Abstract
The protease inhibitor (PI) ritonavir is used as a strong inhibitor of cytochrome P450 3A4, which boosts the activities of coadministered PIs, resulting in augmented plasma PI levels, simplification of the dosage regimen, and better efficacy against resistant viruses. The objectives of the present open-label, multiple-dose study were to determine the steady-state pharmacokinetics of amprenavir administered at 600 mg twice daily (BID) and ritonavir administered at 100 mg BID in human immunodeficiency virus type 1 (HIV-1)-infected adults treated with different antiretroviral combinations including or not including a nonnucleoside reverse transcriptase inhibitor (NNRTI). Nineteen patients completed the study. The steady-state mean minimum plasma amprenavir concentration (Cmin,ss) was 1.92 μg/ml for patients who received amprenavir and ritonavir without an NNRTI and 1.36 μg/ml for patients who received amprenavir and ritonavir plus efavirenz. For patients who received amprenavir-ritonavir without an NNRTI, the steady-state mean peak plasma amprenavir concentration (Cmax,ss) was 7.12 μg/ml, the area under the concentration-time curve from 0 to 10 h (AUC0-10) was 32.06 μg · h/ml, and the area under the concentration-time curve over a dosing interval (12 h) at steady-state (AUCss) was 35.74 μg · h/ml. Decreases in the mean values of Cmin,ss (29%), Cmax,ss (42%), AUC0-10 (42%), and AUCss (40%) for amprenavir occurred when efavirenz was coadministered with amprenavir-ritonavir. No unexpected side effects were observed. As expected, coadministration of amprenavir with ritonavir resulted in an amprenavir Cmin,ss markedly higher than those previously reported for the marketed dose of amprenavir. When amprenavir-ritonavir was coadministered with efavirenz, amprenavir-ritonavir maintained a mean amprenavir Cmin,ss above the mean 50% inhibitory concentration of amprenavir previously determined for both wild-type HIV-1 isolates and HIV-1 strains isolated from PI-experienced patients. These data support the use of low-dose ritonavir to enhance the level of exposure to amprenavir and increase the efficacy of amprenavir.
Human immunodeficiency virus (HIV) protease inhibitors and nonnucleoside reverse transcriptase inhibitors (NNRTIs) are primarily metabolized by cytochrome P450 isoenzymes. Consequently, they are susceptible to pharmacokinetic drug-drug interactions (3). Coadministration of some combinations of these drugs can result in altered or favorable pharmacokinetics compared with those of the drugs taken alone. Of the HIV protease inhibitors, ritonavir (Norvir; Abbott Laboratories) is the most potent competitive inhibitor of cytochrome P450, and its coadministration at subtherapeutic doses with some protease inhibitors has led to beneficial pharmacokinetic effects of those protease inhibitors in HIV-infected patients (8, 10). For example, notable increases in steady-state minimum drug concentrations in plasma (Cmin,ss) have been reported for indinavir and saquinavir when they are coadministered with ritonavir (1, 9, 11).
Amprenavir is a potent HIV type 1 (HIV-1) protease inhibitor and is both a substrate and an inhibitor of cytochrome P450 3A4 (2). When amprenavir is coadministered with ritonavir, the amprenavir Cmin,ss and the area under the concentration-time curve over a dosing interval at steady state (AUCss) for amprenavir markedly increase, with variable effects on the maximum concentration (12). This allows patients to significantly reduce the number of amprenavir (Agenerase; GlaxoWellcome) capsules that they take each day when the drugs are coadministered. Conversely, the NNRTI efavirenz induces the metabolism of amprenavir, leading to decreased plasma amprenavir concentrations and a subsequent need to consider the use of increased doses of amprenavir by patients who take both compounds (7). However, previous studies have suggested that coadministration of amprenavir with ritonavir at either 100 or 200 mg twice daily (BID) can overcome the concentration-reducing effect of efavirenz (6; O. Degen, M. Kurowski, J. Van Lunzen, and H. J. Stellbrink, 1st Int. Workshop Clin. Pharmacol. HIV Ther., abstr. 2.12, 2000; S. Piscitelli, C. Bechtel, B. Sadler, and J. Falloon, 7th Conf. Retrovir. Opportun. Infect., abstr. 234, 2000).
