Abstract
The authors developed a new, simple, and reliable PCR/restriction fragment length polymorphism technique, using amplification of the hyphal wall protein 1 gene of Candida albicans and its gene homologue in Candida dubliniensis, to differentiate the two species of Candida. Performed with a new primer set, CRR-f/CRR-r, PCR produced two different fragments: one of 1,180 bp for C. albicans, and one of 930 bp for C. dubliniensis.
In 1995, Sullivan et al. (14) described for the first time a new species of Candida called Candida dubliniensis. Like Candida albicans, C. dubliniensis isolates produce germ tubes and chlamydospores and cannot be differentiated using conventional methods (9, 12, 13). Because phenotypic methods may not unequivocally differentiate C. albicans from C. dubliniensis, more-reliable tests based on molecular techniques such as specific PCR are used for discriminating between these two Candida species (2, 4, 5, 8).
Hyphal wall protein 1 (HWP1) is a gene that is required for virulence in systemic candidiasis (10). This gene encodes a surface protein that has been demonstrated to serve as a substrate for mammalian transglutaminase, which cross-links C. albicans to epithelial cells (10). An HWP1 homologue is also present in C. dubliniensis, as shown in the GenBank database. We decided to compare the nucleotide sequence of the HWP1 gene of C. albicans (GenBank accession number U64206) and its homologue in C. dubliniensis (GenBank accession number AJ632273) to differentiate between these two organisms.
Twenty strains of C. albicans were obtained from various body sites of patients hospitalized at the OORR of Reggio Calabria, Italy. A total of 16 C. dubliniensis strains from the oral cavities of human immunodeficiency virus-infected individuals came from different geographic locations. Three strains of C. dubliniensis (V3, V4, and V5) were obtained from the laboratory of José Ponton of the Universidad del Paìs Vasco, Bilbao, Spain; four strains (CD33, CD36, CD519, and CAN6) were kind gifts from Derek J. Sullivan of the Dublin Dental School and Hospital, University of Dublin, Ireland; six strains (05-87 h, 05-110 h, 05-111 h, 05-112 h, 05-131 h, and 05-139 h) were obtained from stock cultures of V. Vidotto of the Department of Medical and Surgical Sciences, University of Turin, Italy; and three strains (MAL CD1, MAL CD2, and MAL CD3) were isolated from patients hospitalized at the OORR, Reggio Calabria, Italy. C. albicans ATCC 10231 and C. dubliniensis CBS 7987 were used as reference strains. The identity of all the strains was determined by the ID 32C system (bioMérieux, Marcy l'Etoile, France) and other phenotypic (1, 3, 7, 11, 15) and molecular methods (5, 8).
The Vector NTI program (version 9.0.0; Invitrogen, San Giuliano Milanese, Italy) was used for gene alignment and primer design. Using the new primers, we calculated the lengths of the resulting PCR products as 1,180 bp for C. albicans and 930 bp for C. dubliniensis. The 1,180-bp fragment of C. albicans contains a restriction site specific for BamHI, a restriction enzyme which cuts the C. albicans PCR product into two fragments of the expected lengths of 793 and 387 bp. BamHI does not digest the 930-bp PCR product of C. dubliniensis.
DNA was extracted from yeast isolates as described by Hoskins (6), with the following small modifications made to enhance the speed of growth of the yeast strains: the yeast cells were cultured in 10 ml of yeast extract-peptone-dextrose broth and incubated overnight at 37°C under shaking conditions (C25 incubator shaker; New Brunswick Scientific, Edison, N.J.).
The primers used for PCR were CRR-f 5′-GTTTTTGCAACTTCTCTTTGTA-3′ and CRR-r 5′-ACAGTTGTATCATGTTCAGT-3′. The PCR mixture (total volume, 100 μl) contained 3 μl of genomic DNA template (2 μg ml−1), 0.2 mM (each) deoxynucleoside triphosphate, 100 mM (each) primer, 5 U of EuroTaq polymerase (Euroclone, Pero-Milan, Italy), 1× reaction buffer, and 1.5 mM MgCl2. Amplification was performed after denaturation at 95°C for 5 min followed by 34 cycles of denaturation at 94°C for 45 s, primer annealing at 50°C for 60 s, and an extension at 72°C for 90 s, followed by a final extension at 72°C for 10 min in a GeneAmp PCR 2400 system (Perkin Elmer, Monza-Milan, Italy).
