Abstract
The Nef protein of primate lentiviruses is a unique protein that has evolved in several ways to manipulate the biology of an infected cell to support viral replication, immune evasion, pathogenesis, and viral spread. Nef is a small (25- to 34-kDa), myristoylated protein that binds to a collection of cellular factors and acts as an adaptor to generate novel protein interactions to accomplish specific functions. Of the many biological activities attributed to Nef, the reduction of surface levels of the viral receptor (CD4) and antigen-presenting molecules (major histocompatibility complex class I) has been intensely examined; recent evidence demonstrates that Nef utilizes multiple, distinct pathways to affect these proteins. To accomplish this, Nef promotes the formation of multiprotein complexes, recruiting host adaptor proteins to commandeer intracellular vesicular trafficking routes. The altered trafficking of several other host molecules has also been reported, and an emerging theory suggests that Nef generates pleiotrophic effects in the secretory and endocytic pathways that reprogram intracellular protein trafficking and may ultimately provide an efficient platform for viral assembly. This review critically discusses some of the major findings regarding the impact of human immunodeficiency virus type 1 Nef on host protein transport and addresses some emerging directions in this area of human immunodeficiency virus biology.
INTRODUCTION
The Role of HIV-1 Nef in Progression to AIDS
Human immunodeficiency virus type 1 (HIV-1) establishes a chronic infection in humans that ultimately leads to the demise of the immune system. The clinical manifestations of AIDS are caused by the decline of circulating CD4+ T cells (to <200 cells/μl blood), which in turn leads to increased susceptibility to opportunistic infections. When the viral load, or number of viral genomes present in the blood, is monitored over the course of HIV infection, a classic disease progression profile emerges: shortly following an exposure, the viral load rises sharply in the blood, until the host acquires HIV-specific cytotoxic T lymphocytes (CTLs) and anti-HIV antibodies; the viral load then settles out to a low-level equilibrium value, termed the viral set point. The relative value of the set point correlates with the rate of progression to AIDS (i.e., a high viral set point typically results in a rapid disease course, and vice versa) (108). Thus, knowledge of the interplay between cellular and viral factors involved in the maintenance of the set point is critical to the understanding of disease progression.
The HIV accessory protein Nef has been extensively studied and appears to be a key determinant for viral pathogenesis. One striking example of the pathogenic potential of Nef is a cohort infected with an aberrant HIV strain that contained a large deletion in the nef open reading frame. These individuals, designated long-term nonprogressors, have not displayed the typical clinical manifestations of AIDS (45), although after an extended time (14 to 18 years) some have shown some reduction in CD4 counts, consistent with the effects of HIV disease (19, 90).
In addition, rhesus macaques infected with an engineered strain of simian immunodeficiency virus (SIV) that lacked a functional Nef protein (SIVΔNef) also did not progress to clinical disease in a timely fashion (85). In fact, SIVΔNef strains have been proposed as candidates for vaccination trials with live attenuated vaccine in the simian model system. While this virus does provide some immune protection against challenge with wild-type virus (40), it can also sometimes cause AIDS itself, particularly in neonatal macaques (8, 9). Thus, while Nef significantly enhances the ability of HIV to induce AIDS, other HIV factors clearly contribute to the development of disease.
General Properties of HIV-1 and SIV Nef Proteins
Primate lentiviruses have evolved to encode multifunctional accessory proteins that manipulate host biology to promote the viral life cycle. The accessory protein Nef was originally named because it was thought to be a negative factor that inhibited viral replication (29); however, it has become clear that Nef positively affects viral replication and infectivity (109, 110). The HIV-1 and SIV Nefs are small (25- to 34-kDa), myristoylated proteins that reside both in the cytoplasm and in association with the cytosolic face of cellular membranes (48). To date, no enzymatic activity has been directly attributed to the Nef protein; however, extensive studies of Nef biology have revealed several conserved motifs that mediate physical association with cellular factors. Consequently, Nef has been hypothesized to function as a molecular adaptor, altering cellular pathways via multiple protein-protein interactions. Indeed, Nef is able to modulate diverse cellular functions such as protein trafficking events, signal transduction cascades, and apoptotic pathways.
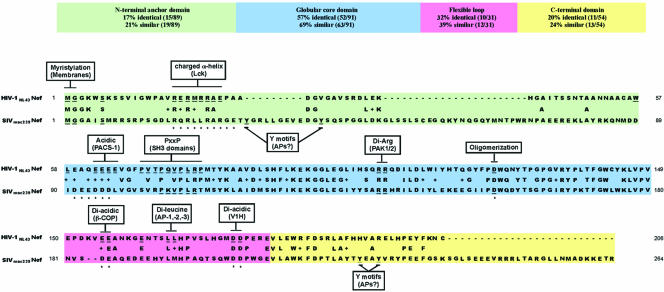
Nef is a relatively extended protein containing a large degree of solvent-exposed surface area with several disordered regions (55). Because of this property, it has been difficult to obtain an accurate three-dimensional structure of the full-length protein. However, the structure of the globular core domain (amino acids 54 to 205 [unless otherwise noted, all amino acid numbering in this paper refers to the NL4-3 Nef allele]) of Nef has been solved using X-ray crystallography (4, 53, 92) and nuclear magnetic resonance (NMR) (53, 67). Additionally, the structure of the N-terminal anchor domain has been solved using NMR spectroscopy, and it appears that this domain adopts a relatively unstructured conformation that becomes partially ordered upon the addition of an N-terminal myristyl group (57). Geyer and colleagues used these known structures to assemble a structural prediction of the conformation of the full-length Nef polypeptide (58). This model predicts that the surface of Nef consists of a linear array of potential protein-protein interaction domains and that this surface is quite flexible (Fig. 1). Interestingly, it has been speculated that the overall flexibility of Nef enables the protein to switch between multiple conformations and that the structural organization of Nef may be dictated by its binding partner(s) (for a review, see reference 6).
FIG. 1.
Sequence alignment and protein-protein interaction domains in HIV-1 and SIV Nef. The HIV-1NL4-3 Nef and SIVmac239 Nef sequences are aligned. Boxes indicate protein motifs and their cellular binding partners (in parentheses). Residues important for protein interactions are underlined. The asterisks indicate residues in SIVmac239 Nef that are homologous to important residues in HIVNL4-3 Nef that have not been tested for functional homology.
In addition to HIV-1, HIV-2 and SIV also carry the Nef gene, and while several regions are highly conserved, there are also many distinctions. As is evident from Fig. 1, the core domains of HIV-1 and SIV Nef are relatively well conserved and are predicted to fold into a discrete globular domain. In contrast, the amino and carboxy termini are less conserved, and these regions are thought to contain extended loop sequences. In addition, these sequences are enriched in short, linear signal sequences (i.e., tyrosine-based motifs, dileucine motifs, and diacidic motifs) that are known to be recognized by components of vesicular coats. SIV Nef contains more amino acids (and more recognition motifs) in both of its flexible termini (Fig. 1).
BIOLOGICAL ACTIVITIES OF HIV-1 Nef
HIV-1 Nef contributes to HIV pathogenesis by several mechanisms. Nef promotes viral infection by activating CD4+ T lymphocytes, which makes them more susceptible to infection. To accomplish this, Nef alters signal transduction pathways downstream of the T-cell receptor (14, 142). Specifically, Nef has been shown to affect molecules that participate in T-cell receptor signaling, such as Vav (50), p21-activated kinase 2 (49, 113, 127), Rac (157), CDC42 (99), and the DOCK2/ELMO1 complex (75). Interactions with the T-cell signaling pathway also lead to the upregulation of Fas ligand (FasL) on the cell surface (166), which may protect infected cells by promoting apoptosis of neighboring CTLs. To prevent FasL (and tumor necrosis factor alpha [ΤNF-α]) (10) from causing premature death of the infected cell, Nef may bind to and suppress the activity of ASK1, a kinase that is responsible for transducing apoptotic signals from FasL and the TNF receptor (54). In addition, Nef inhibits p53-mediated apoptosis (66) and blocks apoptosis via an association with p21-activated kinase and phosphatidylinositol 3-kinases (PI3-kinases) (165). Nef also protects infected cells from CTLs by reducing the cell surface expression of major histocompatibility complex class I (MHC-I), which is required for CTL recognition.
There is a great deal of evidence demonstrating that Nef promotes infection by enhancing the production of infectious HIV virions (51, 60) by at least two mechanisms. First, Nef reduces the expression of the HIV receptor CD4, which can interfere with viral budding and release (87, 132), and second, Nef increases particle infectivity by another, undefined (but CD4-independent) mechanism (101).
In addition, Nef may play a role in the spread of HIV-1 through its effects on dendritic cells (DCs). This cell type can capture HIV-1 particles through a DC-specific receptor (DC-SIGN) and later transmit the virus to target cells without becoming productively infected. There is also separate evidence that in some cases, the DCs can themselves become infected (24, 61). In DCs that have become infected, Nef can upregulate DC-SIGN to promote the efficient spread of HIV infection in cocultures of DCs and T cells (143). In macrophages, Nef induces the production of the CC chemokines macrophage inflammatory protein 1α and 1β and other soluble factors to recruit T cells and facilitate productive transmission of HIV infection (153, 154).
Clearly, there are multiple biological effects associated with expression of the HIV Nef protein, many of which result in enhanced viral replication and spread. More studies are needed to clearly distinguish which of these activities are most important for viral disease pathogenesis in vivo. This review will focus on two of the best understood activities of Nef, its effects on the intracellular trafficking of CD4 and MHC-I.
Nef ALTERS INTRACELLULAR TRAFFICKING PATHWAYS
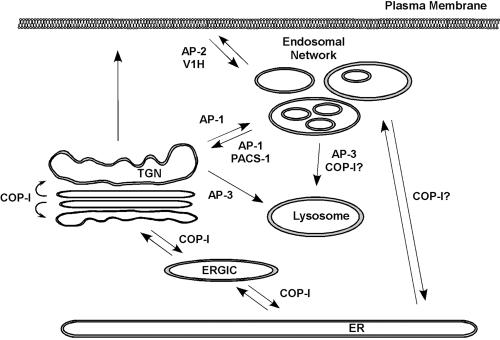
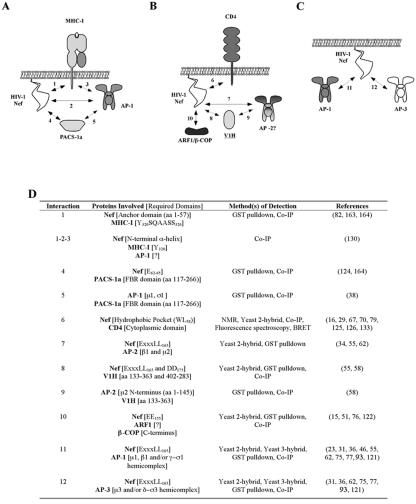
It has been known for some time now that Nef interacts with a number of proteins associated with intracellular trafficking. Figure 2 highlights the proteins that are thought to bind to Nef and presents their normal role in trafficking. A brief overview of the biology of some of these proteins and their association with HIV-1 Nef is given below. A summary of Nef-dependent protein-protein interactions that have been identified is presented in Fig. 3.
FIG. 2.
HIV-1 Nef can associate with components of intracellular vesicular transport pathways. The cartoon outlines the major known trafficking pathways between intracellular organelles. Vesicle coat proteins that bind to HIV-1 Nef are indicated at their proposed sites of cargo selection.
FIG. 3.
Nef-associated protein complexes. (A to C) Schematic diagrams of protein-protein interactions that have been reported for Nef-mediated disruption of MHC-I trafficking (A), CD4 downregulation by Nef (B), and AP binding mediated by the Nef dileucine motif (C). (D) Summary of the experimental evidence supporting the numbered protein-protein interactions depicted in A to C.
Adaptor Protein Complexes
The heterotetrameric clathrin adaptor protein (AP) complexes (AP-1, AP-2, AP-3, and AP-4) are components of vesicle coats that mediate intracellular trafficking events in the cell (for a review, see reference 129). APs selectively recruit cargo protein by binding to canonical linear motifs in the cytoplasmic domains, which are typically either a tyrosine-based motif (YXXΦ) or a dileucine motif ([E/D]XXXL[L/I]) (where X is any amino acid and Φ is a large hydrophobic amino acid). The specificity of AP recruitment relies in part on the subcellular distribution of the AP complex. For example, AP-1 localizes to the trans-Golgi network (TGN), AP-2 to the plasma membrane, and AP-3 to the endosomes (44, 116, 155, 158), and they are thought to mediate cargo selection from that location (Fig. 2).
