Abstract
Sporothrix schenckii causes sporotrichosis, a disease that most commonly presents as a subacute or chronic skin infection. An unusually high incidence of clinical cases of sporotrichosis occurred in the southwest of Western Australia over the last 5 years. Anecdotal accounts from patients implicated contact with hay prior to infection. Isolates of S. schenckii from hay and clinical cases were investigated by traditional phenotypic methods and pulsed-field gel electrophoresis (PFGE). The phenotypic evaluation separated S. schenckii from Ophiostoma spp. A DNA macrorestriction method using SfiI and NotI macrorestriction digestion by PFGE was developed to investigate the epidemiological connections. BioNumerics software was used to analyze the results. DNA macrorestriction digestion patterns for the recent Western Australian clinical isolates and four hay isolates were indistinguishable. Eastern state clinical isolates, national Quality Assurance Program isolates, and other environmental isolates gave different macrorestriction patterns. Clinical isolates from the southwest of Western Australia collected in the 1980s and 1990s were also characterized using PFGE. The patterns generated were indistinguishable from those of the recent clinical isolates. PFGE showed that the dominant strain of S. schenckii causing sporotrichosis in Western Australia is present in hay, has caused sporotrichosis for at least 15 years, and is a different strain from the strains found in other parts of Australia.
Between 2000 and 2004, 62 isolates of Sporothrix schenckii from 62 cases of sporotrichosis were processed at PathWest laboratories in Western Australia. Thirty-six of these cases were from the Busselton/Margaret River region in the southwest of Western Australia. These cases represented an increase compared to 1997 to 1999, when eight cases occurred, none of which came from the Busselton/Margaret River region. Telephone interviews by the Western Australia Department of Health with the patients infected between 2000 and 2004 established that many patients remembered contact with hay prior to the onset of sporotrichosis. In 2004, 50 environmental samples of hay were collected from the properties of six of the patients and from two commercial suppliers of hay.
Hay in Western Australia is often supplied by farming districts that have large amounts of rainfall and bountiful hay crops to areas with less rainfall and inadequate feed for cattle. Hay can be stored for many years before use. Recently, most cases of sporotrichosis have arisen in vineyard areas of the state, whereas in the 1980s and 1990s, most cases arose in the wheat-growing and grazing areas. With the increase in hobby farms and organic farming, hay or straw is often purchased as mulch to improve soil quality, to suppress weeds, and to feed semidomesticated farm animals.
S. schenckii is saprophytic to plants and decaying vegetation, existing as a mycelial mold form in nature at 26°C and as a parasitic yeast form in humans at 35°C to 37°C. No sexual stage has been observed, but it has been proposed that Ophiostoma stenoceras is the sexual or perfect stage of S. schenckii. Environmental isolates were classified as S. schenckii, Ophiostoma stenoceras, or Ophiostoma nigrocarpum.
Various molecular methods of typing S. schenckii, such as karyotyping (10), restriction fragment length polymorphism analysis (3, 9, 11), and random amplified polymorphic DNA analysis (8), have been used previously. Pulsed-field gel electrophoresis (PFGE) can be used to type some fungi (1, 7) and is regarded as the “gold standard” of molecular typing methods for investigations of many bacteria (12). The restriction enzyme SfiI is frequently used for typing of fungi by PFGE. The restriction enzyme NotI was tested because it is cheaper and easier to work with than SfiI and because it is an infrequent cutter. Thus, in conjunction with traditional identification, PFGE was tested on 70 clinical and environmental isolates, using both restriction enzymes.
One purpose of this study was to see if PFGE discriminated between different clinical strains of S. schenckii. For that purpose, clinical isolates obtained from other Australian culture collections were also tested. The main purpose of performing pulsed-field gel electrophoresis was to look for the environmental source of the cluster in order to find ways to reduce the number of clinical cases.
MATERIALS AND METHODS
The isolates included in the study were from the PathWest Mycology culture collection. Clinical isolates were from Western Australian patients with a diagnosis of sporotrichosis. Eastern State clinical isolates were provided by The Australian National Reference Laboratory in Medical Mycology (AMMRL) at Royal North Shore Hospital, NSW, Australia. RCPA Quality Assurance Program (QAP) strains were from dispatches 1993:3:5C, 1996:3:6A, 1999:8:8A, and 2002:2:7B (Table 1).
TABLE 1.
