Abstract
Many bacteria produce antimicrobial peptides, which are also referred to as peptide bacteriocins. The class IIa bacteriocins, often designated pediocin-like bacteriocins, constitute the most dominant group of antimicrobial peptides produced by lactic acid bacteria. The bacteriocins that belong to this class are structurally related and kill target cells by membrane permeabilization. Despite their structural similarity, class IIa bacteriocins display different target cell specificities. In the search for new antibiotic substances, the class IIa bacteriocins have been identified as promising new candidates and have thus received much attention. They kill some pathogenic bacteria (e.g., Listeria) with high efficiency, and they constitute a good model system for structure-function analyses of antimicrobial peptides in general. This review focuses on class IIa bacteriocins, especially on their structure, function, mode of action, biosynthesis, bacteriocin immunity, and current food applications. The genetics and biosynthesis of class IIa bacteriocins are well understood. The bacteriocins are ribosomally synthesized with an N-terminal leader sequence, which is cleaved off upon secretion. After externalization, the class IIa bacteriocins attach to potential target cells and, through electrostatic and hydrophobic interactions, subsequently permeabilize the cell membrane of sensitive cells. Recent observations suggest that a chiral interaction and possibly the presence of a mannose permease protein on the target cell surface are required for a bacteria to be sensitive to class IIa bacteriocins. There is also substantial evidence that the C-terminal half penetrates into the target cell membrane, and it plays an important role in determining the target cell specificity of these bacteriocins. Immunity proteins protect the bacteriocin producer from the bacteriocin it secretes. The three-dimensional structures of two class IIa immunity proteins have been determined, and it has been shown that the C-terminal halves of these cytosolic four-helix bundle proteins specify which class IIa bacteriocin they protect against.
INTRODUCTION
Bacteriocins are antimicrobial peptides or proteins produced by bacteria (140). Peptide bacteriocins produced by lactic acid bacteria (LAB) are categorized into three different classes according to their biochemical and genetic properties (Table 1) (53, 138). Class I peptides are the lantibiotics, which are small, posttranslationally modified peptides that contain unusual amino acids such as lanthionine. Class II includes unmodified bacteriocins which are subdivided into three subclasses, namely, class IIa (pediocin-like bacteriocins), class IIb (two-peptide bacteriocins), and IIc (other [i.e., non-pediocin-like], one-peptide bacteriocins). The designation pediocin-like bacteriocins refers to pediocin PA-1/AcH, which was the first class IIa bacteriocin characterized (19, 141). The class III peptides are thermosensitive proteins (110). The inhibitory spectrum of LAB bacteriocins is relatively narrow compared to that of the antimicrobial peptides produced by eukaryotic cells, such as pleurocidin, which is active against both gram-negative and gram-positive bacteria (37). On the other hand, LAB-produced bacteriocins kill bacteria at much lower concentrations than eukaryotic antimicrobial peptides, probably because they interact with a specific receptor present on target cells (143). The present review focuses on the class IIa bacteriocins.
TABLE 1.