There is some overlap in the distribution and activity of APs, and therefore, additional accessory proteins likely add to the specificity. For example, amphiphysin, endophilin, epsin, AP180, and Hip1/Hip1R are associated with AP-2 (105). EpsinR (74), enthoprotin (161), and possibly Golgi-associated, γ-ear-containing, ADP-ribosylation factor-binding proteins (44) participate with AP-1. These multiprotein complexes allow AP-1 and AP-2 and their respective accessory proteins to link cargo molecules to clathrin coats to facilitate budding. In contrast, AP-3 appears to mediate the budding of both clathrin-coated and non-clathrin-coated vesicles (114). Finally, the localized lipid composition is also thought to play a part in AP recruitment, and accordingly, lipid kinases have been implicated in vesicle transport at several intracellular locations (reviewed in reference 42).
Using the yeast two-hybrid system (37, 47, 78, 94, 122), glutathione S-transferase-Nef pull-down assays (23, 76, 94, 122), or overexpression of a Nef-CD8 chimera in 293 cells (23), a number of investigators have found that the HIV-1 Nef protein interacts with adaptin subunits and whole adaptin complexes. A consensus binding domain for adaptin protein binding (D/EXXXLL) (21) can be found in Nef, and this motif is required for glutathione S-transferase-Nef to pull down AP-1 complexes from mammalian cell lysates (23, 76). In addition, the leucine residues in this motif are required for Nef to downmodulate CD4 but not MHC-I (63, 103). Thus, the dileucine-dependent binding to adaptins appears to be important for Nef's effects on CD4.
V1H
An interaction between Nef and the subunit H (V1H) of the vacuolar membrane ATPase was first noted when this subunit was pulled out of a yeast two-hybrid screen that was set up to find Nef-interacting proteins (100). Subsequent experiments revealed that this subunit interacts with Nef via the dileucine and diacidic motifs in the C-terminal flexible loop of Nef (59, 100). Substitution of this loop with the V1H protein creates a fusion protein that actively promotes the destabilization of CD4 at the cell surface (59). V1H also has a binding site for the μ2 chain of AP-2, and it has been proposed that it functions by linking CD4 to clathrin via AP-2 (56).
PACS-1a
Phosphofurin acid cluster sorting protein 1 (PACS-1) was discovered by using a yeast two-hybrid screen to identify proteins that interacted with the cytoplasmic tail of furin, a TGN-resident protease (159). Later, it was shown that PACS-1 binds to AP-1 and AP-3 complexes and that a PACS-1 mutant that did not bind to APs could act in a dominant negative manner to disrupt the trafficking of furin and mannose 6-phosphate recepter (39). Additionally, there is evidence that PACS-1 and AP-1 bind to vesicle-associated membrane protein 4 (73), an R-SNARE protein that mediates the transport of vesicles between the TGN and endosomes (144). Hence, it was proposed that PACS-1 mediates TGN targeting via a retrograde retrieval mechanism in cooperation with AP-1.
The HIV-1 Nef acidic domain also interacts with PACS-1, and dominant negative mutants of PACS-1 reduce the accumulation of Nef and MHC-I in the juxtanuclear region of certain cell lines, suggesting that PACS-1 may be an important cellular partner of Nef (20, 125).
COP-I
COP-I coatomers play a relatively well-defined role in the retrograde targeting of endoplasmic reticulum (ER) resident proteins, as well as in the sorting of vesicles that transport between stacks of the Golgi cisternae (for a review, see reference 12). Another intriguing yet poorly defined activity of COP-I is its role in the endosomal pathway. COP-I has been reported to bind to endosomal membranes in a luminal pH-dependent manner (3, 70), and it may be involved in endocytic recycling (41), the biogenesis of multivesicular compartments (69), phagosome formation (18, 22), and retrograde transport from the plasma membrane to the ER (81, 98).
An interaction between Nef and β-COP, a component of COP-I coatomers, was initially identified using a two-hybrid screen (15). The domains of Nef that interact with β-COP have not yet been well defined, and a stable interaction may require other cellular proteins such as ARF1 (52, 77, 123). There is evidence that the interaction between Nef and β-COP is important for targeting of Nef and CD4 to acidic late endosomes (52).
Given the number of Nef binding partners, it is apparent that Nef has the potential to control intracellular protein transport at multiple levels. In fact, several groups have reported that Nef induces gross abnormalities in endosomal morphology (47, 76, 101) and that Nef expression increases the amounts of endosomes, lysosomes, and multivesicular bodies (101, 136, 139, 143, 146, 147, 152, 156).
Nef-MEDIATED DISRUPTION OF MHC-I EXPRESSION
Immune Evasion
Antigen presentation by MHC-I provides a mechanism by which a cell can communicate with the extracellular environment—specifically, an infected cell can present foreign antigens to signal the presence of a viral infection. CTLs circulate through the body and survey peptide-loaded MHC-I on the surface of cells via the T-cell receptor. If a foreign antigen-MHC-I complex is detected, several responses in the CTL are triggered (including the release of perforins, granzymes, and proapoptotic factors), which lead to the lysis of the infected cell (for a review, see reference 17).
As with many pathogens that establish a chronic infection, HIV has established ways to subvert the host immune response. HIV-1 Nef reduces the surface expression of MHC-I (141), thus preventing the exposure of viral antigens on the surface of HIV-infected cells. This function allows HIV-infected cells to escape recognition and lysis by anti-HIV CTLs in vitro (33), and there is evidence that the ability of Nef to disrupt MHC-I antigen presentation is very important for viral disease pathogenesis in vivo (26, 111, 149).
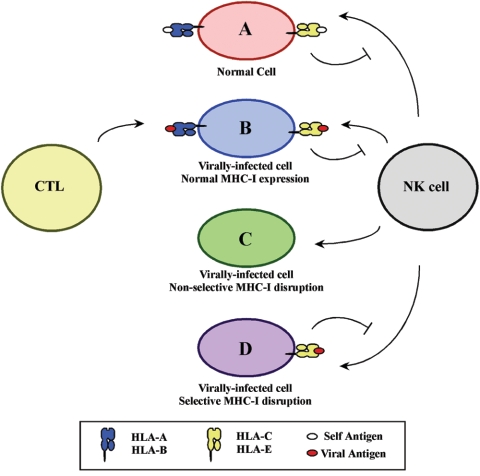
Reducing the cell surface expression of MHC-I is beneficial in avoiding CTL recognition; however, MHC-I also provides inhibitory signals for natural killer (NK) cells. Thus, infected cells that lack sufficient surface MHC-I expression may become lysed by NK cells (for a review, see reference 112). To perhaps avoid this problem, HIV-1 Nef selectively affects some MHC-I allotypes, while ignoring others. Specifically, Nef preferentially disrupts HLA-A and HLA-B expression (65) but not that of HLA-C and HLA-E (31, 164). The preservation of these molecules on the cell surface may provide the proper inhibitory signal to avoid viral detection by NK cells (31). This differential modulation of MHC-I expression can be mapped to a tyrosine-based sequence (YSQAASS) in the cytoplasmic domains of HLA-A and HLA-B allotypes (164) that is not present in HLA-C and HLA-E. As a consequence, Nef may protect the HIV-infected cell from both adaptive and innate cell-mediated immunity (Fig. 4).
FIG. 4.
Differential effects on MHC-I allotypes enable HIV-1 Nef to escape both CTL and NK cell surveillance. (A) A normal cell that does not harbor any viral antigens is not affected by cell-mediated immune surveillance. (B) Virally infected cells are recognized and lysed due to CTL recognition of the antigen-MHC-I complex. (C) Virally infected cells that have reduced levels of all MHC-I allotypes (nonselective disruption) are lysed by NK cells due to lack of inhibitory MHC-I allotypes (HLA-C and HLA-E). (D) The selective removal of surface HLA-A and HLA-B allotypes prevents CTL recognition, while the maintenance of HLA-C and HLA-E expression protects the infected cell from NK cell lysis.
Molecular Determinants of MHC-I Trafficking Disruption by Nef
Two studies in 1989 first reported the phenomenon of MHC-I downregulation in HIV-infected T cells (84, 137). Seven years later, Schwartz and colleagues provided the first study describing a possible mechanism for the loss of MHC-I surface expression in HIV-infected T cells and, importantly, determined that HIV-1 Nef was the accessory protein responsible for this effect (141). It was subsequently demonstrated that this effect of Nef was of sufficient magnitude in primary T cells to protect infected cells from anti-HIV CTL killing (33).
Mutagenesis studies have revealed functional domains in Nef that are required for its effects on MHC-I trafficking. Disruption of an amphipathic α-helix containing a critical methionine residue (R17ERM20RRAEPA26), an acidic region (E62-65), or a polyproline helix (P69/72/75/78) (1, 64, 103) dramatically reduces Nef activity with respect to MHC-I trafficking. Disruptions in these domains also abrogate the ability of Nef to associate with the MHC-I cytoplasmic tail domain (163), highlighting the importance of this interaction. However, it is also possible that these domains serve another role as well. The N-terminal α-helix is required for a physical interaction between Nef and the CD4-associated tyrosine kinase Lck (13), the acidic region has been shown to mediate an association with PACS-1 (discussed below) (125), and the polyproline domain binds with high affinity to the SH3 domains of Src kinases (134). While these cellular binding partners have been identified, the specific role of these interactions in MHC-I trafficking remains unclear.
Sequences in the MHC-I cytoplasmic tail are also required for Nef activity. Mutation of Y320, A323, and D327 abrogates responsiveness to Nef. These required residues are present in HLA-A and HLA-B allotypes. However, MHC-I allotypes that do not respond to Nef (i.e., HLA-C and HLA-E) lack one or more of these residues (31, 94) and fail to bind Nef (164).
Trafficking of MHC-I in Nef-Expressing Cells
Many studies from a variety of laboratories have contributed to our understanding of how Nef disrupts MHC-I trafficking, although many key questions remain unanswered. Under normal conditions, MHC-I heavy and light chains are assembled in the endoplasmic reticulum with a short peptide antigen. The concerted efforts of a number of protein chaperones and a specialized peptide transporter ensure that this process occurs efficiently. The properly folded molecules then exit the ER and are further modified in the Golgi apparatus. The mature molecules are then phosphorylated on their cytoplasmic tails in a post-TGN compartment.
In HIV-infected primary T cells, immunoprecipitates of endogenous HLA-A2 contain Nef (130). The binding site on MHC-I that interacts with Nef has been mapped to the same region that is required for responsiveness to Nef (YSQAASS) in the cytoplasmic domains of HLA-A and HLA-B allotypes (164). It is thought that Nef initially binds to MHC-I molecules in the ER, because Nef is found in a complex with immature forms of MHC-I and the ER chaperone tapasin (83). However, despite the binding of Nef in the ER, the rate of MHC-I transport through the ER and medial Golgi apparatus is not affected by Nef expression (130, 141). The lack of effect of Nef binding on MHC-I trafficking through the early secretory compartment can be explained by the requirement for a host factor that binds the Nef-MHC-I complex later in the secretory pathway and is consistent with the model that AP-1 binds the Nef-MHC-I complex in the TGN (130). The MHC-I cytoplasmic tail is not phosphorylated in the ER (46, 95), and because Nef preferentially binds to unphosphorylated MHC-I cytoplasmic tails, it has been proposed that Nef specifically targets early forms of MHC-I that are being newly loaded with peptide antigens (83).
Multiple studies have demonstrated that in HIV Nef-expressing cells, MHC-I accumulates in the region of the TGN, especially in γ-adaptin-positive structures (Fig. 5) (64, 94). However, it remained unclear for some time whether MHC-I accumulated in the TGN as a result of retrograde transport of internalized MHC-I, whether HIV Nef disrupted transport of newly synthesized MHC-I and blocked its exit to the cell surface, or whether HIV Nef affected both pathways. Data from a number of groups have documented that HIV Nef decreases the half-life of cell surface MHC-I. These investigators have utilized different HIV nef alleles, different methods of expressing HIV Nef, and different cell lines to demonstrate this. An effect of HIV Nef on the half-life of cell surface MHC-I was first demonstrated in a CEM T-cell line selected to stably express the HIV nef AO1 allele, and cell surface MHC-I was detected with an antibody that is “pan-MHC-I” in that it detects all the class I heterodimers (including HLA-C and HLA-E, which do not respond to Nef) (140). In separate studies, IMR90 fibroblasts (64) and 293 human embryonic kidney cells (125) transiently transfected with Na7 Nef were found to take up surface MHC-I bound to a pan-MHC-I antibody as detected by a nonquantitative, indirect immunofluorescence assay. Additionally, HeLa cells transiently transfected with LAI Nef were found to internalize the MHC-I allotype HLA-A2 as detected by an allotype-specific monoclonal antibody. However, in quantitative comparative studies, those authors demonstrated that the Nef-dependent internalization rate of HLA-A2 was small compared to the constitutive internalization rate of an HLA-A2 mutant with a canonical endocytosis signal (93), More recent studies revealed that HeLa cells expressing HXB-2D Nef introduced via a vaccinia virus internalize total surface biotinylated MHC-I more rapidly than control cells. However, after 15 min, only about 10% of total MHC-I had been internalized, compared with 60% of CD4 internalized over that same period (20). Two studies have shown that NL4-3 Nef (89) and NA7 Nef (151) introduced into Jurkat cells either with murine retroviral vectors (89) or by transient transfection (151) decreased the half-life of total MHC-I on the cell surface. However, in these studies the effect seems modest, as only 15 to 20% of the total MHC-I was internalized. Similarly, CEM T cells transiently expressing NL4-3 or HXB Nef via replication-defective adenovirus vectors or via bona fide HIV infection internalized the HLA-A2 allotype of MHC-I to a limited degree (82, 83, 163).
