Abstract
The immediate lack of market-dominating commercial products (Vitek or MicroScan) for susceptibility testing of the new glycolipopeptide, dalbavancin, requires a surrogate marker agent to assist microbiologists in the correct categorization of potentially indicated species (staphylococci and streptococci). Error-rate analyses for 16,749 isolates using vancomycin or teicoplanin results to categorize dalbavancin susceptibilities demonstrated that both glycopeptide agents were highly predictive of dalbavancin-susceptible results (nearly 100%) with only a rare minor error. Vancomycin test results most reliably predict dalbavancin susceptibility until validated commercial reagents become available for direct testing in clinical practice.
A recurrent problem with modern antimicrobial susceptibility testing is the delay in the approval of commercial susceptibility testing products containing new drugs after their release for clinical use by the US Food and Drug Administration. The appearance of new antimicrobials in commonly used automated systems (Vitek or Vitek 2; bioMerieux, Hazelwood, MO; MicroScan WalkAway; Dade Berhing, West Sacramento, CA) can lag by 6 to 18 months, compromising utilization of these agents in clinical practice and their entry into hospital formularies, where they may have significant favorable impact. In contrast, manual diffusion testing products (disks or Etest; AB BIODISK, Solna, Sweden) have more immediate utility along with published interpretive criteria that can be found in the reagent or antimicrobial product package inserts (Food and Drug Administration). To facilitate the prompter use of newer antimicrobial agents, clinical microbiologists could select an agent available on a commercial system in the same or a similar class to act as a “surrogate marker,” e.g., the so-called “class” disk or drug (1). Such practices have resulted in groupings of very similar agents in the standard documents (M2 or M7) (Table 1) of the Clinical and Laboratory Standards Institute (CLSI) (formerly National Committee for Clinical Laboratory Standards) (4, 5) or via independent publications recommending the use of surrogates until the new antimicrobial becomes available in widely applied diagnostic products (1, 11, 14, 18). Examples include the previous recommendations of testing levofloxacin or ciprofloxacin to predict gatifloxacin susceptibility (11), cefoxitin to predict cefotetan susceptibility (1), and ceftriaxone to predict susceptibility to the orally administered cephem, cefpodoxime (14).
TABLE 1.
Comparison of dalbavancin MIC results to those of vancomycin and teicoplanin tested against four gram-positive organism groupsa
| Organism | No. of strains tested
|
Dalbavancin MIC (μg/ml)e | No. (%) of strains for which vancomycin MIC (μg/ml) wasf:
|
No. (%) of strains for which teicoplanin MIC (μg/ml) was:
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vancomycin | Teicoplanin | ≤1 | 2 | 4 | ≤8 | 16 | ≥32 | |||||||||
| S. aureusb | 11,867 | 11,867 | ≤0.5 | 11,472 (96.7) | 394 (3.3) | 1 (<0.1) | 11,865 (>99.9) | 2 (<0.1) | ||||||||
|
|
1 |
|
||||||||||||||
| Coagulase-negative staphylococcic | 3,450 | 3,450 | ≤0.5 | 2,040 (59.1) | 1,388 (40.2) | 17 (0.5) | 3,329 (96.4) | 96 (2.8) | 20 (0.6) | |||||||
| 1 | 3 (<0.1) | 2 (<0.1) | 4 (0.1) | 1 (<0.1) | ||||||||||||
| β-Hemolytic streptococcid | 1,051 | 1,050 | ≤0.5 | 1,051 (100.0) | 1,050 (100.0) | |||||||||||
|
|
1 |
|
||||||||||||||
| Viridans group streptococcid | 381 | 380 | ≤0.5 | 381 (100.0) | 380 (100.0) | |||||||||||
| 1 | ||||||||||||||||
Susceptibility testing was by CLSI methods (4), and a total of 16,749 strains were tested.
For S. aureus, the CLSI susceptibility breakpoint for vancomycin is 2 μg/ml, and that for teicoplanin is ≤8 μg/ml (5).
For coagulase-negative staphylococci, the CLSI susceptibility breakpoint for vancomycin is 4 μg/ml, and that for teicoplanin is ≤8 μg/ml (5).
For β-hemolytic streptococci, the CLSI susceptibility breakpoint for vancomycin is ≤1 μg/ml (5), and intermediate and resistant categories have not been defined. No CLSI interpretive criteria for teicoplanin have been published for streptococcal isolates.
Two possible breakpoints (≤0.5 and ≤1 μg/ml) for dalbavancin were used. Higher concentrations (2 and ≥4 μg/ml) of dalbavancin were also tested, but the MIC of this drug was not higher than 1 μg/ml for any strain.