Classification of LAB bacteriocins
| Main category | Characteristics | Subcategory | Examples | Reference(s) |
|---|---|---|---|---|
| Class I | Lantibiotics (containing lanthionine and β-lanthionine) | Type A (elongated molecules; molecular mass, <4 kDa) | Nisin A | 23 |
| Nisin Z | 135 | |||
| Subtilin | 10 | |||
| Epidermin | 165 | |||
| Type B (globular molecules; molecular mass, 1.8 to 2.1 kDa) | Mersacidin | 111 | ||
| Actagardin | 109 | |||
| Mutacin II | 190 | |||
| Class II | Nonmodified heat-stable bacteriocins containing peptides with molecular masses of <10 kDa | Subclass IIa (antilisterial pediocin-like bacteriocins) | See Table 3 | |
| Subclass IIb (two-peptide bacteriocins) | Plantaricin EF | 3, 48 | ||
| Plantaricin JK | 3, 48 | |||
| Subclass IIc (other peptide bacteriocins) | Lactococcin 972 | 122 | ||
| Class III | Protein bacteriocins with molecular masses of >30 kDa | Helveticin J | 96 | |
| Millericin B | 16 |
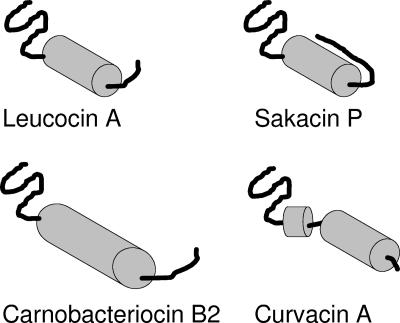
The past few years have seen the emergence of class IIa bacteriocins produced by LAB as one of the most interesting groups of antimicrobial peptides for use in food preservation (36) and in medicine, as antibiotic complements in treating infectious diseases (92) or as antiviral agents (186, 187). Some of these peptides inhibit growth of gram-positive food spoilage and pathogenic bacteria such as Bacillus cereus, Clostridium perfringens, Staphylococcus aureus, and Listeria monocytogenes. Class IIa bacteriocins are often described as listericidal, small (<10-kDa), heat-stable, unmodified peptides of 37 (leucocin A and mesentericin Y105) to 48 (carnobacteriocin B2 and enterocin SE-K4) amino acids and having a net positive charge, with pI values ranging from 8 to 10. Sequence alignment of class IIa bacteriocins reveals that they consist of a highly conserved hydrophilic and charged N-terminal part harboring the consensus sequence YGNGV(X)C(X)4C(X)V(X)4A (X denotes any amino acid) (36, 55, 110) (Fig. 1) and a more variable hydrophobic and/or amphiphilic C-terminal part. Based on amino acid sequence alignments, further division of the class IIa bacteriocins into three or four subgroups has been suggested (62, 134). So far it seems that bacteriocins from the different class IIa subgroups have somewhat different three-dimensional (3D) structures, which in turn may reflect differences in their target cell specificities. Today, the 3D structures of four class IIa bacteriocins have been elucidated by nuclear magnetic resonance (NMR) spectroscopy; they are carnobacteriocin B2 (189), curvacin A (79), leucocin A (67), and sakacin P and a structurally stabilized sakacin P variant (176). Briefly, the class IIa bacteriocins consist of an N-terminal β-sheet-like domain which is structurally stabilized by the conserved disulfide bridge and a C-terminal domain consisting of one or two α helices, often ending with a structurally extended C-terminal tail (63) (Fig. 2). In the C-terminal part, a few class IIa bacteriocins, such as sakacin G, plantaricin 423, pediocin PA-1/AcH, divercin V41, and enterocin A, contain an additional C-terminal disulfide bridge which plays an important role in stabilizing the 3D structure of the C-terminal domain (63, 176). Often, but not always, these structurally stabilized bacteriocins display higher antimicrobial potencies than those containing only one disulfide bridge, especially at higher temperatures (52, 60).
FIG. 1.
Sequence alignment of mature class IIa bacteriocins. Underlined cysteine residues are those involved in disulfide bond formation.
FIG. 2.
Schematic presentation of the four class IIa bacteriocins for which 3D structures have been determined by NMR, i.e., leucocin A (67), carnobacteriocin B2 (189), sakacin P (176), and curvacin A (79). Due to sequence similarities among these class IIa bacteriocins, it is assumed that the 3D structures of their N-terminal beta-sheet-like structures are relatively similar despite the fact that their NMR structures display some variation in this region. It is also assumed that the C-terminal tails following the central alpha helices of leucocin A and carnobacteriocin B2 most likely form a hairpin-like structure together with the alpha helix, despite the fact that this could not be judged from the NMR structural analyses. This hairpin-like structure has been seen only for the structurally stabilized sakacin P variant (176).
The bacteriocin producers express immunity proteins to protect themselves from the bacteriocin they produce, and until recently little was known about these proteins. The 3D structures of two class IIa immunity proteins, EntA-im (99) and CbnB2-imm (170), have been determined. Despite having little sequence identity, they display basically the same overall left-handed four-helix bundle protein structure. It has been shown that class IIa immunity proteins display selectivity for which bacteriocin they display immunity against, and the determinant for this selectivity seems to be located in the C-terminal parts of the immunity proteins.
Much new information regarding class IIa bacteriocins has emerged, and it is timely to summarize the most relevant insights obtained during the last 4 years. With this aim, the present review provides a wealth of new information that covers most aspects of class IIa bacteriocins.