Strains used in this study
| Source | Isolate no. |
|---|---|
| Clinical strains from Western Australia | |
| 2000-2004 | 951, 966, 1054, 1074, 1084, 1094, 1117, 1169, 1192, 1221, 1222, 1228, 1238, 1300, 1311, 1343 |
| 1988-1999 | 148, 150, 248, 249, 425, 460, 538, 542, 547, 552, 590 |
| AMMRL strains from Eastern Australia | 1390 (15.13), 1391 (15.17), 1392 (15.18), 1393 (15.26), 1394 (15.32), 1395 (15.35) |
| QAP strains | 493 (1993:3:5C), 707 (1996:3:6A), 881 (1999:8:8A), 1051 (2002:2:7B) |
| Hay isolates | 1318 (Hay15), 1319 (Hay18), 1320 (Hay24), 1321 (Hay33#2), 1322 (Hay12), 1323 (Hay13), 1324 (Hay39#2), 1325 (Hay45), 1326 (Hay43), 1327 (Hay39#2), 1328 (Hay4), 1329 (Hay29), 1330 (Hay30), 1331 (Hay33#1), 1332 (Hay34), 1333 (Hay 46) |
Hay samples were collected by the SouthWest Population Unit, Department of Health, Government of Western Australia.
The environmental hay samples were processed in the following manner. One cubic centimeter of hay was coarsely chopped, diluted in 30 ml of sterile water supplemented with antibiotics (5,000 U/ml penicillin G and 1 mg/ml streptomycin), and shaken for 1 hour at room temperature. Volumes of 10 μl and 1 μl of this fluid were plated onto Mycosel plates (BBL, Becton Dickinson and Company) supplemented with gentamicin (final antibiotic concentrations: chloramphenicol, 0.05 g/liter; cycloheximide, 0.4 g/liter; and gentamicin, 5.3 g/liter) and incubated at 30°C for up to 10 days (6). All presumptive isolates had the consistent microscopic characteristics of oval conidia on small cylindrical denticles in a sympodial arrangement on lateral apically swollen conidiophores. The presence of perithecia was noted. Isolates were identified as S. schenckii, O. stenoceras, or O. nigrocarpum (5). Phenotypic characteristics associated with pathogenicity were noted. These included conidial shape, sleeves of dematiaceous conidia, melanin production, conversion to yeast phase at 35°C, and the ability to grow at 37°C. Isolates were divided into groups in a similar manner to that described by Dixon et al. (6).
Clinical isolates were obtained from swabs, skin scrapings, and tissue punch biopsies collected by general practitioners and dermatologists. Laboratory processing of these samples included direct microscopy with Parker's ink and potassium hydroxide, as well as periodic acid-Schiff staining. Samples were inoculated onto brain heart infusion agar supplemented with chloramphenicol, Mycosel slopes, and Sabouraud agar slopes. To confirm thermal dimorphism, duplicate slopes were inoculated and incubated at both 26°C and 35°C for up to 4 weeks. Colonies of S. schenckii were selected that were white and yeast-like on brain heart infusion agar at 35°C and glabrous on Mycosel, developing a wrinkled surface that became black with age, at 26°C. Microscopy of the mold form showing clusters of ovoid, denticulate conidia produced sympodially on short conidiophores confirmed the identity of S. schenckii (5).
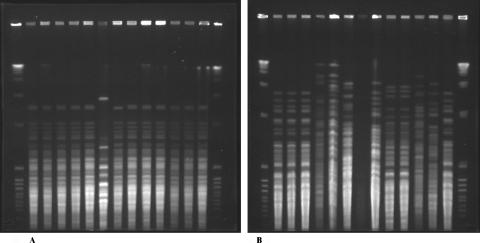
Pulsed-field gel electrophoresis was performed by using a modification of the method of Tateishi et al. (11). Isolates were grown in brain heart infusion broth at either 37°C or 28°C with shaking for 5 days and then centrifuged and washed twice with 50 mM EDTA (BDH, Poole, England), pH 7.5. After 2 h at −80°C, the pellets were heat shocked at 60°C for 2 min and recentrifuged. Pellets (approximately 50 μl) were suspended in 140 μl of 50 mM EDTA and 120 μl of 4-mg/ml lyticase (Sigma, Castle Hill, Australia). An equal amount of 1% agarose (SeaKem Gold; Cambrex Bio Science) was added to make four plugs. Plugs were added to 1 ml of TE buffer (500 mM EDTA, 100 mM Tris-HCl [Sigma], pH 7.5) containing a further 10 μl of lyticase (Sigma) and incubated in a shaking water bath overnight at 37°C. The TE buffer was then removed, and the plugs were incubated at 52°C for 48 h with shaking in 1 ml of digestion buffer (1% lauryl sarcosine [Sigma] in 500 mM EDTA, pH 8) containing 80 μl of 20-mg/ml proteinase K (Promega, Madison, Wis.). After four 1-h washes at 50°C in 50 mM EDTA and three washes in 100 mM Tris-HCl with 5 mM MgCl2, macrorestriction cuts were performed with 50 units/plug of NotI and 40 units/plug of SfiI (Promega). Pulse times were 1 to 8 seconds for NotI digests and 1 to 10 seconds for SfiI digests (Fig. 1). Gels (Seakem Gold; Cambrex Bio Science) were run for 20 h at 200 V on a CHEF DRIII apparatus (Bio-Rad). The size markers used were a lambda ladder (Bio-Rad) and an 8- to 48-kilobase ladder (Bio-Rad).