Higher concentrations (8, 16, and ≥32 μg/ml) of vancomycin were also tested, but the MIC of this drug was not higher than 4 μg/ml for any strain.
In this report, the results from simultaneous reference MIC testing of dalbavancin, vancomycin, and teicoplanin were analyzed to validate a potential “surrogate marker” agent for dalbavancin activity against indicated species. Dalbavancin is a novel injectable, bactericidal glycolipopeptide with enhanced activity against gram-positive cocci (3, 9, 16, 17), most similar to that demonstrated by teicoplanin and vancomycin. These MIC results from an international study platform (2001 to 2004) (7, 10, 12, 21) allowed the direct comparisons of reference MICs from 21,887 strains, both quantitatively and by interpretive category (4, 5, 18).
A total of 16,749 gram-positive cocci were entered into the final analysis (Table 1), each from a documented clinical infection within international surveillance trials (7, 10, 12, 21) monitoring organisms from Europe, North America, and Latin America. These organisms were distributed as follows for direct comparison of dalbavancin MICs with those of vancomycin or teicoplanin, respectively: Staphylococcus aureus, 11,867 or 11,867 strains; coagulase-negative staphylococci (CoNS), 3,450 or 3,450 strains; β-hemolytic streptococci, 1,051 or 1,050 strains; and viridans group streptococci, 381 or 380 strains (Table 1). Streptococcus pneumoniae (3,707 or 727 strains) and Enterococcus spp. (4,131 or 4,128 strains) were also tested, but their results were not included among skin and soft-tissue pathogen data presented here (8, 20, 21).
Validated broth microdilution reference test panels were utilized in trays produced by TREK Diagnostics (Cleveland, OH) (13), conforming to CLSI methods (4, 5). All quality control MIC determinations for CLSI-recommended strains (S. aureus ATCC 29213 and Enterococcus faecalis ATCC 29212) were within published ranges originally derived from studies that used 0.002% polysorbate 80-containing Mueller-Hinton broth for dalbavancin testing (4, 5).
Categorical interpretations for vancomycin and teicoplanin found in M100-S16 (2006) (5) were used for comparisons to dalbavancin quantitative test results (Table 1). Potential breakpoint concentrations for dalbavancin were based on published pharmacokinetic and pharmacodynamic studies (6, 15), as well as results from additional pharmacodynamic target (area under the concentration-time curve/MIC and time of concentration above MIC) attainment studies with Monte Carlo simulations that suggested conservative susceptible breakpoints ranging from ≤0.5 to ≤1 μg/ml (Pfizer Inc., unpublished data).
All MICs for teicoplanin and vancomycin (in μg/ml) were compared to those of dalbavancin by regression statistics and by scattergram plots (see Fig. 1 and 2). The error-rate bounding method used to minimize errors between drug interpretations was also applied (18). Errors in this analysis were defined as false-susceptible (very major) errors (susceptible to the reference agent surrogate but resistant to dalbavancin); false-resistant (major) errors (resistant to the reference surrogate glycopeptide but susceptible to dalbavancin); and minor errors (an intermediate result for one of the two compared agents; surrogate or dalbavancin). Error rates (as percentages) were calculated using all organisms as the denominator, but further analyses were limited by the variety of contemporary gram-positive isolates observed to be nonsusceptible to these agents (exceptions: teicoplanin versus CoNS, all agents versus enterococci). Generally, serious interpretative errors (very major or major) should be minimized (≤1.5% and ≤3%, respectively) while achieving an absolute categorical agreement between drugs at ≥90%; however, >95% agreement would be preferred.
FIG. 1.
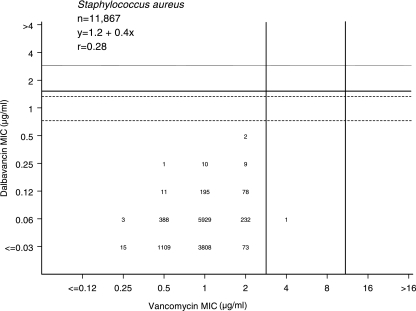
Scattergram plot comparing the dalbavancin and vancomycin MIC results for 11,867 S. aureus isolates tested by the broth microdilution method (4). Vertical solid lines indicate vancomycin CLSI (2006) interpretive criteria for susceptibility (≤2 μg/ml) and resistance (≥16 μg/ml). Solid and broken horizontal lines show potential dalbavancin MIC breakpoints for ≤1 and ≤0.5 μg/ml, respectively.
FIG. 2.
