Abstract
Bartonella henselae, a worldwide fastidious bacterium, has a feline reservoir and is pathogenic for humans. However, the relationship between human and cat isolates of B. henselae, as well as its population dynamics and geographic heterogeneity, is not fully understood, in part because of the absence of appropriate typing methods. Multilocus sequence typing (MLST), the most discriminatory genotyping method for B. henselae, identified seven genotypes and suggested that human isolates arose from a limited number of cat isolates. Herein, we estimated the discriminatory power of multispacer typing (MST) by studying 126 B. henselae cat isolates from various areas of Europe, Asia, and the United States. We identified the nine most variable intergenic spacers conserved by both B. henselae and Bartonella quintana genomes. By comparing the sequences obtained from these nine spacers for each studied isolate, we identified 39 MST genotypes. The distribution of isolates into MST genotypes matched their phylogenetic organization into four clusters. MST showed that European and Asian isolates were different, in contrast with American isolates, but failed to identify pandemic strains. Our study demonstrated that MST is a powerful method for genotyping B. henselae at the strain level and may serve in studying the population dynamics of this bacterium and understanding the relationships between cat and human isolates. Finally, we provide a free-access MST-Rick online software program (http://ifr48.timone.univ-mrs.fr/MST_BHenselae/mst) that investigators may use to compare their own MST sequences to our database.
Bartonella henselae is a gram-negative, fastidious bacterium associated with cats. Its transmission among cats is mediated by the cat flea, Ctenocephalides felis (7). Infected cats may remain bacteremic for long periods, thus playing a major role as a reservoir for the bacterium (6, 24). Human infection occurs through cat scratches or bites (22) and presents as cat scratch disease (2), bacillary angiomatosis (23), peliosis hepatis (32), endocarditis (18), or a variety of other, less frequent manifestations (14).
Although criteria exist for classifying Bartonella isolates as new species (27), there is a need for a method able to reliably identify B. henselae at the strain level. Such a method would allow investigation of the relationships between cat and human isolates, the question of whether epidemic strains occur in cats, and the geographic heterogeneity of B. henselae isolates. Various methods have been proposed for typing Bartonella isolates (10, 12, 19, 20, 26, 29, 34). Of these, sequence-based methods have the advantages of being applicable to clinical or environmental specimens and producing reproducible and comparable results. On the basis of comparison of 16S rRNA gene sequences, B. henselae isolates were classified into two main genotypes, i.e., types I and II. This gene was considered a useful delineation among isolates because the two genotypes also exhibited different serotypes and possessed consistently distinguishable protein profiles (26). Sequences from the ftsZ (12), gltA (10), 35-kDa protein-encoding (26), groEL and pap31 (34) genes, and from the 16S-23S intergenic spacer (20), later permitted the identification of three, two, two, four, and six genotypes, respectively, that did not exactly match 16S rRNA gene types. To date, the most discriminatory typing method for B. henselae isolates is multilocus sequence typing (MLST) incorporating nine genes (21). This method distinguished seven genotypes among 37 human and cat isolates and suggested that lateral gene transfer occurs among B. henselae isolates (21). Although these investigators and others suggested that human infection is caused by a limited number of specific B. henselae genotypes (4, 10, 21), the discriminatory power of the genotyping methods that they used and the small number of B. henselae isolates that they studied were insufficient to allow any statistically significant conclusions to be drawn. Therefore, a genotyping tool with greater discriminatory power for genotyping B. henselae at the strain level is needed to investigate the diversity and population structure of this bacterium.
Recently, we applied a new genotyping method to Bartonella quintana, i.e., multispacer typing (MST) (13). This method allows genotyping of bacteria at the strain level. MST, initially developed for Yersinia pestis (11), was also applied with success to strains of other human pathogens, including Rickettsia conorii (15), Rickettsia prowazekii (35) and Coxiella burnetii (16). MST was developed with the assumption that intergenic spacers are more variable than genes for genotyping bacteria at the strain level. In this study, to estimate the usefulness of MST for studying the population genetics of B. henselae, we applied it to a large collection of cat isolates.
MATERIALS AND METHODS
Study design.
One hundred twenty-six B. henselae cat isolates of various geographic origins were incorporated in this study (Table 1). All 38 European isolates were grown in our laboratory. For the other 88 isolates, from the United States and Asia, we studied DNA extracted by two of the authors (B.B.C. and L.G.) from their isolates.
TABLE 1.
