Abstract
A user-friendly self-sampling method for collecting representative cervical cell material would lower the threshold for women to respond to the invitation for cervical screening. In the present article, we introduce such a device; we have evaluated its sensitivity and specificity to detect high-grade cervical intraepithelial neoplasia (CIN), via high-risk human papillomavirus (hrHPV) detection and liquid-based cytology (LBC), compared to endocervical brush samples obtained by gynecologists. Women who had a cervical smear reading of moderate dyskaryosis or worse or a repeat equivocal Pap smear result in the cervical screening program (n = 64) and healthy volunteers (n = 32) took a self-obtained sample at home prior to their visit to the gynecological outpatient department. At the outpatient department, an endocervical brush smear was taken, followed by colposcopy and biopsy whenever applicable. Both self-obtained samples and endocervical brush samples were immediately collected in Surepath preservation solution and used for LBC and hrHPV testing (by general primer-mediated GP5+/6+ PCR). hrHPV test results showed a good concordance between the two sample types (87%; κ = 0.71), with sensitivities for prevalent high-grade CIN that did not differ significantly (92% and 95%; P = 1.0). The hrHPV test on self-obtained samples proved to be at least as sensitive for high-grade CIN as cytology on endocervical brush samples (34/37 versus 31/37; P = 0.5). LBC showed a poor concordance between self-obtained and endocervical brush samples (60%; κ = 0.27). In conclusion, self-obtained samples taken by this novel device are highly representative of the hrHPV status of the cervix. In combination with hrHPV testing, the use of this device may have implications for increasing the attendance rate for cervical screening programs.
Population-based screening for cervical cancer at present is based on exfoliative cytology that allows early detection of the premalignant stages. The premalignant lesions can be treated fairly easily and without major side effects. However, two major problems need to be overcome. Firstly, the specificity and sensitivity of cytological screening are subject to improvement. Given the causal relation between a persistent infection with high-risk human papillomavirus (hrHPV) and the development of cervical cancer (33) and its precursor lesions, additive testing for hrHPV is being considered for increasing the sensitivity and specificity of population-based cervical screening (8, 22).
Secondly, the compliance rate of current population-based cervical screening programs is not optimal. Annually, in The Netherlands, 30% of the invited women do not participate in the cervical screening program, and as is the case in the United Kingdom and the United States (23, 25, 26), half of the cervical cancers are diagnosed in this group of women (1, 30). A user-friendly self-sampling method for collecting representative cervical cell material at home would lower the threshold for women to participate in the screening. Recently, we conducted a study among more than 2,500 women who even after a recall had not responded to the invitation for screening (A. F. Bais, F. J. van Kemenade, J. Berkhof, R. H. M. Verheijen, P. J. F. Snijders, F. Voorhorst, M. Babovic, M. van Ballegooijen, T. J. M. Helmerhorst, and C. J. L. M. Meijer, submitted for publication). When offered the possibility of using a self-sampling method, almost 30% of these women responded actively by sending a self-obtained vaginal sample to the test laboratory. Vaginal self-sampling methods may not only be useful to increase participation of nonresponders to the cervical screening program but may also increase women's compliance to follow up in longitudinal studies of the natural history of cervical HPV infections or the effect of prophylactic vaccines (12).
Cervicovaginal self-obtained samples are highly representative for detection of cervical intraepithelial neoplasia (CIN) by HPV testing using PCR (12, 21) and Hybrid Capture 2 (9, 14, 27, 34). Ideally, cervicovaginal self-obtained samples should also be suitable for the detection of CIN by (reflex) cytological examination. The recent introduction of liquid-based cytology (LBC) media enables preservation of both cellular morphology and nucleic acids. Theoretically, this allows cytological examination and HPV testing on the same sample (18, 24, 35) because the DNA is also preserved.
In the present study, we introduce a novel, user-friendly cervicovaginal self-sampling device. We evaluated its sensitivity and specificity to detect high-grade CIN (CIN2 and CIN3) via hrHPV detection and LBC, compared to cervical scrapes obtained by gynecologists using an endocervical brush. Our results show that self-obtained samples are highly representative of the hrHPV status of the cervix and that the sensitivities of hrHPV testing for the detection of high-grade CIN are equal for self-obtained samples and endocervical brush samples. Although the concordance between LBC on endocervical brush samples and cervicovaginal self-obtained samples was poor, referral for colposcopy-directed biopsy of women with hrHPV positive and cytologically abnormal self-obtained samples would be an obvious recommendation because of the high number of high-grade CIN lesions in this group.
MATERIALS AND METHODS
Study cohort and sampling.
