Abstract
The microbiological, clinical, and epidemiological features of most non-Candida albicans Candida species are well known, but much less is known about species such as Candida guilliermondii, an uncommon pathogen causing a variety of deep-seated infections in immunocompromised hosts. To characterize C. guilliermondii fungemia in patients with hematological malignancies and its susceptibility to antifungal drugs, all cases of C. guilliermondii fungemia diagnosed in our department between 1983 and 2005 were retrospectively analyzed and the literature was reviewed. C. guilliermondii caused 29/243 (11.7%) candidemia episodes diagnosed during the study period. Central venous catheters were the documented sources of candidemia in 19/29 episodes (65.5%), and invasive tissue infections were documented in 2 (6.9%). In the remaining eight, the catheter was not removed and the source of the fungemia remained obscure. Seven episodes ended in death, but only one could be attributed to invasive C. guilliermondii infection. Molecular typing data reveal no evidence of common infection sources. Isolates displayed high rates of in vitro susceptibility to amphotericin B (100%), voriconazole (95%), and fluconazole (90%) and lower rates of in vitro susceptibility to flucytosine (86%), itraconazole (76%), and caspofungin (33%). Our literature review confirms that C. guilliermondii is a significantly more frequent cause of candidemia among cancer patients compared with the general hospital population. It accounted for <1% of the total number of Candida bloodstream isolates reported in the articles we reviewed, with higher rates in Europe (1.4%) and Asia (1.8%) compared with North America (0.3%).
Non-Candida albicans Candida species have been recognized as emerging pathogens in cancer patients, particularly those with hematological malignancies. Not only are serious infections caused by these yeast species increasing in frequency, but in a number of cases the strains responsible for the infection display tolerance or resistance to antimycotics (13, 41, 67). The microbiological, clinical, and epidemiological features of Candida parapsilosis, Candida tropicalis, Candida krusei, and Candida glabrata are well known, but much less is known about other non-C. albicans Candida species. The few reports in the literature on Candida guilliermondii infections suggest that they are associated with poor clinical outcomes. This species has caused a variety of deep-seated infections in immunocompromised hosts and, less frequently, intravenous drug users. Like Candida lusitaniae, it is one of the fungal pathogens most likely to display in vitro resistance to amphotericin B and fluconazole (8, 19, 20, 28, 33, 49, 60, 63, 68). The present study was an attempt to learn more about the clinical characteristics of infections caused by C. guilliermondii and its antifungal susceptibility pattern. All cases of candidemia diagnosed in our department over the past 22 years were retrospectively analyzed to identify the prevalence and clinical features of C. guilliermondii fungemia in patients with hematological malignancies, risk factors for these infections, and their probable susceptibility to treatment with commonly used antimycotic agents. We also reviewed the literature to evaluate the epidemiological impact of this fungal pathogen.
MATERIALS AND METHODS
Definitions of fungemia.
The cases analyzed in this study were collected from the medical records of the Institute of Hematology, Dipartimento di Biotecnologie Cellulari ed Ematologia, of the University La Sapienza of Rome Medical Center. These records were retrospectively reviewed to identify all patients (inpatients and outpatients) with hematological diseases who were diagnosed with candidemia between September 1983 and August 2005. Cases were included only when the diagnosis was confirmed by isolation of Candida spp. from one or more blood cultures which had been performed with Trypticase soy broth (BCG System, Roche, and Sygnal System, Oxoid, Hants, United Kingdom) and examined daily for at least 2 weeks. Yeast isolates had been identified at the species level with the VITEK and API yeast biochemical systems (BioMérieux Italia, Rome, Italy). Surveillance culture of sputum, urine, and stool specimens were performed weekly for all patients with fungemia.
All episodes of fungemia caused by C. guilliermondii were selected for detailed analysis. Patient charts (including autopsy data when present) were analyzed to characterize the fungemic episode, including its duration, presentation, and treatment; the presence of deep-seated C. guilliermondii tissue infections; outcome, etc. Particular attention was focused on its possible association with a central venous catheter (CVC). Cases were thus analyzed to determine whether the patient had a CVC when the fungemia was diagnosed and whether or not it was removed. In those cases where the CVC was removed, the results of semiquantitative cultures of the catheter tip (26) and the patient's response in terms of fever curves and candidemia clearance after CVC removal were noted. Isolates of C. guilliermondii that had been recovered from these patients and stored (as water suspensions) in the Clinical Microbiology Laboratory of the University La Sapienza of Rome Medical Center were subjected to independent blind testing in a second laboratory to confirm the original species level identification. Most of these strains underwent additional testing as described in the following sections.
Antifungal susceptibility tests.
Twenty-one isolates of C. guilliermondii were available for in vitro antifungal susceptibility testing. Prior to testing, each isolate was passaged at least twice on Sabouraud dextrose agar.
Quality control strains C. albicans ATCC 90028 and C. parapsilosis ATCC 22019 were included in every test run.
Susceptibility to voriconazole (Pfizer, Inc., New York, NY), fluconazole (Pfizer), itraconazole (Janssen, Beerse, Belgium), amphotericin B, and flucytosine (both from Sigma, St. Louis, MO) was tested by the broth microdilution method in accordance with the M27-A2 protocol published by the Clinical and Laboratory Standards Institute (CLSI [formerly NCCLS]) (31). Results were read after 48 h of incubation at 35°C. The lowest drug concentration producing complete growth inhibition (for amphotericin B) or inhibition of 50% or more compared with control growth (for fluconazole, itraconazole, voriconazole, and flucytosine) was recorded as the MIC.
Caspofungin could not be tested by the broth microdilution method because a standard powder preparation of the drug could not be obtained from the manufacturer. Susceptibility to this drug was thus assessed by the E-test method (AB Biodisk, Solna, Sweden) and RPMI 1640-2% glucose agar (Difco Laboratories) in accordance with the manufacturer's instructions. This approach has displayed 100% concordance with the CLSI microdilution method for evaluation of caspofungin susceptibility in C. guilliermondii isolates (39).
Interpretive breakpoints established by the CLSI were used to define susceptibility to fluconazole (MIC, ≤8 μg/ml), itraconazole (MIC, ≤0.125 μg/ml), flucytosine (MIC, ≤4μg/ml) (31), and voriconazole (MIC, ≤1 μg/ml; minutes of the CLSI Antifungal Subcommittee meeting, 2005) (46). Since CLSI-validated breakpoints have not been established for amphotericin B or caspofungin, we adopted the criteria proposed by Pfaller et al. (40, 44), who considered MICs of ≤1 μg/ml indicative of susceptibility to these drugs.
Genotypic characterization.