The pharmacokinetics of amprenavir administered in combination with ritonavir BID were determined in HIV-1-infected patients treated with various stable antiretroviral combinations, whatever their immunological or virological status. This study included patients who received the same doses of amprenavir-ritonavir in combination with an NNRTI, efavirenz or nevirapine.
(Data from this study were presented in part at the 5th International Congress on Drug Therapy in HIV Infection, Glasgow, United Kingdom, 22 to 26 October 2000 [C. Goujard et al., 5th Int. Congr. Drug Ther. HIV Infect., abstr. 182, 2000] and at the ASCPT Meeting, Orlando, Fla., 7 to 9 March 2001 [A. M. Taburet et al., ASCPT Meet., abstr. PII-63, 2001].)
MATERIALS AND METHODS
Patients.
We recruited HIV-1-infected patients ages 18 years or older with no active opportunistic infections. The patients had to weigh 45 to 100 kg and be within 15% of their ideal body weight according to Metropolitan Life Insurance tables. Standard laboratory parameters for all patients had to be within the normal range or judged to be not clinically significant by the investigator. Patients had to have been receiving amprenavir at 600 mg BID plus ritonavir at 100 mg BID in combination with reverse transcriptase inhibitors for a minimum of 2 weeks before inclusion in the study. Women had to test negative for pregnancy to enter the study. All patients gave written informed consent to participate in the study.
Patients were not included in the study if they were taking any other protease inhibitor or any drug which could interact significantly with cytochrome P450 3A4. Patients were ineligible for the study if they had general poor health or if they had experienced an acute illness in the week before the study. Patients with clinically active substance abuse or with clinically significant alcohol abuse were excluded from the study. Any predisposing condition that might have interfered with the absorption, distribution, metabolism, and/or excretion of drugs and any biological abnormalities of AIDS Clinical Trials Group grade 2 or above were recorded.
Study design.
The Ethical Review Committee of Cochin Hospital, Paris, France, reviewed and approved the study protocol. The study lasted from 21 March 2000 to 24 May 2000 and was done in accordance with the version of the Declaration of Helsinki applicable at the time. This was an open-label, multiple-dose, descriptive study done in two clinical study units. Patients were screened as outpatients. At the screening visit (visit 1), a medical history was recorded for each patient. The investigator conducted a physical examination, measured vital signs, and took blood for standard hematological and blood chemistry assessments. Patients who were successfully screened continued their current treatments, which included amprenavir at 600 mg BID (four 150-mg soft gelatin capsules BID) and ritonavir at 100 mg BID (one 100-mg soft gelatin capsule BID). Patients also took nucleoside reverse transcriptase inhibitors and NNRTIs but not other protease inhibitors. Within 15 days of screening, patients went to one of the two clinical study units for visit 2. Between visits 1 and 2, patients kept a diary card to record the date and times at which they took their medications to assess adherence.
At visit 2, patients stayed at the clinical study unit for sampling over 10 h for pharmacokinetic analyses. Fasting patients took their morning dose 12 ± 2 h after they had taken their last dose on the previous day. On the morning of the visit, vital signs were measured and a blood sample was drawn for determination of the CD4+-cell count and the plasma HIV-1 RNA concentration. Adherence to the prescribed treatment was analyzed by inspection of patient diary cards. Blood samples for determination of plasma amprenavir and ritonavir concentrations were drawn before dosing and at 0.5, 1, 2, 4, 6, 8, and 10 h after dosing. Blood samples were drawn and placed in 5-ml dried heparinized Vacutainer tubes by standard technique. The date and exact time of each sample were recorded, and within 45 min of collection each sample was centrifuged at 2,400 × g for 10 min in a refrigerated (4°C) centrifuge to separate the plasma from cells. The plasma was then transferred to a labeled Nunc polypropylene storage tube, which was stored upright at −20°C or less.
