Abstract
Normal human serum inhibits Acanthamoeba (encephalitis isolate) binding to and cytotoxicity of human brain microvascular endothelial cells, which constitute the blood-brain barrier. Zymographic assays revealed that serum inhibits extracellular protease activities of acanthamoebae. But it is most likely that inhibition of specific properties of acanthamoebae is a consequence of the initial amoebicidal-amoebistatic effects induced by serum. For example, serum exhibited amoebicidal effects; i.e., up to 50% of the exposed trophozoites were killed. The residual subpopulation, although viable, remained static over longer incubations. Interestingly, serum enhanced the phagocytic ability of acanthamoebae, as measured by bacterial uptake. Overall, our results demonstrate that human serum has inhibitory effects on Acanthamoeba growth and viability, protease secretions, and binding to and subsequent cytotoxicity for brain microvascular endothelial cells. Conversely, Acanthamoeba phagocytosis was stimulated by serum.
Acanthamoebae are free-living amoebae that can cause fatal Acanthamoeba granulomatous encephalitis (AGE), which is predominantly associated with immunocompromised patients such as human immunodeficiency virus (HIV)-AIDS patients (reviewed in references 9, 11, and 15). In addition, patients suffering from other diseases such as diabetes, malignancies, malnutrition, or alcoholism or who have debilitated immune systems because of immunosuppressive therapy or other complications are also susceptible to AGE infections. With the growing HIV pandemic, it is reasonable to predict an increase in the number of opportunistic infections. This is particularly worrying in developing countries, where HIV patients have limited or no access to novel antiretroviral therapies. Thus, there is a need for continued efforts to (i) increase awareness, (ii) develop rapid diagnostic methods, and (iii) understand basic molecular mechanisms of host-parasite interactions, which should help develop preventative and/or therapeutic strategies. One of the major steps in AGE is invasion of the bloodstream by amoebae, followed by their hematogenous spread (12). Acanthamoeba entry into the central nervous system most likely occurs at blood-brain barrier sites (12; personal communication with the late A. J. Martinez, University of Pittsburgh School of Medicine). Recent studies have shown that acanthamoebae exhibit multifactorial properties to produce damage of human brain microvascular endothelial cells (HBMEC), which constitute the blood-brain barrier (1-3, 16). However, the effects of serum on Acanthamoeba interactions with HBMEC are not known and were the objectives of the present study.
Acanthamoeba castellanii (ATCC 50494) belonging to the T1 genotype was originally isolated from an AGE patient and routinely grown in PYG medium (0.75% Proteose Peptone, 0.75% yeast extract, 1.5% glucose) at 30°C, and the medium was refreshed 17 to 20 h prior to experiments as previously described (16). This resulted in more than 95% acanthamoebae in the trophozoite form. For in vitro assays, primary HBMEC were isolated from human brain tissue as described previously (16, 18) and routinely grown in RPMI 1640 medium containing 10% fetal bovine serum (heat inactivated), 10% NuSerum, 2 mM glutamine, 1 mM pyruvate, penicillin and streptomycin, nonessential amino acids, and vitamins (16, 18).
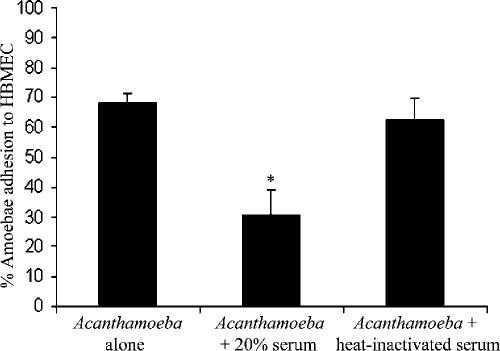
Serum from healthy individuals inhibits Acanthamoeba adhesion to HBMEC.