Genotyping was performed on 19 isolates of C. guilliermondii. Isolates from 48- to 72-h cultures were suspended in 20 ml yeast peptone glucose (1% peptone yeast extract, 2% glucose, 2% Bacto Peptone). After 24 h of incubation with agitation at 35°C, yeasts were harvested by centrifugation and genomic DNAs were extracted as described by Scherer and Stevens (58). DNA typing was performed by random amplification of polymorphic DNA (RAPD) with primers RSD12 (5′CCGCAGCCA3′) (57) and OPE03 (5′CCAGATGCAC3′) (50) (M-Medical/Genenco, Florence, Italy). Thermocycling was performed with a Gene Amp PCR System 9700 (Applied Biosystems, Monza, Italy). PCR was performed with a 50-μl volume of PCR master mix containing approximately 200 ng of yeast DNA as the template, 5 μl of 10× PCR buffer (200 mM Tris-HCl [pH 8.4], 500 mM KCl), 200 μM deoxynucleoside triphosphates, 25 mM MgCl2, 1 μM primer, and 1.5 U of Taq polymerase (Life Technologies). The PCR conditions used have been described elsewhere (58). The PCR products were electrophoresed in an agarose gel (1.2%) for approximately 2 h at room temperature in Tris-borate-EDTA buffer (89 mM Tris, 89 mM boric acid, 2.5 mM EDTA [pH 8.0]), stained with ethidium bromide, and visualized with UV light.
Review of the literature.
We conducted a MEDLINE-based search of the English language literature published since 1966 to identify articles containing the term “candidemia,” “fungemia,” and/or “Candida/fungal bloodstream” in the title and abstract. All articles describing studies including at least 150 cases of candidemia were reviewed to estimate the relative weight of C. guilliermondii fungemia.
RESULTS
Prevalence of C. guilliermondii fungemia.
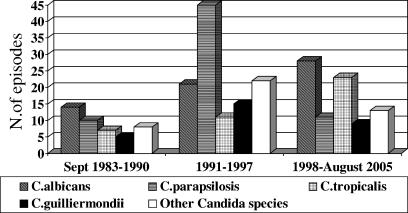
During the 22-year period considered in this study, 247 Candida bloodstream isolates were recovered from patients treated by our staff for hematological malignancies (Fig. 1). They were responsible for a total of 243 episodes of candidemia, 29 (11.7%) of which were caused by C. guilliermondii. No case was observed in patients with nononcologic hematological diseases. Since 1988, when the first case occurred, the incidence of C. guilliermondii fungemia has been 2 per 1,000 admissions. Corresponding values for C. parapsilosis, C. albicans, and C. tropicalis fungemia are 4.5, 3.9, and 2.4 per 1,000 admissions, respectively.
FIG. 1.
Prevalence of Candida species causing fungemia in patients with hematologic malignancies in three different periods at the Dipartimento di Biotecnologie Cellulari ed Ematologia of the University La Sapienza of Rome.
Patients and predisposing factors.
Tables 1 and 2 summarize the patient characteristics, clinical features, and outcomes of the 29 episodes of C. guilliermondii fungemia. As shown in Table 1, more than half the cases occurred in patients with acute nonlymphoid leukemia and almost all were diagnosed during periods of hospitalization (1 to 60 days after admission; mean, 23 days). However, four (cases 4, 8,12, and 25 in Table 2) occurred while the patient was at home (12, 25, 40, and 65 days after the most recent hospital discharge, respectively) and were treated on an outpatient basis. When the fungemia was diagnosed, well over half of the patients had neutropenia, which had been present for 5 to 60 days (mean, 20.8 days). Most patients were receiving antibiotic therapy, and antifungal prophylaxis was being administered in 13 cases; in 7/29 (24.1%) cases, the patient was receiving fluconazole (mean dosage, 400 mg/day) and 6 (20.7%) were taking oral nonabsorbable amphotericin B (mean dosage, 2,000 mg/day). Colonization with C. guilliermondii was documented in only one episode (3.5%) (stool culture positivity in case 2).
TABLE 1.
Patient characteristics in 29 episodes of fungemia caused by C. guilliermondii
| Patient characteristic | No./total (%) |
|---|---|
| Total no. of episodesa | 29 (100) |
| Males | 21/29 (72) |
| Inpatients | 25/29 (86) |
| Hematological malignancies | |
| Acute nonlymphoid leukemia | 16/29 (55) |
| Acute lymphoid leukemia | 7/29 (24) |
| Non-Hodgkin's lymphoma | 4/29 (14) |
| Multiple myeloma | 2/29 (7) |
| Treatments | |
| Chemotherapy | 16/29 (55) |
| Allogeneic blood stem cell transplantation | 5/29 (17) |
| Autologous blood stem cell transplantation | 7/29 (24) |
| Supportive therapy | 1/29 (4) |
| Neutropeniab | 18/29 (62) |
| CVC | 29/29 (100) |
| Total parenteral nutrition | 9/29 (31) |
| Fluconazole prophylaxis | 7/29 (24) |
| Previous antibiotic therapy | 23/29 (79) |
| Colonization by same organism | 1/29 (3) |
Two patients experienced a second episode of fungemia during the study period. For this analysis, their characteristics are listed twice (once for each episode).
Defined as <500 polymorphonuclear cells/mm3.
TABLE 2.
