Abstract
Penetration of cerebrospinal fluid (CSF) by artesunate and DHA was assessed in six adults with cerebral or severe malaria. Lumbar punctures were performed on admission and during convalescence, at 15 min (patient 1), 30 min (patient 2), 45 min (patient 3), 60 min (patient 4), 90 min (patient 5), and 120 min (patient 6) after intravenous administration of 120 mg of artesunate. No artesunate was detectable in CSF. In both studies, DHA levels in CSF increased with time while dihydroartemisinin levels in plasma fell. Dihydroartemisinin might accumulate in CSF during frequent artesunate dosing.
Artemisinin derivatives are potent antimalarial drugs, but there are concerns that they may be neurotoxic. Artemisinin-associated brain stem injury occurs in animals (4, 5, 11, 18), but despite the widespread use of artemisinin derivatives, evidence of human neurotoxicity is weak (10, 14). Nevertheless, inhibition of neuronal development by artemisinin drugs in vitro occurs at concentrations achieved in plasma during treatment of malaria (21).
An understanding of artemisinin-associated neurotoxicity in vivo requires knowledge of the penetration of the cerebrospinal fluid (CSF) by these drugs. Such data are sparse. Artemisinin derivatives cross the blood-brain barrier in rats (17). Concentrations of artemether in the CSF of dogs were low, and there was no detectable penetration by its active metabolite, dihydroartemisinin (DHA) (5). The pharmacokinetics of artesunate in CSF are unknown, even though it is the only derivative that can be given intravenously to severely ill patients, including those with cerebral involvement. There have been no studies of the pharmacokinetics of artemisinin derivatives in human CSF. We have therefore measured artesunate and DHA concentrations in plasma and CSF during initial artesunate treatment of cerebral malaria.
We studied six Vietnamese adults with severe falciparum malaria (25). Five had cerebral malaria (25), and the sixth underwent lumbar puncture to investigate meningism (Table 1). None of the study subjects had been treated with artesunate within 8 h of admission or with artemisinin or artemether within 24 h. Attendant first-degree relatives gave informed consent to the patients' participation in the acute-phase study. The patients themselves consented to follow-up lumbar puncture. The protocol was approved by the Ethics Committee of Cho Ray Hospital and the Vietnamese Ministry of Health.
TABLE 1.
Demographic, anthropometric, and laboratory data for the six study patients at the time of hospital admissiona
| Parameter | Median (range) |
|---|---|
| Age (yr) | 27 (17-62) |
| No. of men/no. of women | 5/1 |
| Weight (kg) | 53 (42-60) |
| Oral temp (°C) | 37.8 (37.0-38.3) |
| Glasgow coma score | 10 (5-15) |
| Venous hematocrit level (%) | 36.5 (21.5-38.5) |
| Parasitemia (no. of parasites/μl) | 2,530 (165-28,905) |
| Glucose concn in plasma (mmol/liter) | 6.0 (3.7-16.6) |
| Creatinine concn in serum (μmol/liter) | 159 (44-389) |
| Bilirubin concn in serum (μmol/liter) | 142 (58-282) |
| Aspartate aminotransferase concn in | 82 (48-195) |
| serum (U/liter) | |
| Parasite clearance time (h) | 10 (3-20) |
| Fever clearance time (h) | 54 (18-84) |
| Coma recovery time (days; n = 5) | 3 (1-7) |
Clinical and parasitologic measurements of treatment response are also shown.
After rehydration and resuscitation, a blood sample was taken for artesunate and DHA assay (0 min) and 120 mg of artesunate was injected intravenously. Lumbar punctures were performed on patient 1 at 15 min after artesunate administration, on patient 2 at 30 min, on patient 3 at 45 min, on patient 4 at 60 min, on patient 5 at 90 min, and on patient 6 at 120 min. CSF aliquots were taken for biochemistry, microscopy, and drug assay. A second blood sample was drawn at the same time as CSF sampling. Patients were monitored intensively, and complications were managed as previously described (25). Parasite counts were determined at least twice daily until parasite clearance. Further 60- to 120-mg artesunate doses were given intravenously at 24-h intervals until oral medication could be taken. Patients underwent repeat lumbar punctures when they were fully conscious, afebrile, and aparasitemic and had not received artesunate for >8 h. All blood and CSF samples were collected into chilled fluoride-oxalate tubes. Blood samples were centrifuged, and the plasma was separated promptly. All samples were stored and transported at temperatures of less than −20°C.