PCR products were separated on a 1.3% (wt/vol) agarose gel, stained with ethidium bromide, and compared to the DNA size marker (2-Log DNA Ladder [0.1-10.0 kb]; BioLabs, Pero-Milan, Italy). The PCR products were digested for 3 h at 37°C with BamHI (MBI Fermentas, St. Leon-Rot, Germany) (20 U per 10 μl of PCR product). The digested products were run on a 1.3% (wt/vol) agarose gel and analyzed using a transilluminator (Foto/PrepI; Fotodyne-Celbio, Milan, Italy). PCR and restriction fragment length polymorphism (RFLP) were repeated three times.
All examined isolates produced chlamydospores on cornmeal agar with 1% Tween 80 and germ tubes in bovine serum (3 h at 37°C). Twenty clinical isolates and C. albicans ATCC 10231 showed a carbohydrate assimilation profile that is typical of C. albicans, whereas 7 of 17 (41.2%) C. dubliniensis strains identified by ID 32C (version 2.0 database) did not correspond to any C. dubliniensis profiles in the database. Four strains showed an identification profile that matched that of C. sake (7142-7100-15 and 7342-7100-15), and three strains showed an unacceptable profile (7145-3004-15, 7147-1006-15, and 7343-7000-15). Excellent identification (99.9%) results were obtained for 10 C. dubliniensis isolates: 6 strains showed a 7042-1000-15 profile, 3 strains a 7142-1000-15 profile, and 1 strain a 7042-1000-11 profile. Further phenotypic and molecular identification results are shown in Table 1.
TABLE 1.
Comparison of phenotypic and molecular methods to differentiate C. albicans from C. dubliniensis
| Species and strain | Origin | Length of PCR product (bp) | Length of RLFP product fragments (bp) | Appearance of colony on Pal's agar | Color of colony on TTC agarc |
|---|---|---|---|---|---|
| C. albicans ATCC 10231a | Reference straina | 1,180 | 793, 387 | Smooth | Pale pink to whitish |
| C. albicans L 30390 | Ascitic liquid | 1,180 | 793, 387 | Smooth | Pale pink to whitish |
| C. albicans L 18907 | Ascitic liquid | 1,180 | 793, 387 | Smooth | Pale pink to whitish |
| C. albicans T 215123 | Oral swab | 1,180 | 793, 387 | Smooth | Pale pink to whitish |
| C. albicans T 25780 | Oral swab | 1,180 | 793, 387 | Smooth | Pale pink to whitish |
| C. albicans T 30378 | Oral swab | 1,180 | 793, 387 | Smooth | Not grown |
| C. albicans T 215140 | Oral swab | 1,180 | 793, 387 | Smooth | Pale pink to whitish |
| C. albicans T 25781 | Oral swab | 1,180 | 793, 387 | Smooth | Pale pink to whitish |
| C. albicans T-V1 | Oral swab | 1,180 | 793, 387 | Smooth | Pale pink to whitish |
| C. albicans TF 29361 | Wound swab | 1,180 | 793, 387 | Hyphal fringe | Pale pink to whitish |
| C. albicans TR 29562 | Rectal swab | 1,180 | 793, 387 | Smooth | Pale pink to whitish |
| C. albicans E 18894 | Sputum | 1,180 | 793, 387 | Smooth | Pale pink to whitish |
| C. albicans E 30384 | Sputum | 1,180 | 793, 387 | Smooth | Pale pink to whitish |
| C. albicans E 29351 | Sputum | 1,180 | 793, 387 | Smooth | Not grown |
| C. albicans E 29459 | Sputum | 1,180 | 793, 387 | Smooth | Pale pink to whitish |
| C. albicans E 18522 | Sputum | 1,180 | 793, 387 | Hyphal fringe | Pale pink to whitish |
| C. albicans AG 29355 | Gastric aspirate | 1,180 | 793, 387 | Smooth | Pale pink to whitish |
| C. albicans A 29463 | Gastric aspirate | 1,180 | 793, 387 | Smooth | Pale pink to whitish |
| C. albicans MAL 012 | Vaginal swab | 1,180 | 793, 387 | Smooth | Pale pink to whitish |
| C. albicans MAL 139 | Vaginal swab | 1,180 | 793, 387 | Smooth | Pale pink to whitish |
| C. albicans S 28274 | Blood | 1,180 | 793, 387 | Smooth | Pale pink to whitish |
| C. dubliniensis CBS 7987b | Reference strainb | 930 | 930 | Hyphal fringe | Red to maroon |
| C. dubliniensis V3 | Oral swab | 930 | 930 | Hyphal fringe | Red to maroon |
| C. dubliniensis V4 | Oral swab | 930 | 930 | Hyphal fringe | Red to maroon |
| C. dubliniensis V5 | Oral swab | 930 | 930 | Hyphal fringe | Red to maroon |