FIG. 5.
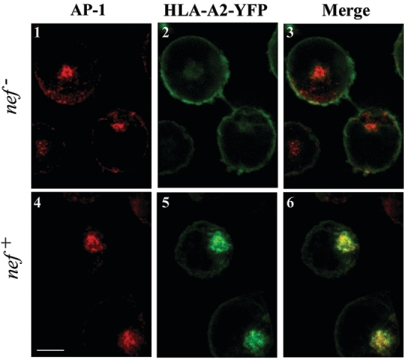
MHC-I and AP-1 colocalize in Nef-expressing T cells. CEM T cells that stably expressed yellow fluorescent protein-tagged HLA-A2 (green) were transduced with a control adenovirus (nef−) or an adenovirus that expressed HIV-1 Nef (nef+). Cells were adhered to glass slides, and the gamma subunit of AP-1 (γ-adaptin, red) was detected by indirect immunofluorescence. Individual Z sections through the middle of the cell were collected using a Zeiss LSM 510 confocal microscope. Scale bar = 5 μm.
Because the dramatic effect of NL4-3 and HXB Nef on steady-state levels of HLA-A2 in HIV-infected primary T cells (33) seemed inconsistent with the modest effects of Nef on surface MHC-I half-life, additional experiments were performed. Direct comparison of the degree to which HXB and NL4-3 nef alleles affected MHC-I cell surface stability versus transport of newly synthesized MHC-I to the cell surface revealed that Nef had more dramatic effects on the transport of MHC-I to the cell surface (82, 83, 130, 163). Indeed, according to these studies, transport of newly synthesized HLA-A2 to the cell surface was reduced by 80 to 90% in Nef-expressing cells. These comparative studies were performed with T-cell lines and primary T cells expressing NL4-3 or HXB Nef in various ways, including via replication-defective adenovirus vectors and bona fide HIV infection (82, 83, 130, 163).
In sum, although the most recent evidence suggests that HIV Nef primarily affects the forward transport of MHC-I to the cell surface, the comparatively small effect of HIV Nef on MHC-I cell surface half-life likely also contributes to the reduction of MHC-I levels in infected cells. In addition, it is important to note that additional studies may reveal that other cell types that are natural targets of HIV infection (such as macrophages) or other HIV or SIV nef alleles may utilize these two pathways to different relative degrees.
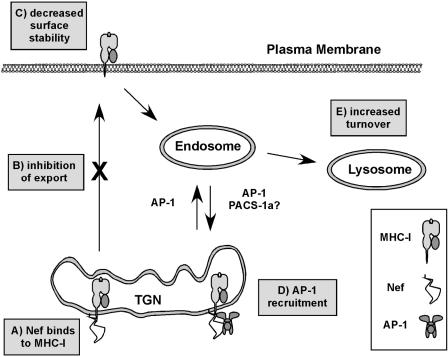
One manifestation of Nef's effects on MHC-I forward transport is that Nef expression dramatically inhibits phosphorylation of MHC-I (83), which normally occurs in a post-TGN compartment (25, 46, 95). MHC-I molecules that escape Nef's effects at the TGN and reach the cell surface do not bind Nef well, presumably because they are phosphorylated on their cytoplasmic tail domains (83). The MHC-I molecules that bind Nef are ultimately not simply blocked from TGN exit but rather directed from the TGN to lysosomes for degradation (130, 141) (Fig. 6).
FIG. 6.
Model for MHC-I transport in Nef-expressing cells. (A) Nef binds to MHC-I early in the secretory pathway. (B) Nef inhibits the transport of newly synthesized MHC-I to the cell surface. (C) MHC-I that escapes the transport block can be rerouted from the cell surface. (D) Nef and MHC-I accumulate in the TGN at steady state, where they cooperate to recruit AP-1. (E) MHC-I is delivered to acidic compartments for degradation.
Nef Reroutes MHC-I via AP-1 Recruitment
The intracellular trafficking pathway followed by MHC-I in Nef-expressing cells suggested that Nef may affect sorting of MHC-I at the TGN by recruiting a cellular trafficking protein that normally sorts proteins in this organelle. For example, the clathrin adaptor protein complex AP-1 normally functions by binding to signals in the cytoplasmic tails of proteins in the TGN, thereby sorting these proteins into specific clathrin-coated transport vesicles destined for the endolysosomal pathway (Fig. 6). Consistent with such a model, depletion of AP-1 by RNA interference (RNAi) blocks the Nef-mediated disruption of MHC-I transport in astrocytic cells, T cells, and HeLa cells (130). Moreover, AP-1 coprecipitates with MHC-I and Nef in HIV-infected primary T cells (130).
The involvement of AP-1 in Nef-mediated MHC-I trafficking was in some ways quite unexpected, because several studies have reported that Nef binds to AP-1 via a dileucine motif (EXXXLL165) (23, 37, 47, 63, 76, 78, 94, 122) and this motif is dispensable for Nef-mediated MHC-I transport (103). However, the explanation for this apparent discrepancy was that recruitment of AP-1 to the Nef-MHC-I complex requires a novel AP-1 binding site composed of sequences derived from both the cytoplasmic domain of MHC-I and the N-terminal α-helix in Nef (130). Thus, Nef appears to have multiple ways of recruiting AP-1, either directly via its dileucine motif or via novel sites that become activated upon binding to other cellular partners.
Cell Type Specificity
Although Nef recruits AP-1 to the MHC-I cytoplasmic tail to reroute MHC-I from the TGN to lysosomes in T cells, in other cells types (for example, in HeLa cells), Nef does not efficiently block MHC-I export (20, 82, 83), nor does Nef induce MHC-I degradation (20). Thus, in these cells, the endocytic pathway appears to be the primary route that Nef utilizes to affect MHC-I (20, 82). Accordingly, the effect of Nef on MHC-I surface expression is much less in these cells than in T cells, which utilize both pathways (82). While this endocytic effect of Nef has been the primary focus of Nef activity in the literature, it does not appear to be the primary mechanism by which MHC-I is eliminated from the surface of HIV-infected T cells (82, 83, 130, 163).
The explanation for why Nef is able to disrupt MHC-I transport more efficiently in T cells than in HeLa cells is related to differences in the rate at which MHC-I is transported through the secretory pathway (83). In T cells, MHC-I is transported more slowly from the ER and through the Golgi apparatus, whereas in HeLa cells, this process happens much more quickly (83). When transport rates are reduced in HeLa cells (by reducing the temperature at which they are cultured), Nef acquires the ability to disrupt MHC-I transport to the cell surface, and interestingly, this disruption of MHC-I export correlates with the efficient recruitment of AP-1 to the MHC-I cytoplasmic tail (83). Thus, though other explanations remain possible, the data are consistent with the model that recruitment of AP-1 in the Golgi apparatus is rate limiting and that low transport rates allow this process to occur more efficiently.
Because Nef appears to affect MHC-I both in the secretory pathway and at the cell surface, it is important to know whether the two pathways are functionally related. Indeed, the depletion of AP-1 by RNAi inhibits Nef activity in both pathways (83). The mechanism for AP-1 action in the secretory pathway has been discussed; however, the apparent role of AP-1 at the plasma membrane is unclear. Because AP-1 has no reported role in endocytosis, a more likely explanation might be that Nef binds MHC-I in an internal compartment (i.e., early/recycling endosome) and the recruitment of AP-1 sequesters MHC-I and prevents its recycling. MHC-I that enters this route could then be transported to the TGN or targeted to an acidic compartment for degradation. From the perspective of viral immune evasion, the utilization of multiple pathways to eliminate surface MHC-I would be extremely beneficial to the virus. Any molecules that might escape the early transport block exerted by Nef could be removed by Nef activity at the plasma membrane, providing a backup to ensure effective suppression of antigen presentation.
Other Cellular Factors
The Nef acidic domain is required for Nef to disrupt MHC-I trafficking and is required for Nef to coprecipitate with MHC-I (163). This domain is also necessary for Nef to bind to the cellular adaptor protein PACS-1a (125), a protein required for the TGN localization of furin (159). Furthermore, a dominant negative mutant of PACS-1a blocks Nef activity on MHC-I in HeLa cells (39). Thus, it is tempting to speculate that this protein might contribute to the steady-state accumulation of MHC-I and/or Nef in the TGN (Fig. 6). PACS-1a also binds to AP-1 (39); however, it does not appear that PACS-1a links AP-1 to the Nef-MHC-I complex, because the acidic motif in Nef is dispensable for AP-1 recruitment in the context of an A2/Nef fusion protein (130). This result does not completely rule out the possibility that PACS-1a may participate in the stabilization of the Nef-MHC-I-AP-1 complex when Nef and MHC-I are not fused together. Additional experiments are necessary to understand the specific involvement of PACS-1a in this pathway.
Studies performed with HeLa cell lines have suggested that Nef may target MHC-I into a clathrin-independent pathway from the cell surface that is mediated by the GTPase ARF6 (20) and which is involved in the turnover of MHC-I from the cell surface in other systems. The requirement for ARF6 appears to be independent of the status of its associated guanine nucleotide (89). Interestingly, other evidence suggests that Nef can associate with another related GTPase (ARF1) independently of GTP loading/hydrolysis (52). Because studies have suggested that the major effect of Nef on MHC-I in T cells is to redirect trafficking from the TGN (82, 83, 130, 163), the significance of ARF6 for HIV biology in T cells requires further study. It is possible that other natural targets of HIV infection (e.g., macrophages or dendritic cells) or other nef alleles utilize this pathway to a greater degree.
In addition, the Nef polyproline helix (P69/72/75/78) is needed for Nef to disrupt MHC-I (but not CD4) trafficking and for Nef to coprecipitate with MHC-I (64, 103, 163). This domain is also needed for the high-affinity binding and activation of Src family kinase members (4, 13, 91, 134). Interestingly, the overexpression of the Hck SH3 domain inhibits the effect of Nef on MHC-I trafficking (27). However, an important role for tyrosine kinases in Nef-mediated MHC-I transport seems unlikely, because inhibition of tyrosine kinase activity has no effect on Nef-dependent MHC-I transport in T cells (103). In addition, mutation of P78, which is not essential for SH3 domain binding, has a more dramatic effect on MHC-I trafficking than mutation of the other prolines (128, 163, 167). It has been reported that binding of the SH3 domains of either c-Src or Hck to Nef has a major impact on the conformation of the N-terminal anchor domain of Nef (5). Thus, the polyproline domain in Nef may have an important structural role that could also affect protein stability (36). Because higher Nef levels are required for Nef to affect MHC-I compared with CD4 (96), structural defects may affect these activities differently. Finally, the polyproline helix is located along a solvent-exposed region directly downstream of the acidic motif (E62-65) (55). Thus, mutagenesis of the polyproline domain could have allosteric effects on the conformation of the acidic stretch (or vice versa).
PI3-kinase has also been implicated in the effect of Nef on trafficking of MHC-I (20, 89, 148). Extended treatments with chemical inhibitors of PI3-kinase (i.e., wortmannin or LY294,002) restore MHC-I expression to Nef-expressing cells (148). In shorter assays, inhibitors of PI3-kinase did not rescue MHC-I transport to the cell surface from the TGN (82), but they did change the distribution of MHC-I localization within intracellular compartments (89). Interestingly, the defect caused by mutation of the central polyproline helix in Nef was rescued in HeLa cells by fusion of the mutant to a constitutively active PI3-kinase (20). Thus, PI3-kinase activity appears to be required for an as-yet-undefined transport step within the endolysosomal pathway that ultimately leads to MHC-I retention and degradation. In the absence of PI3-kinase activity, MHC-I trafficking is disrupted, but in such a way that MHC-I can eventually find its way back to the cell surface. Clearly, the exact role of lipid kinase activity in Nef-dependent MHC-I transport is complex and requires further study.