ORGANIZATION OF THE GENE CLUSTERS INVOLVED IN THE PRODUCTION AND IMMUNITY OF CLASS IIa BACTERIOCINS
Most class IIa bacteriocin genes are plasmid encoded, except those encoding enterocin A (8), divercin V41 (126), sakacin P (90), and carnobacteriocins B2 and BM1 (152), for which genes are located on chromosomes. The class IIa bacteriocin genes are most often arranged in one or a few operons, and their common organization has been compared and reviewed by Ennahar et al. (55). However, an exception to the common gene organization is seen for the divercin V41 bacteriocin genes, which present a particular organization attributed to DNA rearrangements (55). Overall, genetically characterized class IIa bacteriocin gene clusters are composed of one or three gene modules. In the cases of pediocin PA-1, plantaricin 423, and coagulin, all four genes needed for bacteriocin production and secretion are located in one operon. In other cases the genes are spread on several operons, where one operon carries the structural and immunity genes, a second operon carries genes for bacteriocin secretion, and a third operon carries genes involved in regulation of bacteriocin production (55). Genes involved in biosynthesis and production of class IIa bacteriocins are not necessarily located on the same locus or even the same DNA determinant. As recently reported, the structural and immunity genes on one hand and the genes for bacteriocin secretion on the other hand were found to coreside on individual plasmids in Pediococcus parvulus and Lactobacillus plantarum WHE92 (129).
As described above, the organizations of genes involved in the production of plantaricin 423, pediocin PA-1/AcH, and coagulin are almost identical (181). More specifically, the coagulin-codifying DNA (coaABCD operon) is identically organized and displays high sequence similarity to that of the pap operon encoding the pediocin PA-1/AcH genes (129), despite the fact that coagulin is produced by the relatively unrelated non-LAB Bacillus coagulans I4 (117). This finding could be attributed to a plasmid-borne transfer between Pediococcus acidilactici and B. coagulans, since horizontal gene/operon transfer between bacteria seems to be more common than was originally thought (46). Finally, the genetic organization of the mundticin KS gene cluster (mun locus) has been established (107). It consists of three genes encoding the 58-amino-acid mundticin precursor (munA), a 674-amino-acid polypeptide with similarity to the ABC transporter proteins EntT (138) and CbnT (144) (munB), and mundticin KS immunity (munC).
BIOSYNTHESIS AND EXPORT
At least four genes are required for the production of class IIa bacteriocins (55, 140). They are (i) the structural bacteriocin gene, encoding a prebacteriocin; (ii) the immunity gene, encoding an immunity protein that protects the bacteriocin producer from its own bacteriocin; (iii) a gene encoding an ABC (ATP-binding cassette) transporter necessary for secretion; and (iv) a gene encoding an accessory protein of unknown function. How these genes are organized in operons has been discussed in a previous review (55). A few class IIa bacteriocins (bacteriocin 31, enterocin P, and listeriocin 431) are secreted by the general sec-dependent export system, and for these the exact number of genes required for bacteriocin production is unknown (35, 86, 102, 174).
The class IIa bacteriocins are produced as prebacteriocins having an N-terminal extension. This presequence is removed by site-specific proteolytic cleavage during export, and the mature bacteriocin is then secreted (80). The presequence of the bacteriocin seems to play a dual role in bacteriocin biosynthesis. It may play a protective role at the cytosolic side of the cell membrane by keeping the bacteriocin inactive and thereby preventing the bacteriocin producer from being attacked by its newly synthesized bacteriocin, and it may also play a role as a recognition signal during export and thus be important for the trafficking of the prebacteriocins to the correct ABC transporter.
Pre-leucocin A (67), pre-mesentericin Y105 (18), and pre-carnobacteriocin B2 (149), but not pre-pediocin PA-1 (154), display reduced activity compared to their mature bacteriocins when tested on sensitive cells, suggesting an inactivating role for the presequence. A recent report on the 3D structure of pre-carnobacteriocin B2, studied in a membrane-mimicking environment by the use of NMR (169), revealed that the presequence most likely interferes with bacteriocin-membrane interactions. The presequence was shown to contain a 10-residue-long amphiphilic alpha helix that folded back on the alpha-helical membrane-interacting part of the bacteriocin. The NMR data also revealed that neither the C-terminal alpha-helical nor the N-terminal part of carnobacteriocin B2 was structurally changed in the presence of the presequence (169).