List of B. henselae isolates incorporated in this study and corresponding genotypes
| Isolate | Origin | No. of genotypesa
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | MST | ||
| Amber | USAb | 5 | 1 | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 5 |
| Aron | USA | 7 | 2 | 5 | 4 | 1 | 2 | 1 | 1 | 3 | 7 |
| BisQuick | USA | 5 | 2 | 6 | 5 | 2 | 2 | 2 | 1 | 1 | 35 |
| Budda | USA | 5 | 1 | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 5 |
| Buster Brown | USA | 5 | 1 | 1 | 2 | 2 | 2 | 2 | 1 | 1 | 25 |
| Cleo | USA | 5 | 1 | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 5 |
| Kody | USA | 4 | 5 | 5 | 5 | 1 | 2 | 1 | 1 | 3 | 27 |
| Earl Grey | USA | 5 | 1 | 1 | 2 | 2 | 2 | 2 | 1 | 1 | 25 |
| Erick | USA | 5 | 1 | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 5 |
| Faleen | USA | 5 | 1 | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 5 |
| Gigi | USA | 4 | 5 | 5 | 4 | 1 | 2 | 1 | 1 | 3 | 15 |
| Jackie | USA | 5 | 2 | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 16 |
| Junior | USA | 5 | 1 | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 5 |
| Kelly | USA | 8 | 2 | 5 | 4 | 1 | 2 | 1 | 1 | 3 | 8 |
| Kodie | USA | 5 | 1 | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 5 |
| Lathious | USA | 5 | 2 | 6 | 5 | 2 | 2 | 2 | 1 | 1 | 35 |
| Levi | USA | 5 | 1 | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 5 |
| Mew Mew | USA | 7 | 2 | 5 | 4 | 1 | 2 | 1 | 1 | 3 | 7 |
| Mitzi | USA | 5 | 1 | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 5 |
| Mokka | USA | 5 | 1 | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 5 |
| Molly | USA | 5 | 1 | 1 | 2 | 2 | 2 | 2 | 1 | 1 | 25 |
| Norman | USA | 5 | 1 | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 5 |
| Patches | USA | 7 | 2 | 5 | 4 | 1 | 2 | 1 | 1 | 3 | 7 |
| Pyewacket | USA | 5 | 1 | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 5 |
| Rafiki | USA | 5 | 1 | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 5 |
| Rocket | USA | 7 | 2 | 5 | 4 | 1 | 2 | 1 | 1 | 3 | 7 |
| Rum Tum | USA | 5 | 1 | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 5 |
| Sabrina | USA | 5 | 2 | 6 | 5 | 2 | 2 | 2 | 1 | 1 | 35 |
| Sadie | USA | 4 | 5 | 5 | 4 | 1 | 2 | 1 | 1 | 3 | 15 |
| Saki | USA | 5 | 1 | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 5 |
| Sam | USA | 9 | 2 | 5 | 4 | 1 | 2 | 1 | 1 | 3 | 9 |
| Samantha | USA | 5 | 1 | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 5 |
| Sassy | USA | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 1 |
| Shannon | USA | 5 | 1 | 1 | 5 | 2 | 2 | 2 | 1 | 1 | 26 |
| Simba | USA | 3 | 1 | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 3 |
| Sinbad | USA | 5 | 1 | 1 | 1 | 4 | 2 | 2 | 1 | 1 | 29 |
| Spaz | USA | 5 | 1 | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 5 |
| Sunday | USA | 5 | 2 | 1 | 5 | 2 | 2 | 2 | 1 | 1 | 28 |
| Sweetie | USA | 5 | 1 | 1 | 2 | 2 | 2 | 2 | 1 | 1 | 25 |
| Tabatha | USA | 5 | 1 | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 5 |
| Tasha | USA | 3 | 1 | 6 | 3 | 5 | 4 | 4 | 3 | 2 | 18 |
| Timothy | USA | 5 | 1 | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 5 |
| Toby | USA | 5 | 1 | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 5 |
| Tori | USA | 4 | 5 | 5 | 5 | 1 | 2 | 1 | 1 | 3 | 27 |
| Zipper | USA | 5 | 2 | 6 | 5 | 2 | 2 | 2 | 1 | 1 | 35 |
| Zoe | USA | 5 | 1 | 1 | 1 | 2 | 3 | 2 | 1 | 1 | 30 |
| Newmans | USA | 4 | 2 | 5 | 4 | 1 | 2 | 2 | 1 | 3 | 33 |
| White | USA | 5 | 2 | 6 | 5 | 2 | 2 | 2 | 1 | 1 | 35 |
| Lavery | USA | 5 | 7 | 6 | 1 | 2 | 2 | 1 | 2 | 1 | 17 |
| Rae | USA | 5 | 7 | 6 | 1 | 2 | 2 | 2 | 2 | 1 | 34 |
| Fairminer | USA | 5 | 2 | 6 | 5 | 2 | 2 | 2 | 1 | 1 | 35 |
| Shaw-Lamon | USA | 5 | 2 | 6 | 5 | 2 | 2 | 1 | 1 | 1 | 24 |
| Moyle | USA | 5 | 7 | 6 | 1 | 2 | 2 | 1 | 2 | 1 | 17 |
| Linnan | USA | 5 | 2 | 6 | 5 | 2 | 2 | 1 | 1 | 1 | 24 |
| Silcock | USA | 5 | 2 | 6 | 5 | 2 | 2 | 2 | 1 | 1 | 35 |
| Hunt | USA | 5 | 2 | 6 | 5 | 2 | 2 | 2 | 1 | 1 | 35 |
| Eichtais | USA | 5 | 2 | 6 | 5 | 2 | 2 | 2 | 1 | 1 | 35 |
| Taylor | USA | 5 | 2 | 6 | 5 | 2 | 2 | 2 | 1 | 1 | 35 |
| Ramm | USA | 5 | 2 | 6 | 5 | 2 | 2 | 2 | 1 | 1 | 35 |
| USA1 | USA | 5 | 1 | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 5 |
| USA4 | USA | 4 | 1 | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 4 |
| USA6 | USA | 4 | 5 | 5 | 4 | 1 | 2 | 1 | 1 | 3 | 15 |
| USA7 | USA | 3 | 1 | 6 | 3 | 5 | 4 | 4 | 3 | 2 | 18 |