The study cohort was recruited between February and October 2004 and consisted of 96 women, 64 of whom were referred to the gynecologist for colposcopy-directed biopsy either because of a cervical smear reading of moderate dyskaryosis or worse or because of a repeat equivocal Pap smear result in the cervical screening program. The remaining 32 women were healthy volunteers. The median age of the women was 35 years (range, 18 to 59). The women were asked to take a self-obtained sample at home within the week before their visit to the gynecological outpatient department. All women complied with this request.
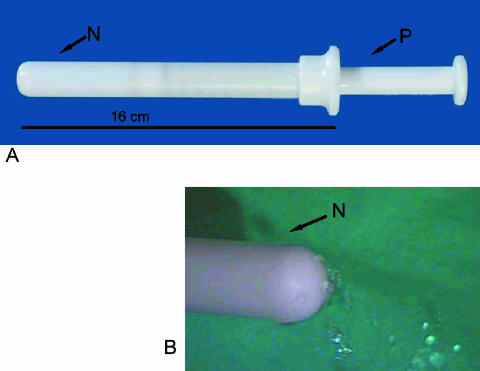
The novel self-sampling device (Mermaid) used in this study is shown in Fig. 1. The cervicovaginal self-obtained sample taken by this device was immediately collected in 10 ml Surepath preservation solution and used for LBC (Tripath, Burlington, NC) and hrHPV testing.
FIG. 1.
(A) The Mermaid cervicovaginal self-sampling method. The instrument comes ready-filled with 5 ml saline. After the insertion of the nozzle (N) into the vagina, the plunger (P) is pushed in order to release the saline via small holes in the nozzle (B) and rinse the upper vagina and the cervix. Then by releasing the plunger, the saline is aspirated back into the instrument. Upon removal of the instrument from the vagina, the saline now containing the sampled cells is released into a dedicated collection vial containing 10 ml SurePath fluid.
During the subsequent visit to the outpatient department an endocervical brush smear of the cervix was taken by the gynecologist followed by colposcopy, whenever applicable. Endocervical brush samples were also collected in 10 ml Surepath preservation solution and used for LBC and hrHPV testing. The time between sample collection and sample processing was at maximum two weeks; samples were mailed at ambient temperature, and upon receipt, they were stored at 4°C. All self-obtained samples and endocervical brush samples were sent to the laboratory with a four-digit code as the sole means of identification. In this way, the test laboratory was blinded for the sampling method and the participants. When a cervical lesion was observed during colposcopy, a biopsy specimen was taken for histological evaluation.
All LBC slides were judged by trained cytologists and a cytopathologist using the CISOE-A classification as used in The Netherlands (13), the interpretation of which can be easily translated into the Bethesda system (2, 29). All slides with a suspicious diagnosis were reevaluated by a cytopathologist (Folkert van Kemenade).
hrHPV testing.
The test for the presence of hrHPV was done by general primer-mediated GP5+/6+ PCR-enzyme immunoassay (PCR-EIA), which detects the 15 hrHPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, 73, and 82 (20), the putative high-risk types HPV 26, 53, and 66 (21), and HPV type 67, which is phylogenetically related to alpha-9 hrHPV types (11). In addition, PCR products of EIA positive women were subjected to HPV genotyping using reverse line blotting (RLB). In addition to detecting the high-risk HPV types detected in the PCR-EIA, the RLB system can separately detect the low-risk HPV types 6, 11, 40, 42, 43, 44, 54, 55, 57, 61, 70, 71, 72, 81, and cp6108; it also contains one lane of cocktail probe to detect the low-risk types 32, 83, 84, 85, 86, and jc9710. PCR-EIA and RLB protocols were described previously (31). Because the SurePath medium does not allow direct PCR, DNA was extracted from the samples by using the High Pure PCR template purification system (Roche, Basel, Switzerland). Briefly, 1 ml of the SurePath suspension was centrifuged for 10 min at 13,000 rpm, the SurePath medium was removed, and the pellet was resuspended in 200 μl 10 mM Tris-HCl, pH 8, and processed according to the manufacturer's instructions. The elution volume was 100 μl. Prior to the study, reconstruction experiments were performed using serial dilutions of HPV16-positive SiHa cells in HPV-negative keratinocytes suspended in SurePath medium. These experiments revealed the sample analytical sensitivity and specificity as that obtained from the same cell mixtures suspended in phosphate-buffered saline (data not shown).