Validated high-performance liquid chromatography (2) was used for analysis of artesunate and DHA in plasma and artesunate in CSF. Assay of DHA in CSF was done by gas chromatography-mass spectrometry (15). For plasma assays, the relative standard deviations [RSDs] have been published (2, 3), while RSDs between runs were ≤11% over the ranges of artesunate and DHA concentrations found in our patients. The limits of detection were 80 nmol/liter for artesunate and 70 nmol/liter for DHA. For DHA in CSF, the RSDs within runs were ≤7.8% and the RSDs between runs were ≤16.0%. The limit of quantitation of DHA in CSF was 3 nmol/liter. Statistical analysis was done by nonparametric methods (SPSS for Windows; SPSS Inc., Chicago, Ill.). The CSF/plasma DHA concentration ratio was calculated from the areas under the plasma and CSF concentration-time curves from 0 to 120 min.
All patients recovered and were discharged an average of 7 days postadmission. One deeply comatose patient required assisted ventilation for 24 h, and two others required peritoneal dialysis. None of the patients suffered a convulsion or developed residual neurological sequelae. Clinical and parasitologic indices of response are summarized in Table 1. Follow-up lumbar punctures were performed an average of 5 days postadmission. Erythrocyte and white cell counts and protein concentrations in CSF and CSF/plasma glucose ratios were within the ranges reported for cerebral malaria (26).
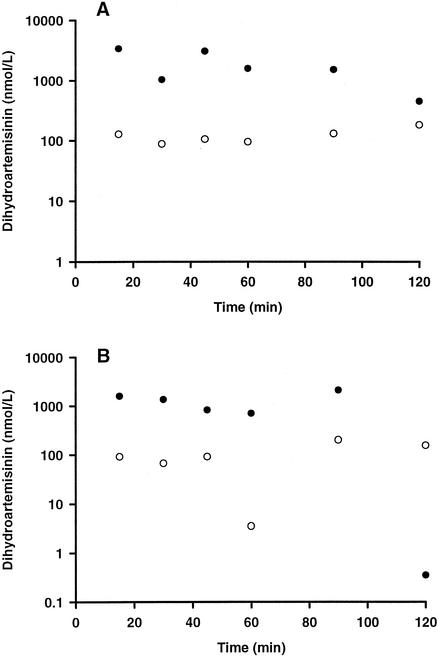
Artesunate was detected, at a low concentration (315 nmol/liter), in only the 15-min plasma sample of the acute-phase study. None of the CSF samples contained artesunate. DHA profiles in plasma and CSF are shown in Fig. 1. In the acute-phase study, DHA concentrations in CSF increased progressively as DHA concentrations in plasma fell. A similar, more variable pattern was seen at follow-up. There were no differences between the DHA levels in plasma and CSF when paired values from the acute-phase and follow-up studies were compared (P > 0.18). CSF/plasma DHA ratios were 0.095 and 0.072 for the acute-phase and follow-up studies, respectively.
FIG. 1.
DHA concentrations in paired plasma (•) and CSF (○) samples from six individual patients with severe falciparum malaria treated with 120 mg (312 μmol) of artesunate given as an intravenous injection. Panel A shows data for samples taken during the acute phase of the infection, and panel B shows data for samples taken during the follow-up study, when the patients had recovered consciousness and were blood slide negative for malaria.