Normal human serum was obtained from Harlan Sera-Lab, Leicester, United Kingdom (tested negative for HIV antibody and hepatitis B surface antigen). To determine whether normal human serum possesses Acanthamoeba-specific antibodies, Western blotting assays were performed. Using whole-cell lysates, we observed that normal human serum possesses antibodies against Acanthamoeba antigens (data not shown). To determine the effects of human serum on Acanthamoeba binding to HBMEC, adhesion assays were performed. Briefly, HBMEC were grown to confluence in 24-well plates. Acanthamoebae (4 × 105 amoebae/well) were preincubated with and without 20% normal human serum or heat-inactivated serum (65°C for 30 min) in RPMI medium. Finally, the suspensions (amoebae with or without serum) were transferred to HBMEC monolayers and plates were incubated at 37°C in a 5% CO2 incubator. After 1 h of incubation, the unbound amoebae in the supernatant were counted with a hemocytometer and the numbers of bound amoebae were calculated as follows: number of unbound amoebae/total number of amoebae × 100 = percent unbound amoebae. The numbers of bound amoebae were deduced as follows: percent unbound amoebae − 100 = percent bound amoebae. We observed that serum inhibited amoeba binding to HBMEC monolayers >50% (Fig. 1) (P < 0.05 by t test, paired, one-tail distribution). This was not surprising, as the serum contained anti-Acanthamoeba antibodies. Heat inactivation abolished the serum effects, indicating that factors which inhibit amoeba binding to HBMEC are proteinaceous in nature (Fig. 1).
FIG. 1.
Serum significantly (*, P < 0.05) inhibited Acanthamoeba adhesion to HBMEC. These results are representative of three independent experiments performed in triplicate. Bars represent standard errors.
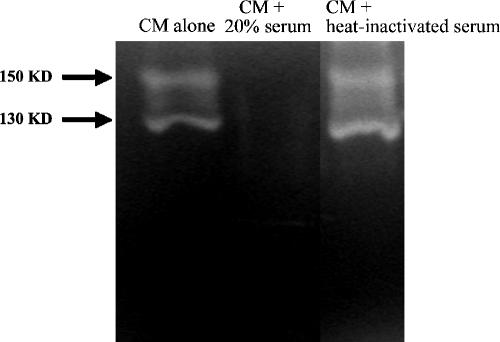
Human serum inhibits Acanthamoeba protease secretion.
Recent studies have shown that Acanthamoeba extracellular serine proteases play important roles in HBMEC monolayer perturbations (2). This highlights a role for extracellular proteases in facilitating the migration of acanthamoebae from the systemic circulation into the deeper-lying tissues of the central nervous system. To determine the effects of serum on Acanthamoeba proteases, conditioned medium (CM) was produced by incubating acanthamoebae in the presence or absence of normal human serum in RPMI medium for 24 h. Cell-free supernatant (i.e., CM) was collected by centrifugation and analyzed for protease activity with zymographic assays as previously described (7). In the absence of serum, we observed two distinct proteases, i.e., 130-kDa and 150-kDa protease bands (Fig. 2). The 130-kDa protease is sensitive to phenylmethylsulfonyl fluoride (a serine protease inhibitor), indicating that it is a serine protease, and the 150-kDa protease is sensitive to 1,10-phenanthroline (a metalloprotease inhibitor), indicating that it is a metalloprotease (2). In contrast, CM prepared in the presence of 20% human serum exhibited minimal or no protease activity under the test conditions used. Again, heat inactivation abolished the inhibitory effects of human serum and protease activities were restored (Fig. 2).
FIG. 2.
Serum inhibits extracellular proteases of acanthamoebae. CM was collected, and proteolytic activities were determined by zymographic assays. Note that serum abolished Acanthamoeba proteases. These results are representative of three independent experiments. KD, kilodaltons.