As a quality control for the presence of DNA and absence of PCR inhibitors in the isolated material, we performed a 209-bp PCR for the beta-globin gene as described previously (10). As positive controls for PCR-EIA and RLB, a dilution series containing 10 ng, 1 ng, and 100 pg SiHa cell line DNAs in a background of 100 ng human placental DNA was used. Negative controls consisted of High Pure PCR template purification system isolation blanks and PCR blanks, both inserted before and after sample aliquoting.
Statistical analysis.
Kappa statistics were used to assess the agreement between the cytology results and hrHPV test results obtained with the corresponding self-obtained and endocervical brush samples. Differences in sensitivity and specificity between self-obtained and endocervical brush samples were assessed using chi-square tests (McNemar). The significance level was set at 0.05. Confidence intervals of proportions were calculated based on binomial distribution. All analyses were performed using SPSS 9.0 software.
RESULTS
hrHPV test.
The overall hrHPV data obtained from self-obtained samples versus endocervical brush samples are given in Table 1. hrHPV test results showed a good concordance between self-obtained and endocervical brush samples (87%, κ = 0.71). Table 1 shows hrHPV test results of the two sampling methods for women with or without CIN2, CIN3, or cervical cancer. The agreement between both methods was 92% (κ = 0.36). The sensitivity of hrHPV testing for underlying CIN2, CIN3, or cervical cancer did not differ significantly between the self-obtained and endocervical brush samples (34/37 versus 35/37; P = 1.0). In addition, the specificity of hrHPV testing did not differ significantly between the two sampling methods (56% and 54%, respectively; P = 1.0). Compared to LBC, the hrHPV test revealed a significantly higher sensitivity for CIN2, CIN3, or cervical cancer on self-obtained samples (34/37 versus 24/37; P = 0.002). The hrHPV test on self-obtained samples proved to be at least as sensitive for CIN2, CIN3, and cervical cancer as cytology on endocervical brush samples (34/37 versus 31/37; P = 0.5).
TABLE 1.
hrHPV test results for corresponding cervicovaginal self-obtained samples and endocervical brush samplesa
| Group and self-sampling method result | No. of endocervical brush samples with result that was:
|
Total no. of samples per group with indicated condition | ||
|---|---|---|---|---|
| Positive | Negative | NA | ||
| Women with: | ||||
| CIN1 or no CIN | ||||
| Positive | 25 | 5 | 1 | 31 |
| Negative | 4 | 20 | 2 | 26 |
| NA | 2 | 2 | ||
| Total | 29 | 25 | 5 | 59 |
| ≥CIN2 | ||||
| Positive | 33b | 1 | 34 | |
| Negative | 2 | 1 | 3 | |
| Total | 35 | 2 | 37 | |
| Overall | ||||
| Positive | 53 | 5 | 1 | 59 |
| Negative | 7 | 26 | 2 | 35 |
| NA | 2 | 2 | ||
| Total | 60 | 31 | 5 | 96 |
≥CIN2 signifies CIN2, CIN3, or cervical cancer. NA, not assessable.
For the three women with invasive carcinomas, both the self-obtained sample and the endocervical brush sample were hrHPV positive.
A combination of hrHPV testing and cytology yielded a specificity for CIN2, CIN3, and cervical cancer of 89% for the self-sampling method versus 63% for the endocervical brush sampling method (P = 0.001).
HPV genotyping.
The frequencies of the HPV genotypes detected in the self-obtained and endocervical brush samples are summarized in Table 2.
TABLE 2.
Distribution of hrHPV and probably hrHPV types in cervicovaginal self-obtained samples and endocervical brush samples from women who showed hrHPV PCR-EIA positivity in both samples
| hrHPV type | Infection frequencya
|
|
|---|---|---|
| Self-obtained samples | Endocervical brush samples | |
| 16 | 23 | 26 |
| 18 | 8 | 8 |
| 26 | 0 | 0 |
| 31 | 12 | 14 |
| 33 | 2 | 2 |
| 35 | 2 | 1 |
| 39 | 5 | 5 |
| 45 | 0 | 0 |
| 51 | 3 | 3 |
| 52 | 1 | 1 |
| 53 | 3 | 3 |
| 56 | 7 | 8 |
| 58 | 2 | 1 |
| 59 | 1 | 0 |
| 66 | 3 | 2 |
| 67b | 3 | 3 |
| 68 | 2 | 2 |
| 73 | 0 | 0 |
| 82 | 0 | 0 |
| Xc | 2 | 2 |
| Multiple hrHPV infections | 16 | 16 |
Frequencies indicated here include presence of types both in single and multiple infections. Values are numbers of samples.
HPV67 risk factor is at present unknown. It is classified here as high risk because of homology with alpha-9 HPV types (such as HPV16).