Our data are the first relating to the penetration of CSF by artemisinin compounds in humans. In our single-dose study, the absence of artesunate in CSF was consistent with its low lipid solubility and the rapid fall in its concentration in plasma after intravenous injection (7). Artesunate is converted stoichiometrically to DHA, concentrations of which in plasma peak at 10 min (3, 7). DHA is highly lipid soluble and has a low molecular mass (284 Da), factors favoring penetration of CSF (13). At 15 min postinjection, the DHA concentration in CSF was 4% of that in plasma. The concentration in CSF increased with time, while that in plasma fell, suggesting continuing influx but a slower efflux of DHA. This may reflect a sink effect of DHA uptake transfer by lipid-rich brain structures (13), but the rate and extent of the movement of DHA from the CSF to the brain parenchyma are unknown. The transmembrane drug transporters multidrug resistance protein 1 (27) and P glycoprotein (19) may also influence DHA kinetics in CSF by preventing influx and efflux, respectively, even though the parent drug, artemisinin, is not a P-glycoprotein substrate (22). Increased blood-brain barrier permeability enhances penetration of the CSF by a drug (20). Since the acute- and convalescent-phase profiles of DHA in CSF were similar, our data are consistent with previous reports of normal cerebral capillary permeability in severe malaria (8).
The average CSF/plasma ratio from limited area under the concentration-time curve data (0.08 for both studies combined) shows that the concentration of DHA in CSF was generally <10% of that in plasma during the study. This concentration, 0.11 ± 0.02 μmol/liter (mean ± standard error of the mean; n = 12), is only 10% of that required to inhibit neurite outgrowth in vitro, 1 μmol/liter (21). We did not sample beyond 120 min, as concentrations of DHA in plasma after intravenous administration of artesunate are very low at this time (3, 7). By contrast, the CSF concentration-time profile suggests that DHA could persist in CSF beyond 120 min. Frequent and prolonged dosing could, therefore, promote DHA accumulation in CSF. The conventional artesunate regimen in severe malaria is 120 mg, followed by 60 mg at 4, 24, and 48 h (1). The long gaps between the later doses and the limited total duration of treatment should safeguard against human neurotoxicity, in contrast to the more intensive dosing regimens used in animal studies (4, 5, 11, 18). Artemether has greater neurotoxicity than artesunate in animals (9) and a longer half-life (16). It would be interesting to determine the pharmacokinetics of artemether and DHA in the CSF of humans.
It is reassuring that there has been no evidence of neuropathology in large-scale artemether treatment trials (12, 23), in neurophysiologic studies of patients who received multiple courses of artemisinin drugs (24), and in brain stem sections from an artesunate-treated male who died of severe malaria (6). Our preliminary data provide evidence that this reflects the use of short-course therapy. Nevertheless, accumulation of artemisinin compounds such as artemether and DHA in CSF could explain the neurotoxicity found in vitro and in animal models. Prolonged frequent dosing of long-half-life compounds such as artemether might be inadvisable in severe malaria.
Acknowledgments
We are indebted to Truong Van Viet and staff at Cho Ray Hospital for supporting this study.
This work was supported by a project grant from the National Health and Medical Research Council of Australia (T.M.E.D. and K.F.I.).