| C. dubliniensis CD33 | Oral swab | 930 | 930 | Hyphal fringe | Red to maroon |
| C. dubliniensis CD36 | Oral swab | 930 | 930 | Hyphal fringe | Red to maroon |
| C. dubliniensis CD519 | Oral swab | 930 | 930 | Hyphal fringe | Red to maroon |
| C. dubliniensis CAN6 | Oral swab | 930 | 930 | Hyphal fringe | Red to maroon |
| C. dubliniensis 05-87h | Oral swab | 930 | 930 | Hyphal fringe | Red to maroon |
| C. dubliniensis 05-110h | Oral swab | 930 | 930 | Hyphal fringe | Red to maroon |
| C. dubliniensis 05-111h | Oral swab | 930 | 930 | Hyphal fringe | Red to maroon |
| C. dubliniensis 05-112h | Oral swab | 930 | 930 | Hyphal fringe | Red to maroon |
| C. dubliniensis 05-131h | Oral swab | 930 | 930 | Hyphal fringe | Red to maroon |
| C. dubliniensis 05-139h | Oral swab | 930 | 930 | Hyphal fringe | Pale pink |
| C. dubliniensis MAL CD1 | Oral swab | 930 | 930 | Hyphal fringe | Pale pink |
| C. dubliniensis MAL CD2 | Oral swab | 930 | 930 | Hyphal fringe | Red to maroon |
| C. dubliniensis MAL CD3 | Oral swab | 930 | 930 | Hyphal fringe | Red to maroon |
The reference strain C. albicans ATCC 10231 is from the American Type Culture Collection.
The reference strain C. dubliniensis CBS 7987 is from the Centraalbureau voor Schimmelcultures.
TTC, triphenyltetrazolium chloride (15).
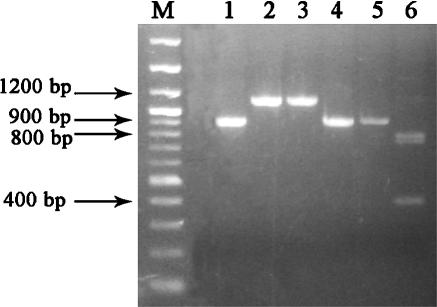
A fragment of 1,180 bp identical to that of the reference strain C. albicans ATCC 10231 was detected in all the C. albicans isolates, whereas all the C. dubliniensis strains produced a fragment of 930 bp. In addition, the 1,180-bp fragment of C. albicans produced two fragments (793 and 387 bp) after digestion with the BamHI restriction enzyme for 3 h at 37°C. No restriction fragments were recovered from the 930-bp PCR product of C. dubliniensis (Fig. 1). Unequivocal PCR and RFLP profiles were obtained in all the assayed strains.
FIG. 1.
Differentiation of C. dubliniensis and C. albicans by the PCR/RFLP method. Lanes 1 and 4, PCR products of C. dubliniensis CBS 7987; lanes 2 and 3, PCR products of C. albicans ATCC 10231; lane 5, PCR products of C. dubliniensis CBS 7987 digested with the BamHI restriction enzyme; lane 6, PCR products of C. albicans ATCC 10231 digested with the BamHI restriction enzyme; lane M, molecular size marker.
Several research groups have described rapid and reliable PCR for the identification of C. dubliniensis, with specific primers that bind a specific gene and amplify one fragment in one of the two species (2, 8). In addition, Donnelly et al. (4) have developed a discriminative PCR that uses C. dubliniensis-specific primers producing a fragment of 288 bp, but universal fungal primers are also used as an internal positive control. Our method uses only a pair of primers that produces different DNA fragments in the two Candida species. It excludes the possibility of false-negative results, avoiding misidentification due to possible experimentation errors, particularly when many strains have to be examined.
Graf et al. (5) have recently described a simple, rapid, and inexpensive PCR/RFLP method to discriminate between C. albicans and C. dubliniensis. After PCR this method uses digestion with the HpyF10VI restriction enzyme to discriminate between the two species of Candida. In our study, the RFLP method can be omitted because discrimination between C. albicans and C. dubliniensis is already made on analysis of the PCR products. RFLP analysis performed with the BamHI restriction enzyme could represent a useful tool for further confirmation in cases of doubtful PCR results.
To our knowledge, this is the first report that uses the HWP1 gene of C. albicans and its homologue from C. dubliniensis as targets for reliable and unequivocal discrimination between the two Candida species. The present study shows a new, relevant role for the HWP1 gene as an important target in rapid, unequivocal differentiation of C. dubliniensis and C. albicans.