Safety.
Only serious adverse events related to study participation were recorded.
Pharmacokinetic measurements and analyses.
Plasma amprenavir and ritonavir concentrations were determined by validated reverse-phase high-performance liquid chromatography (HPLC) assay methods with UV detection. After addition of internal standard and alkaline buffer to plasma samples, both drugs were isolated by liquid-liquid extraction. The internal standard for amprenavir quantification was 6,7-demethyl-2,3-di-(2-pyridyl)-quinoxaline. Abbott A86093.0 was used as an internal standard for the ritonavir assay. The chromatographic separations were accomplished with a C18 reversed-phase column (Lichrospher 100 RP-18 end-capped 125-4, 5-μm column; Merck) with a pH 7.5 mobile phase containing water-acetonitrile-sodium hydroxide-orthophosphoric acid-triethylamine (650/350/0.9/0.7/0.5; vol/vol/vol/vol/vol) for amprenavir and a mobile phase containing phosphate buffer (pH 5.6)-acetonitrile (550/450; vol/vol) for ritonavir. A washing step with hexane was included in the ritonavir assay to decrease the lower limit of quantification. Amprenavir and ritonavir were detected at 210 and 220 nm, respectively. HPLC data were collected with Shimadzu class LC 10A software. The methods were validated over concentration ranges of 40 to 8,390 ng/ml for amprenavir and 12.5 to 2,500 ng/ml for ritonavir. The limits of quantification were 40 ng/ml for amprenavir and 12.5 ng/ml for ritonavir. Calculated quality control concentrations of amprenavir were <10.8% of the nominal value. The interrun coefficients of variation of the quality controls ranged from 3.9 to 8.1% (n = 11). Quality control concentrations of ritonavir were <2.5% of the nominal value. The interrun coefficients of variation of the quality controls ranged from 3.7 to 11.2% (n = 7).
Pharmacokinetic analysis.
Steady-state pharmacokinetic parameters were calculated by noncompartmental methods. The steady-state maximum concentration in plasma (Cmax,ss) and the time to Cmax,ss (Tmax,ss) were taken from raw data. Cmin,ss, which corresponds to the concentration predosing, was taken from the raw data. The average area under the concentration-time curve from 0 to 10 h (AUC0-10) was calculated for all the patients by using a combination of linear (up) and logarithmic (down) trapezoidal methods. The AUCss for amprenavir was calculated by using the predose concentration as the value at 12 h because concentrations were not measured at 12 h postdosing and extrapolation was not possible for many patients. The AUCss for ritonavir was calculated by extrapolation whenever possible. The apparent oral clearance from plasma (CL/F) was also calculated.
Statistical methods.
This was a descriptive study; the sample size was decided without use of a power calculation. The all-patient population contained all patients included in the study, and the pharmacokinetic population contained all patients for whom data on the pharmacokinetic parameters were evaluable.
All statistical analyses were conducted with data for the pharmacokinetic population by using SAS software (version 6.12; SAS Institute, Cary, N.C.). Cmax,ss, Cmin,ss, Tmax,ss, AUCss, AUC0-10, and CL/F were summarized by using descriptive statistics. Subgroup analyses were done for patients who received amprenavir-ritonavir plus efavirenz and patients who took amprenavir-ritonavir without an additional NNRTI. A linear regression calculation was used for correlation analysis.
RESULTS
Patient disposition and baseline characteristics.
The pharmacokinetic population comprised 19 patients. Baseline characteristics for the patients in the pharmacokinetic population are presented in Table 1. All 19 patients had been heavily pretreated and switched to an amprenavir-containing regimen as salvage therapy. Ten patients received amprenavir and ritonavir without an NNRTI, seven patients received amprenavir and ritonavir plus efavirenz, and two patients received amprenavir and ritonavir plus nevirapine.
TABLE 1.