HPVX means that the hrHPV cocktail EIA was positive but no signal was obtained with any of the separate probes in the RLB analysis.
For 53 women, both self-obtained and endocervical brush samples were hrHPV positive by the EIA. Fifty (94%) of these women showed concordant results for at least one HPV type (95% confidence interval, 84 to 99%) on both samples. Full type concordance was observed in 36 (68%) women. One woman had discordant typing results, showing HPV type 59 in the self-obtained sample and a multiple infection of types 16, 31, and 56 and low-risk types 42 and 55 in the endocervical brush sample. LBC showed normal cytology in her self-obtained sample, whereas the endocervical brush sample revealed mild dyskaryosis. The sample pairs of the remaining two women were hrHPV positive by the EIA but revealed no HPV genotype by RLB. Therefore, their HPV types were classified as “X” and type concordance could be neither confirmed nor excluded.
Cytology.
The cytological results of LBC samples obtained with the cervicovaginal self-obtained sampler and the endocervical brush are summarized in Table 3. Overall, the cervicovaginal self-sampling method showed 61 cases of normal cytology, 27 cases of borderline to mild dyskaryosis (BMD), and 8 cases worse than BMD. For the endocervical brush samples, these numbers were 35, 35, and 25, respectively, whereas one endocervical brush sample could not be judged.
TABLE 3.
Comparison of cytology results from cervicovaginal self-obtained samples and endocervical brush samplesa
| Self-sampling method result | No. of endocervical brush samples with result that was:
|
Total | |||
|---|---|---|---|---|---|
| NA | Normal | BMD | >BMD | ||
| Normal | 1 | 29 | 24 | 7 | 61 |
| BMD | 6 | 10 | 11 | 27 | |
| >BMD | 0 | 1 | 7 | 8 | |
| Total | 1 | 35 | 35 | 25 | 96 |
NA, not accessable; >BMD, worse than BMD.
The agreement between LBC results (normal cytology versus “borderline dyskaryosis or worse”) of the two sampling methods was poor (60%; κ = 0.27).
Histological data were available for 61 of the 96 women. High-grade CIN or cervical cancer was observed in 37 of these women (3 with invasive carcinomas, 21 with CIN3, and 13 with CIN2). Low-grade CIN (CIN1) was present in 8 women, and 16 women had no CIN. The remaining 35 women for whom no histological data were available either did not undergo colposcopy (n = 30) or had no visible abnormalities at the squamocolumnar junction at the time of colposcopy (n = 5). Therefore, no biopsy specimens were taken.
The cytological results of the two sampling methods in women with or without high-grade CIN or cervical cancer are given in Tables 4 and 5. Figure 2 shows the cytology of three pairs of self-obtained and endocervical brush samples where concordance was good. The agreement between the two sampling methods in women with CIN2, CIN3, or cervical cancer was poor (65%; κ = 0.12). The sensitivity of cytology for underlying CIN2, CIN3, and cervical cancer tended to be lower for the self-obtained cervicovaginal samples than for the endocervical brush samples, but this difference was not statistically significant (24/37 versus 31/37; P = 0.09). However, the specificity of cytology for CIN2, CIN3, and cervical cancer among self-obtained samples was significantly higher than that among endocervical brush samples (81% versus 50%; P = 0.0003), because of its lower sensitivity for CIN2, CIN3, or cervical cancer.
TABLE 4.
Cytology results from cervicovaginal self-obtained samples and endocervical brush samples from women with CIN2 or CIN3a
| Self-sampling method result | No. of endocervical brush samples with result that was:
|
Total | |
|---|---|---|---|
| Normal | ≥BMD | ||
| Normal | 3 | 10 | 13 |
| ≥BMD | 3 | 21b | 24 |
| Total | 6 | 31 | 37 |
≥BMD, BMD or worse dyskaryosis.
For the three women with invasive carcinomas, both the self-obtained sample and the endocervical brush sample read ≥BMD.
TABLE 5.
Cytology results from cervicovaginal self-obtained samples and endocervical brush samples from healthy women and women with CIN1a
| Self-sampling method result | No. of endocervical brush samples with result that was:
|
Total | ||
|---|---|---|---|---|
| NA | Normal | ≥BMD | ||
| Normal | 1 | 26 | 21 | 48 |
| ≥BMD | 3 | 8 | 11 | |
| Total | 1 | 29 | 29 | 59 |
NA, not assessable; ≥BMD, BMD or worse dyskaryosis.