The second putative role of the presequence is related to the recognition of the bacteriocin by the secretion machinery, the dedicated ABC transporter. Among bacterial ABC transporters the bacteriocin exporters make up a small subfamily (43, 80), which is unique in that they have two protein domains (the C-terminal and N-terminal domains) on the cytosolic side anchored to the membrane by an intervening transmembrane region (80). The C-terminal cytosolic domain contains the ATP-binding cassette, which upon ATP hydrolysis energizes the secretion of the peptide out of the cell, whereas the N-terminal cytosolic protein domain contains the proteolytic activity necessary for cleavage and maturation of the prebacteriocin (4, 80). The presequence is cleaved at the C-terminal side of a double Gly motif, thereby liberating the mature bacteriocin (65), and it is believed that the transmembrane domain of the ABC transporter and the accessory protein are important for translocation of the mature bacteriocin through the membrane (55). Several ABC transporters may be present in a single cell at the same time (43), and specific recognition signals are necessary to ensure effective export by the correct exporter. It has been suggested that the recognition between the prebacteriocin and the N-terminal proteolytic domain of the ABC transporter occurs by interactions between hydrophobic residues on the amphiphilic alpha-helical presequence and hydrophobic residues near the catalytic site in the N-terminal proteolytic domain of the ABC transporter protein (4). Supporting this is the fact that the class IIa bacteriocins that depend on the sec-dependent exporters have very different N-terminal presequences (also lacking the double-Gly motif) and are thus not recognized by the bacteriocin ABC transporters (140). A recent study has shown that the secretion rate and proteolytic efficiency of the ABC transporters depend to some extent on the amino acid sequence of the presequence (4). By site-directed mutagenesis, it was shown that some point mutations in the presequence, including the double-Gly motif, could block the secretion completely. However, some mutations in the double-Gly cleavage site could be tolerated. The tolerance of bacteriocin ABC transporters to foreign presequences was demonstrated by interchanging complete double-Gly presequences between different bacteriocins (5, 179). Likewise, it has been shown that a double-Gly leader of class IIa bacteriocins may be replaced by a sec leader with only minor changes in bacteriocin secretion (18, 125).
REGULATION OF BIOSYNTHESIS
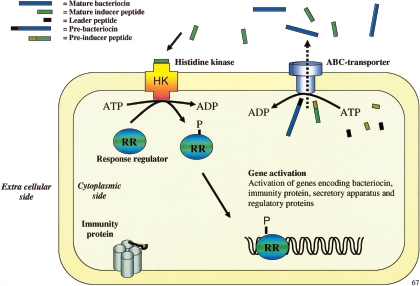
Quorum sensing is a widespread bacterial mechanism for monitoring the cell density of a bacterial population (130). Basically, a signal molecule (pheromone) is secreted at a low but constant rate by most cells in the bacterial population. The pheromone concentration thus reflects the cell density during growth, and, at a certain pheromone concentration (cell density), the pheromone-dependent regulatory system is activated and cellular processes are initiated (130). Quorum-sensing systems used for the regulation of class IIa bacteriocin production are composed of three gene products and are accordingly termed three-component regulatory systems (139). The three components are (i) the inducer peptide (a peptide pheromone), (ii) the transmembrane histidine protein kinase (pheromone receptor), and (iii) the cytosolic response regulator (139).