Baseline characteristics for pharmacokinetic populationa
| Characteristic | Value |
|---|---|
| Mean ± SD age (yr)b | 42.5 ± 7.6 |
| Mean ± SD wt (kg)b | 67.9 ± 7.6 |
| Mean ± SD ht (cm)b | 175.1 ± 7.9 |
| No. (%) of patients of male gender | 19 (100) |
| No. (%) of patients of white ethnicity | 15 (79) |
| Median (range) duration of APV-RTV treatment (mo) | 5.8 (0.5-19.2) |
| No. (%) of patients taking two RTIs | 12 (63) |
| No. (%) of patients taking three RTIs | 7 (37) |
| Median (range)c CD4 cell count (no. of cells/mm3) | 233 (12-426) |
| Median (range)c plasma HIV-1 RNA; load (log10 copies/ml) | 3.80 (<1.3-5.76) |
Abbreviations: SD, standard deviation; APV, amprenavir; RTV, ritonavir; RTIs, reverse transcriptase inhibitors, including efavirenz and nevirapine. A total of 19 patients were included in the pharmacokinetic population.
Geometric mean ± standard deviation.
Median interquantile range.
All patients were considered to have adhered to the prescribed treatment when patient diary cards were analyzed. Administrations occurred at intervals of approximately 12 h.
Pharmacokinetic results.
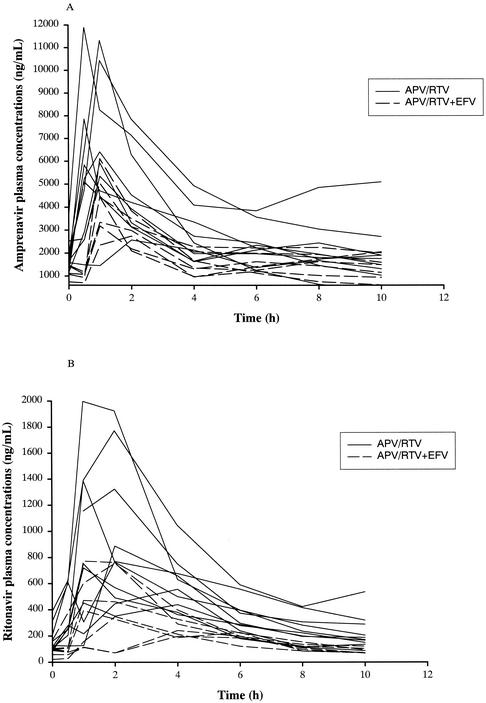
Median plasma amprenavir and ritonavir concentrations were higher in patients who took amprenavir and ritonavir without an NNRTI than in patients who took amprenavir and ritonavir plus efavirenz (Fig. 1).
FIG. 1.
Individual amprenavir (A) and ritonavir (B) concentration-time profiles for patients who received amprenavir at 600 mg BID plus ritonavir at 100 mg BID with (dotted lines) or without (solid lines) efavirenz at 600 mg once daily. APV, amprenavir; RTV; ritonavir; EFV, efavirenz.
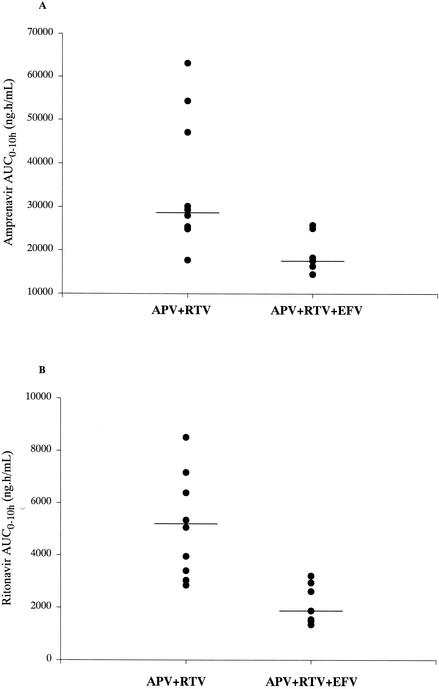
The geometric mean amprenavir Cmin,ss was higher for patients who took amprenavir at 600 mg BID and ritonavir at 100 mg BID without an NNRTI (1.92 μg/ml) than for those who took amprenavir and ritonavir plus efavirenz (1.36 μg/ml) (Table 2). There were decreases in the geometric mean values of Cmin,ss (29%), Cmax,ss (42%), AUC0-10 (42%), and AUCss (40%) for patients who took amprenavir and ritonavir in combination with efavirenz than for patients who took amprenavir and ritonavir without an NNRTI. Individual AUC0-10s showed large variability between patients (Fig. 2). The Cmin,ss and AUC0-10 for amprenavir for the two patients who took amprenavir and ritonavir with nevirapine were 0.84 μg/ml and 16.2 μg · h/ml, respectively, and 2.55 μg/ml and 29.5 μg · h/ml, respectively.