| USA8 | USA | 5 | 1 | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 5 |
| USA11 | USA | 4 | 1 | 5 | 4 | 1 | 2 | 1 | 1 | 3 | 19 |
| USA12 | USA | 3 | 3 | 6 | 3 | 5 | 4 | 4 | 1 | 2 | 11 |
| USA15 | USA | 4 | 2 | 5 | 4 | 1 | 2 | 1 | 1 | 3 | 14 |
| USA16 | USA | 3 | 1 | 6 | 3 | 5 | 4 | 4 | 3 | 2 | 18 |
| USA17 | USA | 5 | 1 | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 5 |
| UR.BH.M.NHC.32 | France | 3 | 5 | 4 | 5 | 2 | 2 | 2 | 1 | 3 | 22 |
| UR.BH.M.NHC.33 | France | 3 | 5 | 4 | 5 | 2 | 2 | 2 | 1 | 3 | 22 |
| UR.BH.M.NHC.34-H | France | 3 | 5 | 4 | 5 | 2 | 2 | 2 | 1 | 3 | 22 |
| UR.BH.M.NHC.35 | France | 3 | 5 | 4 | 5 | 2 | 2 | 2 | 1 | 3 | 22 |
| UR.BH.M.NHC.50-H | France | 3 | 5 | 4 | 5 | 2 | 2 | 2 | 1 | 3 | 22 |
| UR.BH.M.NHC.52-H | France | 3 | 5 | 4 | 5 | 2 | 2 | 2 | 1 | 3 | 22 |
| UR.BH.M.NHC.54-H | France | 3 | 5 | 4 | 5 | 2 | 2 | 2 | 1 | 3 | 22 |
| UR.BH.M.NHC.55-H | France | 3 | 5 | 4 | 5 | 2 | 2 | 2 | 1 | 3 | 22 |
| UR.BH.M.NHC 56-H | France | 3 | 5 | 4 | 5 | 2 | 2 | 2 | 1 | 3 | 22 |
| UR.BH.M.NHC.57-M | France | 3 | 6 | 2 | 3 | 5 | 4 | 4 | 3 | 2 | 13 |
| UR.BH.M.NHC.58-H | France | 3 | 5 | 4 | 5 | 2 | 2 | 2 | 1 | 3 | 22 |
| UR.BH.M.NHC 59-H | France | 3 | 5 | 4 | 5 | 2 | 2 | 2 | 1 | 3 | 22 |
| UR.BH.M.NHC.67 | France | 5 | 1 | 1 | 1 | 2 | 2 | 4 | 2 | 1 | 31 |
| UR.BH.M.NHC.72-H | France | 3 | 5 | 4 | 5 | 2 | 2 | 2 | 1 | 3 | 22 |
| UR.BH.M.NHC 77-M | France | 5 | 1 | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 5 |
| UR.BH.M.NHC.78-M | France | 5 | 1 | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 5 |
| UR.BH.M.NHC.79-H | France | 3 | 5 | 4 | 5 | 2 | 2 | 2 | 1 | 3 | 22 |
| UR.BH.M.NHC 80-H | France | 3 | 5 | 4 | 5 | 2 | 2 | 2 | 1 | 3 | 22 |
| UR.BH.M.NHC 82-M | France | 5 | 1 | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 5 |
| UR.BH.M.NHC.84-M | France | 2 | 4 | 1 | 1 | 3 | 1 | 3 | 3 | 2 | 2 |
| UR.BHM.M.NHC.128 | France | 3 | 6 | 2 | 3 | 5 | 4 | 4 | 3 | 2 | 13 |
| UR.BHM.M.NHC.129 | France | 3 | 6 | 2 | 3 | 5 | 4 | 4 | 3 | 2 | 13 |
| UR.BHM.M.NHC.130 | France | 3 | 6 | 2 | 3 | 5 | 4 | 4 | 3 | 2 | 13 |
| UR.BH.M.NHC 154 | France | 2 | 4 | 1 | 1 | 3 | 1 | 3 | 3 | 2 | 2 |
| UR.BH.M.NHC 155 | France | 2 | 4 | 1 | 1 | 3 | 1 | 3 | 3 | 2 | 2 |
| UR.BH.M.NHC 156 | France | 2 | 4 | 1 | 1 | 3 | 1 | 3 | 3 | 2 | 2 |
| UR.BH.M.NHC 159 | France | 2 | 4 | 1 | 1 | 3 | 1 | 3 | 3 | 2 | 2 |
| UR.BH.M.NHC 161 | France | 2 | 4 | 1 | 1 | 3 | 1 | 3 | 3 | 2 | 2 |
| FR96/BK7 | Germany | 3 | 5 | 4 | 5 | 2 | 2 | 2 | 1 | 3 | 22 |
| FR96/BK26II | Germany | 5 | 1 | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 5 |
| FR96/BK36 | Germany | 3 | 5 | 4 | 5 | 2 | 2 | 2 | 1 | 3 | 22 |
| FR96/BK36II | Germany | 5 | 1 | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 5 |
| FR96/BK75 | Germany | 5 | 1 | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 5 |
| FR96/BK77 | Germany | 2 | 4 | 1 | 1 | 3 | 1 | 3 | 3 | 2 | 2 |
| FR96/BK78 | Germany | 2 | 4 | 1 | 1 | 3 | 1 | 3 | 3 | 2 | 2 |
| FR96/BK79 | Germany | 2 | 4 | 1 | 1 | 3 | 1 | 3 | 3 | 2 | 2 |
| ZF-1 | France | 3 | 5 | 3 | 5 | 2 | 2 | 2 | 1 | 3 | 12 |
| FIZZ | Switzerland | 5 | 1 | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 5 |
| J1 | Japan | 5 | 2 | 6 | 5 | 2 | 2 | 2 | 1 | 1 | 35 |
| J4 | Japan | 5 | 2 | 5 | 4 | 1 | 2 | 1 | 1 | 3 | 23 |
| J5 | Japan | 8 | 2 | 5 | 4 | 1 | 2 | 2 | 1 | 3 | 32 |
| J6 | Japan | 5 | 2 | 6 | 5 | 2 | 2 | 2 | 1 | 1 | 35 |
| J7 | Japan | 5 | 2 | 6 | 5 | 2 | 2 | 2 | 1 | 1 | 35 |
| J8 | Japan | 5 | 2 | 6 | 5 | 2 | 2 | 2 | 1 | 1 | 35 |
| P1 | Philippines | 5 | 2 | 6 | 5 | 2 | 2 | 2 | 2 | 1 | 38 |
| P2 | Philippines | 5 | 2 | 6 | 5 | 2 | 2 | 2 | 2 | 1 | 38 |
| P4 | Philippines | 5 | 2 | 6 | 5 | 2 | 2 | 2 | 2 | 1 | 38 |
| P5 | Philippines | 5 | 2 | 6 | 5 | 2 | 2 | 1 | 2 | 1 | 37 |
| P6 | Philippines | 4 | 2 | 5 | 4 | 1 | 2 | 1 | 2 | 3 | 36 |
| P7 | Philippines | 3 | 2 | 5 | 5 | 2 | 2 | 2 | 1 | 1 | 10 |
| P8 | Philippines | 3 | 2 | 6 | 5 | 2 | 2 | 2 | 1 | 1 | 21 |
| T1 | Thailand | 5 | 2 | 6 | 5 | 2 | 2 | 2 | 1 | 3 | 39 |
| T3 | Thailand | 8 | 2 | 5 | 4 | 1 | 2 | 2 | 1 | 3 | 32 |
| T5 | Thailand | 6 | 2 | 5 | 4 | 1 | 2 | 2 | 1 | 3 | 6 |
| T6 | Thailand | 4 | 2 | 5 | 4 | 1 | 2 | 2 | 1 | 3 | 33 |
| T7 | Thailand | 5 | 2 | 6 | 5 | 2 | 2 | 2 | 2 | 1 | 38 |
| T8 | Thailand | 5 | 1 | 6 | 5 | 2 | 2 | 2 | 2 | 1 | 20 |
The description of intergenic spacers S1 to S9 and the primers used for their amplification and sequencing are given in Table 2.