Clinical features and outcomes of 29 episodes of C. guilliermondii fungemia in patients with hematological malignancies
| Case no. | Onset date | Clinical presentation | Genotype | CVC tip culturea | Antifungal therapy | Fungemia outcome (duration [days]) | Type of fungemia | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | Sept. 1988 | Fever | NTb | + | None | Cleared (11) | CVC related | Survival |
| 2 | March 1989 | Fever, multiple organ failure | NT | NRc | None | Present at death (7) | Secondary | Death due to C. guilliermondii infection |
| 3 | Dec. 1989 | Fever | NT | + | Fluconazole | Cleared (82) | CVC related | Death due to underlying malignancy |
| 4d | May 1990 | Fever | NT | + | Fluconazole | Cleared (2) | CVC related | Survival |
| 5 | May 1990 | Fever | NT | + | Fluconazole | Cleared (7) | CVC related | Survival |
| 6 | Sept. 1992 | Fever, pulmonary aspergillosis | I | NR | AmBe | Present at death (3) | Unknown | Death due to pulmonary aspergillosis |
| 7 | Dec. 1992 | Fever | II | NR | AmB | Cleared (2) | Unknown | Survival |
| 8d | April 1993 | Fever | III | + | Fluconazole | Cleared (8) | CVC related | Survival |
| 9 | Oct. 1993 | Fever | I | + | AmB | Cleared (7) | CVC related | Survival |
| 10 | Nov. 1993 | Fever | I | NR | Fluconazole-flucytosine | Cleared (7) | Unknown | Survival |
| 11 | Jan. 1994 | Fever | IV | + | Fluconazole | Cleared (30) | CVC related | Survival |
| 12d | Sept. 1994 | Fever | IV | + | Fluconazole | Cleared (6) | CVC related | Survival |
| 13 | March 1995 | Fever | NT | + | Fluconazole | Cleared (17) | CVC related | Survival |
| 14 | March 1995 | Fever | III | + | Fluconazole | Cleared (12) | CVC related | Survival |
| 15 | April 1995 | Fever | II | NR | Fluconazole | Cleared (5) | Unknown | Survival |
| 16 | June 1995 | Fever | III | + | Fluconazole | Cleared (3) | CVC related | Survival |
| 17 | Aug. 1995 | Fever, CVC exit site infection, multiple skin lesions | I | + | Fluconazole | Cleared (19)f | Secondary | Survival |
| 18 | July 1996 | Fever, septic shock | I | NR | None | Present at death (4) | Unknown | Death due to gram-negative septicemia (role of Candida infection unknown) |
| 19 | June 1997 | Fever | III | + | Fluconazole | Cleared (6) | CVC related | Survival |
| 20 | Dec. 1997 | Fever | NT | NR | Fluconazole | Cleared (1) | Survival | |
| 20a | Feb. 1998 | Fever | NT | + | Fluconazole | Cleared (6) | CVC related | Survival |
| 21 | Jan. 1998 | Fever | II | + | Fluconazole | Cleared (24) | CVC related | Survival |
| 22 | March 1998 | Fever | II | + | Fluconazole | Cleared (8) | CVC related | Survival |
| 23 | Aug. 1999 | Fever | III | + | Fluconazole | Cleared (6) | CVC related | Survival |
| 24 | Jan. 2000 | Fever, pulmonary aspergillosis | NT | + | Fluconazole-AmB | Cleared (10) | CVC related | Death due to invasive aspergillosis |
| 25d | Oct. 2000 | Fever | III | + | Itraconazole | Cleared (16) | CVC related | Survival |
| 26 | Jan. 2001 | Fever | I | + | Fluconazole-AmB | Cleared (8) | CVC related | Survival |
| 26a | April 2001 | Fever | IV | NR | No | Present at death (5) | Unknown | Death due to underlying malignancy |
| 27 | Sept. 2003 | Fever, pulmonary aspergillosis | NT | NR | AmB | Present at death (3) | Unknown | Death due to invasive aspergillosis (no Candida infection at autopsy) |
CVCs were present at diagnosis in all episodes. Culture of the removed CVCs always grew C. guilliermondii (+).
NT, not tested.
NR, CVC was not removed.
Candidemia was documented while the patient was not hospitalized; the remaining 25 cases occurred during hospitalization.
AmB, amphotericin B.
Candidemia cleared 5 days after CVC removal. In all other cases where CVCs were removed, candidemia cleared 24 h later.
Clinical characteristics and outcome of C. guilliermondii fungemia.
The mean duration of candidemia (from the first positive blood culture to negative blood culture or death) was 11 days (range, 1 to 82 days), and a mean of seven positive blood cultures were collected per episode (range, 1 to 25). As shown in Table 2, all 29 episodes were associated with fever. Two (6.9%) were considered secondary to invasive tissue infections, i.e., case 17, which was associated with skin lesions (culture positive for C. guilliermondii) and cellulitis at the CVC insertion site, and case 2, in which there was multiorgan failure due to disseminated C. guilliermondii candidiasis. In the other 27 (93.1%), there was no clinical or microbiological evidence of invasive C. guilliermondii tissue infection. In 19 of these episodes, the CVC was removed, and within 24 h both the fever and candidemia had cleared. Semiquantitative cultures of the catheter tips were all positive for C. guilliermondii. These 19 cases (65.5% of the total series) were considered CVC related. In the remaining eight cases (type unknown), the CVC could not be removed and no other source of infection could be identified. In four of these, blood cultures became negative (after 1, 2, 5, and 7 days of antifungal therapy); in the remaining four, the candidemia persisted until death. Overall, fatal outcomes were recorded for 7 (24.1%) of the 29 episodes. In five, fungemia was still present at the time of death, but only one of these deaths was clearly attributed to C. guilliermondii infection (case 2) (Table 2).
In vitro antifungal susceptibility.
Table 3 summarizes the antifungal susceptibilities of the 21 C. guilliermondii isolates tested. All were fully susceptible to amphotericin B (MIC, <1 μg/ml). Eighteen (86%) isolates were susceptible to flucytosine. All but two (91%) were susceptible to fluconazole. Sixteen (76%) isolates were susceptible to itraconazole. Susceptibility to voriconazole was documented in all but one strain (95%). For 14 (66%) of the isolates, the caspofungin MICs were indicative of probable resistance (>1 μg/ml).
TABLE 3.
Antifungal susceptibilities of 21 strains of C. guilliermondii
| Agent | No. susceptible/total no. of strains (%) | MIC range (μg/ml) |
|---|---|---|
| Amphotericin Ba | 21/21 (100) | <0.03-0.125 |
| Flucytosinea | 18/21 (86) | <0.125->64 |
| Fluconazolea | 19/21 (91) | 0.5->64 |
| Itraconazolea | 16/21 (76) | <0.03->16 |
| Voriconazolea | 20/21 (95) | <0.03-4 |
| Caspofunginb | 7/21 (33) | 0.5->32 |
Susceptibility was evaluated by the CLSI broth microdilution method.
Susceptibility was evaluated by the E-test method with RPMI-2% glucose agar.
Genotypic characterization.
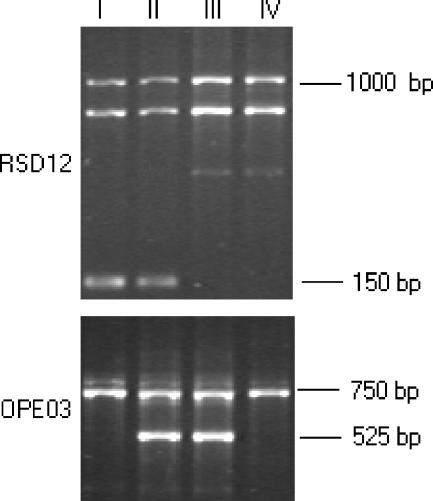
Nineteen isolates were genotyped by RAPD with the RSD12 and OPE03 primers (Fig. 2). On the basis of the combined results obtained with the two primers, four DNA types were identified; types I and III included six strains each, four isolates were type II, and three were type IV (Table 2).