FIG. 1.
(A) SfiI-generated macrorestriction patterns of hay isolates (lanes 7 and 11) and recent and past (lane 2) clinical isolates of S. schenckii. Lanes 1 and 15, combined 48.5- to 970-kbp lambda ladder and 8- to 48-kbp ladder; lane 2, isolate 249; lane 3, isolate 966; lane 4, isolate 1074; lane 5, isolate 1192; lane 6, isolate 1343; lane 7, isolate 1331; lane 8, isolate 1169; lane 9, isolate 1201; lane 10, isolate 1228; lane 11, isolate 1321; lane 12, isolate 1094; lane 13, isolate 1300; lane 14, isolate 1311. (B) NotI-generated macrorestriction patterns of Western Australian clinical isolates (lanes 2, 3, and 14), hay isolates (lanes 4 to 7), and Eastern Australian isolates (lanes 8 to 13). Lanes 1 and 15, combined 48.5- to 970-kbp lambda ladder and 8- to 48-kbp ladder; lane 2, isolate 1217; lane 3, isolate 1222; lane 4, isolate 1318; lane 5, isolate 1326; lane 6, isolate 1328; lane 7, isolate 1330; lane 8, untypable isolate 1390; lane 9, isolate 1391; lane 10, isolate 1392; lane 11, isolate 1393; lane 12, isolate 1394; lane 13, isolate 1395; lane 14, isolate 1300.
To look for epidemiological links, all of the related fungal isolates from hay and 38 of the 62 clinical isolates were tested by PFGE. The infrequently cutting restriction enzymes NotI and SfiI (2) were used. Isolates from the Eastern States of Australia and quality assurance strains were run to exclude the possibility that S. schenckii only produced one macrorestriction pattern. To determine if the macrorestriction pattern from recent Western Australian isolates belonged to a new strain of S. schenckii, isolates from patients from Western Australia in the 1980s and 1990s were also tested by PFGE.
The results were analyzed using the BioNumerics software package (Applied Maths, Kortrijk, Belgium). The dendrograms were calculated by using the unweighted-pair group method using average linkages and either the categorical or Dice coefficient.
RESULTS
Clinical isolates were identified as S. schenckii. Environmental isolates were identified as S. schenckii, O. stenoceras, and O. nigrocarpum on the basis of their phenotypic characteristics. S. schenckii isolates that exhibited the same characteristics as the clinical S. schenckii isolates were obtained from hay at three of the six patients' farms. Each of these patients had purchased hay from the same supplier. S. schenckii was also isolated from the hay collected from that supplier. S. schenckii exhibiting other characteristics and Ophiostoma spp. were isolated from 30% of the remaining hay samples. Hay bales were reported as being visually moldy. This agrees with the observations made during the outbreak of sporotrichosis linked to hay in Queensland in 1995 (2).