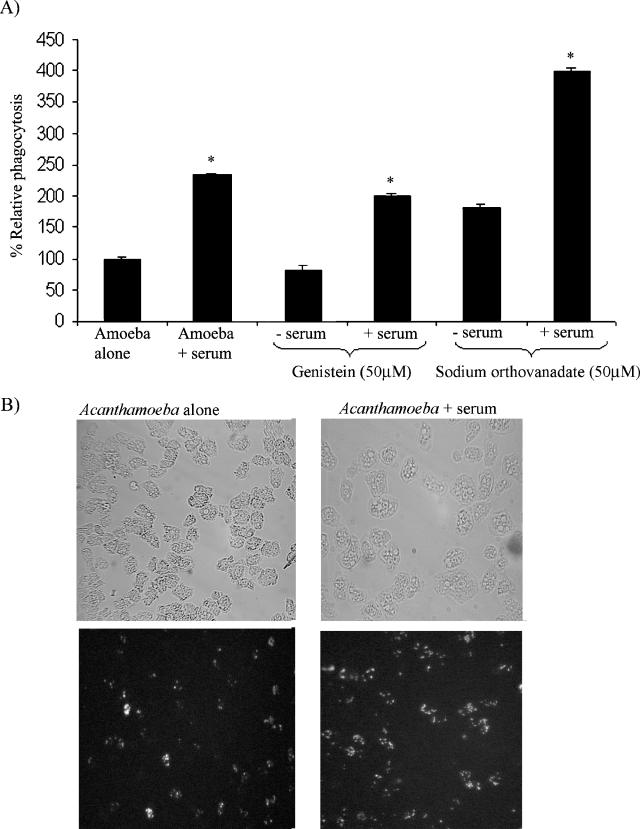
Serum enhances Acanthamoeba phagocytosis.
Previous studies have shown that phagocytosis is an important virulence factor in the pathogenesis of Acanthamoeba infections (8, 13, 14). To determine the effects of serum on Acanthamoeba phagocytosis with live Escherichia coli K-12, phagocytosis assays were performed. Briefly, acanthamoebae were incubated with and without 20% serum in RPMI medium at room temperature for 30 min. Acanthamoebae were washed with phosphate-buffered saline to remove any residual serum, and live E. coli K-12 cells (107/well) were added. Plates were incubated for 45 min to allow phagocytic uptake, followed by addition of gentamicin (final concentration, 100 μg/ml) and another 45 min of incubation to kill extracellular E. coli. The numbers of acanthamoebae were determined by hemocytometer counting. Finally, acanthamoebae were solubilized with 0.5% sodium dodecyl sulfate and E. coli counts were determined by inoculating lysates onto nutrient agar plates. This allowed the determination of intracellular E. coli. The level of Acanthamoeba phagocytosis was determined as follows: number of E. coli CFU/total number of acanthamoebae × 100 = percent phagocytosis. Results are expressed as relative phagocytosis (the percent phagocytosis in untreated acanthamoebae was considered 100%, and levels of phagocytosis in serum- and/or inhibitor-treated acanthamoebae are shown as percent change). We demonstrated that acanthamoebae exhibited a significant increase in bacterial uptake in response to serum (P < 0.05) (Fig. 3A). In an attempt to dissect the molecular mechanisms underlying the serum-mediated increase in amoeba phagocytosis, acanthamoebae were pretreated with genistein (a protein tyrosine kinase inhibitor) and sodium orthovanadate (a tyrosine phosphatase inhibitor) prior to the addition of serum. We observed that in the absence of serum, genistein partially decreased amoeba phagocytosis. However, in the presence of serum, the inhibitory effects of genistein were reversed and serum enhanced the phagocytic uptake of E. coli K-12 (Fig. 3A). Similarly, serum enhanced phagocytosis in sodium orthovanadate (a tyrosine phosphatase inhibitor)-treated acanthamoebae (Fig. 3A). To visualize the effects of human serum on Acanthamoeba phagocytosis, we performed phagocytosis assays with fluorescein isothiocyanate (FITC)-labeled, heat-killed E. coli K-12 as previously described (3). As shown in Fig. 3B, we observed that human serum stimulated the phagocytic uptake of FITC-labeled E. coli. Overall, these data suggest that serum enhances Acanthamoeba phagocytosis by modulating protein tyrosine kinase pathways.
FIG. 3.
(A) Serum enhances Acanthamoeba phagocytosis with live E. coli K-12. Untreated acanthamoebae were used as 100% phagocytosis, and levels of phagocytosis in serum and/or inhibitor-treated acanthamoebae are shown as percent change. Serum exhibits a significant (*, P < 0.05) increase in Acanthamoeba phagocytosis. These results are representative of three independent experiments performed in triplicate. Bars represent standard errors. (B) Representative effects of serum on Acanthamoeba uptake of heat-killed, FITC-labeled E. coli. Note that serum enhanced Acanthamoeba uptake of FITC-labeled E. coli. Magnification, ×200.