REFERENCES
- 1.Barradell, L. B., and A. Fitton. 1995. Artesunate. A review of its pharmacology and therapeutic efficacy in the treatment of malaria. Drugs 50:714-741. [DOI] [PubMed] [Google Scholar]
- 2.Batty, K. T., T. M. E. Davis, L. T. A. Thu, T. Q. Binh, T. K. Anh, and K. F. Ilett. 1986. Selective high-performance liquid chromatographic determination of artesunate and alpha- and beta-dihydroartemisinin in patients with falciparum malaria. J. Chromatogr. B 677:345-350. [DOI] [PubMed] [Google Scholar]
- 3.Batty, K. T., L. T. A. Thu, T. M. E. Davis, K. F. Ilett, T. X. Mai, N. C. Hung, N. P. Tien, S. M. Powell, H. V. Thein, T. Q. Binh, and N. V. Kim. 1998. A pharmacokinetic and pharmacodynamic study of intravenous vs oral artesunate in uncomplicated falciparum malaria. Br. J. Clin. Pharmacol. 45:123-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brewer, T. G., S. J. Grate, J. O. Peggins, P. J. Weina, J. M. Petras, B. S. Levine, M. H. Heiffer, and B. G. Schuster. 1994. Fatal neurotoxicity of arteether and artemether. Am. J. Trop. Med. Hyg. 51:251-259. [DOI] [PubMed] [Google Scholar]
- 5.Classen, W., B. Altmann, P. Gretener, C. Souppart, P. Skelton Stroud, and G. Krinke. 1999. Differential effects of orally versus parenterally administered qinghaosu derivative artemether in dogs. Exp. Toxicol. Pathol. 51:507-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis, T. M. E., F. X. Breheny, P. A. Kendall, F. Daly, K. T. Batty, A. Singh, and K. F. Ilett. 1997. Severe falciparum malaria with hyperparasitaemia treated with intravenous artesunate. Med. J. Aust. 166:416-418. [DOI] [PubMed] [Google Scholar]
- 7.Davis, T. M. E., H. L. Phuong, K. F. Ilett, N. C. Hung, K. T. Batty, V. D. B. Phuong, S. M. Powell, H. V. Thien, and T. Q. Binh. 2001. Pharmacokinetics and pharmacodynamics of intravenous artesunate in severe falciparum malaria. Antimicrob. Agents Chemother. 45:181-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis, T. M. E., Y. Suputtamongkol, J. Spencer, S. Ford, N. Chienkul, W. Schulenburg, and N. J. White. 1992. Measures of capillary permeability in acute falciparum malaria: relation to severity of infection and treatment. Clin. Infect. Dis. 15:256-266. [DOI] [PubMed] [Google Scholar]
- 9.Dayan, A. D. 1998. Neurotoxicity and artemisinin compounds do the observations in animals justify limitation of clinical use? Med. Trop. (Mars) 58:32-37. [PubMed] [Google Scholar]
- 10.Elias, Z., E. Bonnet, B. Marchou, and P. Massip. 1999. Neurotoxicity of artemisinin: possible counseling and treatment of side effects. Clin. Infect. Dis. 28:1330-1331. [DOI] [PubMed] [Google Scholar]
- 11.Genovese, R. F., D. B. Newman, J. M. Petras, and T. G. Brewer. 1998. Behavioral and neural toxicity of arteether in rats. Pharmacol. Biochem. Behav. 60:449-458. [DOI] [PubMed] [Google Scholar]
- 12.Hien, T. T., N. P. J. Day, H. P. Nguyen, T. H. Nguyen, T. H. Tran, P. L. Pham, X. S. Dinh, V. C. Ly, V. Ha, D. Waller, T. E. Peto, and N. J. White. 1996. A controlled trial of artemether or quinine in Vietnamese adults with severe falciparum malaria. N. Engl. J. Med. 335:76-83. [DOI] [PubMed] [Google Scholar]
- 13.Kearney, B. P., and F. T. Aweeka. 1999. The penetration of anti-infectives into the central nervous system. Neurol. Clin. N. Am. 17:883-900. [DOI] [PubMed] [Google Scholar]
- 14.Miller, L. G., and C. B. Panosian. 1997. Ataxia and slurred speech after artesunate treatment for falciparum malaria. N. Engl. J. Med. 336:1328.. [DOI] [PubMed] [Google Scholar]