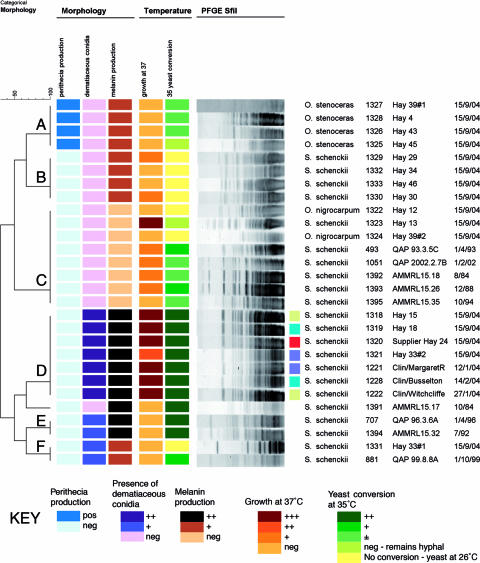
Morphological characteristics divided the clinical and environmental isolates into six groups, A, B, C, D, E, and F (Fig. 2). Five groups contained S. schenckii isolates. Four of the environmental isolates were in group D, along with all the Western Australian clinical isolates. Groups A and B were made up of only environmental isolates, and groups C, D, and F contained the Eastern Australian clinical isolates and some environmental isolates.
FIG. 2.
Phenotypic groups formed and their associated macrorestriction patterns. The small colored squares link clinical isolates with the hay isolates from the patients' properties. The red square shows the isolate from the supplier.
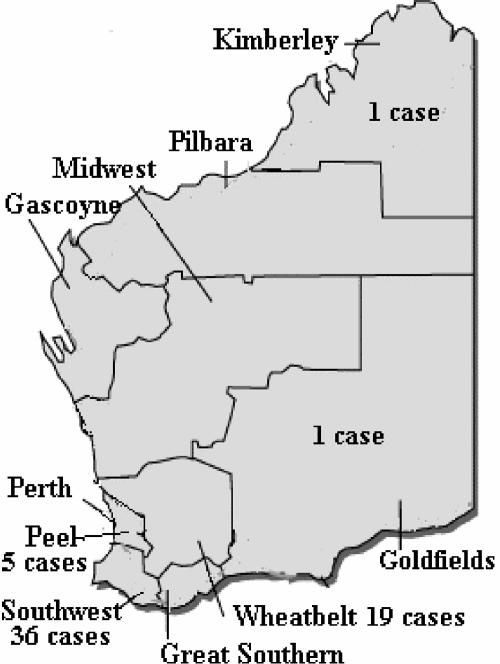
Indistinguishable PFGE patterns were generated from all but one (1169) of the Western Australian clinical isolates and four of the S. schenckii isolates from hay (1318, 1319, 1320, and 1321). This linked three clinical cases of sporotrichosis with S. schenckii isolated from hay on the patients' own properties. The fourth hay isolate with this PFGE pattern was collected from hay from one of the two hay suppliers (1320). The clinical cases were spread over a large area of farming districts (Fig. 3). The Eastern State clinical isolates had a variety of different macrorestriction patterns with both restriction enzymes (Fig. 2). The restriction enzymes were successful in discriminating between the clinical isolates from Western Australia and the clinical isolates and QAP strains from the Eastern States of Australia. Other hay sample isolates also generated different macrorestriction patterns from those for the clinical isolates of S. schenckii.
FIG. 3.
Areas of Western Australia affected by the outbreak of sporotrichosis.
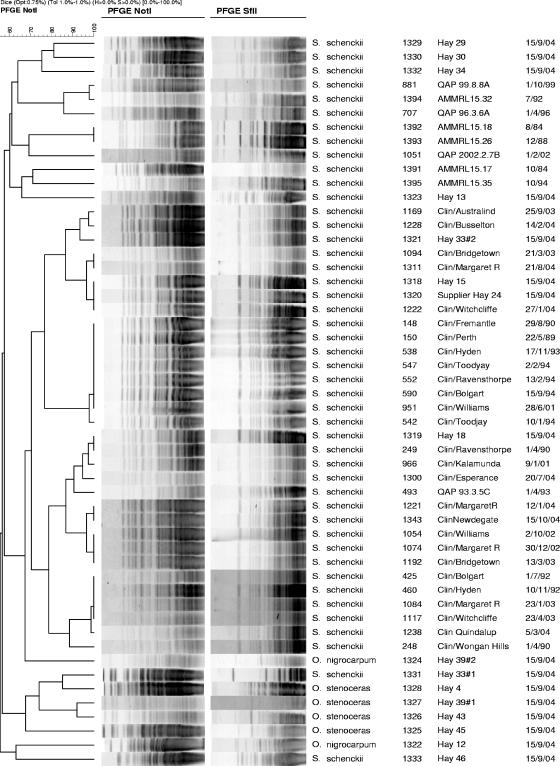
The PFGE patterns generated from the clinical samples collected in the 1980s and 1990s were indistinguishable from those of the recent Western Australian clinical isolates (Fig. 4).
