Abstract
Methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus faecium were exposed to linezolid (MIC of 2 mg/liter) under aerobic or anaerobic conditions in an in vitro pharmacodynamic model. Drug concentration and half-life were adjusted to simulate clinical dosing (600 mg twice daily) of linezolid. Linezolid produced a 2-log10 killing at 24 h, and rates of killing against each of these facultative organisms as measured by mean survival time appeared similar under aerobic and anaerobic conditions.
Linezolid is an oxazolidinone approved by the Food and Drug Administration for the treatment of infections due to vancomycin-resistant Enterococcus faecium (VREF) and nosocomial pneumonia and skin and skin structure infections due to methicillin-resistant Staphylococcus aureus (MRSA), among others (linezolid [Zyvox] product information, January 2001; Pharmacia & Upjohn Co., Kalamazoo, Mich.). The recommended dose for infection with VREF, nosocomial pneumonia, complicated skin and skin structure infections, and community-acquired pneumonia is 600 mg intravenously or orally every 12 h, and the recommended dose for uncomplicated skin and skin structure infections is 400 mg orally every 12 h. Linezolid has demonstrated activity against these facultative organisms in an aerobic environment, and an in vitro study found the agent to be active against strict anaerobes implicated in skin infections after animal and human bites (1). The relative activity of linezolid against S. aureus and E. faecium, which can cause infections in aerobic, anaerobic, and microaerophilic environments, is unknown. A recent in vitro study found a difference in the rates of killing of S. aureus by vancomycin under aerobic and anaerobic conditions (4). The purpose of this study was to determine whether a difference in the rate and extent of bacterial killing exists between aerobic and anaerobic environments with standard dosing of linezolid with an in vitro pharmacodynamic model.
A series of experiments were performed in a previously described in vitro pharmacodynamic model (5) with two clinical isolates—one strain of MRSA and one strain of VREF. Time-kill curves were generated after MRSA and VREF were exposed to linezolid in both aerobic and anaerobic environments. All experiments were performed in duplicate for a 24-h duration. The model consisted of a 610-ml sealed glass chemostat, representing the central compartment, filled with cation-supplemented Mueller-Hinton broth (CAMHB; Ca2+, 50 mg/liter; Mg2+, 25 mg/liter) and fitted with input and output tubing. Linezolid, obtained from Pharmacia & Upjohn, was prepared according to the manufacturer's specifications and stored at −4°C until use. To simulate dosing of 600 mg orally every 12 h in humans, linezolid was instilled via bolus into the chemostat at time zero and 12 h to achieve peak concentrations of 20 μg/ml (linezolid product information). As linezolid is less than 50% protein bound (linezolid product information), we chose to simulate total serum drug concentrations in the model, as the relationship between protein-binding values below 85 to 90% and the effect on tissue penetration and clinical impact is unclear (3). By pumping antibiotic-free medium into the system at a rate of 1.2 ml/min with a peristaltic pump, an equal volume of antibiotic-containing medium was displaced. This resulted in the simulation of a monexponential pharmacokinetic process with a desired linezolid half-life of 6 h. To test if targeted pharmacokinetics were achieved, samples were withdrawn from the model to determine linezolid concentration by high-performance liquid chromatography (HPLC). Placing the entire apparatus in a Bactron IV anaerobic chamber (Sheldon Manufacturing., Cornelius, Oreg.) created an anaerobic environment. Medium was prereduced in anaerobic experiments.
A suspension of each organism was allowed to grow overnight and diluted 1:10 in fresh medium prior to the experiment. The diluted suspension was reincubated for approximately 1 h to allow organisms to attain exponential growth. Upon comparison with a 0.5 McFarland equivalence turbidity standard, an appropriate portion of the bacterial culture was added to the chemostat, producing an initial bacterial inoculum of 106 CFU/ml. The in vitro pharmacodynamic model was placed in a monitored 37°C water bath. Placing a magnetic stirring bar in the bottom of each chamber ensured constant mixing of the microorganisms and antibiotic. One-milliliter samples were taken at selected time intervals, serially diluted, and plated onto Trypticase soy agar (Becton Dickinson, Cockeysville, Md.) with 5% sheep blood. Antibiotic carryover was addressed by using saline dilution techniques. Following incubation for 24 h at 37°C, agar plates containing 30 to 300 bacterial colonies were counted to construct time-kill curves. The lower limit of bacterial detection in our laboratory has been determined to be 3 × 102 CFU/ml.
The MIC of linezolid for each organism was determined by broth microdilution techniques both prior to and after antibiotic exposure. CAMHB was used for all susceptibility testing. MICs were determined in quadruplicate by using an inoculum size of 105 to 106 CFU/ml and incubation for 16 to 20 h at 37°C. Quality control monitoring was done with S. aureus ATCC 29213 and Enterococcus faecalis ATCC 29212.