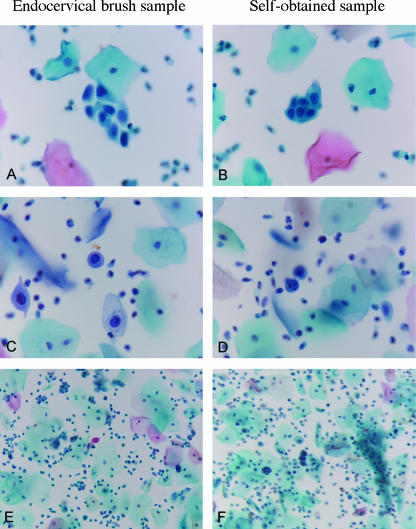
FIG. 2.
Representative thin layer sample pairs read as having conditions worse than BMD obtained from endocervical brush samples (A, C, and E) and the corresponding self-obtained samples (B, D, and F). (A through D) Objective, ×40; (E and F) objective, ×20.
DISCUSSION
We evaluated a novel self-sampling device for its sensitivity and specificity to detect CIN2, CIN3, or cervical cancer via hrHPV detection and LBC, compared to endocervical brush samples obtained by gynecologists. The cervicovaginal self-obtained samples obtained with this method are highly representative of the hrHPV status of the cervix—which is in line with previous studies (9, 12, 14, 21, 27, 34)—but not for cytology. In particular, the sensitivity of cytology on self-obtained samples is low, which is probably due to the fact that self-obtained samples mostly contain vaginal cells and only a few cervical cells. However, the specificity of cytology on cervicovaginal self-obtained samples for the detection of CIN2, CIN3, or cervical cancer is quite high, especially when combined with hrHPV testing (81% and 89%, respectively). This indicates that abnormal cytology (worse than BMD) in an hrHPV-positive cervicovaginal self-obtained sample warrants referral for colposcopy-directed biopsy.
The good HPV-type concordance between the corresponding self-obtained and endocervical brush samples indicates that the self-obtained samples are representative of the HPV types which infect the cervix. A likely explanation for this good HPV-type concordance is the presence of viral particles or viral DNA fragments in the vagina due to exfoliation of infected cells from the cervix. An alternative explanation is that the vaginal epithelium itself is infected with HPV. This would be in line with the previous finding that a vaginal hrHPV infection can apparently be maintained without the need of the transformation zone, although only the transformation zone is susceptible to HPV-mediated carcinogenesis (6). The good representation of the prevalent HPV types in the self-obtained samples is especially interesting in the light of recent studies (3, 4, 5, 16). These studies show that infections with HPV types 16 and 18 require extra follow-up because these HPV types confer an increased risk of high-grade cervical lesions compared to other types.
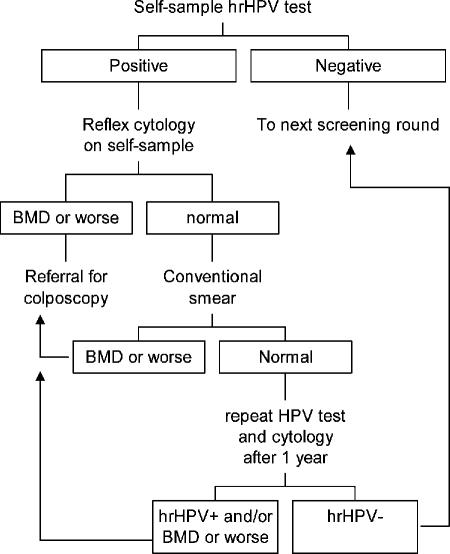
A possible algorithm of using the HPV and cytology results of cervicovaginal self-obtained material as a primary screening tool is depicted schematically in Fig. 3. Although hrHPV testing followed by reflex cytology on self-obtained cervicovaginal material results in the detection of more than half of the high-grade CIN lesions, a disadvantage of this possible algorithm is the relatively low positive predictive value of hrHPV testing and thus the large number of repeat smears needed for hrHPV-positive women with normal cytology. At present, markers to improve risk stratification of these women with hrHPV-positive cytologically normal smears are under evaluation. For example, analysis of hrHPV load analysis proved to be predictive of the risk of CIN2, CIN3, or cervical cancer even in cytologically normal smears (15, 28, 32). In addition, hrHPV oncogene E6/E7 mRNA assessment may be a useful marker for risk assessment, probably because it distinguishes a transcriptionally active hrHPV infection from a clinically irrelevant infection (7, 19). Finally, p16INK4a immunocytochemistry (17) may be a useful tool to prevent false negativity in cytology because it facilitates the detection of dysplastic cells. Whether staining for p16INK4a is also applicable on self-obtained samples remains to be investigated.
FIG. 3.