FIG. 4.
PFGE patterns for Eastern State clinical isolates (AMMRL), environmental isolates (hay), and a selection of Western Australian clinical isolates (by date).
Dendrograms generated with BioNumerics software for the two enzymes, NotI and SfiI, gave almost identical groupings. The two enzymes also discriminated between other fungi, such as Ophiostoma spp. There were 5 different PFGE patterns among the Ophiostoma spp. and 12 different patterns for the S. schenckii isolates with either restriction enzyme. NotI distinguished between 22 and 26 bands, with a size range between 20 and 194 kbp, and SfiI distinguished between 11 and 20 bands, also with a size range between 20 and 194 kbp (Fig. 4). The PFGE results were reproducible, and only 1 of 70 isolates was nontypeable (1390) with either restriction enzyme, giving the methods a typability index of >98%.
DISCUSSION
PFGE and phenotypic tests successfully confirmed the anecdotal link between contact with hay and cases of sporotrichosis. The environmental strain isolated from the relevant hay samples had an indistinguishable PFGE pattern from that of the clinical isolates of S. schenckii that caused the increase in incidence of sporotrichosis. Our results indicate that this strain of S. schenckii has caused sporotrichosis in Western Australia since 1988. It appears that either the way the hay is handled or the appearance of this strain in the hay has led to the increase in this condition rather than the appearance of a new strain. Some of the patients from 2000 onwards linked their condition to the handling of hay. No data are available from patients regarding the probable cause of sporotrichosis before 2000. The wide area of rural properties that were affected appears to be due to the distribution of hay from one area over a large area of the southwest of Western Australia. The years 2000-2004 were, on average, drier than the preceding years of 1997-1999 (Bureau of Meteorology, Perth, Australia, personal communication). The fact that the strain of S. schenckii causing sporotrichosis was isolated from 4 of the 50 hay samples probably indicates that hay is frequently contaminated with this strain. It is possible that S. schenckii present in the hay multiplies during storage. Sphagnum moss caused a large outbreak of sporotrichosis in the United States in 1988 (6). Both hay and sphagnum moss can be stored for long periods of time.
Sporotrichosis is not a notifiable disease and does not require laboratory diagnosis for initiation of treatment. This disease is likely underreported, as there is no central laboratory collecting clinical isolates from other laboratories. The figures reported here are probably a fraction of the numbers involved in this outbreak.
PFGE appears to be able to separate different strains of the closely related species S. schenckii and O. stenoceras. It proved useful as an epidemiological tool with these fungal isolates. Besides the Western Australian clinical pattern, there were six other macrorestriction patterns among the S. schenckii environmental isolates from hay. None of these other patterns were seen among any of the clinical isolates. This could mean that there are differences between S. schenckii strains that can cause sporotrichosis and those that cannot. The nonclinical S. schenckii isolates exhibited different macrorestriction patterns, similar to the case in another study (3) that reported the restriction fragment length polymorphism profiles of a nonclinical group to be very heterogeneous.
Mariat (7) proposed that O. stenoceras is the perfect stage of S. schenckii. Suzuki et al. (9), however, concluded that because the different species had different banding patterns, O. stenoceras is not the perfect stage of S. schenckii. In this study, O. stenoceras and S. schenckii did not exhibit the same macrorestriction patterns with either restriction enzyme, even when isolated from the same hay bale.
DeBeer et al. (4) stated that O. nigrocarpum and O. stenoceras were more closely related to each other than to S. schenckii and that S. schenckii may be more than one species. Their tree divided the S. schenckii strains into two groups, clinical and environmental.
The work of Cooper et al. (3) concluded that the results of molecular typing corroborated the results of traditional phenotypic methods carried out during a sporotrichosis epidemic in the United States in 1988 (6). Our results showed that phenotypic methods would have established a link between cases of sporotrichosis and hay in Western Australia. Other Australian clinical isolates were not within the same phenotypic group as the Western Australian isolates. This is different from Dixon et al.'s group 1, which contained all clinical isolates (6). All of the Western Australian clinical isolates were in group D. Groups D and E correspond loosely to Dixon et al.'s group 1. Eastern Australian clinical isolates were in groups C, E, and F (Fig. 2).