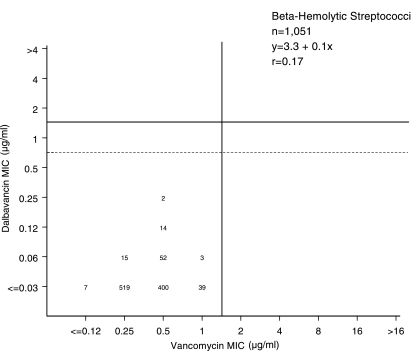
Scattergram plot comparing the dalbavancin and vancomycin MIC results for 1,051 β-hemolytic streptococcus isolates tested by the broth microdilution method (4). The single vertical line shows the susceptible-only breakpoint for vancomycin (≤1 μg/ml). Solid and broken horizontal lines show potential dalbavancin breakpoints of ≤1 and ≤0.5 μg/ml, respectively.
Table 1 lists the comparative MIC results for 11,867 S. aureus strains showing all dalbavancin MICs at ≤0.5 μg/ml. Only one and two strains had nonsusceptible vancomycin (MIC, >2 μg/ml) or teicoplanin (MIC, >8 μg/ml) results, respectively (5). Greater detail for S. aureus results can be observed in Fig. 1, where the potency of dalbavancin (MIC at which 90% of bacteria tested are inhibited [MIC90], 0.06 μg/ml) was 16-fold greater than that of vancomycin (MIC90, 1 μg/ml), and only one minor, false-intermediate, error (0.008%) was detected for a vancomycin-intermediate S. aureus (VISA) strain using the recently changed CLSI breakpoint (5). Since these results were from nearly 12,000 routine, contemporary clinical strains of S. aureus, we also determined the comparative potencies of vancomycin and dalbavancin when testing VISA (16 strains), hetero-VISA (78 strains), and vancomycin-resistant S. aureus (VRSA) (five strains) isolates. The results for vancomycin and dalbavancin (MIC range [MIC at which 50% of bacteria tested are inhibited] in μg/ml) were the following: for VISA, 4 to 8 (4) and 0.25 to 1 (0.5); for h-VISA, 0.5 to 2 (2) and 0.03 to 1 (0.12); and for VRSA, >16 (>16) and 1 to 16 (1). Only one VRSA strain among these 99 special S. aureus isolates had dalbavancin MIC results at ≤1 μg/ml. Teicoplanin was equally accurate in predicting dalbavancin susceptibility (99.8%) at either breakpoint concentration suggested.
For CoNS (3,450 strains), dalbavancin MICs ranged up to 1 μg/ml (only five occurrences) and vancomycin MICs to 4 μg/ml (the CLSI susceptible breakpoint) (5). Again, dalbavancin activity was significantly greater than that of vancomycin, with comparative MIC90 results of 0.12 and 2 μg/ml (16-fold), respectively (data not shown). Use of a dalbavancin breakpoint of ≤1 μg/ml achieved complete categorical agreement for vancomycin as a predictor of dalbavancin susceptibility, whereas the superior activity of dalbavancin and vancomycin against these CoNS species produced more-numerous, but acceptable, levels of conservative, major (0.7%; false resistant) and minor (2.8%) errors compared to that of teicoplanin. However, using teicoplanin to predict dalbavancin susceptibility had a 96.5% accuracy using the ≤0.5-μg/ml breakpoint and 96.6% accuracy at the ≤1-μg/ml breakpoint; all errors were false intermediate or false resistant.
Also listed in Table 1 are the comparisons of MIC results for the three glycopeptides tested against 1,432 streptococci. Teicoplanin breakpoints are not available (5) for comparisons with potential dalbavancin-susceptible breakpoint concentrations for streptococci. For β-hemolytic streptococci (including Streptococcus pyogenes), as well as α-hemolytic species, all had dalbavancin MIC90s at ≤0.03 μg/ml, compared to 0.5 or 1 μg/ml for vancomycin, e.g., ≥8-fold-greater activity (Fig. 2). When using vancomycin MICs to predict dalbavancin susceptibility, all isolates of β-hemolytic streptococci (Table 1; Fig. 2) were susceptible to vancomycin (MIC, ≤1 μg/ml) and had dalbavancin MICs at ≤0.25 μg/ml. Dalbavancin potency against viridans group streptococci was similarly predicted by vancomycin test results.
The dalbavancin spectrum of activity against enterococci most resembles that of teicoplanin, generally being inactive against vanA-type vancomycin-resistant enterococci (VRE) but harboring some residual potency versus vanB vancomycin-resistant enterococcus strains (7, 9, 10, 12, 21). Only false-intermediate (minor) or false-resistant (major) errors occurred using vancomycin as the surrogate to predict dalbavancin susceptibility at either selected breakpoint. Absolute categorical agreement between vancomycin and dalbavancin (≤1 μg/ml as susceptible, ≥4 μg/ml as resistant) was 94.3%, with no false-susceptible error. Teicoplanin as the surrogate marker for dalbavancin showed 95.9% absolute categorical agreement, but a small percentage of very major errors (0.2%; acceptable level) was detected (data not shown).