Acknowledgments
We thank G. Bolignano and his staff of the OORR, Reggio Calabria, Italy; J. Ponton, Departemento de Microbiologia y Inmunologia Facultad de Medicina y Odontologia, Universidad del Pais Vasco, Bilbao, Spain; V. Vidotto, Department of Medical and Surgical Sciences, University of Turin, Italy; and D. J. Sullivan, Dublin Dental School and Hospital, University of Dublin, Ireland, for providing C. albicans and C. dubliniensis strains. The authors are also grateful to Derek J. Sullivan for useful comments on the manuscript.
REFERENCES
- 1.Al Mosaid, A., D. J. Sullivan, and D. C. Coleman. 2003. Differentiation of Candida dubliniensis from Candida albicans on Pal's Agar. J. Clin. Microbiol. 41:4787-4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biswas, S. K., K. Yokoyama, L. Wang, K. Nishimura, and M. Miyaji. 2001. Identification of Candida dubliniensis based on the specific amplification of mitochondrial cytochrome b gene. Nippon Ishinkin Gakkai Zasshi. 42:95-98. [DOI] [PubMed] [Google Scholar]
- 3.Boerlin, P., F. Boerlin-Petzold, C. Durussel, M. Addo, J. L. Pagani, J. P. Chave, and J. Bille. 1995. Cluster of oral atypical Candida albicans isolates in a group of human immunodeficiency virus-positive drug users. J. Clin. Microbiol. 33:1129-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donnelly, S. M., D. J. Sullivan, D. B. Shanley, and D. C. Coleman. 1999. Phylogenetic analysis and rapid identification of Candida dubliniensis based on analysis of ACT1 intron and exon sequences. Microbiology 145:1871-1882. [DOI] [PubMed] [Google Scholar]
- 5.Graf, B., A. Trost, J. Eucker, U. B. Göbel, and T. Adam. 2004. Rapid and simple differentation of C. dubliniensis from C. albicans. Diagn. Microbiol. Infect. Dis. 48:149-151. [DOI] [PubMed] [Google Scholar]
- 6.Hoskins, L. 16 October 1998. Quick yeast DNA prep: isolation of total DNA (genomic and plasmid). Hahn Laboratory [Online.] http://www.fhcrc.org/labs/hahn/methods/mol_bio_meth/yeast_quick_dna.html.
- 7.Kurtzman, C. P., and J. W. Fell. 1998. The yeasts, a taxonomy study, 4th ed., p. 476-479. Elsevier Science B.V., Amsterdam, The Netherlands.
- 8.Kurzai, O., W. J. Heinz, D. J. Sullivan, D. C. Coleman, M. Frosch, and F. A. Mühlschlegel. 1999. Rapid PCR test for discriminating between Candida albicans and Candida dubliniensis isolates using primers derived from the pH-regulated PHR1 and PHR2 genes of C. albicans. J. Clin. Microbiol. 37:1587-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pincus, D. H., D. C. Coleman, W. R. Pruitt, A. A. Padhye, I. F. Salkin, M. Geimer, A. Bassel, D. J. Sullivan, M. Clarke, and V. Hearn. 1999. Rapid identification of Candida dubliniensis with commercial yeast identification systems. J. Clin. Microbiol. 37:3533-3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Staab, J. F., S. D. Bradway, P. L. Fidel, and P. Sundstrom. 1999. Adhesive and mammalian transglutaminase substrate properties of Candida albicans Hwp1. Science 283:1535-1538. [DOI] [PubMed] [Google Scholar]
- 11.Staib, P., and J. Morschhäuser. 1999. Chlamydospore formation on Staib agar as species-specific characteristic of Candida dubliniensis. Mycoses 42:521-524. [DOI] [PubMed] [Google Scholar]
- 12.Sullivan, D., G. P. Moran, and D. C. Coleman. 2005. Candida dubliniensis: ten years on. FEMS Microbiol. Lett. 253:9-17. [DOI] [PubMed] [Google Scholar]
- 13.Sullivan, D., and D. Coleman. 1997. Candida dubliniensis: an emerging opportunistic pathogen. Curr. Top. Med. Mycol. 8:15-25. [PubMed] [Google Scholar]
- 14.Sullivan, D. J., T. J. Westerneng, K. A. Haynes, D. E. Bennett, and D. C. Coleman. 1995. Candida dubliniensis sp. nov: phenotypic and molecular characterization of a novel species associated with oral candidosis in HIV-infected individuals. Microbiology 141:1507-1521. [DOI] [PubMed] [Google Scholar]
- 15.Velegraki, A., and M. Logotheti. 1998. Presumptive identification of an emerging yeast pathogen, Candida dubliniensis (sp. nov.) reduces 2,3,5-triphenyltetrazolium chloride. FEMS Immunol. Med. Microbiol. 20:239-241. [DOI] [PubMed] [Google Scholar]