DISRUPTION OF CD4 TRAFFICKING BY Nef
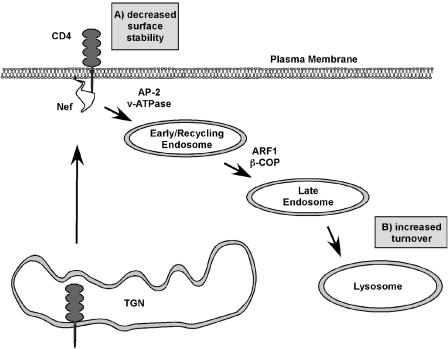
The CD4 protein is a coreceptor required for HIV infection. However, its continued presence on the surface of an HIV-infected cell after viral entry is problematic for several reasons. Because of their capacity to form a complex, coexpression of CD4 and the viral envelope disrupts the trafficking of both proteins. Moreover, the presence of CD4 on the cell membrane reduces the ability of the newly formed particle to properly bud and escape the infected cell and therefore reduces viral infectivity. HIV-1 counteracts this effect with the activities of two viral proteins, Vpu and Nef (87). There is general agreement, based on work from a number of labs, that Nef does not have much of an effect on the transport of CD4 to the cell surface but that it dramatically decreases the half-life of CD4 that has reached the cell surface. Ultimately, Nef targets CD4 for degradation in an acidic compartment. The experiments underlying this model have been the subject of other excellent reviews (7, 43, 124) and so will not be discussed in detail here.
Nef Binds to CD4
To decrease the half-life of CD4 on the cell surface, it is thought that Nef binds the cytoplasmic tail of CD4. Indeed, Nef has been shown to bind to the CD4 cytoplasmic tail in yeast two-hybrid assay systems (133), in purified protein assay systems (68, 71, 126), in cell lysates (80), and in living cells (16, 30). An NMR structural analysis demonstrated that the direct physical interaction occurred between a hydrophobic pocket in the Nef core domain and a 13-amino-acid peptide (QIKRLLSEKKT) from the CD4 cytoplasmic tail (68). This in vitro interaction was quite weak (with a dissociation constant of 1 mM) but highly specific, as the disruption of the dileucine motif in this peptide abrogated the association (68). A complex of full-length Nef with the CD4 cytoplasmic tail was found to be more stable, with a dissociation complex in the submicromolar range, and suggested that other amino acids in the N terminus contributed (126). In vivo there may be other factors that further stabilize and alter this interaction, because the dileucine motif was not needed when the interaction was detected in vivo (16, 30).
The Nef Flexible Loop and Its Binding Partners
There are three domains in the C-terminal flexible loop that are required for Nef's ability to downregulate CD4: a dileucine motif (EXXXLL165) and two diacidic motifs (EE155 and DD175). The roles of the dileucine motif and the diaspartic acid motif are clear: the mutation of these domains completely abrogates Nef activity. However, the role of the diglutamic acid motif is controversial—one paper reported a requirement for this sequence, while another study saw no effect (77, 123). Several models for Nef-mediated CD4 transport exist, each based on a familiar premise: Nef binds to the cytoplasmic tail of CD4 and recruits a cellular factor(s) to transport CD4 from the cell surface to lysosomes for degradation. Several potential cellular factors have been proposed, based on the correlative data that mutations that effect their binding are also necessary for Nef activity: V1H binding requires the dileucine and diaspartic acid motifs (56, 59, 102), AP-2 binding requires the dileucine motif (37), and both ARF1 binding and β-COP binding require the diglutamic acid motif (52, 123) (although not all investigators have observed this dependency [77]).
To address which of these interactions are functionally important, investigators have begun measuring the effect of reducing protein expression by RNA interference. Unfortunately, using this approach, different conclusions have been made by different investigators as to whether or not AP-2 expression is required for Nef's effects on CD4. Rose and colleagues reported that eliminating AP-2 by RNAi does not affect CD4 downregulation by HIV-1 Nef, even though this treatment inhibited CD4 downregulation by SIV Nef and blocked transferrin receptor internalization (131). The fact that SIV Nef responded differently might be explained by the presence of N-terminal tyrosine residues that bind AP-2 (102, 122), which are absent from HIV-1 Nef (Fig. 1). In contrast, two other studies reported that AP-2 expression does affect HIV Nef activity (79, 145), although Jin et al. observed a dependency on AP-2 only when Eps-15 activity was also inhibited (79).
The role of V1H may be linked to that of AP-2, as V1H binds to the medium chain of AP-2 and may connect Nef to the endocytic machinery by this route (59). The effect of reducing V1H protein expression on Nef activity has not yet been reported.
The role of β-COP in normal or Nef-mediated endosomal transport is not well understood. There is evidence that the late endosomal targeting of CD4 and Nef depended on the diglutamic acid motif (52, 123). In addition, the coprecipitation of β-COP, ARF1, and Nef was detected in HeLa cells by using overexpressed proteins, and this complex was partially reduced when the Nef diglutamic acid motif was mutated (52). However, because not all investigators have observed this dependency (77), further work is required to clearly understand the function of β-COP in this pathway. Specifically, this model would be greatly strengthened by the demonstration that these molecules are actually recruited to the CD4 tail in Nef-expressing cells.
Several experiments examining the mechanism of Nef-dependent CD4 transport have been performed with adherent cell lines that do not express Lck, a primary regulator of normal CD4 surface expression (104, 106, 107, 117-121). There is evidence that nonlymphoid cells (and lymphoid cells lacking Lck) constitutively internalize and recycle CD4, whereas in T cells, the expression of Lck anchors CD4 to the cell membrane (117, 120). Although the details are unclear, several studies have reported that the CD4-Lck physical interaction may affect or be affected by Nef binding (11, 62, 86), and interestingly, the exogenous expression of Lck has been reported to affect Nef activity either positively in Jurkat and U937 cells (11) or negatively when overexpressed in COS-7 cells (60). Thus, the CD4-Lck complex at the plasma membrane may be a critical target for Nef in this pathway. (Alternatively, it is possible that the dynamics of the CD4-Lck complex may change upon T-cell activation in such a way that it has less of an impact.) The HIV-infected, activated primary T cell presents a rather unique subcellular environment and is the most relevant system to understand Nef-mediated CD4 trafficking. Accordingly, the multiple events in CD4 transport that have been observed in Nef-expressing adherent cells may become more clear when fundamental differences in experimental systems are accounted for.
In sum, Nef is a multifunctional protein that binds to a number of cellular factors using protein domains that must also be intact for Nef to affect CD4 expression. Direct evidence that these cellular proteins exist in a complex with CD4 in Nef-expressing cells is needed to provide substance to the proposed models. A summary of the proposed mechanisms by which Nef affects CD4 expression is presented in Fig. 7.
FIG. 7.
Summary of the proposed mechanisms for Nef-induced CD4 downregulation. (A) Nef targets CD4 molecules from the cell surface, resulting in their enhanced internalization. (B) Nef causes the degradation of CD4 in acidic compartments.
PERSPECTIVES AND FUTURE DIRECTIONS
The results of the intense study of HIV-1 Nef have raised several important questions about its effects on protein trafficking. The next few sections will discuss some of the important issues that arise regarding Nef activity.
Nef Affects the Transport of Multiple Cellular Proteins
The list of proteins affected by Nef has continued to grow over the past several years. To date, Nef has been reported to decrease the cell surface expression of MHC-I, MHC-II, CD4, CD28, transferrin receptor, mannose receptor, CD80, CD86, CD8, and CCR5 and to increase the expression of TNF, LIGHT, DC-SIGN, and the invariant chain (2, 28, 88, 101, 139, 141, 143, 145, 147, 152, 156). The modulation of some of these proteins (e.g., MHC-I and CD4) clearly has an impact on HIV biology. However, a complete understanding of the biological significance and the mechanism by which Nef affects the expression of each of these proteins will require further study.
Nef-Induced Alterations in Protein Transport and HIV Infectivity
It is clear that Nef commandeers several pathways of intracellular protein trafficking. Because HIV assembles and buds from cellular membranes, does Nef modulate protein transport to play an active role in viral assembly? This question has not been explored in great detail, but interestingly, Nef increases the infectivity of viral particles, and this phenotype requires residues in the C-terminal flexible loop that are known to be involved in binding to APs (35, 101, 102, 109). Reduction of CD4 expression on the producer cell (and on the budding viral particles) by Nef clearly augments viral infectivity. However, because effects of Nef on viral infectivity can be demonstrated in CD4-negative viral producer cells, Nef must serve another, as yet poorly understood role (115). Nef is incorporated into HIV particles (162), indicating that Nef is present at the site of particle production. Nef associates with lipid rafts, and it appears that this may contribute to the Nef-dependent increase in viral infectivity (168, 169). Thus, it is possible that Nef affects the protein transport of HIV factors (i.e., Gag and Env) or cellular factors to promote viral assembly and/or budding. In support of this idea, a recent study reported that Nef affected the membrane trafficking and proteolytic processing of HIV Env (138). Also, it has been reported that Nef binds to Gag-Pol via the C-terminal flexible loop (34). Finally, Nef was recently shown to increase the localization of viral glycoproteins on endosomal membranes in producer cells and to also increase the incorporation of these molecules into viral particles (135). More experiments should be performed to monitor the intracellular trafficking of Gag, Env, and cellular proteins that play a role in virus production and release.
Recruitment of Adaptor Proteins by Nef
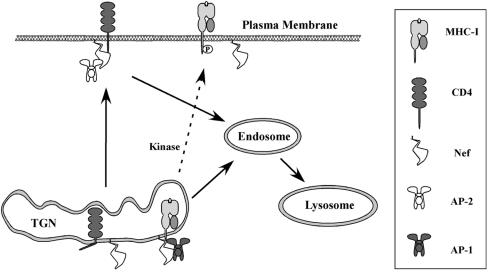
Nef binds to the cytoplasmic domains of both MHC-I and CD4, yet Nef affects MHC-I primarily by disrupting its transport to the cell surface (82, 83, 130, 148, 163), whereas Nef's effects on CD4 result largely from accelerating the internalization and degradation of CD4 molecules that have already arrived on the cell surface (7, 43). What are the factors that distinguish between these pathways, given that they both rely on Nef binding to a cytoplasmic tail? One possibility could be that Nef affects proteins differently depending on where it is able to bind them. For example, Nef binds hypophosphorylated MHC-I early in its biosynthesis and before it has reached the cell surface (83). Thus, Nef may promote MHC-I association with AP-1, because AP-1 is also found within the secretory pathway. Likewise, Nef might recognize CD4 only at the plasma membrane (perhaps in association with Lck), and thus binding to it would logically promote its association with the adaptor protein found at this location, AP-2 (Fig. 8). Another possibility might be that adaptor protein recruitment is dictated by sequences in Nef as well as in its binding partner(s). Consistent with this model, it has been shown that sequences within MHC-I are required for a Nef-MHC-I fusion protein to recruit AP-1 to the Nef-MHC-I complex (130). In addition, reduction of the T-cell receptor CD3 complex by SIV Nef (HIV Nef is not known to have this activity) requires a cooperative interaction between SIV Nef, AP-2, and the CD3-zeta cytoplasmic tail domain. Thus, the use of sequences within the host protein to facilitate recruitment of trafficking partners may be a general paradigm that potentially helps explain how Nef proteins are able to affect multiple different host proteins in different ways (150).
FIG. 8.
Proposed mechanism for differential effects of Nef on CD4 and MHC-I. In this model Nef preferentially binds to hypophosphorylated MHC-I molecules early in the secretory pathway (83) and recruits AP-1 at its normal localization in the Golgi. In contrast, Nef may preferentially associate with plasma membrane forms of CD4 and recruits AP-2 at this location.
The Nef dileucine motif and AP binding.
A perplexing issue regarding CD4 downregulation by HIV-1 Nef is the exact role(s) of the Nef dileucine motif. This domain is clearly required for Nef activity; however, many different APs have been reported to interact with this domain, and so it is unclear exactly which is required. The isolation of a protein complex containing Nef, CD4, and a dileucine binding protein would be extremely helpful in this regard. Unfortunately, such a complex may be transient, making it difficult to detect. The reduction of AP-1 and AP-3 by RNA interference does not affect Nef-mediated downregulation of CD4 in astrocytes or T cells (130), and (as discussed above) the reduction of AP-2 expression by RNA interference has yielded conflicting results. Thus, it is possible that a single adaptor protein alone is not responsible for disruption of CD4 trafficking but that multiple proteins are recruited via the Nef dileucine motif, serving either redundant or sequential roles in CD4 trafficking.
AP-1 recruitment to the Nef-MHC-I complex.
At this stage, the recruitment of AP-1 by the Nef-MHC-I complex is only minimally understood. For example, the N-terminal α-helix in Nef and tyrosine 320 in the cytoplasmic domain of HLA-A2 are necessary for AP-1 binding to a Nef-MHC-I fusion protein and are also needed for Nef expressed in trans to associate with the MHC-I tail (130). At this stage it is unclear which and how many amino acids directly participate in binding, and it is unclear exactly how the three-way complex forms. It is possible that (i) residues from Nef and MHC-I cooperatively form a novel AP-1 binding site, (ii) Nef (or MHC-I) may recruit other factors that mediate AP-1 binding indirectly, or (iii) the interaction between Nef and MHC-I may induce conformational changes to reveal a cryptic AP-1 binding site. In addition, it will be important to determine whether the cytoplasmic tail domains of other cellular partners of Nef participate in the recruitment of adaptor proteins.