USA, United States.
Bartonella henselae culture and DNA extraction.
B. henselae isolates were cultivated on Columbia agar with 5% sheep blood (BioMerieux, Marcy l'Etoile, France) at 37°C in 5% CO2 (Genbag CO2 system; BioMerieux). Genomic DNA of B. henselae strains was extracted by using the Chelex procedure as previously described (9) or the QIAmp Tissue kit following the manufacturer's recommendations (QIAGEN, Hilden, Germany).
Selection of target sequences.
We aligned the genomic sequences of B. henselae (GenBank accession number BX897699) and B. quintana (BX897700) by using the BLASTn (1) and GenomeComp (33) software programs to identify conserved pairs of consecutive genes. Then, intergenic sequences were aligned using the CLUSTAL W program (31). We classified intergenic spacers conserved by both genomes, with sizes ranging from 150 to 600 bp, by degree of similarity and then selected the 20 most variable spacers (detailed in Table 2).
TABLE 2.
The 20 most variable intergenic spacers conserved by both B. henselae and B. quintana and primers used for amplification and sequencing
| Spacer namea | Spacer position on the genomeb | Spacer size (bp)b | PCR product size (bp)b | Forward primer | Reverse primer |
|---|---|---|---|---|---|
| tRNA-Ala/GCA-tRNA-Ile/AUC (S1)c | 1412349-1412683 | 335 | 414 | TTGCAAAGCAGGTGCTCTCC | TAAGCGTGAGGTCGGAGGTT |
| BH2865724-dut (S2) | 1685859-1686289 | 431 | 602 | GGTTTTTGCCACGGGTATTT | GGAAGTTCTAAACCTTGTCCATGG |
| dnaJ related protein-cobS (S3) | 1828960-1829320 | 361 | 490 | CAATGGAGGCAACCGTTCTT | GTGATATCGGGTACATTTTCAACTG |
| pssA-oxidoreductase (S4) | 609654-610228 | 575 | 709 | GATTTTTCTTCCGTGTAGCTTTGT | TGTGCGTAAAAATCGATTCATG |
| carB-cold shock protein (S5) | 1292681-1293066 | 386 | 509 | AGAAGCTATCGAAGCACTCACAAA | TGAATGAACCCGAAACCTTTAGT |
| alr-gcvP (S6) | 1431110-1431442 | 333 | 540 | TCAAAGAGGTGATTGGGTAGAGC | CTGTTTCACGTATTGATAATGTTGC |
| ftsK-oxidoreductase (S7) | 1799482-1799984 | 503 | 594 | GCGAACCTTGAGAACTCTGCA | GGGTTTTACACCTTCATTGAGATCA |
| BH2864883-BH2864884 (S8) | 1594026-1594377 | 352 | 524 | TAACCACATCATCCCCTCCTCT | GAAATAATCATGAAACGCATAAGC |
| acpP2-malate oxidoreductase (S9) | 853898-854063 | 166 | 296 | CAACTTCACTGATTTCTGCGATAA | CGAGGAGTGGTTAATATGACAGCT |
| BH16140-BH16150 (S10) | 1864960-1865467 | 508 | 508 | CTCATTACAGAGCAAAACGGATATC | TTATCAAGGTTTGCTTCTACAGCG |
| dapE-hemN (S11) | 76032-76228 | 197 | 395 | ATGCATATGGTGGATGAGTGTGT | GATTTACAACAACAAGGGCTGGT |
| phoH-BH02260 (S12) | 302238-302400 | 163 | 327 | CTTATTTTCTCTTTAACGCGCTTT | TCACCTTGGCTTTTACCTGTTGT |
| Glutathione S-transferase- dapB (S13) | 1383473-1383792 | 320 | 441 | CTTCTTTTCGCCCTCTTTTAACA | TCGCGTCCCATTCTTCCAT |
| rpmF-ispA (S14) | 1751167-1751490 | 324 | 394 | GATGGAGAGGTTTTTCGTTTAGG | TGGGCGTGTTTTGCAAGAA |
| asd-BH12900 (S 15) | 1441922-1442299 | 378 | 636 | TACGCGATGCACCAGGCT | CCGTGTTGTGACCTATCCTGCT |
| recO-panC (S16) | 596596-596744 | 149 | 438 | TTGTGCAAAGAACTGTTCGTCC | ACCAAACCAATCGAAAATCCTAA |
| BH16010-rpsP (S17) | 1846327-1846669 | 343 | 461 | AGACTGGGAAATTAAAGGCCG | CGTATAGCAGCAGCAAAGCAAG |
| pgk-gap (S18) | 1729282-1729787 | 506 | 590 | GAACACGTTTTCCTGTGACATCA | GTGATACGGCTGTGGCTTTTG |
| uvrC-BH05560 (S19) | 653261-653650 | 390 | 532 | AGCTTTTCTTGCTCATTTTCGG | AGCTCAGTCCCTTTCTTATCGC |
| trwL4-trwL5 (S20) | 1805508-1805660 | 153 | 280 | AGATACATTCGTACGGTGGGGA | CCTGTTGTTATTTTTTGATTGGAG |
Intergenic spacer names consist of the name of the 5′-flanking gene combined (-) with the name of the 3′-flanking gene. Flanking open reading frames encoding putative proteins of unknown function are named after their open reading frame number within the B. henselae genome (GenBank accession number BX897699).