TABLE 2.
Amprenavir pharmacokinetic parametersa
| Treatment | Cmax,ss (μg/ml) | Cmin,ss (μg/ml) | Tmax,ss (h) | AUC0-10 (μg · h/ml) | AUCss (μg · h/ml) | CL/F (liters/h) |
|---|---|---|---|---|---|---|
| All patients (n = 19) | 5.71 (4.57, 7.13) | 1.64 (1.29, 2.09) | 1.00 (0.48-2.03) | 25.25 (20.62, 30.91) | 28.34 (23.13, 34.71) | 21.17 (17.28, 25.94) |
| APV-RTV (n = 10) | 7.12 (5.11, 9.93) | 1.92 (1.32, 2.79) | 0.95 (0.50-2.00) | 32.06 (24.04, 42.76) | 35.74 (26.62, 47.98) | 16.79 (12.51, 22.54) |
| APV-RTV-EFV (n = 7) | 4.13 (3.14, 5.43) | 1.36 (0.95, 1.97) | 1.00 (0.48-2.03) | 18.70 (15.22, 22.98) | 21.31 (17.23, 26.35) | 28.16 (22.78, 34.82) |
Abbreviations: APV, amprenavir; RTV, ritonavir; EFV, efavirenz. CI, confidence interval. Summary statistics are given for patients who received amprenavir and ritonavir and for patients who received amprenavir and ritonavir plus efavirenz. Values are geometric means (95% confidence intervals) for all parameters except Tmax,ss, which is given as the median (range).
FIG. 2.
Individual amprenavir (A) and ritonavir (B) AUC0-10s for patients who were administered amprenavir at 600 mg BID and ritonavir at 100 mg BID in combination with nucleoside analogs or nucleoside analogs and efavirenz (n = 7). APV, amprenavir; RTV; ritonavir; EFV, efavirenz.
For ritonavir, the geometric mean Cmin,ss was 0.15 μg/ml (95% confidence interval, 0.11, 0.22 μg/ml) and the geometric mean Cmax,ss was 0.95 μg/ml (95% confidence interval, 0.67, 1.36 μg/ml) (Table 3). The concentration-reducing effect of efavirenz was more pronounced on ritonavir metabolism than amprenavir metabolism; Cmin,ss decreased by 47% and AUC0-10 decreased by 58% in patients taking amprenavir and ritonavir plus efavirenz compared with the values for those taking amprenavir and ritonavir without efavirenz.
TABLE 3.
Ritonavir pharmacokinetic parametersa
| Treatment | Cmax,ss (μg/ml) | Cmin,ss (μg/ml) | Tmax,ss (h) | AUC0-10 (μg · h/ml) | AUCss (μg · h/ml) |
|---|---|---|---|---|---|
| All patients (n = 19) | 0.62 (0.45, 0.86) | 0.11 (0.08, 0.15) | 1.00 (0.83-4.02) | 2.94 (2.09, 4.13) | 3.13 (2.63, 4.86) |
| APV-RTV (n = 10) | 0.95 (0.67, 1.36) | 0.15 (0.11, 0.22) | 1.50 (0.92-4.02) | 4.89 (3.72, 6.42) | 4.90 (3.73, 6.81) |
| APV-RTV-EFV (n = 7) | 0.41 (0.25, 0.66) | 0.08 (0.04, 0.14) | 2.00 (0.92-4.02) | 2.03 (1.46, 2.82) | 2.22 (1.62, 3.02) |
Abbreviations: APV, amprenavir; RTV, ritonavir; EFV, efavirenz. Summary statistics are given for patients who received amprenavir and ritonavir and for patients who received amprenavir and ritonavir plus efavirenz. Values are geometric means (95% confidence intervals) for all parameters except Tmax,ss, which is given as the median (range).