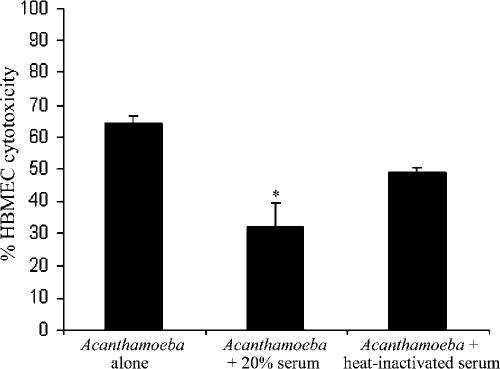
Human serum inhibits Acanthamoeba-mediated HBMEC cytotoxicity.
To determine the effects of human serum on Acanthamoeba-mediated HBMEC death, cytotoxicity assays were performed as previously described (16). Briefly, acanthamoebae with and without human serum (20%) were added to HBMEC monolayers and plates were incubated at 37°C in a 5% CO2 incubator for up to 24 h. At the end of this incubation period, supernatants were collected and cytotoxicity was determined by measuring lactate dehydrogenase release as previously described (16) (cytotoxicity detection kit; Roche Applied Science, Lewes, East Sussex, United Kingdom). In the absence of serum, acanthamoebae produced severe HBMEC cell cytotoxicity (up to 70%) within 24 h (Fig. 4). However, Acanthamoeba-mediated HBMEC cytotoxicity was inhibited in the presence of serum (Fig. 4). Heat inactivation of human serum did not completely abolish serum effects (Fig. 4). Overall, these data show that human serum partially inhibits Acanthamoeba-mediated HBMEC cytotoxicity.
FIG. 4.
Serum significantly (*, P < 0.05) inhibited Acanthamoeba-mediated HBMEC cytotoxicity. Heat inactivation partially restored Acanthamoeba-mediated HBMEC cytotoxicity (not significant). These results are representative of three independent experiments performed in triplicate. Bars represent standard errors.
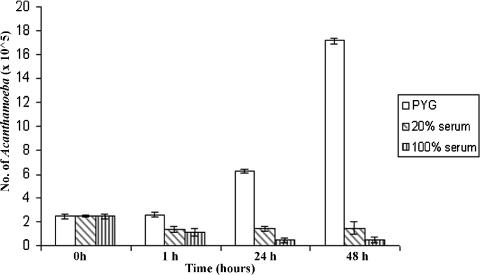
Human serum induces an initial amoebicidal effect, followed by amoebistatic activity.
To determine whether the effects of serum on Acanthamoeba properties are mediated via distinct molecular mechanisms or simply an effect secondary to the amoebistatic and amoebicidal properties of serum, assays were performed. Briefly, various concentrations of serum were added to acanthamoebae in 24-well plates (2.5 × 105 amoebae/well) and the plates were incubated for various times. The effects of serum on amoeba growth and viability were determined by hemocytometer counting and trypan blue exclusion testing. For controls, normal growth rates of acanthamoebae were determined with growth medium alone, i.e., PYG. We observed that normal human serum exhibited an initial amoebicidal effect; approximately 50% of the exposed trophozoites were killed, supporting the previous findings that normal human serum exhibits amoebicidal effects (Fig. 5) (4). However, a subpopulation of amoebae remained viable but cultures were stationary over longer incubations (Fig. 5). The fact that serum exhibited approximately 50% inhibition of amoeba binding to HBMEC (similar to amoebicidal effects) suggests that the effects of serum on the properties of acanthamoebae are most likely secondary to the amoebicidal and amoebistatic effects. Our findings that a subpopulation of acanthamoebae resisted serum-mediated killing support previous studies of Toney and Marciano-Cabral (19) in that pathogenic acanthamoebae resist serum killing. For such isolates, it is likely that both humoral and cellular immune responses work in conjunction to exert their amoebicidal effects (17). Of interest, similar findings were obtained with a keratitis isolate of acanthamoebae (T4 genotype), suggesting that our findings may be relevant to other species of Acanthamoeba (data not shown).