The positions of the spacers on the genome, the spacer size, and the PCR product size were deduced from B. henselae (BX897699).
Spacers S1 to S9 were numbered in descending order of variability.
PCR amplification and sequencing.
Primers were designed to amplify the 20 most variable spacers fulfilling the above criteria using the Primer 3.0 software (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi). Primers for amplifying the 20 most variable spacers were selected within genes flanking the selected spacers and are listed in Table 2. All primers were obtained from Eurogentec (Seraing, Belgium). Their specificity was predicted by comparison with GenBank using the BLASTn software (1). PCRs were carried out in a PTC-200 automated thermal cycler (MJ Research, Waltham, Mass.). One nanomolar concentration of each DNA preparation was amplified in a 25-μl reaction mixture containing 50 pM of each primer; 200 μM (each) dATP, dCTP, dGTP, and dTTP (Invitrogen, Gaithersburg, Md.); 1 U of eLONGase polymerase (Invitrogen); 1 μl of eLONGase buffer A; and 4 μl of eLONGase buffer B. The following conditions were used for amplification: an initial 3 min of denaturation at 94°C was followed by 40 cycles of denaturation for 30 s at 94°C, annealing for 30 s at 52°C, and extension for 1 min at 68°C. Amplification was completed by holding the reaction mixture for 10 min at 68°C to allow complete extension of the PCR products. PCR products were purified by using the MultiScreen PCR filter plate (Millipore, Saint-Quentin en Yvelines, France) as recommended by the manufacturer. PCR products were sequenced in both directions by using the d-Rhodamine Terminator Cycle Sequencing Ready Reaction kit with Amplitaq polymerase FS (Perkin-Elmer, Coignieres, France) as described by the manufacturer. Sequencing products were resolved using an ABI 3100 automated sequencer (Perkin-Elmer). Sterile water was used as a negative control in each PCR assay. Sequences from each genotype were checked twice in both directions to ensure the reliability of the MST method.
Sequence analysis and phylogenetic analysis.
Nucleotide sequences were edited using the Autoassembler package (Perkin-Elmer). For each intergenic spacer, a genotype was defined as a sequence exhibiting unique mutations. MST genotypes were defined as unique combinations of spacer genotypes. Multiple alignment of sequences was carried out using the CLUSTAL W software (31). Phylogenetic analysis of the studied isolates was obtained using the neighbor-joining and maximum parsimony methods within the MEGA 3 software (25). For this purpose, sequences of the selected spacers were concatenated. To facilitate sequence comparison with our MST sequences, we developed an online site named MST-Rick. This site contains a local BLAST to help scientists compare their sequences to our database.
Statistical tests.
The genotypic variability of B. henselae isolates according to their geographic origin was estimated using Fisher's exact test. A difference was considered significant when P was <0.05.
Nucleotide sequence accession numbers.
The different genotypes for the discriminatory spacers have been deposited in the GenBank database under accession numbers DQ383226 to DQ383270.
RESULTS
MST genotyping.
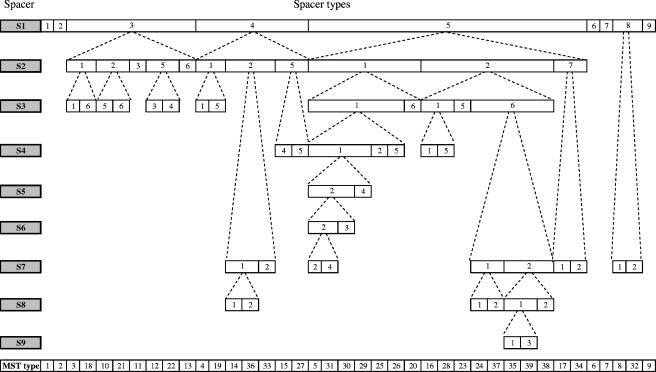
One thousand four hundred thirteen intergenic spacers were found conserved by B. henselae and B. quintana genomes. Among them, 293 had a size ranging from 150 to 600 bp. We tested the 20 most variable (S1 to S20) of these 293 spacers among the 126 B. henselae cat isolates available. Nine of the spacers (S1 to S9) were found highly variable among these isolates (Table 2). The tRNA-Ala/GCA-tRNA-Ile/AUC spacer (S1), flanked by two tRNA genes, was found to be the most variable spacer among the nine tested, with five variable nucleotide positions and a 15-bp sequence fragment presenting either as a single copy or repeated up to five times, depending on the isolate (Fig. 1; Table 3). Sequences from the S1 spacer classified the 126 isolates into nine genotypes. The BH2865724-dut spacer (S2), with 14 variable nucleotide positions, was the second most variable spacer and allowed the 126 tested isolates to be classified into seven genotypes (Table 3). The dnaJ-related protein-cobS spacer (S3) held eight variable nucleotide positions and classified the 126 isolates into six genotypes (Table 3). The pssA-oxidoreductase (S4) and carB-cold shock protein (S5) spacers had nine and five variable nucleotide positions, respectively, and classified the 126 isolates into five genotypes each (Table 3). The alr-gcvP (S6) and ftsK-oxidoreductase spacers (S7) contained eight variable nucleotide positions each and classified the 126 isolates into four genotypes each (Table 3). The BH2864883-BH2864884 (S8) and acpP2-malate oxidoreductase (S9) spacers harbored eight and four variable nucleotide positions, respectively, and classified the 126 isolates into three genotypes each (Table 3). In total, 69 variable nucleotide positions were found within the nine intergenic spacers (Table 3). Each variable nucleotide was checked three times to ensure the reliability of MST. Only two alleles at each variable position were found, with the exception of position 256 within the alr-gcvP spacer. At this position, 117 isolates had a thymine (types 2 and 3), compared to a cytosine in five European isolates (type 1) and a guanine in four American isolates (type 4) (Table 3). By combining the genotypes obtained from each variable spacer, the 126 tested isolates could be classified into 39 MST genotypes (Table 1). Each of the 39 genotypes was identified based on sequence specificities from either a single spacer or a combination of a maximum of seven spacers (Fig. 2). Sequences from each genotype from the nine spacers were added to the MST-Rick database (http://ifr48.timone.univ-mrs.fr/MST_BHenselae/mst).