- 15.Mohamed, S. S., S. A. Khalid, S. A. Ward, T. S. Wan, H. P. Tang, M. Zheng, R. K. Haynes, and G. Edwards. 1999. Simultaneous determination of artemether and its major metabolite dihydroartemisinin in plasma by gas chromatography-mass spectrometry-selected ion monitoring. J. Chromatogr. B 731:251-260. [DOI] [PubMed] [Google Scholar]
- 16.Murphy, S., E. Mberu, D. Muhia, M. English, J. Crawley, C. Waruiru, B. Lowe, C. Newton, P. Winstanley, K. Marsh, and W. Watkins. 1997. The disposition of intramuscular artemether in children with cerebral malaria: a preliminary study. Trans. R. Soc. Trop. Med. Hyg. 91:331-334. [DOI] [PubMed] [Google Scholar]
- 17.Nui, X. Y., L. Y. Ho, Z. H. Ren, and Z. Y. Song. 1985. Metabolic fate of qinghaosu in rats: a new TLC densitometric method for its determination in biological material. Eur. J. Drug Metab. Pharmacokinet. 10:55-59. [DOI] [PubMed] [Google Scholar]
- 18.Petras, J. M., D. E. Kyle, M. Gettayacamin, G. D. Young, R. A. Bauman, H. K. Webster, K. D. Corcoran, J. O. Peggins, M. A. Vane, and T. G. Brewer. 1997. Arteether: risks of two-week administration in Macaca mulatta. Am. J. Trop. Med. Hyg. 56:390-396. [DOI] [PubMed] [Google Scholar]
- 19.Rao, V. V., J. L. Dahlheimer, M. E. Bardgett, A. Z. Snyder, R. A. Finch, A. C. Sartorelli, and D. Piwnica-Worms. 1999. Choroid plexus epithelial expression of MDR1 P glycoprotein and multidrug resistance-associated protein contribute to the blood-cerebrospinal-fluid drug-permeability barrier. Proc. Natl. Acad. Sci. USA 96:3900-3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scheld, W. M. 1989. Drug delivery to the central nervous system: general principles and relevance to therapy for infections of the central nervous system. Rev. Infect. Dis. 11:S1669-S1690. [DOI] [PubMed] [Google Scholar]
- 21.Smith, S. L., C. J. Sadler, C. C. Dodd, G. Edwards, S. A. Ward, B. K. Park, and W. G. McLean. 2001. The role of glutathione in the neurotoxicity of artemisinin derivatives in vitro. Biochem. Pharmacol. 61:409-416. [DOI] [PubMed] [Google Scholar]
- 22.Svensson, U. S., R. Sandstrom, O. Carlborg, H. Lennernas, and M. Ashton. 1999. High in situ rat intestinal permeability of artemisinin unaffected by multiple dosing and with no evidence of P-glycoprotein involvement. Drug Metab. Dispos. 27:227-232. [PubMed] [Google Scholar]
- 23.Van-Hensbroek, M. B., E. Onyiorah, S. Jaffar, G. Schneider, A. Palmer, J. Frenkel, G. Enwere, S. Forsk, A. Nusmeijer, S. Bennett, B. Greenwood, and D. Kwiatkowski. 1996. A trial of artemether or quinine in children with cerebral malaria. N. Engl. J. Med. 335:69-75. [DOI] [PubMed] [Google Scholar]
- 24.Van Vugt, M., B. J. Angus, R. N. Price, C. Mann, J. A. Simpson, C. Poletto, S. E. Htoo, S. Looareesuwan, N. J. White, and F. Nosten. 2000. A case-control auditory evaluation of patients treated with artemisinin derivatives for multidrug-resistant Plasmodium falciparum malaria. Am. J. Trop. Med. Hyg. 62:65-69. [DOI] [PubMed] [Google Scholar]
- 25.Warrell, D. A., M. Molyneux, and P. Beales. 1990. Severe and complicated malaria. Trans. R. Soc. Trop. Med. Hyg. 84(Suppl. 2):1-69. [Google Scholar]
- 26.White, N. J., and S. Looareesuwan. 1989. Cerebral malaria, p. 118-143. In G. T. Strickland (ed.), Infections of the nervous system. Saunders, London, England.
- 27.Wijnholds, J., E. C. de Lange, G. L. Scheffer, D. J. van den Berg, C. A. Mol, M. van der Valk, A. H. Schinkel, R. J. Scheper, D. D. Breimer, and P. Borst. 2000. Multidrug resistance protein 1 protects the choroid plexus epithelium and contributes to the blood-cerebrospinal fluid barrier. J. Clin. Investig. 105:279-285. [DOI] [PMC free article] [PubMed] [Google Scholar]