To fully understand the nature of the Nef-MHC-I-AP-1 complex, it will also be necessary to better understand the requirements for AP-1 recruitment and functional activity. For example, is the normal YXXΦ binding site on AP-1 (72) utilized? Is the activity of the GTPase, which is normally required for adaptor protein association with membranes (ARF1), required? Do associated membranes require a particular phosphatidylinositide content (38)? Is there a role for the Golgi resident phosphatidylinositol 4-kinase (PI4KIIα), which generates PI(4)P in the TGN, to facilitate AP-1 recruitment (160)? Further definition of the factors that mediate AP-1 recruitment and vesicle targeting (i.e., SNARE proteins or additional adaptors) would be extremely helpful to fully understand Nef activity as well as the normal cell biology of AP-1.
In addition, it would be very interesting to determine whether Nef targets MHC-I into a completely novel pathway or whether there are certain circumstances in which MHC-I normally utilizes an AP-1-dependent pathway. The same tyrosine residue in MHC-I that participates in AP-1 recruitment in the presence of Nef is also required for efficient cross-priming (antigen presentation of exogenous peptides). Thus, there may be certain circumstances in which there is AP-1-dependent transport of MHC-I into phagolysosomal compartments in antigen-presenting cells (97).
Nef stabilizes the membrane association of APs.
Brefeldin A (BFA) is a fungal metabolite that uncouples the ARF1 GDP-GTP cycle. In cells treated with BFA, AP-1 and AP-3 become cytosolic due to the inability of ARF1 to recruit these complexes to the membrane. However, Nef promotes the stable association of AP-1 and AP-3 with membranes even in the presence of BFA, suggesting that Nef can recruit APs independently of ARF1 activity or that it can stabilize the recruited molecules after ARF-dependent attachment of the complexes to membranes (32, 76). The abnormal association of APs with membranes has been proposed to alter endosomal morphology and possibly to increase viral infectivity. In support of this idea, the dileucine motif is required for both the persistent AP membrane association and Nef-dependent increase in viral infectivity in cells that lack CD4 (101). Further experiments are necessary to determine the relevance of this correlation and to determine the role of AP membrane stabilization in Nef-mediated protein trafficking.
Molecular Therapeutics Targeting Nef Activity
It is clear from work from many laboratories that the biological function(s) of Nef is likely to increase the pathogenicity of HIV. Thus, pharmaceutical reagents aimed at blocking Nef activity are likely to be effective treatments. For example, the targeted disruption of the Nef-MHC-I complex would block the inhibition of antigen presentation in HIV-infected cells, and as a consequence, the HIV-specific CTL response may be able to better control HIV infection. In conjunction with highly active antiretroviral therapy, inhibition of Nef could increase our chances of achieving our long-term goal of providing a cure for those suffering from HIV disease.
Acknowledgments
This work was funded by NIH grants AI046998 and AI051192.
REFERENCES
- 1.Akari, H., S. Arold, T. Fukumori, T. Okazaki, K. Strebel, and A. Adachi. 2000. Nef-induced major histocompatibility complex class I down-regulation is functionally dissociated from its virion incorporation, enhancement of viral infectivity, and CD4 down-regulation. J. Virol. 74:2907-2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, S., D. C. Shugars, R. Swanstrom, and J. V. Garcia. 1993. Nef from primary isolates of human immunodeficiency virus type 1 suppresses surface CD4 expression in human and mouse T cells. J. Virol. 67:4923-4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aniento, F., F. Gu, R. G. Parton, and J. Gruenberg. 1996. An endosomal beta COP is involved in the pH-dependent formation of transport vesicles destined for late endosomes. J. Cell Biol. 133:29-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arold, S., P. Franken, M. P. Strub, F. Hoh, S. Benichou, R. Benarous, and C. Dumas. 1997. The crystal structure of HIV-1 Nef protein bound to the Fyn kinase SH3 domain suggests a role for this complex in altered T cell receptor signaling. Structure 5:1361-1372. [DOI] [PubMed] [Google Scholar]
- 5.Arold, S., R. O'Brien, P. Franken, M. P. Strub, F. Hoh, C. Dumas, and J. E. Ladbury. 1998. RT loop flexibility enhances the specificity of Src family SH3 domains for HIV-1 Nef. Biochemistry 37:14683-14691. [DOI] [PubMed] [Google Scholar]
- 6.Arold, S. T., and A. S. Baur. 2001. Dynamic Nef and Nef dynamics: how structure could explain the complex activities of this small HIV protein. Trends Biochem. Sci. 26:356-363. [DOI] [PubMed] [Google Scholar]
- 7.Arora, V. K., B. L. Fredericksen, and J. V. Garcia. 2002. Nef: agent of cell subversion. Microbes Infect. 4:189-199. [DOI] [PubMed] [Google Scholar]
- 8.Baba, T. W., Y. S. Jeong, D. Pennick, R. Bronson, M. F. Greene, and R. M. Ruprecht. 1995. Pathogenicity of live, attenuated SIV after mucosal infection of neonatal macaques. Science 267:1820-1825. [DOI] [PubMed] [Google Scholar]
- 9.Baba, T. W., V. Liska, A. H. Khimani, N. B. Ray, P. J. Dailey, D. Penninck, R. Bronson, M. F. Greene, H. M. McClure, L. N. Martin, and R. M. Ruprecht. 1999. Live attenuated, multiply deleted simian immunodeficiency virus causes AIDS in infant and adult macaques. Nat. Med. 5:194-203. [DOI] [PubMed] [Google Scholar]
- 10.Badley, A. D., D. Dockrell, M. Simpson, R. Schut, D. H. Lynch, P. Leibson, and C. V. Paya. 1997. Macrophage-dependent apoptosis of CD4+ T lymphocytes from HIV-infected individuals is mediated by FasL and tumor necrosis factor. J. Exp. Med. 185:55-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bandres, J. C., A. S. Shaw, and L. Ratner. 1995. HIV-1 Nef protein downregulation of CD4 surface expression: relevance of the lck binding domain of CD4. Virology 207:338-341. [DOI] [PubMed] [Google Scholar]
- 12.Barlowe, C. 2000. Traffic COPs of the early secretory pathway. Traffic 1:371-377. [DOI] [PubMed] [Google Scholar]
- 13.Baur, A. S., G. Sass, B. Laffert, D. Willbold, C. Cheng-Mayer, and B. M. Peterlin. 1997. The N-terminus of Nef from HIV-1/SIV associates with a protein complex containing Lck and a serine kinase. Immunity 6:283-291. [DOI] [PubMed] [Google Scholar]
- 14.Baur, A. S., E. T. Sawai, P. Dazin, W. J. Fantl, C. Cheng-Mayer, and B. M. Peterlin. 1994. HIV-1 Nef leads to inhibition or activation of T cells depending on its intracellular localization. Immunity 1:373-384. [DOI] [PubMed] [Google Scholar]
- 15.Benichou, S., M. Bomsel, M. Bodeus, H. Durand, M. Doute, F. Letourneur, J. Camonis, and R. Benarous. 1994. Physical interaction of the HIV-1 Nef protein wih β-cop, a component of non-clathrin coated vesicles essential for membrane traffic. J. Biol. Chem. 269:30073-30076. [PubMed] [Google Scholar]
- 16.Bentham, M., S. Mazaleyrat, and M. Harris. 2003. The di-leucine motif in the cytoplasmic tail of CD4 is not required for binding to human immunodeficiency virus type 1 Nef, but is critical for CD4 down-modulation. J. Gen. Virol. 84:2705-2713. [DOI] [PubMed] [Google Scholar]
- 17.Berke, G. 1995. The CTL's kiss of death. Cell 81:9-12. [DOI] [PubMed] [Google Scholar]
- 18.Beron, W., L. S. Mayorga, M. I. Colombo, and P. D. Stahl. 2001. Recruitment of coat-protein-complex proteins on to phagosomal membranes is regulated by a brefeldin A-sensitive ADP-ribosylation factor. Biochem. J. 355:409-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Birch, M. R., J. C. Learmont, W. B. Dyer, N. J. Deacon, J. J. Zaunders, N. Saksena, A. L. Cunningham, J. Mills, and J. S. Sullivan. 2001. An examination of signs of disease progression in survivors of the Sydney Blood Bank Cohort (SBBC). J. Clin. Virol. 22:263-270. [DOI] [PubMed] [Google Scholar]
- 20.Blagoveshchenskaya, A. D., L. Thomas, S. F. Feliciangeli, C. H. Hung, and G. Thomas. 2002. HIV-1 Nef downregulates MHC-I by a PACS-1- and PI3K-regulated ARF6 endocytic pathway. Cell 111:853-866. [DOI] [PubMed] [Google Scholar]
- 21.Bonifacino, J. S., and L. M. Traub. 2003. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 72:395-447. [DOI] [PubMed] [Google Scholar]
- 22.Botelho, R. J., D. J. Hackam, A. D. Schreiber, and S. Grinstein. 2000. Role of COPI in phagosome maturation. J. Biol. Chem. 275:15717-15727. [DOI] [PubMed] [Google Scholar]
- 23.Bresnahan, P. A., W. Yonemoto, S. Ferrell, D. Williams-Herman, R. Geleziunas, and W. C. Greene. 1998. A dileucine motif in HIV-1 Nef acts as an internalization signal for CD4 downregulation and binds the AP-1 clathrin adaptor. Curr. Biol. 8:1235-1238. [DOI] [PubMed] [Google Scholar]
- 24.Burleigh, L., P. Y. Lozach, C. Schiffer, I. Staropoli, V. Pezo, F. Porrot, B. Canque, J. L. Virelizier, F. Arenzana-Seisdedos, and A. Amara. 2006. Infection of dendritic cells (DCs), not DC-SIGN-mediated internalization of human immunodeficiency virus, is required for long-term transfer of virus to T cells. J. Virol. 80:2949-2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Capps, G. G., and M. C. Zuniga. 2000. Phosphorylation of class I MHC molecules in the absence of phorbol esters is an intracellular event and may be characteristic of trafficking molecules. Mol. Immunol. 37:59-71. [DOI] [PubMed] [Google Scholar]
- 26.Carl, S., T. C. Greenough, M. Krumbiegel, M. Greenberg, J. Skowronski, J. L. Sullivan, and F. Kirchhoff. 2001. Modulation of different human immunodeficiency virus type 1 Nef functions during progression to AIDS. J. Virol. 75:3657-3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang, A. H., M. V. O'Shaughnessy, and F. R. Jirik. 2001. Hck SH3 domain-dependent abrogation of Nef-induced class 1 MHC down-regulation. Eur. J. Immunol. 31:2382-2387. [DOI] [PubMed] [Google Scholar]
- 28.Chaudhry, A., S. R. Das, A. Hussain, S. Mayor, A. George, V. Bal, S. Jameel, and S. Rath. 2005. The Nef protein of HIV-1 induces loss of cell surface costimulatory molecules CD80 and CD86 in APCs. J. Immunol. 175:4566-4574. [DOI] [PubMed] [Google Scholar]
- 29.Cheng-Mayer, C., P. Iannello, K. Shaw, P. A. Luciw, and J. A. Levy. 1989. Differential effects of nef on HIV replication: implications for viral pathogenesis in the host. Science 246:1629-1632. [DOI] [PubMed] [Google Scholar]
- 30.Cluet, D., C. Bertsch, C. Beyer, L. Gloeckler, M. Erhardt, J. P. Gut, J. L. Galzi, and A. M. Aubertin. 2005. Detection of human immunodeficiency virus type 1 Nef and CD4 physical interaction in living human cells by using bioluminescence resonance energy transfer. J. Virol. 79:8629-8636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen, G. B., R. T. Gandhi, D. M. Davis, O. Mandelboim, B. K. Chen, J. L. Strominger, and D. Baltimore. 1999. The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity 10:661-671. [DOI] [PubMed] [Google Scholar]
- 32.Coleman, S. H., D. Hitchin, C. M. Noviello, and J. C. Guatelli. 2006. HIV-1 Nef stabilizes AP-1 on membranes without inducing ARF1-independent de novo attachment. Virology 345:148-155. [DOI] [PubMed] [Google Scholar]
- 33.Collins, K., B. Chen, S. Kalams, B. Walker, and D. Baltimore. 1998. HIV-1 Nef protein protects infected primary human cells from killing by cytotoxic T lymphocytes. Nature 391:397-401. [DOI] [PubMed] [Google Scholar]