A possible algorithm for follow-up of hrHPV positive self-obtained samples. These samples could be used for reflex cytology, and if this turns out to be abnormal, women should be referred for colposcopy-directed biopsy because the specificity of combined hrHPV testing and cytology on cervicovaginal self-obtained samples is quite high for CIN2, CIN3, and cervical cancer. If cytology of an hrHPV-positive cervicovaginal self-obtained sample is normal, an endocervical brush smear should be taken for cytology. If this smear is cytologically abnormal (BMD or worse dyskaryosis), referral for colposcopy is warranted. If the smear is cytologically normal, advisement for a repeat cervical smear after 1 year could be given for hrHPV testing and cytology. If this repeat smear is cytologically normal and hrHPV negative, women can go to the next screening round. Women should be referred for colposcopy-directed biopsy if the repeat smear is hrHPV positive and/or cytologically abnormal (BMD or worse dyskaryosis).
In summary, self-obtained cervicovaginal samples taken by this novel Mermaid device are particularly representative for hrHPV analysis. In combination with hrHPV testing, the use of this device may have implications for increasing the attendance rate for cervical screening programs. The value of self-sampling methods in population-based screening and its putative role for evaluation of effectiveness of preventive vaccination studies are presently under investigation.
Acknowledgments
This study has received approval (no. 0335) by the institutional review board on human studies at the Máxima Medical Center (Veldhoven, The Netherlands).
REFERENCES
- 1.Bulk, S., O. Visser, L. Rozendaal, R. H. Verheijen, and C. J. Meijer. 2005. Cervical cancer in The Netherlands 1989-1998: decrease of squamous cell carcinoma in older women, increase of adenocarcinoma in younger women. Int. J. Cancer 113:1005-1009. [DOI] [PubMed] [Google Scholar]
- 2.Bulk, S., F. J. van Kemenade, L. Rozendaal, and C. J. Meijer. 2004. The Dutch CISOE-A framework for cytology reporting increases efficacy of screening upon standardisation since 1996. J. Clin. Pathol. 57:388-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bulk, S., J. Berkhof, N. W. Bulkmans, G. D. Zielinski, L. Rozendaal, F. J. van Kemenade, P. J. Snijders, and C. J. Meijer. 2006. Preferential risk of HPV16 for squamous cell carcinoma and of HPV18 for adenocarcinoma of the cervix compared to women with normal cytology in The Netherlands. Br. J. Cancer 94:171-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bulkmans, N. W., M. C. Bleeker, J. Berkhof, F. J. Voorhorst, P. J. Snijders, and C. J. Meijer. 2005. Prevalence of types 16 and 33 is increased in high-risk human papillomavirus positive women with cervical intraepithelial neoplasia grade 2 or worse. Int. J. Cancer 117:177-181. [DOI] [PubMed] [Google Scholar]
- 5.Castle, P. E., D. Solomon, M. Schiffman, and C. M. Wheeler. 2005. Human papillomavirus type 16 infections and 2-year absolute risk of cervical precancer in women with equivocal or mild cytologic abnormalities. J. Natl. Cancer Inst. 97:1066-1071. [DOI] [PubMed] [Google Scholar]
- 6.Castle, P. E., M. Schiffman, M. C. Bratti, A. Hildesheim, R. Herrero, M. L. Hutchinson, A. C. Rodriguez, S. Wacholder, M. E. Sherman, H. Kendall, R. P. Viscidi, J. Jeronimo, J. E. Schussler, and R. D. Burk. 2004. A population-based study of vaginal human papillomavirus infection in hysterectomized women. J. Infect. Dis. 190:458-467. [DOI] [PubMed] [Google Scholar]
- 7.Cuschieri, K. S., M. J. Whitley, and H. A. Cubie. 2004. Human papillomavirus type specific DNA and RNA persistence-implications for cervical disease progression and monitoring. J. Med. Virol. 73:65-70. [DOI] [PubMed] [Google Scholar]
- 8.Cuzick, J., A. Szarewski, G. Terry, L. Ho, A. Hanby, P. Maddox, M. Anderson, G. Kocjan, S. T. Steele, and J. Guillebaud. 1995. Human papillomavirus testing in primary cervical screening. Lancet 345:1533-1536. [DOI] [PubMed] [Google Scholar]