FIG. 5.
Human serum exhibits amoebicidal and amoebistatic effects. At a concentration of 20%, serum exhibited an initial amoebicidal effects, followed by an amoebistatic effect. In contrast, 100% serum exhibited an amoebicidal effect for up to 24 h, followed by an amoebistatic effect. These results are representative of three independent experiments performed in triplicate. Bars represent standard errors.
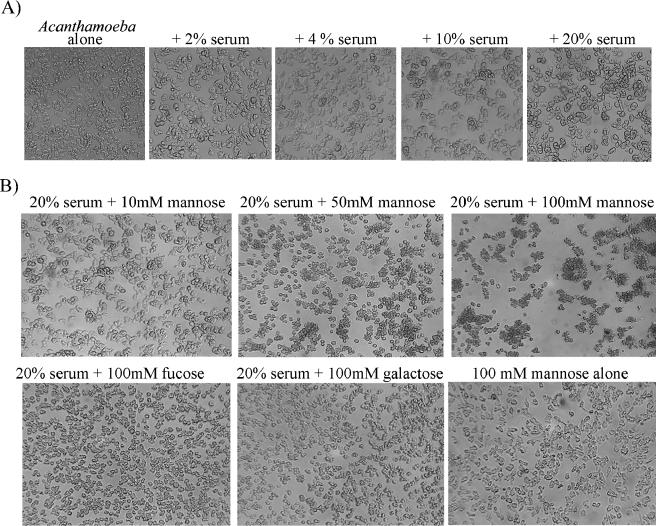
Human serum induces Acanthamoeba clustering.
An interesting observation was that human serum induces acanthamoebae to form aggregates and/or clusters in a concentration-dependent manner (Fig. 6A). Moreover, exogenous mannose, but not other sugars, in the presence of serum induced strong clustering of acanthamoebae in a concentration-dependent manner (Fig. 6B), suggesting a possible role for mannan-binding lectin (MBL) in Acanthamoeba clustering in response to serum. Other sugars such as fucose and galactose did not exhibit such effects; therefore, this was not an osmotic effect of sugars. In human serum, MBL is a collectin that plays a role in the innate immune response by binding to carbohydrates, specifically mannose, on the surface of microbes, activating the lectin complement pathway. Interestingly, MBL levels are significantly lower in HIV patients compared with healthy individuals (6), and MBL deficiency has been associated with increased susceptibility to infectious diseases. Of interest, we tested the role of MBL in serum-mediated clustering effects on acanthamoebae. Our preliminary studies revealed that anti-MBL antibody inhibited the clustering effects of serum (data not shown).
FIG. 6.
(A) Serum induced Acanthamoeba clustering in a concentration-dependent manner. (B) Mannose, but not other sugars, enhanced serum-induced clustering of acanthamoebae in a concentration-dependent manner. These results are representative of three independent experiments.
In conclusion, these and other studies suggest that serum factors in the presence of neutrophils or macrophages may play important roles in targeting acanthamoebae (4, 5, 10, 17, 19) and that normal human serum is adept at inhibiting amoeba-mediated HBMEC cytotoxicity and support the idea that a healthy immune response is sufficient to control and/or eradicate these life-threatening pathogens. Future studies will further clarify the mechanisms associated with Acanthamoeba pathogenesis, which may help develop preventative and/or therapeutic interventions.
Acknowledgments
This work was partially supported by grants from the Faculty Research Fund, Central Research Fund, University of London; the British Council for Prevention of Blindness; and The Royal Society.