Overall, there was a statistically significant (P < 0.05) correlation between the results for amprenavir and ritonavir for Cmin,s, and AUC0-10, although this relationship was very weak (r2 = 0.28).
Safety.
There were no serious adverse events in this study.
DISCUSSION
In this study, Cmin,sss for HIV-infected patients receiving amprenavir at 600 mg BID and ritonavir at 100 mg BID were higher than previously reported values after the administration of amprenavir at 1,200 mg BID (2). The addition of low-dose ritonavir to amprenavir at 600 mg BID led to a marked sevenfold increase in amprenavir Cmin,ss compared with the value resulting from the standard amprenavir dose of 1,200 mg BID. When efavirenz at 600 mg once daily was added to the amprenavir and ritonavir regimen, the Cmin,ss was reduced, but it was still higher than the previously reported values achieved after the administration of amprenavir at 1,200 mg BID. In contrast, amprenavir Cmax,sss were similar to historical values for patients receiving amprenavir and ritonavir. The addition of efavirenz to the amprenavir and ritonavir regimen led to decreases in the extent of exposure to amprenavir (AUC) and an increase in amprenavir clearance. Conclusions on the effect of nevirapine could not be drawn because of the limited number of patients and the variability of the results obtained.
Ritonavir improved the pharmacokinetic profile of amprenavir due mainly to its inhibition of CYP3A4 metabolism, as the inhibition of P glycoprotein, which is capable of pumping protease inhibitors across plasma membranes and out of target cells, appeared to be weak (5, 10, 13).
Unlike saquinavir, the first-pass effect in the gut appears to be minimal, as it was previously shown that grapefruit juice does not impair the pharmacokinetics of amprenavir (4). Ritonavir exerts its effect through inhibition of hepatic clearance. Thus, the selective effect of ritonavir on clearance has the benefit of increasing trough amprenavir concentrations, which are associated with antiretroviral activity. The lack of concomitant increases in peak concentrations avoids exposure of patients to higher maximum concentrations after drug coadministration.
Efavirenz and nevirapine are known inducers of cytochrome P450 enzymes (3). Consequently, our data show that the combination of efavirenz or nevirapine with an amprenavir and ritonavir regimen leads to a decrease in the amprenavir Cmin,ss, despite the potent inhibition of amprenavir metabolism by ritonavir. However, the Cmin,ss remained higher than that achieved after administration of the standard dose of amprenavir (1,200 mg BID).
Amprenavir is 90% bound to alpha 1 glycoprotein in plasma (2). The concentration of amprenavir required to inhibit the growth of wild-type HIV-1 by 50% (IC50), with adjustment for protein binding, was 0.146 μg/ml, as calculated by using 334 clinical isolates taken from protease inhibitor-naïve patients (D. Stein, B. Sadler, and M. Sale, 1st Int. Workshop Clin. Pharmacol. HIV Ther., abstr. 5.2, 2000). The Cmin,ss for viruses from our patients receiving amprenavir and ritonavir without efavirenz was over 13-fold higher than the amprenavir IC50 for wild-type virus, and the Cmin,ss for viruses from our patients receiving amprenavir and ritonavir plus efavirenz was over ninefold higher. The adjusted IC50 of amprenavir was 0.903 μg/ml when it was calculated for 284 clinical isolates from patients who had experienced virological failure after multiple prior treatment regimens that included a protease inhibitor (indinavir, nelfinavir, ritonavir, or saquinavir) (Stein et al., 1st Int. Workshop Clin. Pharmacol. HIV Ther., abstr. 5.2, 2000). The amprenavir Cmin,ss for patients receiving amprenavir and ritonavir with or without efavirenz in this study was at least twofold higher than the amprenavir IC50 measured for virus resistant to multiple protease inhibitors. Unfortunately, the concentration-effect relationship could not be assessed in our patients, as resistance tests were not scheduled when this pharmacokinetic study was designed. This accounts for the heterogeneity of the virological and immunological results at the time that this study was performed.