Concentrations of linezolid were determined by a validated HPLC assay that was developed to measure concentrations in plasma but adapted to measure concentrations in CAMHB. Samples were measured with a system consisting of a Waters 515 HPLC pump (Milford, Mass.) with a model 680 gradient controller and a solvent select valve, a Spectra Physics (San Jose, Calif.) model 8875 fixed-volume autosampler, a Waters model 486 UV detector, a Macintosh 7100 computer (Apple Computers Inc., Cupertino, Calif.), and the Rainin (Woburn, Mass.) Dynamax HPLC data management system. The plasma and CAMHB standard curves for linezolid ranged from 0.5 to 30 μg/ml. The absolute recovery of linezolid from plasma was 95%. The within-sample precision (percent coefficient of variation) of validation of a single standard concentration was 0.69%, and the overall validation precision across all standards was 1.04 to 4.39%. Similar results for the study samples were obtained with either the plasma or CAMHB standard curves. Plasma quality control samples were nearly identical when calculated by using the two standard curves. In most cases, the targeted concentration was within 5% of the predicted value; on average, the targeted concentration was within 0.5% of the predicted value, indicating that the pump system produced the desired concentrations.
Time-kill curves were plotted as declines in CFU per milliliter versus time. Extent of killing was determined by visual inspection. The area under the kill curve (AUBKC) and the area under the kill times curve (AUBKTC) were calculated by the trapezoidal rule, and a mean survival time (MST) was calculated with the equation AUBKTC/AUBKC (2). This measure of effect allows for a simple comparison of linezolid activities under aerobic and anaerobic conditions. MSTs were compared by one-way analysis of variance with Tukey's posttest by using GraphPad Prism version 3.02 for Windows (GraphPad Software, San Diego Calif.). Significance was defined as P < 0.05.
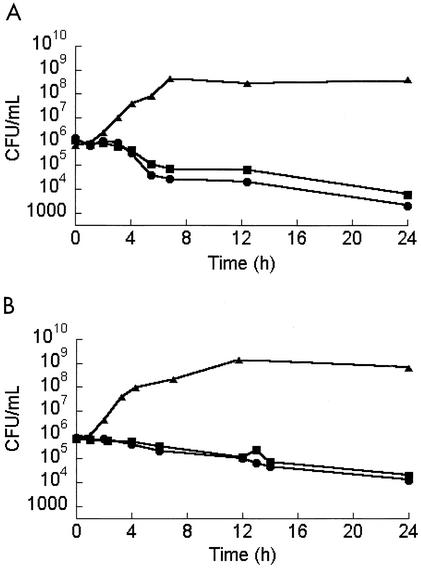
MICs of linezolid for MRSA and VREF were 2 μg/ml, and all postexposure MICs were identical to preexposure MICs. There was no apparent difference in rate or extent of killing of either MRSA or VREF between aerobic and anaerobic environments (Fig. 1). Linezolid achieved a 2-log10 killing at 24 h under aerobic and anaerobic conditions against both strains. Of note, much of the killing was during the log phase of the growth, while bacteria in an abscess may be in lag-phase growth.
FIG. 1.
Activities of linezolid against MRSA (A) and VREF (B) under aerobic (circles) and anaerobic (squares) conditions along with growth control (triangles).
The MSTs ± standard deviations of MRSA under aerobic and anaerobic conditions were 3.59 ± 0.16 and 4.64 ± 0.02 h, respectively. For VREF, the MST under aerobic conditions was 4.78 ± 0.11 h, and that under anaerobic conditions was 5.75 ± 0.55 h. No significant differences were detected in MSTs between aerobic and anaerobic conditions for either species.
In this in vitro model, linezolid demonstrated comparable activities against MRSA and VREF under aerobic and anaerobic conditions. Linezolid did not produce a 3-log10 killing of either MRSA or VREF at 24 h under aerobic or anaerobic conditions despite the fact that the drug concentration was at least 3× the bacterial MIC for the entire duration of the experiments. Linezolid has demonstrated clinical efficacy in the treatment of infections due to VREF and MRSA, and these data indicate that efficacy is not predicated on the presence or absence of oxygen.
Acknowledgments
This study was supported by a grant from Pharmacia, Inc.
REFERENCES
- 1.Goldstein, E. J., D. M. Citron, and C. V. Merriam. 1999. Linezolid activity compared to those of selected macrolides and other agents against aerobic and anaerobic pathogens isolated from soft tissue bite infections in humans. Antimicrob. Agents Chemother. 43:1469-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li, J., J. Turnidge, R. Milne, R. L. Nation, and K. Coulthard. 2001. In vitro pharmacodynamic properties of colistin and colistin methanesulfonate against Pseudomonas aeruginosa isolates from patients with cystic fibrosis. Antimicrob. Agents Chemother. 45:781-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peterson, L. R., and D. N. Gerding. 1980. Influence of protein binding of antibiotics on serum pharmacokinetics and extravascular penetration: clinically useful concepts. Rev. Infect. Dis. 2:340-348. [DOI] [PubMed] [Google Scholar]
- 4.Suller, M. T., and D. Lloyd. 2002. The antibacterial activity of vancomycin towards Staphylococcus aureus under aerobic and anaerobic conditions. J. Appl. Microbiol. 92:866-872. [DOI] [PubMed] [Google Scholar]
- 5.Zabinski, R. A., K. Vance-Bryan, A. J. Krinke, K. J. Walker, J. A. Moody, and J. C. Rotschafer. 1993. Evaluation of activity of temafloxacin against Bacteroides fragilis by an in vitro pharmacodynamic system. Antimicrob. Agents Chemother. 37:2454-2458. [DOI] [PMC free article] [PubMed] [Google Scholar]