- 34.Costa, L. J., Y. H. Zheng, J. Sabotic, J. Mak, O. T. Fackler, and B. M. Peterlin. 2004. Nef binds p6* in GagPol during replication of human immunodeficiency virus type 1. J. Virol. 78:5311-5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Craig, H., M. Pandori, and J. Guatelli. 1998. Interaction of HIV-1 Nef with the cellular dileucine-based sorting pathway is required for CD4 down-regulation and optimal viral infectivity. Proc. Natl. Acad. Sci. USA 95:11229-11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Craig, H. M., M. W. Pandori, N. L. Riggs, D. D. Richman, and J. C. Guatelli. 1999. Analysis of the SH3-binding region of HIV-1 nef: partial functional defects introduced by mutations in the polyproline helix and the hydrophobic pocket. Virology 262:55-63. [DOI] [PubMed] [Google Scholar]
- 37.Craig, H. M., T. R. Reddy, N. L. Riggs, P. P. Dao, and J. C. Guatelli. 2000. Interactions of HIV-1 nef with the mu subunits of adaptor protein complexes 1, 2, and 3: role of the dileucine-based sorting motif. Virology 271:9-17. [DOI] [PubMed] [Google Scholar]
- 38.Crottet, P., D. M. Meyer, J. Rohrer, and M. Spiess. 2002. ARF1.GTP, tyrosine-based signals, and phosphatidylinositol 4,5-bisphosphate constitute a minimal machinery to recruit the AP-1 clathrin adaptor to membranes. Mol. Biol. Cell 13:3672-3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crump, C. M., Y. Xiang, L. Thomas, F. Gu, C. Austin, S. A. Tooze, and G. Thomas. 2001. PACS-1 binding to adaptors is required for acidic cluster motif-mediated protein traffic. EMBO J. 20:2191-2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Daniel, M. D., F. Kirchhoff, S. C. Czajak, P. K. Sehgal, and R. C. Desrosiers. 1992. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science 258:1938-1941. [DOI] [PubMed] [Google Scholar]
- 41.Daro, E., D. Sheff, M. Gomez, T. Kreis, and I. Mellman. 1997. Inhibition of endosome function in CHO cells bearing a temperature-sensitive defect in the coatomer (COPI) component epsilon-COP. J. Cell Biol. 139:1747-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Matteis, M. A., and A. Godi. 2004. PI-loting membrane traffic. Nat. Cell Biol. 6:487-492. [DOI] [PubMed] [Google Scholar]
- 43.Doms, R. W., and D. Trono. 2000. The plasma membrane as a combat zone in the HIV battlefield. Genes Dev. 14:2677-2688. [DOI] [PubMed] [Google Scholar]
- 44.Doray, B., P. Ghosh, J. Griffith, H. J. Geuze, and S. Kornfeld. 2002. Cooperation of GGAs and AP-1 in packaging MPRs at the trans-Golgi network. Science 297:1700-1703. [DOI] [PubMed] [Google Scholar]
- 45.Dyer, W. B., A. F. Geczy, S. J. Kent, L. B. McIntyre, S. A. Blasdall, J. C. Learmont, and J. S. Sullivan. 1997. Lymphoproliferative immune function in the Sydney Blood Bank Cohort, infected with natural nef/long terminal repeat mutants, and in other long-term survivors of transfusion-acquired HIV-1 infection. AIDS 11:1565-1574. [DOI] [PubMed] [Google Scholar]
- 46.Eichholtz, T., P. Vossebeld, M. van Overveld, and H. Ploegh. 1992. Activation of protein kinase C accelerates internalization of transferrin receptor but not of major histocompatibility complex class I, independent of their phosphorylation status. J. Biol. Chem. 267:22490-22495. [PubMed] [Google Scholar]
- 47.Erdtmann, L., K. Janvier, G. Raposo, H. M. Craig, P. Benaroch, C. Berlioz-Torrent, J. C. Guatelli, R. Benarous, and S. Benichou. 2000. Two independent regions of HIV-1 Nef are required for connection with the endocytic pathway through binding to the mu 1 chain of AP1 complex. Traffic 1:871-883. [DOI] [PubMed] [Google Scholar]
- 48.Fackler, O. T., N. Kienzle, E. Kremmer, A. Boese, B. Schramm, T. Klimkait, C. Kucherer, and N. Mueller-Lantzsch. 1997. Association of human immunodeficiency virus Nef protein with actin is myristoylation dependent and influences its subcellular localization. Eur. J. Biochem. 247:843-851. [DOI] [PubMed] [Google Scholar]
- 49.Fackler, O. T., X. Lu, J. A. Frost, M. Geyer, B. Jiang, W. Luo, A. Abo, A. S. Alberts, and B. M. Peterlin. 2000. p21-activated kinase 1 plays a critical role in cellular activation by Nef. Mol. Cell. Biol. 20:2619-2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fackler, O. T., W. Luo, M. Geyer, A. S. Alberts, and B. M. Peterlin. 1999. Activation of Vav by Nef induces cytoskeletal rearrangements and downstream effector functions. Mol. Cell 3:729-739. [DOI] [PubMed] [Google Scholar]
- 51.Fauci, A. S. 1988. The human immunodeficiency virus: infectivity and mechanisms of pathogenesis. Science 239:617-622. [DOI] [PubMed] [Google Scholar]
- 52.Faure, J., R. Stalder, C. Borel, K. Sobo, V. Piguet, N. Demaurex, J. Gruenberg, and D. Trono. 2004. ARF1 regulates Nef-induced CD4 degradation. Curr. Biol. 14:1056-1064. [DOI] [PubMed] [Google Scholar]
- 53.Franken, P., S. Arold, A. Padilla, M. Bodeus, F. Hoh, M. Strub, M. Boyer, M. Jullien, R. Benarous, and C. Dumas. 1997. HIV-1 Nef protein: purification, crystallizations, and preliminary X-ray diffraction studies. Protein Sci. 6:2681-2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Geleziunas, R., W. Xu, K. Takeda, H. Ichijo, and W. C. Greene. 2001. HIV-1 Nef inhibits ASK1-dependent death signalling providing a potential mechanism for protecting the infected host cell. Nature 410:834-838. [DOI] [PubMed] [Google Scholar]
- 55.Geyer, M., O. T. Fackler, and B. M. Peterlin. 2001. Structure-function relationships in HIV-1 Nef. EMBO Rep. 2:580-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Geyer, M., O. T. Fackler, and B. M. Peterlin. 2002. Subunit H of the V-ATPase involved in endocytosis shows homology to beta-adaptins. Mol. Biol. Cell 13:2045-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Geyer, M., C. E. Munte, J. Schorr, R. Kellner, and H. R. Kalbitzer. 1999. Structure of the anchor-domain of myristoylated and non-myristoylated HIV-1 Nef protein. J. Mol. Biol. 289:123-138. [DOI] [PubMed] [Google Scholar]
- 58.Geyer, M., and B. M. Peterlin. 2001. Domain assembly, surface accessibility and sequence conservation in full length HIV-1 Nef. FEBS Lett. 496:91-95. [DOI] [PubMed] [Google Scholar]
- 59.Geyer, M., H. Yu, R. Mandic, T. Linnemann, Y. H. Zheng, O. T. Fackler, and B. M. Peterlin. 2002. Subunit H of the V-ATPase binds to the medium chain of adaptor protein complex 2 and connects Nef to the endocytic machinery. J. Biol. Chem. 277:28521-28529. [DOI] [PubMed] [Google Scholar]
- 60.Goldsmith, M. A., M. T. Warmerdam, R. E. Atchison, M. D. Miller, and W. C. Greene. 1995. Dissociation of the CD4 downregulation and viral infectivity enhancement functions of human immunodeficiency virus type 1 Nef. J. Virol. 69:4112-4121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Granelli-Piperno, A., I. Shimeliovich, M. Pack, C. Trumpfheller, and R. M. Steinman. 2006. HIV-1 selectively infects a subset of nonmaturing BDCA1-positive dendritic cells in human blood. J. Immunol. 176:991-998. [DOI] [PubMed] [Google Scholar]
- 62.Gratton, S., X. J. Yao, S. Venkatesan, E. A. Cohen, and R. P. Sekaly. 1996. Molecular analysis of the cytoplasmic domain of CD4: overlapping but noncompetitive requirement for lck association and down-regulation by Nef. J. Immunol. 157:3305-3311. [PubMed] [Google Scholar]
- 63.Greenberg, M., L. DeTulleo, I. Rapoport, J. Skowronski, and T. Kirchhausen. 1998. A dileucine motif in HIV-1 Nef is essential for sorting into clathrin-coated pits and for downregulation of CD4. Curr. Biol. 8:1239-1242. [DOI] [PubMed] [Google Scholar]
- 64.Greenberg, M., A. Iafrate, and J. Skowronski. 1998. The SH3 domain-binding surface and an acidic motif in HIV-1 Nef regulate trafficking of class I MHC complexes. EMBO J. 17:2777-2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Greenberg, M. E., S. Bronson, M. Lock, M. Neumann, G. N. Pavlakis, and J. Skowronski. 1997. Co-localization of HIV-1 Nef with the AP-2 adaptor protein complex correlates with Nef-induced CD4 down-regulation. EMBO J. 16:6964-6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Greenway, A. L., D. A. McPhee, K. Allen, R. Johnstone, G. Holloway, J. Mills, A. Azad, S. Sankovich, and P. Lambert. 2002. Human immunodeficiency virus type 1 Nef binds to tumor suppressor p53 and protects cells against p53-mediated apoptosis. J. Virol. 76:2692-2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grzesiek, S., A. Bax, G. M. Clore, A. M. Gronenborn, J. S. Hu, J. Kaufman, I. Palmer, S. J. Stahl, and P. T. Wingfield. 1996. The solution structure of HIV-1 Nef reveals an unexpected fold and permits delineation of the binding surface for the SH3 domain of Hck tyrosine protein kinase. Nat. Struct. Biol. 3:340-345. [DOI] [PubMed] [Google Scholar]
- 68.Grzesiek, S., S. J. Stahl, P. T. Wingfield, and A. Bax. 1996. The CD4 determinant for downregulation by HIV-1 Nef directly binds to Nef. Mapping of the Nef binding surface by NMR. Biochemistry 35:10256-10261. [DOI] [PubMed] [Google Scholar]
- 69.Gu, F., F. Aniento, R. G. Parton, and J. Gruenberg. 1997. Functional dissection of COP-I subunits in the biogenesis of multivesicular endosomes. J. Cell Biol. 139:1183-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gu, F., and J. Gruenberg. 2000. ARF1 regulates pH-dependent COP functions in the early endocytic pathway. J. Biol. Chem. 275:8154-8160. [DOI] [PubMed] [Google Scholar]
- 71.Harris, M. P., and J. C. Neil. 1994. Myristoylation-dependent binding of HIV-1 Nef to CD4. J. Mol. Biol. 241:136-142. [DOI] [PubMed] [Google Scholar]
- 72.Heldwein, E. E., E. Macia, J. Wang, H. L. Yin, T. Kirchhausen, and S. C. Harrison. 2004. Crystal structure of the clathrin adaptor protein 1 core. Proc. Natl. Acad. Sci. USA 101:14108-14113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hinners, I., F. Wendler, H. Fei, L. Thomas, G. Thomas, and S. A. Tooze. 2003. AP-1 recruitment to VAMP4 is modulated by phosphorylation-dependent binding of PACS-1. EMBO Rep. 4:1182-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hirst, J., A. Motley, K. Harasaki, S. Y. Peak Chew, and M. S. Robinson. 2003. EpsinR: an ENTH domain-containing protein that interacts with AP-1. Mol. Biol. Cell 14:625-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Janardhan, A., T. Swigut, B. Hill, M. P. Myers, and J. Skowronski. 2004. HIV-1 Nef binds the DOCK2-ELMO1 complex to activate rac and inhibit lymphocyte chemotaxis. PLoS Biol. 2:E6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Janvier, K., H. Craig, D. Hitchin, R. Madrid, N. Sol-Foulon, L. Renault, J. Cherfils, D. Cassel, S. Benichou, and J. Guatelli. 2003. HIV-1 Nef stabilizes the association of adaptor protein complexes with membranes. J. Biol. Chem. 278:8725-8732. [DOI] [PubMed] [Google Scholar]
- 77.Janvier, K., H. Craig, S. Le Gall, R. Benarous, J. Guatelli, O. Schwartz, and S. Benichou. 2001. Nef-induced CD4 downregulation: a diacidic sequence in human immunodeficiency virus type 1 Nef does not function as a protein sorting motif through direct binding to β-COP. J. Virol. 75:3971-3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Janvier, K., Y. Kato, M. Boehm, J. R. Rose, J. A. Martina, B. Y. Kim, S. Venkatesan, and J. S. Bonifacino. 2003. Recognition of dileucine-based sorting signals from HIV-1 Nef and LIMP-II by the AP-1 gamma-sigma1 and AP-3 delta-sigma3 hemicomplexes. J. Cell Biol. 163:1281-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jin, Y. J., C. Y. Cai, X. Zhang, H. T. Zhang, J. A. Hirst, and S. J. Burakoff. 2005. HIV Nef-mediated CD4 down-regulation is adaptor protein complex 2 dependent. J. Immunol. 175:3157-3164. [DOI] [PubMed] [Google Scholar]
- 80.Jin, Y. J., X. Zhang, J. G. Boursiquot, and S. J. Burakoff. 2004. CD4 phosphorylation partially reverses Nef down-regulation of CD4. J. Immunol. 173:5495-5500. [DOI] [PubMed] [Google Scholar]