- 9.Dannecker, C., U. Siebert, C. J. Thaler, D. Kiermeir, H. Hepp, and P. Hillemanns. 2004. Primary cervical cancer screening by self-sampling of human papillomavirus DNA in internal medicine outpatient clinics. Ann. Oncol. 15:863-869. [DOI] [PubMed] [Google Scholar]
- 10.de Roda Husman, A. M., P. J. Snijders, H. V. Stel, A. J. van den Brule, C. J. Meijer, and J. M. Walboomers. 1995. Processing of long-stored archival cervical smears for human papillomavirus detection by the polymerase chain reaction. Br. J. Cancer 72:412-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Villiers, E. M., C. Fauquet, T. R. Broker, H. U. Bernard, and H. zur Hausen. 2004. Classification of papillomaviruses. Virology 324:17-27. [DOI] [PubMed] [Google Scholar]
- 12.Gravitt, P. E., J. V. Lacey, Jr., L. A. Brinton, W. A. Barnes, J. R. Kornegay, M. D. Greenberg, S. M. Greene, O. C. Hadjimichael, L. McGowan, R. Mortel, P. E. Schwartz, R. Zaino, and A. Hildesheim. 2001. Evaluation of self-collected cervicovaginal cell samples for human papillomavirus testing by polymerase chain reaction. Cancer Epidemiol. Biomarkers Prev. 10:95-100. [PubMed] [Google Scholar]
- 13.Hanselaar, A. G. 2002. Criteria for organized cervical screening programs. Special emphasis on The Netherlands program. Acta Cytol. 46:619-629. [DOI] [PubMed] [Google Scholar]
- 14.Hillemanns, P., R. Kimmig, U. Huttemann, C. Dannecker, and C. J. Thaler. 1999. Screening for cervical neoplasia by self-assessment for human papillomavirus DNA. Lancet 354:1970. [DOI] [PubMed] [Google Scholar]
- 15.Josefsson, A. M., P. K. Magnusson, N. Ylitalo, P. Sorensen, P. Qwarforth-Tubbin, P. K. Andersen, M. Melbye, H. O. Adami, and U. B. Gyllensten. 2000. Viral load of human papilloma virus 16 as a determinant for development of cervical carcinoma in situ: a nested case-control study. Lancet 355:2189-2193. [DOI] [PubMed] [Google Scholar]
- 16.Khan, M. J., P. E. Castle, A. T. Lorincz, S. Wacholder, M. Sherman, D. R. Scott, B. B. Rush, A. G. Glass, and M. Schiffman. 2005. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J. Natl. Cancer Inst. 97:1072-1079. [DOI] [PubMed] [Google Scholar]
- 17.Klaes, R., T. Friedrich, D. Spitkovsky, R. Ridder, W. Rudy, U. Petry, G. Dallenbach-Hellweg, D. Schmidt, and D. M. von Knebel. 2001. Overexpression of p16(INK4A) as a specific marker for dysplastic and neoplastic epithelial cells of the cervix uteri. Int. J. Cancer 92:276-284. [DOI] [PubMed] [Google Scholar]
- 18.Manos, M. M., W. K. Kinney, L. B. Hurley, M. E. Sherman, J. Shieh-Ngai, R. J. Kurman, J. E. Ransley, B. J. Fetterman, J. S. Hartinger, K. M. McIntosh, G. F. Pawlick, and R. A. Hiatt. 1999. Identifying women with cervical neoplasia: using human papillomavirus DNA testing for equivocal Papanicolaou results. JAMA 281:1605-1610. [DOI] [PubMed] [Google Scholar]
- 19.Molden, T., J. F. Nygard, I. Kraus, F. Karlsen, M. Nygard, G. B. Skare, H. Skomedal, S. O. Thoresen, and B. Hagmar. 2005. Predicting CIN2+ when detecting HPV mRNA and DNA by PreTect HPV-proofer and consensus PCR: a 2-year follow-up of women with ASCUS or LSIL Pap smear. Int. J. Cancer 114:973-976. [DOI] [PubMed] [Google Scholar]
- 20.Munoz, N., F. X. Bosch, S. de Sanjose, R. Herrero, X. Castellsague, K. V. Shah, P. J. Snijders, and C. J. Meijer. 2003. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N. Engl. J. Med. 348:518-527. [DOI] [PubMed] [Google Scholar]
- 21.Nobbenhuis, M. A., T. J. Helmerhorst, A. J. van den Brule, L. Rozendaal, L. H. Jaspars, F. J. Voorhorst, R. H. Verheijen, and C. J. Meijer. 2002. Primary screening for high risk HPV by home obtained cervicovaginal lavage is an alternative screening tool for unscreened women. J. Clin. Pathol. 55:435-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nobbenhuis, M. A., J. M. Walboomers, T. J. Helmerhorst, L. Rozendaal, A. J. Remmink, E. K. Risse, H. C. van der Linden, F. J. Voorhorst, P. Kenemans, and C. J. Meijer. 1999. Relation of human papillomavirus status to cervical lesions and consequences for cervical-cancer screening: a prospective study. Lancet 354:20-25. [DOI] [PubMed] [Google Scholar]