Plasma ritonavir concentrations were low and subtherapeutic, as the dose used in this study was only 100 mg BID and well below the doses used for antiretroviral activity (600 mg BID). A previous study with 10 HIV-1-infected patients who were administered ritonavir at 600 mg BID found the mean ± standard deviation ritonavir Cmin and Cmax to be 3.03 ± 2.13 and 11.2 ± 3.6 μg/ml, respectively (13). Furthermore, the adjusted IC90 of ritonavir for wild-type HIV-1 isolates is in the 2-μg/ml range. In comparison, the Cmin,ss and the Cmax,ss in our study were 0.15 and 0.95 μg/ml, respectively. In previous studies (1, 9), large interindividual variability in ritonavir pharmacokinetics was reported whether ritonavir at 100 mg BID was combined with indinavir, saquinavir, or amprenavir. An important point which needs further evaluation is the effect of food on the pharmacokinetics of a low dose of ritonavir. In our study, amprenavir and ritonavir were administered to fasted patients, as no food effect has been observed with amprenavir; in constrast, food was demonstrated to enhance the concentrations of ritonavir. This food effect could account for the discrepancies in the results between various studies. Higher ritonavir concentrations were recently reported when ritonavir was coadministered with indinavir and taken with a standard breakfast (1).
In conclusion, this study shows that coadministration of a reduced dose of amprenavir with a low dose of ritonavir results in amprenavir Cmin,sss that are markedly higher than the concentrations achieved with a standard amprenavir dose of 1,200 mg BID, with the added benefit of a reduction in the number of pills that a patient must take each day. The presence of ritonavir in combination with amprenavir counteracted, in part, the negative effect of efavirenz at the doses tested in this study. This amprenavir-ritonavir pharmacokinetic interaction could be beneficial as a salvage antiretroviral therapy in patients infected with multiresistant HIV strains. Additional studies are under way to explore the interaction of amprenavir and ritonavir, including the evaluation of increased doses of ritonavir in the presence of NNRTIs.
Acknowledgments
Funding for the study (PROF1004) was provided by Glaxo Wellcome Research and Development.
We thank Justin Cook for writing and editing assistance during preparation of the manuscript. We thank Abbott Laboratories for providing the ritonavir and the internal standard for the analytical assay.