REFERENCES
- 1.Alsam, S., K. S. Kim, M. Stins, A. O. Rivas, J. Sissons, and N. A. Khan. 2003. Acanthamoeba interactions with human brain microvascular endothelial cells. Microb. Pathog. 35(6):235-241. [DOI] [PubMed] [Google Scholar]
- 2.Alsam, S., J. J. Sissons, S. Jayasekera, and N. A. Khan. 2005. Extracellular proteases of Acanthamoeba castellanii (encephalitis isolate belonging to T1 genotype) contribute to increased permeability in an in vitro model of the human blood-brain barrier. J. Infect. 51(2):150-156. [DOI] [PubMed] [Google Scholar]
- 3.Alsam, S., J. Sissons, R. Dudley, and N. A. Khan. 2005. Mechanisms associated with Acanthamoeba castellanii (T4) phagocytosis. Parasitol. Res. 96(6):402-409. [DOI] [PubMed] [Google Scholar]
- 4.Ferrante, A., and B. Rowan-Kelly. 1983. Activation of the alternative pathway of complement by Acanthamoeba culbertsoni. Clin. Exp. Immunol. 54(2):477-485. [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrante, A. 1991. Immunity to Acanthamoeba. Rev. Infect. Dis. 13:S403-S409. [DOI] [PubMed] [Google Scholar]
- 6.Garred, P., C. Richter, A. B. Andersen, H. O. Madsen, I. Mtoni, A. Svejgaard, and J. Shao. 1997. Mannan-binding lectin in the sub-Saharan HIV and tuberculosis epidemics. Scand. J. Immunol. 46(2):204-208. [DOI] [PubMed] [Google Scholar]
- 7.Khan, N. A., E. L. Jarroll, N. Panjwani, Z. Cao, and T. A. Paget. 2000. Proteases as markers of differentiation of pathogenic and non-pathogenic Acanthamoeba. J. Clin. Microbiol. 38:2858-2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khan, N. A. 2001. Pathogenicity, morphology and differentiation of Acanthamoeba. Curr. Microbiol. 43(6):391-395. [DOI] [PubMed] [Google Scholar]
- 9.Khan, N. A. 2003. Pathogenesis of Acanthamoeba infections. Microb. Pathog. 34:277-285. [DOI] [PubMed] [Google Scholar]
- 10.Marciano-Cabral, F., and D. M. Toney. 1998. The interaction of Acanthamoeba spp. with activated macrophages and with macrophage cell lines. J. Eukaryot. Microbiol. 45(4):452-458. [DOI] [PubMed] [Google Scholar]
- 11.Marciano-Cabral, F., and G. Cabral. 2003. Acanthamoeba spp. as agents of disease in humans. Clin. Microbiol. Rev. 16(2):273-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinez, A. J., and G. S. Visvesvara. 1997. Free-living, amphizoic and opportunistic amebas. Brain Pathol. 7(1):583-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niederkorn, J. Y., H. Alizadeh, H. Leher, and J. P. McCulley. 1999. The pathogenesis of Acanthamoeba keratitis. Microbes Infect. 1(6):437-443. [DOI] [PubMed] [Google Scholar]
- 14.Pettit, D. A., J. Williamson, G. A. Cabral, and F. Marciano-Cabral. 1996. In vitro destruction of nerve cell cultures by Acanthamoeba spp.: a transmission and scanning electron microscopy study. J. Parasitol. 82(5):769-777. [PubMed] [Google Scholar]
- 15.Schuster, F. L., and G. S. Visvesvara. 2004. Free-living amoebae as opportunistic and non-opportunistic pathogens of humans and animals. Int. J. Parasitol. 34(9):1-27. [DOI] [PubMed] [Google Scholar]
- 16.Sissons, J., K. S. Kim, M. Stins, S. Jayasekera, S. Alsam, and N. A. Khan. 2005. Acanthamoeba castellanii induces host cell death via a phosphatidylinositol 3-kinase-dependent mechanism. Infect. Immun. 73:2704-2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stewart, G. L., I. Kim, K. Shupe, H. Alizadeh, R. Silvany, J. P. McCulley, and J. Y. Niederkorn. 1992. Chemotactic response of macrophages to Acanthamoeba castellanii antigen and antibody-dependent macrophage-mediated killing of the parasite. J. Parasitol. 78(5):849-855. [PubMed] [Google Scholar]
- 18.Stins, M. F., F. Gilles, and K. S. Kim. 1997. Selective expression of adhesion molecules on human brain microvascular endothelial cells. J. Neuroimmunol. 76:81-90. [DOI] [PubMed] [Google Scholar]
- 19.Toney, D. M., and F. Marciano-Cabral. 1998. Resistance of Acanthamoeba species to complement lysis. J. Parasitol. 84:338-344. [PubMed] [Google Scholar]