The inducer peptide is ribosomally synthesized at low levels as a prepeptide, which is cleaved and secreted through the dedicated bacteriocin ABC transporter (55, 139). At a certain concentration threshold of the externalized inducer peptide, the transmembrane histidine kinase is activated, and this leads to the autophosphorylation of a conserved histidine residue at the cytosolic side of the transmembrane protein (33, 139) (Fig. 3). The molecular details of how the inducer peptide activates the histidine kinase are unknown, but it has been shown that the plantaricin A inducer peptide interacts extensively with cell membranes and that stereospecific interactions, presumably with the histidine kinase, are necessary to elicit the histidine kinase response (78, 112). Subsequently, the activated histidine kinase interacts with its cognate response regulator protein through transphosphorylation and the phosphate group residing on the histidine residue of the activated histidine kinase is transferred to a conserved Asp residue in the response regulator (33, 139). The phosphorylated, and thus activated, response regulator functions as a transcriptional activator, which binds to bacteriocin gene-specific promoters and stimulates transcription (139) (Fig. 3). The response regulator also activates the genes encoding the three-component system, and a positive feedback circuit is thus initiated (139). At a certain time, essentially all bacteriocin producers in the population are believed to secrete bacteriocins, and this sudden increase in the synthesis of antimicrobials may have great impact on the microbiota.
FIG. 3.
Schematic presentation of the biosynthesis of class IIa bacteriocins. The ribosomally produced prebacteriocin and preinducer peptide are matured and secreted through the ABC transporter. Mature inducer peptide interacts with its receptor (the histidine kinase [HK]), which is autophosphorylated at the cytosolic side. The phosphate group is subsequently transferred to the response regulator (RR), which then becomes active, enabling it to function as a transcriptional activator for bacteriocin-related genes.
The biosyntheses of several class IIa bacteriocins, in addition to the cell density-dependent regulation, have been shown to vary with respect to growth temperature, ionic strength, and pH (35, 60, 118). In some cases, the bacteriocin production seems to be highest at temperatures near 20°C and to drop to zero at temperatures between 35 and 37°C or higher (35, 49, 60, 118). This makes biological sense, since most class IIa bacteriocins display low potency at and above 37°C (60, 106). One known exception to this is pediocin PA-1/AcH, which displays similar antimicrobial activity at 20 and 37°C due to the stabilizing effect of its C-terminal disulfide bridge (60, 106). Interestingly, pediocin PA-1/AcH is produced at the same rate at 20°C and 37°C (60). In the case of the sakacin A producer Lactobacillus sakei 706, which harbors a three-component regulatory system, it has been shown that it is not possible to induce bacteriocin production by adding the inducer peptide when this production has ceased due to growth near 37°C (49, 139). Temperature-based regulation thus seems to overrule the quorum-sensing mechanism (139).
BACTERIOCIN IMMUNITY
Structure and Cellular Location
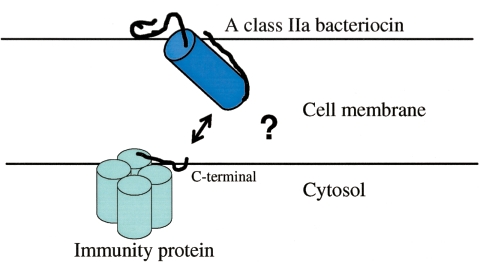
Immunity proteins protect the bacteriocin producer against its cognate bacteriocin. So far, at least 20 putative immunity proteins have been identified from DNA sequences, and they have been subgrouped according to sequence similarities (Fig. 4) (107, 150, 174, 183) (GenBank accession numbers CAF25009 and AAQ95743; also see references cited in reference 61). The sequence similarities between pairs of immunity proteins (Fig. 4) vary from 5 to 85% (52). The proteins are highly charged, with most of them containing 25 to 35% charged residues. They are cytosolic proteins, with a minor fraction of the cellular pool perhaps loosely associated with the inside of the cell membrane (44, 151). When expressed in sensitive cells, they strongly protect against externally added cognate bacteriocins (61, 151). If both immunity proteins and cognate bacteriocins are added extracellularly, however, no protection is seen. Thus, class IIa immunity proteins seem to act inside the cell (151, 170). Similar results have also been obtained for the immunity protein for the non-class IIa bacteriocin lactococcin A (142, 184).
FIG. 4.