- 81.Johannes, L., V. Pezo, F. Mallard, D. Tenza, A. Wiltz, A. Saint-Pol, J. Helft, C. Antony, and P. Benaroch. 2003. Effects of HIV-1 Nef on retrograde transport from the plasma membrane to the endoplasmic reticulum. Traffic 4:323-332. [DOI] [PubMed] [Google Scholar]
- 82.Kasper, M. R., and K. L. Collins. 2003. Nef-mediated disruption of HLA-A2 transport to the cell surface in T cells. J. Virol. 77:3041-3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kasper, M. R., J. F. Roeth, M. Williams, T. M. Filzen, R. I. Fleis, and K. L. Collins. 2005. HIV-1 Nef disrupts antigen presentation early in the secretory pathway. J. Biol. Chem. 280:12840-12848. [DOI] [PubMed] [Google Scholar]
- 84.Kerkau, T., R. Schmitt-Landgraf, A. Schimpl, and E. Wecker. 1989. Downregulation of HLA class I antigens in HIV-1-infected cells. AIDS Res. Hum. Retroviruses 5:613-620. [DOI] [PubMed] [Google Scholar]
- 85.Kestler, H. W., III, D. J. Ringler, K. Mori, D. L. Panicali, P. K. Sehgal, M. D. Daniel, and R. C. Desrosiers. 1991. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell 65:651-662. [DOI] [PubMed] [Google Scholar]
- 86.Kim, Y. H., S. H. Chang, J. H. Kwon, and S. S. Rhee. 1999. HIV-1 Nef plays an essential role in two independent processes in CD4 down-regulation: dissociation of the CD4-p56(lck) complex and targeting of CD4 to lysosomes. Virology 257:208-219. [DOI] [PubMed] [Google Scholar]
- 87.Lama, J., A. Mangasarian, and D. Trono. 1999. Cell-surface expression of CD4 reduces HIV-1 infectivity by blocking Env incorporation in a Nef- and Vpu-inhibitable manner. Curr. Biol. 9:622-631. [DOI] [PubMed] [Google Scholar]
- 88.Lama, J., and C. F. Ware. 2000. Human immunodeficiency virus type 1 Nef mediates sustained membrane expression of tumor necrosis factor and the related cytokine LIGHT on activated T cells. J. Virol. 74:9396-9402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Larsen, J. E., R. H. Massol, T. J. Nieland, and T. Kirchhausen. 2004. HIV Nef-mediated major histocompatibility complex class I down-modulation is independent of Arf6 activity. Mol. Biol. Cell 15:323-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Learmont, J. C., A. F. Geczy, J. Mills, L. J. Ashton, C. H. Raynes-Greenow, R. J. Garsia, W. B. Dyer, L. McIntyre, R. B. Oelrichs, D. I. Rhodes, N. J. Deacon, and J. S. Sullivan. 1999. Immunologic and virologic status after 14 to 18 years of infection with an attenuated strain of HIV-1. A report from the Sydney Blood Bank Cohort. N. Engl. J. Med. 340:1715-1722. [DOI] [PubMed] [Google Scholar]
- 91.Lee, C. H., B. Leung, M. A. Lemmon, J. Zheng, D. Cowburn, J. Kuriyan, and K. Saksela. 1995. A single amino acid in the SH3 domain of Hck determines its high affinity and specificity in binding to HIV-1 Nef protein. EMBO J. 14:5006-5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee, C. H., K. Saksela, U. A. Mirza, B. T. Chait, and J. Kuriyan. 1996. Crystal structure of the conserved core of HIV-1 Nef complexed with a Src family SH3 domain. Cell 85:931-942. [DOI] [PubMed] [Google Scholar]
- 93.Le Gall, S., F. Buseyne, A. Trocha, B. D. Walker, J. M. Heard, and O. Schwartz. 2000. Distinct trafficking pathways mediate Nef-induced and clathrin-dependent major histocompatibility complex class I down-regulation. J. Virol. 74:9256-9266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Le Gall, S., L. Erdtmann, S. Benichou, C. Berlioz-Torrent, L. Liu, R. Benarous, J. M. Heard, and O. Schwartz. 1998. Nef interacts with mu subunit of clathrin adaptor complexes and reveals a cryptic sorting signal in MHC I molecules. Immunity 8:483-495. [DOI] [PubMed] [Google Scholar]
- 95.Lippe, R., E. Luke, Y. T. Kuah, C. Lomas, and W. A. Jefferies. 1991. Adenovirus infection inhibits the phosphorylation of major histocompatibility complex class I proteins. J. Exp. Med. 174:1159-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu, X., J. A. Schrager, G. D. Lange, and J. W. Marsh. 2001. HIV Nef-mediated cellular phenotypes are differentially expressed as a function of intracellular Nef concentrations. J. Biol. Chem. 276:32763-32770. [DOI] [PubMed] [Google Scholar]
- 97.Lizee, G., G. Basha, J. Tiong, J. P. Julien, M. Tian, K. E. Biron, and W. A. Jefferies. 2003. Control of dendritic cell cross-presentation by the major histocompatibility complex class I cytoplasmic domain. Nat. Immunol. 4:1065-1073. [DOI] [PubMed] [Google Scholar]
- 98.Llorente, A., S. U. Lauvrak, B. van Deurs, and K. Sandvig. 2003. Induction of direct endosome to endoplasmic reticulum transport in Chinese hamster ovary (CHO) cells (LdlF) with a temperature-sensitive defect in epsilon-coatomer protein (epsilon-COP). J. Biol. Chem. 278:35850-35855. [DOI] [PubMed] [Google Scholar]
- 99.Lu, X., X. Wu, A. Plemenitas, H. Yu, E. T. Sawai, A. Abo, and B. M. Peterlin. 1996. CDC42 and Rac1 are implicated in the activation of the Nef-associated kinase and replication of HIV-1. Curr. Biol. 6:1677-1684. [DOI] [PubMed] [Google Scholar]
- 100.Lu, X., H. Yu, S. H. Liu, F. M. Brodsky, and B. M. Peterlin. 1998. Interactions between HIV1 Nef and vacuolar ATPase facilitate the internalization of CD4. Immunity 8:647-656. [DOI] [PubMed] [Google Scholar]
- 101.Madrid, R., K. Janvier, D. Hitchin, J. Day, S. Coleman, C. Noviello, J. Bouchet, A. Benmerah, J. Guatelli, and S. Benichou. 2005. Nef-induced alteration of the early/recycling endosomal compartment correlates with enhancement of HIV-1 infectivity. J. Biol. Chem. 280:5032-5044. [DOI] [PubMed] [Google Scholar]
- 102.Mandic, R., O. T. Fackler, M. Geyer, T. Linnemann, Y. H. Zheng, and B. M. Peterlin. 2001. Negative factor from SIV binds to the catalytic subunit of the V-ATPase to internalize CD4 and to increase viral infectivity. Mol. Biol. Cell 12:463-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mangasarian, A., V. Piguet, J.-K. Wang, Y.-L. Chen, and D. Trono. 1999. Nef-induced CD4 and major histocompatibility complex class I (MHC-I) down-regulation are governed by distinct determinants: N-terminal alpha helix and proline repeat of Nef selectively regulate MHC-I trafficking. J. Virol. 73:1964-1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Marsh, M., J. E. Armes, and A. Pelchen-Matthews. 1990. Endocytosis and recycling of CD4. Biochem. Soc. Trans. 18:139-143. [DOI] [PubMed] [Google Scholar]
- 105.Marsh, M., and H. T. McMahon. 1999. The structural era of endocytosis. Science 285:215-220. [DOI] [PubMed] [Google Scholar]
- 106.Marsh, M., I. J. Parsons, P. Reid, and A. Pelchen-Matthews. 1993. Endocytic regulation of the T lymphocyte co-receptor proteins CD4 and CD8. Biochem. Soc. Trans. 21:703-706. [DOI] [PubMed] [Google Scholar]
- 107.Marsh, M., P. Reid, I. Parsons, C. Hermon, and A. Pelchen-Matthews. 1992. Morphological analysis of the regulation of CD4 endocytosis by p56lck. Biochem. Soc. Trans. 20:719-724. [DOI] [PubMed] [Google Scholar]
- 108.Mellors, J. W., C. Rinaldo, P. Gupta, R. M. White, J. A. Todd, and L. A. Kingsley. 1996. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science 272:1167-1170. [DOI] [PubMed] [Google Scholar]
- 109.Miller, M. D., M. B. Feinberg, and W. C. Greene. 1994. The HIV-1 nef gene acts as a positive viral infectivity factor. Trends Microbiol. 2:294-298. [DOI] [PubMed] [Google Scholar]
- 110.Miller, M. D., M. T. Warmerdam, I. Gaston, W. C. Greene, and M. B. Feinberg. 1994. The human immunodeficiency virus-1 nef gene product: a positive factor for viral infection and replication in primary lymphocytes and macrophages. J. Exp. Med. 179:101-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Munch, J., N. Stolte, D. Fuchs, C. Stahl-Hennig, and F. Kirchhoff. 2001. Efficient class I major histocompatibility complex down-regulation by simian immunodeficiency virus Nef is associated with a strong selective advantage in infected rhesus macaques. J. Virol. 75:10532-10536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Natarajan, K., N. Dimasi, J. Wang, R. A. Mariuzza, and D. H. Margulies. 2002. Structure and function of natural killer cell receptors: multiple molecular solutions to self, nonself discrimination. Annu. Rev. Immunol. 20:853-885. [DOI] [PubMed] [Google Scholar]
- 113.Nunn, M. F., and J. W. Marsh. 1996. Human immunodeficiency virus type 1 Nef associates with a member of the p21-activated kinase family. J. Virol. 70:6157-6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Odorizzi, G., C. R. Cowles, and S. D. Emr. 1998. The AP-3 complex: a coat of many colours. Trends Cell Biol. 8:282-288. [DOI] [PubMed] [Google Scholar]
- 115.Pandori, M., H. Craig, L. Moutouh, J. Corbeil, and J. Guatelli. 1998. Virological importance of the protease-cleavage site in human immunodeficiency virus type 1 Nef is independent of both intravirion processing and CD4 down-regulation. Virology 251:302-316. [DOI] [PubMed] [Google Scholar]
- 116.Peden, A. A., V. Oorschot, B. A. Hesser, C. D. Austin, R. H. Scheller, and J. Klumperman. 2004. Localization of the AP-3 adaptor complex defines a novel endosomal exit site for lysosomal membrane proteins. J. Cell Biol. 164:1065-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pelchen-Matthews, A., J. E. Armes, G. Griffiths, and M. Marsh. 1991. Differential endocytosis of CD4 in lymphocytic and nonlymphocytic cells. J. Exp. Med. 173:575-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pelchen-Matthews, A., J. E. Armes, and M. Marsh. 1989. Internalization and recycling of CD4 transfected into HeLa and NIH3T3 cells. EMBO J. 8:3641-3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pelchen-Matthews, A., I. Boulet, D. R. Littman, R. Fagard, and M. Marsh. 1992. The protein tyrosine kinase p56lck inhibits CD4 endocytosis by preventing entry of CD4 into coated pits. J. Cell Biol. 117:279-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pelchen-Matthews, A., R. P. da Silva, M. J. Bijlmakers, N. Signoret, S. Gordon, and M. Marsh. 1998. Lack of p56lck expression correlates with CD4 endocytosis in primary lymphoid and myeloid cells. Eur. J. Immunol. 28:3639-3647. [DOI] [PubMed] [Google Scholar]
- 121.Pelchen-Matthews, A., I. J. Parsons, and M. Marsh. 1993. Phorbol ester-induced downregulation of CD4 is a multistep process involving dissociation from p56lck, increased association with clathrin-coated pits, and altered endosomal sorting. J. Exp. Med. 178:1209-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Piguet, V., Y. L. Chen, A. Mangasarian, M. Foti, J. L. Carpentier, and D. Trono. 1998. Mechanism of Nef-induced CD4 endocytosis: Nef connects CD4 with the mu chain of adaptor complexes. EMBO J. 17:2472-2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Piguet, V., F. Gu, M. Foti, N. Demaurex, J. Gruenberg, J. L. Carpentier, and D. Trono. 1999. Nef-induced CD4 degradation: a diacidic-based motif in Nef functions as a lysosomal targeting signal through the binding of beta-COP in endosomes. Cell 97:63-73. [DOI] [PubMed] [Google Scholar]
- 124.Piguet, V., O. Schwartz, S. Le Gall, and D. Trono. 1999. The downregulation of CD4 and MHC-I by primate lentiviruses: a paradigm for the modulation of cell surface receptors. Immunol. Rev. 168:51-63. [DOI] [PubMed] [Google Scholar]
- 125.Piguet, V., L. Wan, C. Borel, A. Mangasarian, N. Demaurex, G. Thomas, and D. Trono. 2000. HIV-1 Nef protein binds to the cellular protein PACS-1 to downregulate class I major histocompatibility complexes. Nat. Cell Biol. 2:163-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Preusser, A., L. Briese, A. S. Baur, and D. Willbold. 2001. Direct in vitro binding of full-length human immunodeficiency virus type 1 Nef protein to CD4 cytoplasmic domain. J. Virol. 75:3960-3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Renkema, G. H., A. Manninen, D. A. Mann, M. Harris, and K. Saksela. 1999. Identification of the Nef-associated kinase as p21-activated kinase 2. Curr. Biol. 9:1407-1410. [DOI] [PubMed] [Google Scholar]