Takeda et al. (10) and Mesa-Arango et al. (8) concluded that their restriction fragment length polymorphism and random amplified polymorphic DNA profiles of clinical S. schenckii isolates were related to geographical areas. The PFGE macrorestriction patterns observed here were also different for different areas of Australia. Similarly, Mesa-Arango et al. also found that their phenotypic groups aligned with geographical areas. This was also the case in this study, where the phenotypes of clinical S. schenckii isolates were different in Western Australia from those obtained from the Eastern States of Australia.
It appears that S. schenckii has a very stable genome. The Western Australian clinical isolates tested by PFGE stretched over a 16-year period and maintained the same banding pattern. Only 1 of the 45 clinical isolates from Western Australia (1169) showed a one-band difference in PFGE pattern, and then only with one of the restriction enzymes, NotI.
PFGE proved to be the most useful method in this study. It confirmed the link between contact with hay and the increase in incidence of sporotrichosis in Western Australia. As a result of this study, a warning is now issued with hay purchases. It is recommended that if the hay is to be handled, then a long-sleeved shirt and gloves are appropriate. There has been a marked decrease in the number of cases since this caution was first issued.
Acknowledgments
We thank Kerry Weeks at the Australian National Reference Laboratory in Medical Mycology (AMMRL) at Pacific Laboratory Medicine Services, Royal North Shore Hospital of Sydney, NSW, Australia, and Amanda Whittle of the South West Population Unit, Department of Health, Government of Western Australia. We also thank T. J. J. Inglis for proofreading the manuscript.
REFERENCES
- 1.Birren, B., and E. Lai. 1993. Pulsed field gel electrophoresis: a practical guide. Academic Press, San Diego, Calif.
- 2.Conias, S., and P. Wilson. 1998. Epidemic cutaneous sporotrichosis: report of 16 cases in Queensland due to mouldy hay. Aust. J. Dermatol. 39:34-37. [DOI] [PubMed] [Google Scholar]
- 3.Cooper, C. R., B. J. Breslin, D. M. Dixon, and I. F. Salkin. 1992. DNA typing of isolates associated with the 1988 sporotrichosis epidemic. J. Clin. Microbiol. 30:1631-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Beer, Z. W., T. C. Harrington, H. F. Vismer, B. D. Wingfield, and M. J. Wingfield. 2003. Phylogeny of the Ophiostoma stenoceras-Sporothrix schenckii complex. Mycologia 95:434-441. [PubMed] [Google Scholar]
- 5.De Hoog, G. S. 1974. The genera Blastobotrys, Sporothrix, Calcarisporium and Calcarisporiella gen. nov. Institute of the Royal Netherlands Academy of Sciences and Letters, Baarn, The Netherlands.
- 6.Dixon, D. M., I. F. Salkin, R. A. Duncan, N. J. Hurd, J. H. Haines, M. E. Kemna, and F. B. Coles. 1991. Isolation and characterization of Sporothrix schenckii from clinical and environmental sources associated with the largest U.S. epidemic of sporotrichosis. J. Clin. Microbiol. 29:1106-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mariat, F. 1971. Adaptation de Ceratocystis stenoceras (Robak) a la vie parasitaire chez l'animal. Etude de l'acquisition d'un pauvoir pathogene comparable a celui de Sporothrix schenckii. Sabouraudia 9:191-205. [PubMed] [Google Scholar]
- 8.Mesa-Arango, A. C., M. del Rocio Reyes-Montes, A. Perez-Mejia, H. Navarro-Barranco, V. Souza, G. Zuniga, and C. Toriello. 2002. Phenotyping and genotyping of Sporothrix schenckii isolates according to geographic origin and clinical form of sporotrichosis. J. Clin. Microbiol. 40:3004-3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suzuki, K., M. Kawasaki, and H. Ishizaki. 1988. Analysis of restriction profiles of mitochondrial DNA from Sporothrix schenckii and related fungi. Mycopathologia 103:147-151. [DOI] [PubMed] [Google Scholar]
- 10.Takeda, Y., M. Kawasaki, and H. Ishizaki. 1991. Phylogeny and molecular epidemiology of Sporothrix schenckii in Japan. Mycopathologia 116:9-14. [DOI] [PubMed] [Google Scholar]
- 11.Tateishi, T., S. Y. Murayama, F. Otsuka, and H. Yamaguchi. 1996. Karyotyping by PFGE of clinical isolates of Sporothrix schenckii. FEMS Immunol. Med. Microbiol. 13:147-154. [DOI] [PubMed] [Google Scholar]
- 12.Tenover, F. C., R. D. Arbeit, R. V. Goering, et al. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]