Sequence alignment and grouping of putative immunity proteins of class IIa bacteriocins. Regions of sequence similarity are indicated by black and gray boxes. The following amino acids were considered similar: D and E, F and Y, V and L, N and Q, K and R, and S and T. Group A consists of the following (putative) immunity proteins: leucocin A (leuA-im), mesentericin Y105 (mesY-im), divercin V41 (divI-im), enterocin A (entA-im), an open reading frame in the sakacin P locus with no corresponding bacteriocin (orfY-im), pediocin PA-1 (ped-im), and coagulin (coa-im). Group B consists of the following (putative) immunity proteins: an open reading frame in the carnobacteriocin locus with no corresponding bacteriocin (orfβ 3-im), an open reading frame in Lactobacillus sakei LB790 (GenBank accession no. CAF25009) with no corresponding bacteriocin (orf285-im), piscicolin 126 (pisc-im), sakacin 5x (sakX-im), sakacin P (sakP-im), mundticin KS (munKS-im), enterocin CRL35 (entCL35-im), listeriocin 743A (lisA-im), and an open reading frame in the divercin V41 locus with no corresponding bacteriocin (divT2-im). Group C consists of the following (putative) immunity proteins: carnobacteriocin B1 (cbnBM1-im), curvacin A (curA-im), enterocin P (entP-im), bacteriocin 31 (bac31-im), and carnobacteriocin B2 (cbnB2). See the text for references.
Recently, the 3D structures of two class IIa immunity proteins have been elucidated. The carnobacteriocin B2 immunity protein (ImB2) was analyzed using NMR spectroscopy (170), whereas the enterocin A immunity protein (EntA-im) was investigated using X-ray crystallography (42, 99). Interestingly, they both have basically the same 3D structure despite the fact that these two proteins confer resistance on two very different class IIa bacteriocins and have very different amino acid sequences, thus belonging to different subgroups (Fig. 4). They are globular proteins with a left-turning four-helix bundle protein motif. The four antiparallel alpha helices are amphiphilic and connected through short loops. They are oriented relative to each other such that their hydrophobic faces interact and form a hydrophobic core in the center of the protein, whereas the hydrophilic and charged faces of the helices constitute the protein surface. This distribution of residues gives rise to a structurally stable and hydrophilic cytosolic protein. In the case of EntA-im, the melting temperature was found to be close to 80°C, as judged by circular dichroism spectroscopy (98). Although overall the four-helix bundle parts of these two immunity proteins are very similar, there are some minor differences with respect to the angles between their helices (99).
At the C-terminal end following helix 4, the two immunity proteins display some clear structural differences. ImB2 forms a well-defined short alpha helix with a C-terminal extension. This fifth helix orients perpendicular relative to the four-helix bundle and interacts with helix 3 and helix 4 (170). In the case of EntA-im, the crystal structure shows a C-terminal end with no clear secondary structure and is suggested to be relatively flexible (99). Homology modeling of immunity proteins belonging to the same subgroup as EntA-im (Fig. 4) (divI-im, leuA-im, and mesY-im) indicates that the four-helix bundle is a conserved structural motif in class IIa immunity proteins (99). The homology modeling also reveals that the length of the alpha helices is relatively constant and that only some minor differences exist in the loop regions (99). The 3D structures of EntA-im and ImB2 have shown that there are clusters of positively charged residues on the immunity protein surfaces that may be involved in the association with the negatively charged phospholipids in membranes (99, 170). However, circular dichroism structural analyses of EntA-im indicated that no further structuring of the protein was obtained when it was exposed to membrane-mimicking entities, suggesting that class IIa immunity proteins do not interact extensively with the cell membrane (98). This is in contrast to the immunity protein for the non-class IIa bacteriocin lactococcin A, which has been shown to be a membrane-interacting immunity protein (142, 184).
Immunity Proteins Display Specificity towards the Bacteriocins to Which They Confer Resistance
Despite their 3D structural similarities, the immunity proteins display strong specificity with respect to the bacteriocins to which they confer resistance (61, 98, 151). In one study, the specificities of seven immunity genes were evaluated by expressing the genes in three different bacteriocin-sensitive strains and subsequently testing whether the strain had become resistant to various bacteriocins (61). The results revealed that the immunity proteins protected against their cognate bacteriocins and in some cases also (but to a lesser extent) against one or two other class IIa bacteriocins (61). When cross-protection was observed, it was most often directed against closely related bacteriocins. For instance, sakacin P and pediocin PA-1/AcH are two very similar bacteriocins, and thus the immunity proteins for sakacin P and pediocin PA-1/AcH protected against sakacin P and pediocin PA-1/AcH, despite the fact that these two immunity proteins display only 28% similarity and are thus placed in different subgroups (61). Cross-protection is not unique to class IIa immunity proteins. It has been found that the immunity protein for the non-class IIa bacteriocin carnobacteriocin A protects against both carnobacteriocin A and the structurally related bacteriocin enterocin B (66). Despite the fact that the protection pattern of immunity proteins cannot be predicted from their sequence similarities to other class IIa immunity proteins alone, the subgrouping of class IIa immunity proteins gives some indications about which class IIa bacteriocins the immunity proteins protect against (61), and it has been a useful tool for functional analyses of the immunity proteins and the construction of hybrid immunity proteins (98).