- 128.Riggs, N. L., H. M. Craig, M. W. Pandori, and J. C. Guatelli. 1999. The dileucine-based sorting motif in HIV-1 Nef is not required for down-regulation of class I MHC. Virology 258:203-207. [DOI] [PubMed] [Google Scholar]
- 129.Robinson, M. S. 2004. Adaptable adaptors for coated vesicles. Trends Cell Biol. 14:167-174. [DOI] [PubMed] [Google Scholar]
- 130.Roeth, J. F., M. Williams, M. R. Kasper, T. M. Filzen, and K. L. Collins. 2004. HIV-1 Nef disrupts MHC-I trafficking by recruiting AP-1 to the MHC-I cytoplasmic tail. J. Cell Biol. 167:903-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rose, J. J., K. Janvier, S. Chandrasekhar, R. P. Sekaly, J. S. Bonifacino, and S. Venkatesan. 2005. CD4 down-regulation by HIV-1 and simian immunodeficiency virus (SIV) Nef proteins involves both internalization and intracellular retention mechanisms. J. Biol. Chem. 280:7413-7426. [DOI] [PubMed] [Google Scholar]
- 132.Ross, T. M., A. E. Oran, and B. R. Cullen. 1999. Inhibition of HIV-1 progeny virion release by cell-surface CD4 is relieved by expression of the viral Nef protein. Curr. Biol. 9:613-621. [DOI] [PubMed] [Google Scholar]
- 133.Rossi, F., A. Gallina, and G. Milanesi. 1996. Nef-CD4 physical interaction sensed with the yeast two-hybrid system. Virology 217:397-403. [DOI] [PubMed] [Google Scholar]
- 134.Saksela, K., G. Cheng, and D. Baltimore. 1995. Proline-rich (PxxP) motifs in HIV-1 Nef bind to SH3 domains of a subset of Src kinases and are required for the enhanced growth of Nef+ viruses but not for down-regulation of CD4. EMBO J. 14:484-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sandrin, V., and F. L. Cosset. 2006. Intracellular versus cell surface assembly of retroviral pseudotypes is determined by the cellular localization of the viral glycoprotein, its capacity to interact with Gag, and the expression of the Nef protein. J. Biol. Chem. 281:528-542. [DOI] [PubMed] [Google Scholar]
- 136.Sanfridson, A., S. Hester, and C. Doyle. 1997. Nef proteins encoded by human and simian immunodeficiency viruses induce the accumulation of endosomes and lysosomes in human T cells. Proc. Natl. Acad. Sci. USA 94:873-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Scheppler, J., J. Nicholson, D. Swan, A. Ahmed-Ansari, and J. McDougal. 1989. Down-modulation of MHC-I in a CD4+ T cell line, CEM-E5, after HIV-1 infection. J. Immunol. 143:2858-2866. [PubMed] [Google Scholar]
- 138.Schiavoni, I., S. Trapp, A. C. Santarcangelo, V. Piacentini, K. Pugliese, A. Baur, and M. Federico. 2004. HIV-1 Nef enhances both membrane expression and virion incorporation of Env products. A model for the Nef-dependent increase of HIV-1 infectivity. J. Biol. Chem. 279:22996-23006. [DOI] [PubMed] [Google Scholar]
- 139.Schindler, M., S. Wurfl, P. Benaroch, T. C. Greenough, R. Daniels, P. Easterbrook, M. Brenner, J. Munch, and F. Kirchhoff. 2003. Down-modulation of mature major histocompatibility complex class II and up-regulation of invariant chain cell surface expression are well-conserved functions of human and simian immunodeficiency virus nef alleles. J. Virol. 77:10548-10556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Schwartz, O., A. Dautry-Varsat, B. Goud, V. Marechal, A. Subtil, J. M. Heard, and O. Danos. 1995. Human immunodeficiency virus type 1 Nef induces accumulation of CD4 in early endosomes. J. Virol. 69:528-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Schwartz, O., V. Marechal, S. Le Gall, F. Lemonnier, and J. Heard. 1996. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat. Med. 2:338-342. [DOI] [PubMed] [Google Scholar]
- 142.Skowronski, J., D. Parks, and R. Mariani. 1993. Altered T cell activation and development in transgenic mice expressing the HIV-1 nef gene. EMBO J. 12:703-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sol-Foulon, N., A. Moris, C. Nobile, C. Boccaccio, A. Engering, J. P. Abastado, J. M. Heard, Y. van Kooyk, and O. Schwartz. 2002. HIV-1 Nef-induced upregulation of DC-SIGN in dendritic cells promotes lymphocyte clustering and viral spread. Immunity 16:145-155. [DOI] [PubMed] [Google Scholar]
- 144.Steegmaier, M., J. Klumperman, D. L. Foletti, J. S. Yoo, and R. H. Scheller. 1999. Vesicle-associated membrane protein 4 is implicated in trans-Golgi network vesicle trafficking. Mol. Biol. Cell 10:1957-1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Stove, V., I. Van de Walle, E. Naessens, E. Coene, C. Stove, J. Plum, and B. Verhasselt. 2005. Human immunodeficiency virus Nef induces rapid internalization of the T-cell coreceptor CD8αβ. J. Virol. 79:11422-11433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Stumptner-Cuvelette, P., M. Jouve, J. Helft, M. Dugast, A. S. Glouzman, K. Jooss, G. Raposo, and P. Benaroch. 2003. Human immunodeficiency virus-1 Nef expression induces intracellular accumulation of multivesicular bodies and major histocompatibility complex class II complexes: potential role of phosphatidylinositol 3-kinase. Mol. Biol. Cell 14:4857-4870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Stumptner-Cuvelette, P., S. Morchoisne, M. Dugast, S. Le Gall, G. Raposo, O. Schwartz, and P. Benaroch. 2001. HIV-1 Nef impairs MHC class II antigen presentation and surface expression. Proc. Natl. Acad. Sci. USA 98:12144-12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Swann, S. A., M. Williams, C. M. Story, K. R. Bobbitt, R. Fleis, and K. L. Collins. 2001. HIV-1 Nef blocks transport of MHC class I molecules to the cell surface via a PI 3-kinase-dependent pathway. Virology 282:267-277. [DOI] [PubMed] [Google Scholar]
- 149.Swigut, T., L. Alexander, J. Morgan, J. Lifson, K. G. Mansfield, S. Lang, R. P. Johnson, J. Skowronski, and R. Desrosiers. 2004. Impact of Nef-mediated downregulation of major histocompatibility complex class I on immune response to simian immunodeficiency virus. J. Virol. 78:13335-13344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Swigut, T., M. Greenberg, and J. Skowronski. 2003. Cooperative interactions of simian immunodeficiency virus Nef, AP-2, and CD3-ζ mediate the selective induction of T-cell receptor-CD3 endocytosis. J. Virol. 77:8116-8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Swigut, T., A. J. Iafrate, J. Muench, F. Kirchhoff, and J. Skowronski. 2000. Simian and human immunodeficiency virus Nef proteins use different surfaces to downregulate class I major histocompatibility complex antigen expression. J. Virol. 74:5691-5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Swigut, T., N. Shohdy, and J. Skowronski. 2001. Mechanism for down-regulation of CD28 by Nef. EMBO J. 20:1593-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Swingler, S., B. Brichacek, J. M. Jacque, C. Ulich, J. Zhou, and M. Stevenson. 2003. HIV-1 Nef intersects the macrophage CD40L signalling pathway to promote resting-cell infection. Nature 424:213-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Swingler, S., A. Mann, J. Jacque, B. Brichacek, V. G. Sasseville, K. Williams, A. A. Lackner, E. N. Janoff, R. Wang, D. Fisher, and M. Stevenson. 1999. HIV-1 Nef mediates lymphocyte chemotaxis and activation by infected macrophages. Nat. Med. 5:997-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Traub, L. M. 2003. Sorting it out: AP-2 and alternate clathrin adaptors in endocytic cargo selection. J. Cell Biol. 163:203-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Vigerust, D. J., B. S. Egan, and V. L. Shepherd. 2005. HIV-1 Nef mediates post-translational down-regulation and redistribution of the mannose receptor. J. Leukoc. Biol. 77:522-534. [DOI] [PubMed] [Google Scholar]
- 157.Vilhardt, F., O. Plastre, M. Sawada, K. Suzuki, M. Wiznerowicz, E. Kiyokawa, D. Trono, and K. H. Krause. 2002. The HIV-1 Nef protein and phagocyte NADPH oxidase activation. J. Biol. Chem. 277:42136-42143. [DOI] [PubMed] [Google Scholar]
- 158.Waguri, S., F. Dewitte, R. Le Borgne, Y. Rouille, Y. Uchiyama, J. F. Dubremetz, and B. Hoflack. 2003. Visualization of TGN to endosome trafficking through fluorescently labeled MPR and AP-1 in living cells. Mol. Biol. Cell 14:142-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wan, L., S. Molloy, L. Thomas, G. Liu, Y. Xiang, S. Rybak, and G. Thomas. 1998. PACS-1 defines a novel gene family of cytosolic sorting proteins required for trans-Golgi network localization. Cell 94:205-216. [DOI] [PubMed] [Google Scholar]
- 160.Wang, Y. J., J. Wang, H. Q. Sun, M. Martinez, Y. X. Sun, E. Macia, T. Kirchhausen, J. P. Albanesi, M. G. Roth, and H. L. Yin. 2003. Phosphatidylinositol 4 phosphate regulates targeting of clathrin adaptor AP-1 complexes to the Golgi. Cell 114:299-310. [DOI] [PubMed] [Google Scholar]
- 161.Wasiak, S., V. Legendre-Guillemin, R. Puertollano, F. Blondeau, M. Girard, E. de Heuvel, D. Boismenu, A. W. Bell, J. S. Bonifacino, and P. S. McPherson. 2002. Enthoprotin: a novel clathrin-associated protein identified through subcellular proteomics. J. Cell Biol. 158:855-862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Welker, R., H. Kottler, H. R. Kalbitzer, and H. G. Krausslich. 1996. Human immunodeficiency virus type 1 Nef protein is incorporated into virus particles and specifically cleaved by the viral proteinase. Virology 219:228-236. [DOI] [PubMed] [Google Scholar]
- 163.Williams, M., J. F. Roeth, M. R. Kasper, T. M. Filzen, and K. L. Collins. 2005. Human immunodeficiency virus type 1 Nef domains required for disruption of major histocompatibility complex class I trafficking are also necessary for coprecipitation of Nef with HLA-A2. J. Virol. 79:632-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Williams, M., J. F. Roeth, M. R. Kasper, R. I. Fleis, C. G. Przybycin, and K. L. Collins. 2002. Direct binding of human immunodeficiency virus type 1 Nef to the major histocompatibility complex class I (MHC-I) cytoplasmic tail disrupts MHC-I trafficking. J. Virol. 76:12173-12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wolf, D., V. Witte, B. Laffert, K. Blume, E. Stromer, S. Trapp, P. d'Aloja, A. Schurmann, and A. S. Baur. 2001. HIV-1 Nef associated PAK and PI3-kinases stimulate Akt-independent Bad-phosphorylation to induce anti-apoptotic signals. Nat. Med. 7:1217-1224. [DOI] [PubMed] [Google Scholar]
- 166.Xu, X. N., B. Laffert, G. R. Screaton, M. Kraft, D. Wolf, W. Kolanus, J. Mongkolsapay, A. J. McMichael, and A. S. Baur. 1999. Induction of Fas ligand expression by HIV involves the interaction of Nef with the T cell receptor zeta chain. J. Exp. Med. 189:1489-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yamada, T., N. Kaji, T. Odawara, J. Chiba, A. Iwamoto, and Y. Kitamura. 2003. Proline 78 is crucial for human immunodeficiency virus type 1 Nef to down-regulate class I human leukocyte antigen. J. Virol. 77:1589-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zheng, Y. H., A. Plemenitas, C. J. Fielding, and B. M. Peterlin. 2003. Nef increases the synthesis of and transports cholesterol to lipid rafts and HIV-1 progeny virions. Proc. Natl. Acad. Sci. USA 100:8460-8465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zheng, Y. H., A. Plemenitas, T. Linnemann, O. T. Fackler, and B. M. Peterlin. 2001. Nef increases infectivity of HIV via lipid rafts. Curr. Biol. 11:875-879. [DOI] [PubMed] [Google Scholar]