REFERENCES
- 1.Aarnoutse, R. E., K. J. T. Grintjes, D. S. C. Telgt, M. Stek, P. W. H. Hugen, P. Reiss, P. P. Koopmans, Y. A. Hekster, and D. M. Burger. 2002. The influence of efavirenz on the pharmacokinetics of a twice-daily combination of indinavir and low-dose ritonavir in healthy volunteers. Clin. Pharmacol. Ther. 71:57-67. [DOI] [PubMed] [Google Scholar]
- 2.Adkins, J., and D. Faulds. 1998. Amprenavir. Drugs 55:837-842. [DOI] [PubMed] [Google Scholar]
- 3.Barry, M., F. Mulcahy, C. Merry, S. Gibbons, and D. Back. 1999. Pharmacokinetics and potential interactions amongst antiretroviral agents used to treat patients with HIV infection. Clin. Pharmacokinet. 36:289-304. [DOI] [PubMed] [Google Scholar]
- 4.Demarles, D., C. Gillotin, S. Bonaventure-Paci, I. Vincent, S. Fosse, and A. M. Taburet. 2002. Single-dose pharmacokinetics of amprenavir coadministered with grapefruit juice. Antimicrob. Agents Chemother. 46:1589-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drewe, J., H. Gutmann, G. Fricker, M. Torok, C. Beglinger, and J. Huwyler. 1999. HIV protease inhibitor ritonavir: a more potent inhibitor of p-glycoprotein than the cyclosporine analog SDZ PSC 833. Biochem. Pharmacol. 57:1147-1152. [DOI] [PubMed] [Google Scholar]
- 6.Duval, X., V. Le Moing, P. Longuet, C. Leport, J. L. Vilde, C. Lamotte, G. Peytavin, and R. Farinotti. 2000. Efavirenz-induced decrease in plasma amprenavir levels in human immunodeficiency virus-infected patients and correction by ritonavir. Antimicrob. Agents Chemother. 44:2593-2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falloon, J., S. Piscitelli, S. Vogel, B. Sadler, H. Mitsuya, M. F. Kavlick, K. Yoshimura, M. Rogers, S. Lafon, D. J. Manion, H. C. Lane, and H. Masur. 2000. Combination therapy with amprenavir, abacavir, and efavirenz in human immunodeficiency virus (HIV)-infected patients failing a protease-inhibitor regimen: pharmacokinetic drug interactions and antiviral activity. Clin. Infect. Dis. 30:313-318. [DOI] [PubMed] [Google Scholar]
- 8.Kempf, D., K. Marsh, G. Kumar, A. Rodrigues, J. Denissen, E. McDonald, M. J. Kukulka, A. Hsu, G. R. Granneman, P. A. Baroldi, E. Sun, D. Pizzuti, J. J. Plattner, D. W. Norbeck, and J. M. Leonard. 1997. Pharmacokinetic enhancement of inhibitors of the human immunodeficiency virus protease by coadministration with ritonavir. Antimicrob. Agents Chemother. 41:654-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kilby, J., G. Sfakianos, N. Gizzi, P. Siemon-Hryczyk, E. Ehrensing, C. Oo, N. Buss, and M. S. Saag. 2000. Safety and pharmacokinetics of once-daily regimens of soft-gel capsule saquinavir plus minidose ritonavir in human immunodeficiency virus-negative adults. Antimicrob. Agents Chemother. 44:2672-2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koudriakova, T., E. Latsimirskaia, I. Utkin, E. Gangl, P. Vouros, E. Storozhuk, D. Orza, J. Marinina, and N. Gerber. 1998. Metabolism of the human immunodeficiency virus protease inhibitors indinavir and ritonavir by human intestinal microsomes and expressed cytochrome P4503A4/3A5: mechanism-based inactivation of cytochrome P4503A by ritonavir. Drug Metab. Dispos. 26:552-561. [PubMed] [Google Scholar]
- 11.Saah, A. J., G. A. Winchell, M. L. Nessly, M. A. Seniuk, R. R. Rhodes, and P. J. Deutsch. 2001. Pharmacokinetic profile and tolerability of indinavir-ritonavir combinations in healthy volunteers. Antimicrob. Agents Chemother. 45:2710-2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sadler, B. M., P. J. Piliero, S. L. Preston, Y Lou, and D. S. Stein. 2001. Pharmacokinetics and safety of amprenavir and ritonavir following multiple-dose, co-administration to healthy volunteers. AIDS 15:1009-1018. [DOI] [PubMed] [Google Scholar]
- 13.Sven, A., S. Danner, A. Carr, J. M. Leonard, L. M. Lehman, F. Gudiol, J. Gonzales, A. Raventos, R. Rubio, E. Bouza, V. Pintado, A. G. Aguado, J. Garcia de Lomas, R. Delgado, J. C. C. Borleffs, A. Hsu, J. M. Valdes, C. A. B. Boucher, and D. A. Cooper. 1995. A short-term study of the safety, pharmacokinetics, and efficacy of ritonavir, an inhibitor of HIV-1 protease. N. Engl. J. Med. 333:1528-1533. [DOI] [PubMed] [Google Scholar]