Dalbavancin, a potent glycolipopeptide active against many antimicrobial agent-resistant gram-positive cocci (3, 7, 9, 10, 12, 16, 17, 21), has demonstrated clinical efficacy against organisms associated with skin and soft-tissue infections (8, 20). Extensive pharmacokinetic and pharmacodynamic studies have characterized dalbavancin, and Monte Carlo simulations suggest potential susceptible breakpoint concentrations ranging from ≤0.5 μg/ml (staphylococci) to ≤4 μg/ml (streptococci) (Pfizer Inc., unpublished data) (2, 6, 15). Candidate dalbavancin interpretative criteria for MICs of ≤0.5 and ≤1 μg/ml were used in this analysis to determine if currently tested glycopeptides by commercial systems (validated against this reference test) could predict dalbavancin susceptibility with acceptable accuracy. These surrogate marker agents (vancomycin or teicoplanin) were observed to be highly predictive and produced only a single minor error (false-intermediate for a VISA strain) when using the 2006 CLSI vancomycin breakpoint (5).
Because of regulatory/manufacturer delays in the availability of the most-utilized commercial susceptibility products (automated or manual MIC systems), “surrogate marker” agents in the same class or a related class have been successfully applied by clinical laboratories over the last four decades (1, 11, 14). These options are particularly attractive if the new agent has greater potency and/or wider utility against the targeted species; this appears to be the case for dalbavancin (3, 7, 9, 10, 12, 16, 17, 19, 21). Further complicating this testing problem, the dalbavancin disk diffusion test has been observed to be suboptimal due to poor drug diffusion in agar and will not be available as an immediate, simple diagnostic procedure; but another agar diffusion method (Etest; AB BIODISK, Solna, Sweden) has been successfully evaluated for accuracy (Pfizer Inc., unpublished data). As demonstrated here (Table 1; Fig. 1 and 2) for dalbavancin tested against indicated species, very major test errors using the vancomycin surrogate did not occur, and this testing option could be used with relative confidence until commercial systems incorporate a reliable dalbavancin test. Where teicoplanin would be tested and reported, this agent could also be a surrogate for dalbavancin susceptibility with a test accuracy also approaching 100%.
Acknowledgments
We thank the following persons for contributions to statistical analysis or assistance with manuscript preparation: N. O'Mara, M. G. Stilwell, P. Strabala, and J. Ross.
This study was supported by an educational/research grant from Pfizer Inc.
REFERENCES
- 1.Barry, A. L., and R. N. Jones. 1987. Cross susceptibility and absence of cross resistance to cefotetan and cefoxitin. J. Clin. Microbiol. 25:1570-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buckwalter, M., and J. A. Dowell. 2005. Population pharmacokinetic analysis of dalbavancin, a novel lipoglycopeptide. J. Clin. Pharmacol. 45:1279-1287. [DOI] [PubMed] [Google Scholar]
- 3.Candiani, G., M. Abbondi, M. Borgonovi, G. Romano, and F. Parenti. 1999. In-vitro and in-vivo antibacterial activity of BI 397, a new semi-synthetic glycopeptide antibiotic. J. Antimicrob. Chemother. 44:179-192. [DOI] [PubMed] [Google Scholar]
- 4.Clinical and Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Document M7-A7. Clinical and Laboratory Standards Institute, Wayne, Pa.
- 5.Clinical and Laboratory Standards Institute. 2006. Performance standards for antimicrobial susceptibility testing, 16th informational supplement M100-S16. Clinical and Laboratory Standards Institute, Wayne, Pa.