FIG. 1.
Description of the 15-bp repeated sequences within the tRNA-Ala/GCA-tRNA-Ile/AUC spacer. The first column contains the copy number of repeats. Numbers in parentheses indicate the numbers of strains that have the corresponding repeat numbers.
TABLE 3.
Polymorphism characteristics of the nine variable intergenic spacers
| Spacer name | No. of nucleotide variations | No. of genotypes | Spacer polymorphism, with reference to Houston-1 straina |
|---|---|---|---|
| tRNA-Ala/GCA-tRNA-Ile/AUC (S1) | 5 | 9 | G9A, C49T, 203inserT, C256T, 294VNTR |
| BH2865724-dut (S2) | 14 | 7 | T19C, G31A, C92T, C103T, C113T, C142T, A156G, A162G, C169T, G237T, A289G, C310T, T332dele, T339C |
| dnaJ-related protein-cobS (S3) | 8 | 6 | A3G, G12A, A25G, G46A, C84T, T203C, T255C, T264dele |
| pssA-oxidoreductase (S4) | 9 | 5 | A49G, 51inserA, 93inser,b G159T, A274G, A306G, T322C, A362G, T484C |
| carB-cold shock protein (S5) | 5 | 5 | 51inser,e C83A, G145C, T157C, T240C |
| alr-gcvP (S6) | 8 | 4 | C4T, G10A, C60A, G242A, T256G or C, 296dele,d A305G, A306G |
| ftsK-oxidoreductase (S7) | 8 | 4 | C324A, G326A, 362inser,c G370A, A390C, C432T, A436G, C480T |
| BH2864883-BH2864884 (S8) | 8 | 3 | G19C, A60G, A61G, 69inserT, A88G, C102A, C249A, C282T |
| acpP2-malate oxidoreductase (S9) | 4 | 3 | C28T, A40C, G96A, C114T |
| Total (9 spacers) | 69 | 39 |
The numbers show each variable nucleotide position in reference to the Houston-1 strain. The locus before the number is that within Houston-1, and the locus after the number is a possible variable nucleotide within other strains. inser, insertion; dele, deletion; VNTR, variable number of tandem repeats.
Insertion of CCAGAGTGCTATTCATTAAATAAGTTTGCTTTTAAAAAATATTTCTTG.
Insertion of TTCACCTGTTTCATA.
Deletion of TTTTTG.
Insertion of GTAGGGCA.
FIG. 2.
Guidelines for selection of spacers for MST genotyping of B. henselae isolates.
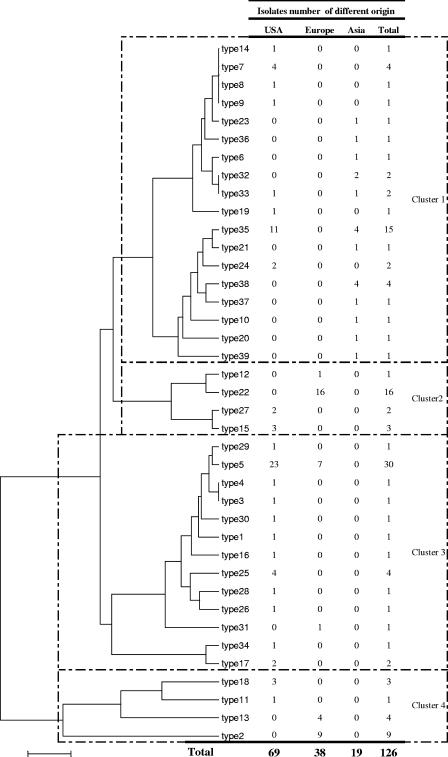
Among the 39 MST types, 24 MST types (types 1, 3, 4, 6, 8 to 12, 14, 16, 19 to 21, 23, 26, 28 to 31, 34, 36, 37, and 39) contained only one isolate each and five MST genotypes (types 17, 24, 27, 32, and 33) contained only two isolates each (Table 1). The 19 Asian isolates were distributed into 12 MST genotypes, compared to 6 (P < 0.01) and 24 (P = 0.03) MST types for the 38 European and 69 American isolates, respectively (Fig. 3). Among the 39 MST genotypes, 10 (types 6, 10, 20, 21, 23, 32, and 36 to 39), 21 (types 1, 3, 4, 7 to 9, 11, 14 to 19, 24 to 30, and 34), and 5 (types 4, 9, 12, 22, and 31) genotypes were specific to Asian, American, and European isolates, respectively.
FIG. 3.
Dendrogram showing the phylogenetic organization of the 39 MST genotypes, constructed using the neighbor-joining method. Sequences from the nine spacers were concatenated. The scale bar represents a 1% nucleotide sequence variation.
A significant difference in genotypic diversity was found between B. quintana (4 MST types out of 71 isolates) (13), and B. henselae (39 MST types among 126 B. henselae isolates; P < 0.01).
Phylogenetic classification of MST types.
Phylogenetic trees obtained from concatenated spacer sequences using the neighbor-joining (Fig. 3) and maximum parsimony methods showed similar phylogenetic classifications. The 126 tested isolates were grouped into four clusters. Asian isolates were grouped into cluster 1. European isolates were grouped into clusters 2 to 4. In contrast, American isolates did not form a coherent cluster but were spread among the four clusters.