FIG. 2.
Representative DNA types (I to IV) of four C. guilliermondii isolates obtained by RAPD with primers RSD12 and OPE03. All amplifications were repeated twice, and the most intense bands in the patterns were reproducible. For comparative analyses, only high-intensity bands (lanes II and III for RSD12 and lanes I and II for OPE03) were considered. The combined results obtained with the two individual primers revealed four different DNA types (I to IV).
Review of the literature.
The literature search yielded 42 articles reporting at least 150 cases of candidemia (1-3, 4-6, 7, 9, 10, 12, 14-18, 21-25, 27, 29, 30, 32, 34-38, 42, 48, 51-55, 59, 61-65, 69) (Table 4). C. guilliermondii accounted for 0.73% of the total number of Candida bloodstream isolates (median, 0.4%; range, 0 to 4.5%) and 1.6% of the non-C. albicans Candida isolates. The percentage of cases caused by C. guilliermondii appeared to be significantly higher in Europe and Asia compared with North America. Rates among cancer patients were also higher than those among the general hospital populations, and even lower rates emerged from the three population-based surveillance studies (7, 12, 15).
TABLE 4.
Incidence of C. guilliermondii fungemia on the basis of data in the literature
| Parameter | No. of candidemia episodes | No. (%) of candidemia episodes caused by C. guilliermondiie |
|---|---|---|
| Totala | 21,504 | 157 (0.73) |
| Geographic distribution | ||
| North America | 12,302 | 38 (0.3)* |
| Europe | 4,574 | 62 (1.4)† |
| Asia | 2,241 | 41 (1.8)‡ |
| Patient populations | ||
| Selected general hospitalsb | 17,526 | 127 (0.7)§ |
| Cancer centersc | 1,647 | 26 (1.6)¶ |
| Population-based | 2,331 | 4 (0.17)‖ |
| surveillanced |
References 1-3, 4-6, 7, 9, 10, 12, 14-18, 21-25, 27, 29, 30, 32, 34-38, 42, 48, 51-55, 59, 61-65, and 69.
References 2, 3, 4-6, 9, 10, 16, 17, 21, 23-25, 27, 29, 30, 32, 34-38, 42, 48, 52-55, 59, 61-63, 65, and 69.
* versus †: P < 0.0001; odds ratio, 4.43; 95% confidence interval, 2.91 to 6.78. * versus ‡: P < 0.0001; odds ratio, 6.01; 95% confidence interval, 3.78 to 9.59. † versus ‡: P = not significant. § versus ¶: P = 0.0002; odds ratio, 2.20; 95% confidence interval, 1.40 to 3.42. § versus ‖: P = 0.002; odds ratio, 0.24; 95% confidence interval, 0.07 to 0.66. ¶ versus ‖: P < 0.0001; odds ratio, 0.11; 95% confidence interval, 0.03 to 0.32.
DISCUSSION
C. guilliermondii is part of the normal flora of human skin and mucosal surfaces, but it is occasionally implicated as a cause of chronic onychomycosis, acute osteomyelitis, septic arthritis, endocarditis, fungemia, and disseminated invasive infections (56). It is one of the opportunistic fungi recovered most frequently from severely immunocompromised patients. Our literature review confirmed that C. guilliermondii is a more common cause of candidemia in cancer patients than it is in general hospital populations, but it is rarely implicated in bloodstream infections occurring in other high-risk categories, such as intensive care unit patients (62). Even among cancer patients, the actual incidence appears to be quite low. A review of 37 reports published between 1952 and 1992 revealed that C. guilliermondii was responsible for only 0.8% of all systemic Candida infections in this risk group (67). The largest reported series includes nine cases (two-thirds occurring in leukemia patients) observed over 11 years (1988 to 1998) at the M. D. Anderson Cancer Center (28).
In comparison, the rates observed in our institute appear fairly high. The first case was observed in 1988, and since then, 28 other episodes have been diagnosed. C. guilliermondii accounted for 11.7% of the Candida bloodstream isolates recovered from our patients during the 22-year study period. Its frequency was inferior only to those of C. parapsilosis, C. albicans, and C. tropicalis. It is important to note, however, that the incidence of C. guilliermondii fungemia in our institute is by no means uniform. Approximately 80% of the cases were observed between 1992 and 2001, and only one has occurred since then.
It is difficult to pinpoint specific reasons for the relatively high frequency of C. guilliermondii candidemia in our institution. Cases of C. guilliermondii fungemia were documented in patients with different hematological malignancies who underwent various chemotherapy treatments and only in a minority of cases received systemic antifungal prophylaxis. Molecular analyses of 19 isolates recovered from our patients from 1992 to 2001 do not support the possibility of a common source of infection. In fact, there were no case clusters during any of the periods considered, and even temporally related candidemia episodes were usually due to genetically different strains. Regional variations have been documented, and European rates are significantly higher than those in North America. We have no interpretation regarding this apparently nonhomogeneous geographic distribution of C. guilliermondii bloodstream infections.
Clinically and epidemiologically, C. guilliermondii fungemia seems to resemble that caused by C. parapsilosis (11, 66). All of our cases occurred in patients with indwelling CVCs, at least 19 of the 29 episodes (65.5%) could be classified as catheter related, and there were only two cases of deep invasive infections. In addition, both types of fungemia increased in frequency between 1991 and 1997 and decreased thereafter. Their close association with central venous access suggests that this trend might be attributed to changes in the use of CVCs in our institute. The number of CVC insertions rose progressively during the first 10 years of the study, and this increase could explain the increasing number of CVC-related candidemias. In the last 10 years, however, CVC placement rates have remained fairly stable and the same type of central line was used. The decreasing frequencies of C. guilliermondii and C. parapsilosis infection during this period might thus reflect improved management of these catheters. Furthermore, it should be noted that the same blood culture method in the same laboratory was used over the years of study.
Catheter removal had a major impact on the outcome of treatment. It was almost always followed by defervescence and candidemia clearance within 24 h. The only exception was a patient with disseminated infection (case 17) whose fungemia persisted for 5 days after CVC removal. It should be noted that when the CVC was removed, this patient had been receiving antifungal therapy for 2 weeks with no sign of resolution of the candidemia. Indeed, most of the other patients had been treated unsuccessfully for more than a week before the CVC was removed. In case 3, the CVC was removed after 82 days of candidemia with no evidence of secondary deep-seated foci. In four of the nine episodes in which the CVC could not be removed, the duration of candidemia was still brief, but in the other five, the fungemia persisted and was still present when death occurred. Two patients experienced a second episode of C. guilliermondii fungemia 2 months (cases 20 and 20a in Table 2) and 3 months (cases 26 and 26a) after the first. Molecular typing was done only for the two isolates recovered from the latter two cases, which were confirmed to be genetically different. Overall, fatal outcomes occurred in 7 (24%) of the 29 episodes, but only one of these deaths could be attributed to C. guilliermondii infection.
