the central nervous system in conjunction with elevated CSF protein and pleocytosis. The absence of apparent risk factors or an immunosuppressive state in a patient should not deter the physician from consideration of this disease entity.
REFERENCES
- 1.Anzil, A. P., R. Chandrakant, M. A. Wrzolek, G. S. Visvesvara, J. H. Sher, and P. B. Kozlowski. 1991. Amebic meningoencephalitis in a patient with AIDS caused by a newly recognized opportunistic pathogen. Arch. Pathol. Lab. Med. 115:21-25. [PubMed] [Google Scholar]
- 2.Bakardjiev, A., P. H. Azimi, N. Ashouri, D. P. Ascher, D. Janner, F. L. Schuster, G. S. Visvesvara, and C. Glaser. 2003. Amebic encephalitis caused by Balamuthia mandrillaris: report of four cases. Pediatr. Infect. Dis. J. 22:447-453. [DOI] [PubMed] [Google Scholar]
- 3.Deetz, T. R., M. H. Sawyer, G. Billman, F. L. Schuster, and G. S. Visvesvara. 2003. Successful treatment of Balamuthia amoebic encephalitis: presentation of 2 cases. Clin. Infect. Dis. 37:1304-1312. [DOI] [PubMed] [Google Scholar]
- 4.Duke, B. J., R. W. Tyson, R. DeBiasi, J. E. Freeman, and K. R. Winston. 1997. Balamuthia mandrillaris meningoencephalitis presenting with acute hydrocephalus. Pediatr. Neurosurg. 26:107-111. [DOI] [PubMed] [Google Scholar]
- 5.Dunnebacke, T. H., F. L. Schuster, S. Yagi, and G. C. Booton. 2004. Balamuthia mandrillaris from soil samples. Microbiology 150:2837-2842. [DOI] [PubMed] [Google Scholar]
- 6.Foreman, O., J. Sykes, L. Ball, N. Yang, and H. De Cock. 2004. Disseminated infection with Balamuthia mandrillaris in a dog. Vet. Pathol. 41:506-510. [DOI] [PubMed] [Google Scholar]
- 7.Galarza, M., V. Cuccia, F. P. Sosa, and J. A. Monges. 2002. Pediatric granulomatous cerebral amebiasis: a delayed diagnosis. Pediatr. Neurol. 26:153-156. [DOI] [PubMed] [Google Scholar]
- 8.Healy, J. F. 2002. Balamuthia amebic encephalitis: radiographic and pathologic findings. AJNR Am. J. Neuroradiol. 23:486-489. [PMC free article] [PubMed] [Google Scholar]
- 9.Huang, Z. H., A. Ferrante, and R. F. Carter. 1999. Serum antibodies to Balamuthia mandrillaris, a free-living amoeba recently demonstrated to cause granulomatous amoebic encephalitis. J. Infect. Dis. 179:1305-1308. [DOI] [PubMed] [Google Scholar]
- 10.Jayasekara S, J. Sissons, J. Tucker, C. Rogers, D. Nolder, D. Warhurst, S. Alsam, J. M. L. White, E. M. Higgins, and N. A. Khan. 2004. Post-mortem culture of Balamuthia mandrillaris from the brain and cerebrospinal fluid of a case of granulomatous amoebic meningoencephalitis, using human brain microvascular endothelial cells. J. Med. Microbiol. 53:1007-1012. [DOI] [PubMed] [Google Scholar]
- 11.Jung, S., R. L. Schelper, G. S. Visvesvara, and H. T. Chang. 2004. Balamuthia mandrillaris meningoencephalitis in an immunocompetent patient: an unusual clinical course and a favorable outcome. Arch. Pathol. Lab. Med. 128:466-468. [DOI] [PubMed] [Google Scholar]
- 12.Kodet, R., E. Nohynkova, M. Tichy, J. Soukup, and G. S. Visvesvara. 1998. Amebic encephalitis caused by Balamuthia mandrillaris in a Czech child: description of the first case from Europe. Pathol. Res. Pract. 194:423-429. [DOI] [PubMed] [Google Scholar]
- 13.Lowichik, A., N. Rollins, R. Delgado, G. S. Visvesvara, and D. K. Burns. 1995. Leptomyxid amebic meningoencephalitis mimicking brain stem glioma. AJNR Am. J. Neuroradiol. 16:926-929. [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez, A. J., and G. S. Visvesvara. 1997. Free-living, amphizoic and opportunistic amebas. Brain Pathol. 7:583-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinez, A. J., and G. S. Visvesvara. 2001. Balamuthia mandrillaris infection. J. Med. Microbiol. 50:205-207. [DOI] [PubMed] [Google Scholar]