DISCUSSION
In this study, we demonstrated that MST is a highly efficient method for genotyping B. henselae at the strain level, with 39 genotypes identified among 126 studied isolates using a combination of nine intergenic spacer sequences. Prior to our study, the most discriminatory genotyping method for B. henselae, i.e., MLST using nine genes, had identified seven genotypes among cat and human isolates of B. henselae (21). Therefore, MST was more discriminatory than MLST for typing B. henselae.
We found B. henselae to be significantly more genotypically variable than B. quintana, a human pathogen previously identified to be mostly clonal (13) (P < 0.01). Such a higher genetic diversity of B. henselae is as yet unexplained, despite the studies conducted on the relationship between cat and human isolates. In Germany and The Netherlands, a majority of human isolates were of 16S rRNA gene type I whereas cat isolates mostly belonged to type II (3, 4, 10, 28, 30). In contrast, in Switzerland, France, and the United States, investigators have demonstrated that most of the human isolates of B. henselae belonged to 16S rRNA gene type II (5, 8, 17). Iredell et al., using MLST identifying seven genotypes, found that human infection is caused by a limited number of genotypes (21). Therefore, the relationship between human and cat isolates of B. henselae remains a puzzling problem. We believe that MST may also be a suitable tool for investigating the dynamics of B. henselae populations in humans.
Among the 126 isolates analyzed in this study, we found a significantly higher genotypic heterogeneity among Asian isolates than among European (P < 0.01) and American (P = 0.03) isolates. This may be explained by the fact that most European isolates originate from only two neighboring countries, France and Germany, and American isolates were mostly obtained from only two states, California and Florida, whereas Asian isolates originate from three countries. However, the phylogenic analysis built by concatenating the nine spacers (Fig. 3) revealed that Asian isolates, despite their apparent genotypic heterogeneity, were phylogenetically homogeneous and were grouped into a single cluster, without any overlap with European isolates. This may suggest that Asian isolates have a more recent common origin. American isolates appeared to be phylogenetically more heterogeneous than other isolates. None of the 39 MST types identified was represented in European, American, and Asian isolates together. Thus, we did not identify any pandemic isolate. However, our data may be updated by future studies incorporating isolates from other geographic origins.
To limit the number of spacers to be sequenced, we propose specific guidelines that facilitate their selection (Fig. 2). In addition, to facilitate usage of MST for genotyping of B. henselae, we created an MST-dedicated, free-access online database, i.e., MST-Rick, to which any investigators may compare their own spacer sequences (http://ifr48.timone.univ-mrs.fr/MST_BHenselae/mst). Although our study is preliminary and includes a limited number of strains, we hope that our method and database will be used and implemented by other investigators, which would allow frequent updating of the data.
In conclusion, MST using nine variable intergenic spacers identified 39 genotypes among 126 B. henselae cat isolates. As such, MST is the most discriminatory genotyping method for B. henselae isolates to date and may be used to investigate the relationships between human and cat isolates of B. henselae. Recently, we successfully used MST for genotyping B. henselae isolates within lymph node biopsy samples from patients with cat scratch disease (unpublished data). As B. henselae is extremely difficult to grow from human specimens, MST might thus serve as both a detection and a genotyping tool.
Acknowledgments
We thank Lina Barassi for her technical help.
All authors have read and approved the final version of the manuscript and do not have any conflict of interest related to this research.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, B., K. Sims, and R. Regnery. 1994. Detection of the Rochalimaea henselae DNA in specimens from cat-scratch disease patients by polymerase chain reaction. J. Clin. Microbiol. 32:942-948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergmans, A. M., C. M. de Jong, G. Van Amerongen, C. S. Schot, and L. M. Schouls. 1997. Prevalence of Bartonella species in domestic cats in The Netherlands. J. Clin. Microbiol. 35:2256-2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergmans, A. M., J. F. Schellekens, J. D. van Embden, and L. M. Schouls. 1996. Predominance of two Bartonella henselae variants among cat-scratch disease patients in the Netherlands. J. Clin. Microbiol. 34:254-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Box, A. T. A., A. Sander, I. Perschil, D. L. Goldenberg, and M. Altwegg. 2000. Cats are probably not the only reservoir for infections due to Bartonella henselae. J. Microbiol. Methods 27:101-102. [Google Scholar]
- 6.Chomel, B. B. 2000. Cat-scratch disease. Rev. Sci. Tech. 19:136-150. [DOI] [PubMed] [Google Scholar]
- 7.Chomel, B. B., R. W. Kasten, K. Floyd-Hawkins, B. Chi, K. Yamamoto, J. Roberts-Wilson, A. N. Gurfield, R. C. Abbott, N. C. Pedersen, and J. E. Koehler. 1996. Experimental transmission of Bartonella henselae by the cat flea. J. Clin. Microbiol. 34:1952-1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dauga, C., I. Miras, and P. A. D. Grimont. 1996. Identification of Bartonella henselae and B. quintana 16S rDNA sequences by branch-, genus- and species-specific amplification. J. Med. Microbiol. 45:192-199. [DOI] [PubMed] [Google Scholar]
- 9.De Lamballerie, X., C. Zandotti, C. Vignoli, C. Bollet, and P. de Micco. 1992. A rare step microbial DNA extraction method using Chelex 100 suitable for gene amplification. Res. Microbiol. 143:785-790. [DOI] [PubMed] [Google Scholar]
- 10.Dillon, B., J. Valenzuela, R. Don, D. Blanckenberg, D. I. Wigney, R. Malik, A. J. Morris, J. M. Robson, and J. Iredell. 2002. Limited diversity among human isolates of Bartonella henselae. J. Clin. Microbiol. 40:4691-4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drancourt, M., V. Roux, L. V. Dang, T. H. C. D. Lam, V. Chenal-Francisque, H. Ogata, P. E. Fournier, E. Crubezy, and D. Raoult. 2004. Genotyping, Orientalis-like Yersinia pestis, and plague pandemics. Emerg. Infect. Dis. 10:1585-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ehrenborg, C., L. Wesslen, A. Jakobson, G. Friman, and M. Holmberg. 2000. Sequence variation in the ftsZ gene of Bartonella henselae isolates and clinical samples. J. Clin. Microbiol. 38:682-687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foucault, C., B. La Scola, H. Lindroos, S. G. Andersson, and D. Raoult. 2005. Multispacer typing technique for sequence-based typing of Bartonella quintana. J. Clin. Microbiol. 43:41-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fournier, P. E., and D. Raoult. 1998. Cat scratch disease and an overview of other Bartonella henselae related infections, p. 32-62. In A. Schmidt (ed.), Bartonella and Afipia species emphasizing Bartonella henselae. Karger, Basel, Switzerland.