It is of interest that 14% of our patients became fungemic at home. C. guilliermondii can be part of the normal flora of the skin and mucosal surfaces, and handling of the CVC by the patient or relatives can thus be a risk for contamination.
Although there are reports of single cases of C. guilliermondii infection displaying in vitro resistance to amphotericin B and/or fluconazole (8, 19, 49, 60, 63), there is no evidence of widespread polyene and azole resistance in this species. Pfaller et al. (43, 45) have recently assessed the in vitro susceptibilities of rare Candida bloodstream isolates recovered in various parts of the world, including a total of 150 isolates of C. guilliermondii. The great majority (85 to 100%) were fully susceptible to amphotericin B, flucytosine, fluconazole, voriconazole, and ravuconazole, but susceptibility to itraconazole was much less common (10%). C. guilliermondii seems to be intrinsically resistant to the echinocandins. High caspofungin MICs (>1 μg/ml) have been reported for more than 95% of the isolates tested (39, 44), suggesting that this drug is unlikely to be effective against C. guilliermondii infections. A recent study showed that C. guilliermondii is also among the Candida species that are the least susceptible to the echinocandin anidulafungin (47). Our data confirm high rates of susceptibility to amphotericin B (100%), voriconazole (95%), fluconazole (90%), and flucytosine (86%), but our isolates displayed lower rates of resistance to itraconazole (24%) and caspofungin (66%) than those observed in previous studies.
In conclusion, C. guilliermondii is a potential cause of fungal bloodstream infections, particularly in patients with hematologic malignancies. The overall incidence of these infections seems to be very low, even in cancer patients, but their distribution is by no means homogeneous and higher frequencies may be observed in certain hematological centers. The incidence of C. guilliermondii bloodstream infections in Asia and Europe is slightly higher than it is in North America. These infections are clinically similar to those caused by C. parapsilosis, and bloodstream invasion by both fungal species is closely related to CVC placement. C. guilliermondii is intrinsically resistant to the echinocandins, but despite isolated reports to the contrary, it is also highly susceptible to amphotericin B and all of the azoles, with the probable exception of itraconazole.
REFERENCES
- 1.Abi-Said, D., E. Anaissie, O. Uzun, I. Raad, H. Pinzcowski, and S. Vartivarian. 1997. The epidemiology of hematogenous candidiasis caused by different Candida species. Clin. Infect. Dis. 24:1122-1128. [DOI] [PubMed] [Google Scholar]
- 2.Asmundsdottir, L. R., H. Erlendsottir, and M. Gottfredsson. 2002. Increasing incidence of candidemia: results from a 20-year nationwide study in Iceland. J. Clin. Microbiol. 40:3489-3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bille, J., L. Stockman, and G. D. Roberts. 1982. Detection of yeasts and filamentous fungi in blood cultures during a 10-year period (1972 to 1981). J. Clin. Microbiol. 16:968-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, Y. C., S. C. Chang, K. T. Luh, and W. C. Hsieh. 2003. Stable susceptibility of Candida blood isolates to fluconazole despite increasing use during the past 10 years. J. Antimicrob. Chemother. 52:71-77. [DOI] [PubMed] [Google Scholar]
- 5.Cheng, M. F., K. W. Yu, R. B. Tang, Y. H. Fan, Y. L. Yang, K. S. Hsieh, M. Ho, and H. J. Lo. 2004. Distribution and antifungal susceptibility of Candida species causing candidemia from 1996 to 1999. Diagn. Microbiol. Infect. Dis. 48:33-37. [DOI] [PubMed] [Google Scholar]
- 6.Chryssanthou, E. 2001. Trends in antifungal susceptibility among Swedish Candida species bloodstream isolates from 1994 to 1998: comparison of the E-test and the Sensititre YeastOne Colorimetric Antifungal Panel with the NCCLS M27-A reference method. J. Clin. Microbiol. 39:4181-4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cuenca-Estrella, M., D. Rodriguez, B. Almirante, J. Morgan, A. M. Planes, M. Almela, J. Mensa, F. Sanchez, J. Ayats, M. Gimenez, M. Salvado, D. W. Warnock, A. Pahissa, and J. L. Rodriguez-Tuleda. 2005. In vitro susceptibilities of bloodstream isolates of Candida species to six antifungal agents: results from a population-based active surveillance programme, Barcelona, Spain, 2002-2003. J. Antimicrob. Chemother. 55:194-199. [DOI] [PubMed] [Google Scholar]
- 8.Dick, J., B. Rosengard, W. Merz, R. K. Stuart, G. M. Hutchins, and R. Saral. 1985. Fatal disseminated candidiasis due to amphotericin B-resistant Candida guilliermondii. Ann. Intern. Med. 102:67-68. [DOI] [PubMed] [Google Scholar]
- 9.Diekema, D. J., S. A. Messer, A. B. Brueggemann, S. L. Coffman, G. V. Doern, L. A. herwaldt, and M. A. Pfaller. 2002. Epidemiology of candidemia: 3-year results from the Emerging Infections and the Epidemiology of Iowa Organisms study. J. Clin. Microbiol. 40:1298-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garbino, J., L. Kolarova, P. Rohner, D. Lew, P. Pichna, and D. Pittet. 2002. Secular trends of candidemia over 12 years in adult patients at a tertiary care hospital. Medicine 81:425-433. [DOI] [PubMed] [Google Scholar]
- 11.Girmenia, C., P. Martino, F. De Bernardis, G. Gentile, M. Boccanera, M. Monaco, G. Antonucci, and A. Cassone. 1996. Rising incidence of Candida parapsilosis fungemia in patients with hematologic malignancies: clinical aspects, predisposing factors, and differential pathogenicity of the causative strains. Clin. Infect. Dis. 23:506-514. [DOI] [PubMed] [Google Scholar]