- 23.Peto, J., C. Gilham, O. Fletcher, and F. E. Matthews. 2004. The cervical cancer epidemic that screening has prevented in the UK. Lancet 364:249-256. [DOI] [PubMed] [Google Scholar]
- 24.Qureshi, M. N., D. Bolick, P. J. Ringer, F. L. Spagler, and G. Zimmerman. 2005. HPV testing in liquid cytology specimens: comparison of analytic sensitivity and specificity for in situ hybridization and chemiluminescent nucleic acid testing. Acta Cytol. 49:120-126. [DOI] [PubMed] [Google Scholar]
- 25.Sasieni, P. D., J. Cuzick, E. Lynch-Farmery, et al. 1996. Estimating the efficacy of screening by auditing smear histories of women with and without cervical cancer. Br. J. Cancer 73:1001-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sawaya, G. F., and D. A. Grimes. 1999. New technologies in cervical cytology screening: a word of caution. Obstet. Gynecol. 94:307-310. [DOI] [PubMed] [Google Scholar]
- 27.Sellors, J. W., A. T. Lorincz, J. B. Mahony, I. Mielzynska, A. Lytwyn, P. Roth, M. Howard, S. Chong, D. Daya, W. Chapman, and M. Chernesky. 2000. Comparison of self-collected vaginal, vulvar and urine samples with physician-collected cervical samples for human papillomavirus testing to detect high-grade squamous intraepithelial lesions. CMAJ 163:513-518. [PMC free article] [PubMed] [Google Scholar]
- 28.Snijders, P. J., C. J. Hogewoning, A. T. Hesselink, J. Berkhof, F. Voorhorst, M. C. Bleeker, and C. J. Meijer. 28 March 2006. Determination of viral load thresholds in cervical scrapings to rule out CIN 3 in HPV 16, 18, 31 and 33-positive women with normal cytology. Int. J. Cancer [Online.] doi: 10.1002/ijc.21956. [DOI] [PubMed]
- 29.Solomon, D., D. Davey, R. Kurman, A. Moriarty, D. O'Connor, M. Prey, S. Raab, M. Sherman, D. Wilbur, T. Wright, Jr., and N. Young. 2002. The 2001 Bethesda system: terminology for reporting results of cervical cytology. JAMA 287:2114-2119. [DOI] [PubMed] [Google Scholar]
- 30.van den Akker-van Marle, M. E., M. van Ballegooijen, and J. D. Habbema. 2003. Low risk of cervical cancer during a long period after negative screening in The Netherlands. Br. J. Cancer 88:1054-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van den Brule, A. J., R. Pol, N. Fransen-Daalmeijer, L. M. Schouls, C. J. Meijer, and P. J. Snijders. 2002. GP5+/6+ PCR followed by reverse line blot analysis enables rapid and high-throughput identification of human papillomavirus genotypes. J. Clin. Microbiol. 40:779-787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Duin, M., P. J. Snijders, H. F. Schrijnemakers, F. J. Voorhorst, L. Rozendaal, M. A. Nobbenhuis, A. J. van den Brule, R. H. Verheijen, T. J. Helmerhorst, and C. J. Meijer. 2002. Human papillomavirus 16 load in normal and abnormal cervical scrapes: an indicator of CIN II/III and viral clearance. Int. J. Cancer 98:590-595. [DOI] [PubMed] [Google Scholar]
- 33.Walboomers, J. M., M. V. Jacobs, M. M. Manos, F. X. Bosch, J. A. Kummer, K. V. Shah, P. J. Snijders, J. Peto, C. J. Meijer, and N. Munoz. 1999. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 189:12-19. [DOI] [PubMed] [Google Scholar]
- 34.Wright, T. C., Jr., L. Denny, L. Kuhn, A. Pollack, and A. Lorincz. 2000. HPV DNA testing of self-collected vaginal samples compared with cytologic screening to detect cervical cancer. JAMA 283:81-86. [DOI] [PubMed] [Google Scholar]
- 35.Yoshida, T., T. Fukuda, T. Sano, T. Kanuma, N. Owada, and T. Nakajima. 2004. Usefulness of liquid-based cytology specimens for the immunocytochemical study of p16 expression and human papillomavirus testing: a comparative study using simultaneously sampled histology materials. Cancer 102:100-108. [DOI] [PubMed] [Google Scholar]