- 15.Fournier, P. E., Y. Zhu, H. Ogata, and D. Raoult. 2004. Use of highly variable intergenic spacer sequences for multispacer typing of Rickettsia conorii strains. J. Clin. Microbiol. 42:5757-5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glazunova, O., V. Roux, O. Freylikman, Z. Sekeyova, G. Fournous, J. Tyczka, N. Tokarevich, E. Kovacava, T. J. Marrie, and D. Raoult. 2005. Coxiella burnetii genotyping. Emerg. Infect. Dis. 11:1211-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gurfield, A. N., H. J. Boulouis, B. B. Chomel, R. Heller, R. W. Kasten, K. Yamamoto, and Y. Piemont. 1997. Coinfection with Bartonella clarridgeiae and Bartonella henselae and with different Bartonella henselae strains in domestic cats. J. Clin. Microbiol. 35:2120-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hadfield, T. L., R. Warren, M. Kass, E. Brun, and C. Levy. 1993. Endocarditis caused by Rochalimaea henselae. Hum. Pathol. 24:1140-1141. [DOI] [PubMed] [Google Scholar]
- 19.Handley, S. A., and R. L. Regnery. 2000. Differentiation of pathogenic Bartonella species by infrequent restriction site PCR. J. Clin. Microbiol. 38:3010-3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Houpikian, P., and D. Raoult. 2001. 16S/23S rRNA intergenic spacer regions for phylogenetic analysis, identification, and subtyping of. Bartonella species. J. Clin. Microbiol. 39:2768-2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iredell, J., D. Blanckenberg, M. Arvand, S. Grauling, E. J. Feil, and R. J. Birtles. 2003. Characterization of the natural population of Bartonella henselae by multilocus sequence typing. J. Clin. Microbiol. 41:5071-5079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koehler, J. E. 1996. Bartonella infections. Adv. Pediatr. Infect. Dis. 11:1-27. [PubMed] [Google Scholar]
- 23.Koehler, J. E., C. A. Glaser, and J. W. Tappero. 1994. Rochalimaea henselae infection: a new zoonosis with the domestic cat as a reservoir. JAMA 271:531-535. [DOI] [PubMed] [Google Scholar]
- 24.Kordick, D. L., K. H. Wilson, D. J. Sexton, T. L. Hadfield, H. A. Berkhoff, and E. B. Breitschwerdt. 1995. Prolonged Bartonella bacteremia in cats associated with cat-scratch disease patients. J. Clin. Microbiol. 33:3245-3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar, S., K. Tamura, and M. Nei. 2004. MEGA 3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief. Bioinform. 5:150-163. [DOI] [PubMed]
- 26.La Scola, B., Z. X. Liang, Z. Zeaiter, P. Houpikian, P. A. D. Grimont, and D. Raoult. 2002. Genotypic characteristics of two serotypes of Bartonella henselae. J. Clin. Microbiol. 40:2002-2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.La Scola, B., Z. Zeaiter, A. Khamis, and D. Raoult. 2003. Gene-sequence-based criteria for species definition in bacteriology: the Bartonella paradigm. Trends Microbiol. 11:318-321. [DOI] [PubMed] [Google Scholar]
- 28.Sander, A., M. Posselt, N. Böhm, M. Ruess, and M. Altwegg. 1999. Detection of Bartonella henselae DNA by two different PCR assays and determination of the genotypes of strains involved in histologically defined cat scratch disease. J. Clin. Microbiol. 37:993-997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sander, A., M. Ruess, S. Bereswill, M. Schuppler, and B. Steinbrueckner. 1998. Comparison of different DNA fingerprinting techniques for molecular typing of Bartonella henselae isolates. J. Clin. Microbiol. 36:2973-2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sander, A., M. Ruess, K. Deichmann, N. Böhm, and W. Bredt. 1998. Two different genotypes of Bartonella henselae in children with cat-scratch disease and their pet cats. Scand. J. Infect. Dis. 30:387-391. [DOI] [PubMed] [Google Scholar]
- 31.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Welch, D. F., D. A. Pickett, L. N. Slater, A. G. Steigerwalt, and D. J. Brenner. 1992. Rochalimaea henselae sp. nov., a cause of septicemia, bacillary angiomatosis, and parenchymal bacillary peliosis. J. Clin. Microbiol. 30:275-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang, J., J. Wang, Z. J. Yao, Q. Jin, Y. Shen, and R. Chen. 2003. GenomeComp: a visualization tool for microbial genome comparison. J. Microbiol. Methods 54:423-426. [DOI] [PubMed] [Google Scholar]
- 34.Zeaiter, Z., P. E. Fournier, and D. Raoult. 2002. Genomic variation of Bartonella henselae strains detected in lymph nodes of patients with cat scratch disease. J. Clin. Microbiol. 40:1023-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu, Y., P. E. Fournier, H. Ogata, and D. Raoult. 2005. Multispacer typing of Rickettsia prowazekii enabling epidemiological studies of epidemic typhus. J. Clin. Microbiol. 43:4708-4712. [DOI] [PMC free article] [PubMed] [Google Scholar]