- 12.Hajjeh, R. A., A. N. Sofair, L. H. Harrison, G. Marshall Lyon, B. A. Arthington-Skaggs, S. A. Mirza, M. Phelan, J. Morgan, W. Lee-Yang, M. A. Ciblak, L. E. Benjamin, L. T. Sanza, S. Huie, S. F. Yeo, M. E. Brandt, and D. W. Warnock. 2004. Incidence of bloodstream infections due to Candida species and in vitro susceptibilities of isolates collected from 1998 to 2000 in a population-based active surveillance program. J. Clin. Microbiol. 42:1519-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hobson, R. P. 2003. The global epidemiology of invasive Candida infections—is the tide turning? J. Hosp. Infect. 55:159-168. [DOI] [PubMed] [Google Scholar]
- 14.Horn, R., B. Wong, T. E. Kiehn, and D. Armstrong. 1985. Fungemia in a cancer hospital: changing frequency, earlier onset, and results of therapy. Rev. Infect. Dis. 7:646-655. [DOI] [PubMed] [Google Scholar]
- 15.Kao, A. S., M. E. Brandt, W. R. Pruitt, L. A. Conn, B. A. Perkins, D. S. Stephens, W. S. Baughman, A. L. Reingold, G. A. Rothrock, M. A. Pfaller, R. W. Pinner, and R. A. Hajjeh. 1999. The epidemiology of candidemia in two United States cities: results of a population-based active surveillance. Clin. Infect. Dis. 29:1164-1170. [DOI] [PubMed] [Google Scholar]
- 16.Karlowsky, J. A., G. G. Zhanel, K. A. Klym, D. J. Hoban, and A. M. Kabani. 1997. Candidemia in a Canadian tertiary care hospital from 1976 to 1996. Diagn. Microbiol. Infect. Dis. 28:5-9. [DOI] [PubMed] [Google Scholar]
- 17.Kawakami, S., Y. Ono, Y. Miyazawa, and H. Yamaguchi. 1998. Survey of fungemia cases during the past seventeen years at Teikyo University Hospital. Kansenshogaku Zasshi 72(2):105-113. [DOI] [PubMed] [Google Scholar]
- 18.Kovacicova, G., Y. Krupova, M. Lovaszova, A. Roidova, J. Trupl, A. Liskova, J. Hanzen, P. Milosovic, M. Lamosova, L. Macekova, Z. Szovenjova, A. Purgelova, T. Obertik, J. Bille, and V. Krcmery. 2000. Antifungal susceptibility of 262 bloodstream yeast isolates from a mixed cancer and non-cancer patient population: is there a correlation between in-vitro resistance and the outcome of fungemia? J. Infect. Chemother. 6:216-221. [DOI] [PubMed] [Google Scholar]
- 19.Kovacicova, G., J. Hanzen, M. Pisarcikova, D. Sejnova, J. Horn, R. Babela, I. Svetlansky, M. Lovaszova, M. Gogova, and V. Krcmery. 2001. Nosocomial fungemia due to amphotericin B-resistant Candida spp. in three pediatric patients after previous neurosurgery for brain tumors. J. Infect. Chemother. 7:45-48. [DOI] [PubMed] [Google Scholar]
- 20.Krcmery, V., S Grausova, M. Mraz, E. Pichova, and L. Jurga. 1999. Candida guilliermondii fungemia in cancer patients: report of three cases. J. Infect. Chemother. 5:58-59. [DOI] [PubMed] [Google Scholar]
- 21.Krcmery, V., and G. Kocacicova. 2000. Longitudinal 10-year prospective survey of fungaemia in Slovak Republic: trends in etiology in 310 episodes. Diagn. Microbiol. Infect. Dis. 36:7-11. [DOI] [PubMed] [Google Scholar]
- 22.Laverdiere, M., C. Restieri, and F. Habel. 2002. Evaluation of the in vitro activity of caspofungin against bloodstream isolates of Candida species from cancer patients: comparison of Etest and NCCLS reference methods. Int. J. Antimicrob. Agents 20:468-471. [DOI] [PubMed] [Google Scholar]
- 23.Lecciones, J. A., L. W. Lee, E. E. Navarro, F. G. Witebsky, D. Marshall, S. M. Steinberg, P. A. Pizzo, and T. J. Walsh. 1992. Vascular catheter-associated fungemia in patients with cancer: analysis of 155 episodes. Clin. Infect. Dis. 14:875-883. [DOI] [PubMed] [Google Scholar]
- 24.Luzzati, R., G. Amalfitano, L. Lazzaroni, F. Soldani, S. Bellino, M. Solbiati, M. C. Danzi, S. Vento, G. Todeschini, C. Vivenza, and E. Concia. 2000. Nosocomial candidemia in non-neutropenic patients at an Italian tertiary care hospital. Eur. J. Clin. Microbiol. Infect. Dis. 19:602-607.11014622 [Google Scholar]
- 25.Macphail, G. L. P., G. D. Taylor, M. Buchanan-Chell, C. Ross, S. Wilson, and A. Kureishi. 2002. Epidemiology, treatment and outcome of candidemia: a five-year review at three Canadian hospitals. Mycoses 45:141-145. [DOI] [PubMed] [Google Scholar]
- 26.Maki, D. G., C. E. Weise, and H. W. Sarafin. 1977. A semiquantitative culture method for identifying intravenous catheter-related infection. N. Engl. J. Med. 296:1305-1309. [DOI] [PubMed] [Google Scholar]
- 27.Marco, F., C. Danes, M Almela, A. Jurado, J. Mensa, J. Puing de la Bellacasa, M. Espansa, J. A. Martinez, and M. T. Jimenez de Anta. 2003. Trends in frequency and in vitro susceptibilities to antifungal agents, including voriconazole and anidulafungin, of Candida bloodstream isolates. Results from a six-year study (1996-2001). Diagn. Microbiol. Infect. Dis. 46:259-264. [DOI] [PubMed] [Google Scholar]
- 28.Mardani, M., H. A. Hanna, E. Girgawy, and I. Raad. 2000. Nosocomial Candida guilliermondii fungemia in cancer patients. Infect. Control Hosp. Epidemiol. 21:336-337. [DOI] [PubMed] [Google Scholar]
- 29.Martin, D., F. Persat, M. A. Piens, and S. Picot. 2005. Candida species distribution in bloodstream cultures in Lyon, France, 1998-2001. Eur. J. Clin. Microbiol. Infect. Dis. 24:329-333. [DOI] [PubMed] [Google Scholar]
- 30.Mathews, M. S., P. R. Samuel, and M. Suresh. 2001. Emergence of Candida tropicalis as the major cause of fungemia in India. Mycoses 44:278-280. [PubMed] [Google Scholar]
- 31.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard. NCCLS document M27-A2. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 32.Nguyen, M. H, J. E. Peacock, D. C. Tanner, A. J. Morris, M. L. Nguyen, D. R. Snydman, M. M. Wagener, and V. L. Yu. 1995. Therapeutic approaches in patients with candidemia. Arch. Intern. Med. 155:2429-2435. [PubMed] [Google Scholar]
- 33.O'Connell, C. J., A. V. Cherry, and J. G. Zoll. 1973. Osteomyelitis of cervical spine: C. guilliermondii. Ann. Intern. Med. 79:748. [DOI] [PubMed] [Google Scholar]