- 16.Matson, D. O., E. Rouah, R. T. Lee, D. Armstrong, J. T. Parke, and C. J. Baker. 1998. Acanthamoeba meningoencephalitis masquerading as neurocysticercosis. Pediatr. Infect. Dis. J. 7:121-124. [DOI] [PubMed] [Google Scholar]
- 17.Reed, R. P., C. M. Cooke-Yarborough, A. L. Jaquiery, K. Grimwood, A. S. Kemp, J. C. Su, and J. R. L. Forsyth. 1997. Fatal granulomatous amoebic encephalitis caused by Balamuthia mandrillaris. Med. J. Aust. 67:82-84. [DOI] [PubMed] [Google Scholar]
- 18.Rowen, J. L., C. A. Doerr, H. Vogel, and C. J. Baker. 1995. Balamuthia mandrillaris: a newly recognized agent for amebic meningoencephalitis. Pediatr. Infect. Dis. J. 14:705-710. [PubMed] [Google Scholar]
- 19.Schumacher, D. J., R. D. Tien, and K. Lane. 1995. Neuroimaging findings in rare amebic infections of the central nervous system. Am. J. Neuroradiol. 16:930-935. [PMC free article] [PubMed] [Google Scholar]
- 20.Schuster, F. L., T. H. Dunnebacke, G. C. Booton, S. Yagi, C. K. Kohlmeier, C. Glaser, D. Vugia, A. Bakardjiev, P. Azimi, M. Maddux-Gonzalez, A. J. Martinez, and G. S. Visvesvara. 2003. Environmental isolation of Balamuthia mandrillaris associated with a case of amebic encephalitis. J. Clin. Microbiol. 41:3175-3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schuster, F. L., S. Honarmand, G. S. Visvesvara, and C. A. Glaser. 2006. Detection of antibodies against free-living amebae Balamuthia mandrillaris and Acanthamoeba spp. in a population of encephalitis patients. Clin. Infect. Dis. 42:1260-1265. [DOI] [PubMed] [Google Scholar]
- 22.Schuster, F. L., C. Glaser, S. Honarmand, J. H. Maguire, and G. S. Visvesvara. 2004. Balamuthia amebic encephalitis risk, Hispanic Americans. Emerg. Infect. Dis. 10:1510-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schuster, F. L., and G. S. Visvesvara. 2004. Opportunistic amoebae: challenges in prophylaxis and treatment. Drug. Resist. Updates 7:41-51. [DOI] [PubMed] [Google Scholar]
- 24.Schuster, F. L., and G. S. Visvesvara. 2004. Free-living amoebae as opportunistic and non-opportunistic pathogens of humans and animals. Int. J. Parasitol. 34:1001-1027. [DOI] [PubMed] [Google Scholar]
- 25.Taratuto, A. L., J. Monges, J. C. Acefe, F. Meli, A. Paredes, and A. J. Martinez. 1991. Leptomyxid amoeba encephalitis: report of the first case in Argentina. Trans. R. Soc. Trop. Med. Hyg. 85:77. [DOI] [PubMed] [Google Scholar]
- 26.Visvesvara, G. S., F. L. Schuster, and A. J. Martinez. 1993. Balamuthia mandrillaris, N. G., N. Sp., agent of amebic meningoencephallitis in humans and other animals. J. Eukaryot. Microbiol. 40:504-514. [DOI] [PubMed] [Google Scholar]
- 27.Visvesvara, G. S., A. J. Martinez, F. L. Schuster, G. J. Leitch, S. V. Wallace, T. K. Sawyer, and M. Anderson. 1990. Leptomyxid ameba, a new agent of amebic meningoencephalitis in humans and animals. J. Clin. Microbiol. 28:2750-2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yagi, S., G. C. Booton, G. S. Visvesvara, and F. L. Schuster. 2005. Detection of Balamuthia mitochondrial 16S rRNA DNA in clinical specimens by PCR. J. Clin. Microbiol. 43:3192-3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.White, J. M. L., R. D. Barker, J. R. Salisbury, A. J. Fife, S. B. Lucas, D. C. Warhurst, and E. M. Higgins. 2004. Granulomatous amoebic encephalitis. Lancet 364:220. [DOI] [PubMed] [Google Scholar]