- 6.Dorr, M. B., D. Jabes, M. Cavaleri, J. Dowell, G. Mosconi, A. Malabarba, R. J. White, and T. J. Henkel. 2005. Human pharmacokinetics and rationale for once-weekly dosing of dalbavancin, a semi-synthetic glycopeptide. J. Antimicrob. Chemother. 55(Suppl. 2):ii25-ii30. [DOI] [PubMed] [Google Scholar]
- 7.Gales, A. C., H. S. Sader, and R. N. Jones. 2005. Antimicrobial activity of dalbavancin tested against Gram-positive clinical isolates from Latin American medical centres. Clin. Microbiol. Infect. 11:95-100. [DOI] [PubMed] [Google Scholar]
- 8.Jauregui, L. E., S. Babazadeh, E. Seltzer, L. Goldberg, D. Krievins, M. Frederick, D. Krause, I. Satilovs, Z. Endzinas, J. Breaux, and W. O'Riordan. 2005. Randomized, double-blind comparison of once-weekly dalbavancin versus twice-daily linezolid therapy for the treatment of complicated skin and skin structure infections. Clin. Infect. Dis. 41:1407-1415. [DOI] [PubMed] [Google Scholar]
- 9.Jones, R. N., D. J. Biedenbach, D. M. Johnson, and M. A. Pfaller. 2001. In vitro evaluation of BI 397, a novel glycopeptide antimicrobial agent. J. Chemother. 13:244-254. [DOI] [PubMed] [Google Scholar]
- 10.Jones, R. N., T. R. Fritsche, H. S. Sader, and B. P. Goldstein. 2005. Antimicrobial spectrum and potency of dalbavancin tested against clinical isolates from Europe and North America (2003): initial results from an international surveillance protocol. J. Chemother. 17:593-600. [DOI] [PubMed] [Google Scholar]
- 11.Jones, R. N., and M. A. Pfaller. 2001. Can antimicrobial susceptibility testing results for ciprofloxacin or levofloxacin predict susceptibility to a newer fluoroquinolone, gatifloxacin?: report from The SENTRY Antimicrobial Surveillance Program (1997-99). Diagn. Microbiol. Infect. Dis. 39:237-243. [DOI] [PubMed] [Google Scholar]
- 12.Jones, R. N., M. G. Stilwell, H. S. Sader, T. R. Fritsche, and B. P. Goldstein. 2006. Spectrum and potency of dalbavancin tested against 3,322 Gram-positive cocci isolated in the United States Surveillance Program (2004). Diagn. Microbiol. Infect. Dis. 54:149-153. [DOI] [PubMed] [Google Scholar]
- 13.Jones, R. N., J. M. Streit, and T. R. Fritsche. 2004. Validation of commercial dry-form broth microdilution panels and test reproducibility for susceptibility testing of dalbavancin, a new very long-acting glycopeptide. Int. J. Antimicrob. Agents 23:197-199. [DOI] [PubMed] [Google Scholar]
- 14.Jones, R. N., and G. E. Zurenko. 1993. Prediction of bacterial susceptibility to cefpodoxime by using the ceftriaxone minimum inhibitory concentration result. Diagn. Microbiol. Infect. Dis. 17:313-316. [DOI] [PubMed] [Google Scholar]
- 15.Leighton, A., A. B. Gottlieb, M. B. Dorr, D. Jabes, G. Mosconi, C. VanSaders, E. J. Mroszczak, K. C. Campbell, and E. Kelly. 2004. Tolerability, pharmacokinetics, and serum bactericidal activity of intravenous dalbavancin in healthy volunteers. Antimicrob. Agents Chemother. 48:940-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin, G., K. Credito, L. M. Ednie, and P. C. Appelbaum. 2005. Antistaphylococcal activity of dalbavancin, an experimental glycopeptide. Antimicrob. Agents Chemother. 49:770-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malabarba, A., and B. P. Goldstein. 2005. Origin, structure, and activity in vitro and in vivo of dalbavancin. J. Antimicrob. Chemother. 55(Suppl. 2):ii15-ii20. [DOI] [PubMed] [Google Scholar]
- 18.National Committee for Clinical Laboratory Standards. 2001. Approved guideline M23-A2: development of in vitro susceptibility testing criteria and quality controls parameters, 2nd ed. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 19.Raad, I., R. Darouiche, J. Vazquez, A. Lentnek, R. Hachem, H. Hanna, B. Goldstein, T. Henkel, and E. Seltzer. 2005. Efficacy and safety of weekly dalbavancin therapy for catheter-related bloodstream infection caused by gram-positive pathogens. Clin. Infect. Dis. 40:374-380. [DOI] [PubMed] [Google Scholar]
- 20.Seltzer, E., M. B. Dorr, B. P. Goldstein, M. Perry, J. A. Dowell, and T. Henkel. 2003. Once-weekly dalbavancin versus standard-of-care antimicrobial regimens for treatment of skin and soft-tissue infections. Clin. Infect. Dis. 37:1298-1303. [DOI] [PubMed] [Google Scholar]
- 21.Streit, J. M., T. R. Fritsche, H. S. Sader, and R. N. Jones. 2004. Worldwide assessment of dalbavancin activity and spectrum against over 6,000 clinical isolates. Diagn. Microbiol. Infect. Dis. 48:137-143. [DOI] [PubMed] [Google Scholar]