- 34.Pappas, P. G., J. H. Rex, J. Lee, R. J. Hamill, R. A. Larsen, W. Powderly, C. A. Kauffman, N. Hyslop, J. E. Mangino, S. Chapman, H. W. Horowitz, J. E. Edwards, and W. E. Dismukes. 2003. A prospective observational study of candidemia: epidemiology, therapy and influences on mortality in hospitalized adult and pediatric patients. Clin. Infect. Dis. 37:634-643. [DOI] [PubMed] [Google Scholar]
- 35.Pfaller, M. A., R. N. Jones, G. V. Doern, H. S. Sader, R. J. Hollis, and S. A. Messer. 1998. International surveillance of blood stream infections due to Candida species: frequency of occurrence and antifungal susceptibility of isolates collected in 1997 in the United States, Canada, and South America for the SENTRY program. J. Clin. Microbiol. 36:1886-1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pfaller, M. A., S. A. Messer, A. Houston, M. S. Rangel-Frausto, T. Wiblin, H. M. Blumberg, J. E. Edwards, W. Jarvis, M. A. Martin, H. C. Neu, L. Saiman, J. E. Patterson, J. C. Dibb, M. C. Roldan, M. G. Rinaldi, and R. P. Wenzel. 1998. National epidemiology of mycoses survey: a multicenter study of strain variation and antifungal susceptibility among isolates of Candida species. Diagn. Microbiol. Infect. Dis. 31:289-296. [DOI] [PubMed] [Google Scholar]
- 37.Pfaller, M. A., R. N. Jones, S. A Messer, M. B. Edmond, and R. P. Wenzel. 1998. National surveillance of nosocomial blood stream infection due to species of Candida other than Candida albicans: frequency of occurrence and antifungal susceptibility in the SCOPE program. Diagn. Microbiol. Infect. Dis. 30:121-129. [DOI] [PubMed] [Google Scholar]
- 38.Pfaller, M. A., R. N. Jones, G. V. Doern, A. C. Fluit, J. Verhoef, H. S. Sader, S. A. Messer, A. Houston, S. Coffman, and R. J. Hollis. 1999. International surveillance of blood stream infections in the European SENTRY program: species distribution and antifungal susceptibility including the investigational triazole and echinocandin agents. Diagn. Microbiol. Infect. Dis. 35:19-25. [DOI] [PubMed] [Google Scholar]
- 39.Pfaller, M. A., S. A. Messer, K. Mills, A. Bolmstrom, and R. N. Jones. 2001. Evaluation of Etest method for determining caspofungin (MK-0991) susceptibilities of 726 clinical isolates of Candida species. J. Clin. Microbiol. 39:4387-4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pfaller, M. A., S. A. Messer, R. J. Hollis, R. N. Jones, and D. J. Diekema. 2002. In vitro activities of ravuconazole and voriconazole compared with those of four approved systemic antifungal agents against 6,970 clinical isolates of Candida spp. Antimicrob Agents Chemother. 46:1723-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pfaller, M. A., and D. J. Diekema. 2002. Role of sentinel surveillance of candidemia: trends in species distribution and antifungal susceptibility. J. Clin. Microbiol. 40:3551-3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pfaller, M. A., D. J. Diekema, R. N. Jones, S. A. Messer, and R. J. Hollis. 2002. Trends in antifungal susceptibility of Candida spp. isolated from pediatric and adult patients with bloodstream infections: SENTRY antimicrobial surveillance program, 1997 to 2000. J. Clin. Microbiol. 40:852-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pfaller, M. A., D. J. Diekema, S. A. Messer, L. Boyken, R. J. Hollis, and R. N. Jones. 2003. In vitro activities of voriconazole, posaconazole, and four licensed systemic antifungal agents against Candida species infrequently isolated from blood. J. Clin. Microbiol. 41:78-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pfaller, M. A., D. J. Diekema, S. A. Messer, R. J. Hollis, and R. N. Jones. 2003. In vitro activities of caspofungin compared with those of fluconazole and itraconazole against 3,959 clinical isolates of Candida spp., including 157 fluconazole-resistant isolates. Antimicrob. Agents Chemother. 47:1068-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pfaller, M. A., D. J. Diekema, S. A. Messer, L. Boyken, R. J. Hollis, and R. N. Jones. 2004. In vitro susceptibilities of rare Candida bloodstream isolates to ravuconazole and three comparative antifungal agents. Diagn. Microbiol. Infect. Dis. 48:101-105. [DOI] [PubMed] [Google Scholar]
- 46.Pfaller, M. A., L. Boyken, S. A Messer, S. Tendolkar, R. J. Hollis, and D. J. Diekema. 2005. Comparison of results of voriconazole disk diffusion testing for Candida species with results from a central reference laboratory in the ARTEMIS global antifungal surveillance program. J. Clin. Microbiol. 43:5208-5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pfaller, M. A., L. Boyken, R. J. Hollis, S. A. Messer, S. Tendolkar, and D. J. Diekema. 2005. In vitro activities of anidulafungin against more than 2,500 clinical isolates of Candida spp., including 315 isolates resistant to fluconazole. J. Clin. Microbiol. 43:5425-5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Poikonen, E., O. Lyytikainen, V. J. Anttila, and P. Ruutu. 2003. Candidemia in Finland, 1995-1999. Emerg. Infect. Dis. 9:985-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Powderly, W., G. Kobayashi, G. Herzig, and G. Medoff. 1988. Amphotericin B-resistant yeast infection in severely immunocompromised patients. Am. J. Med. 84:826-832. [DOI] [PubMed] [Google Scholar]
- 50.Pujol, C., S. Joly, S. R. Lockhart, S. Noel, M. Tibayrenc, and D. R. Soll. 1997. Parity among the randomly amplified polymorphic DNA method, multilocus enzyme electrophoresis, and Southern blot hybridization with the moderately repetitive DNA probe Ca3 for fingerprinting Candida albicans. J. Clin. Microbiol. 35:2348-2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raad, P. G., H. Hanna, M. Boktour, E. Girgawy, H. Danawi, M. Mardani, D. Kontoyiannis, R. Darouiche, R. Hachem, and G. P. Bodey. 2004. Management of central venous catheters in patients with cancer and candidemia. Clin. Infect. Dis. 38:1119-1127. [DOI] [PubMed] [Google Scholar]
- 52.Rennert, G., H. S. Rennert, S. Pitlik, R. Finkelstein, and R. Kitzes-Cohen. 2000. Epidemiology of candidemia. A nationwide survey in Israel. Infection 28:26-29. [DOI] [PubMed] [Google Scholar]
- 53.Rex, J. H., J. E. Bennett, A. M. Sugar, P. G. Pappas, C. M. van del Horst, J. E. Edwards, R. G. Washburn, W. M. Scheld, A. W. Karchmer, A. P. Dine, M. J. Levenstein, and C. D. Webb. 1994. A randomized trial comparing fluconazole with amphotericin B for the treatment of candidemia in patients without neutropenia. N. Engl. J. Med. 331:1325-1330. [DOI] [PubMed] [Google Scholar]
- 54.Rex, J. H., P. G. Pappas, A. W. Karchmer, J. Sobel, J. E. Edwards, S. Hadley, C. Brass, J. A. Vazquez, S. W. Chapman, H. W. Horowitz, M. Zervos, D. McKinsey, J. Lee, T. Babinchak, R. W. Bradsher, J. D. Cleary, D. M Cohen, L. Danziger, M. Goldman, J. Goodman, E. Hilton, N. E. Hyslop, D. H. Kett, J. Lutz, R. H. Rubin, W. M. Scheld, M. Schuster, B. Simmons, D. K. Stein, R. G. Washburn, L. Mautner, T. C. Chu, H. Panzer, R. B. Rosenstein, and J. Booth. 2003. A randomized and blinded multicenter trial of high-dose fluconazole plus placebo versus fluconazole plus amphotericin B as therapy for candidemia and its consequences in nonneutropenic subjects. Clin. Infect. Dis. 36:1221-1228. [DOI] [PubMed] [Google Scholar]
- 55.Richet, H., P. Roux, C. Des Champs, Y. Esnault, and A. Andremont. 2002. Candidemia in French hospitals: incidence rates and characteristics. Clin. Microbiol. Infect. 8:405-412. [DOI] [PubMed] [Google Scholar]
- 56.Rippon, J. W. 1982. Candidiasis and the pathogenic yeasts, p. 565-594. In J. W. Rippon (ed.), Medical mycology: the pathogenic fungi and the pathogenic actinomycetes. W. B Saunders Co., Philadelphia, Pa.
- 57.Samaranayake, Y. H., L. P. Samaranayake, E. H. N. Pow, V. T. Beena, and K. W. S. Yeung. 2001. Antifungal effects of lysozyme and lactoferrin against genetically similar, sequential Candida albicans isolates from a human immunodeficiency virus-infected southern Chinese cohort. J. Clin. Microbiol. 39:3296-3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scherer, S., and D. A. Stevens. 1987. Application of DNA typing methods to epidemiology and taxonomy of Candida species. J. Clin. Microbiol. 25:675-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.St-Germain, G., M. Laverdière, R. Pelletier, A. M. Bourgault, M. Libman, C. Lemieux, and G. Noel. 2001. Prevalence of antifungal susceptibility of 442 Candida isolates from blood and other normally sterile sites: results of a 2-year (1996 to 1998) multicenter surveillance study in Quebec, Canada. J. Clin. Microbiol. 39:949-953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tietz, H. J., V. Czaika, and W. Sterry. 1999. Case report: osteomyelitis caused by high resistant Candida guilliermondii. Mycoses 42:577-580. [DOI] [PubMed] [Google Scholar]
- 61.Tortorano, A. M., E. Biraghi, A. Astolfi, C. Ossi, M. Tejada, C. Farina, S. Perin, C. Bonaccorso, C. capanna, A. Raballo, and A. Grossi. 2002. European Confederation of Medical Mycology (ECMM) prospective survey of candidaemia: report from one Italian region. J. Hosp. Infect. 51:297-304. [DOI] [PubMed] [Google Scholar]
- 62.Trick, W. E., S. K. Fridkin, J. R. Edwards, R. A. Hajjeh, and R. P. Gaynes. 2002. Secular trend of hospital-acquired candidemia among intensive care unit patients in the United States during 1989-1999. Clin. Infect. Dis. 35:627-630. [DOI] [PubMed] [Google Scholar]
- 63.Vazquez, J. A., T. Lundstrom, L. Dembry, P. Chandrasekar, D. Boikov, M. B. Parri, and M. J. Zervos. 1995. Invasive Candida guilliermondii infection: in vitro susceptibility studies and molecular analysis. Bone Marrow Transplant. 16:849-853. [PubMed] [Google Scholar]
- 64.Viscoli, C., C. Girmenia, A. Marinus, L. Collette, P. Martino, B. Vandercam, C. Doyen, B. Lebeau, D. Spence, V. Krcmery, B. de Pauw, and F. Meunier. 1999. Candidemia in cancer patients: a prospective, multicenter surveillance study by the Invasive Fungal Infection Group (IFIG) of the European Organization for Research and Treatment of Cancer (EORTC). Clin. Infect. Dis. 28:1071-1079. [DOI] [PubMed] [Google Scholar]
- 65.Voss, A., J. A. J. W. Kluytmans, J. G. M. Koeleman, L. Spanjaard, C. M. J. E. Vandenbroucke-Grauls, H. A. Verbrugh, M. C. Vos, A. Y. L. Weersink, J. A. A. Hoogkamp-Korstanje, and J. F. G. M. Meis. 1996. Occurrence of yeast bloodstream infections between 1997 and 1995 in five Dutch university hospitals. Eur. J. Clin. Microbiol. Infect. Dis. 15:909-912. [DOI] [PubMed] [Google Scholar]
- 66.Weems, J. J., Jr. 1992. Candida parapsilosis: epidemiology, pathogenicity, clinical manifestations, and antimicrobial susceptibility. Clin. Infect. Dis. 14:756-766. [DOI] [PubMed] [Google Scholar]
- 67.Wingard, J. R. 1995. Importance of Candida species other than C. albicans as pathogens in oncology patients. Clin. Infect. Dis. 20:115-125. [DOI] [PubMed] [Google Scholar]
- 68.Yagupsky, P., R. Dagan, M. Chipman, A. Goldschmied-Reouven, E. Zmora, and M. Karplus. 1991. Pseudooutbreak of Candida guilliermondii fungemia in a neonatal intensive care unit. Pediatr. Infect. Dis. J. 10:928-932. [DOI] [PubMed] [Google Scholar]
- 69.Yamamura, D. L. R., C. Rotstein, L. E. Nicolle, and S. Ioannous. 1999. Candidemia at selected Canadian sites: results from the Fungal Disease Registry, 1992-1994. Can. Med. Ass. J. 160:493-499. [PMC free article] [PubMed] [Google Scholar]