In hybrid immunity proteins, the N- and C-terminal halves of immunity proteins from the same immunity protein subgroup have been interchanged (98). The EntA-im crystal structure revealed that the recombination point of these hybrid proteins was situated in the middle of helix 3. Interestingly, four out of six constructed hybrid immunity proteins displayed activity, and their protection specificity was shown to be determined by the C-terminal parts of the proteins (98). This analysis was further supported by a more comprehensive study including both hybrid class IIa bacteriocins and the four active hybrid immunity proteins (100). Results from this study strongly support a model in which the C-terminal membrane-interacting alpha hairpin domain of class IIa bacteriocins (62) is recognized by the C-terminal parts of the immunity proteins (Fig. 5) (99, 100). According to this model, the bacteriocin and immunity proteins are located on opposite sides of the cell membrane, and there seems to be no direct contact between the two molecules (63). Thus, the membrane itself or a specific component embedded in it seems to play a crucial role as a mediator in the recognition between the bacteriocin and the immunity protein. In principle, the immunity protein could interact with a bacteriocin-induced pore and thereby block leakage, but it is difficult to explain how immunity specificity towards different class IIa bacteriocins occurs. Another possibility is that the immunity proteins interact with a putative bacteriocin receptor and directly or indirectly inhibit its action by altering the receptor conformation or by hiding its bacteriocin-binding site, as has been suggested for the immunity protein for the non-class IIa bacteriocin lactococcin A (184). A mannose permease transmembrane protein has been proposed as the receptor for all class IIa bacteriocins (41, 82). However, if all class IIa bacteriocins interact with the same receptor, it is again difficult to explain how immunity specificity occurs. The mode of action for class IIa immunity proteins is poorly understood, and additional gene products might be required to obtain immunity. This is supported by some recent observations that the functionality of various immunity proteins depends on in which strain they are expressed (61, 98). Thus, the genetic environment, and possibly the presence of additional gene products, may be essential for their successful protection.
FIG. 5.
A model showing a class IIa bacteriocin on the extracellular side of the cell membrane and the cognate four-helix bundle immunity protein on the cytosolic side. The bacteriocin (here sakacin P) has an oblique orientation which is caused in part by two Trp residues (Trp18 and Trp41) located in the water-membrane interface (62). By unknown mechanisms the C-terminal part of the immunity protein recognizes the bacteriocin and protects against it.
Biological Function of Immunity Proteins and Expression of Additional Immunity Genes
Since bacteriocin and immunity genes most often reside on the same operon and are expressed concomitantly, the effect of immunity in a natural genetic environment is easily observed in cases where bacteriocin production is regulated by quorum sensing. For instance, the Lactobacillus sakei LTH673 strain, producing sakacin P, is much more sensitive to various class IIa bacteriocins, including sakacin P, in the bacteriocin-negative state than in the bacteriocin-positive state (61). Thus, the bacteriocin sensitivity of a strain may change according to the bacteriocin production state. Upon changing from a bacteriocin-negative to a bacteriocin-positive state, the Lactobacillus sakei LTH673 strain displayed immunity towards more class IIa bacteriocins than was expected from the results based on the heterologous expression of the sakP-im gene alone (52, 61). The strain was then found to carry an additional immunity gene (orfY) that lacked a bacteriocin partner (22), and this orphan immunity gene was shown to display immunity and protect against several class IIa bacteriocins when heterologously expressed (61). Interestingly, orfY-im displayed a nonoverlapping protection pattern compared to sakP-im that was already present in the sakacin P producer, and it was concluded that when bacteriocin-negative Lactobacillus sakei LTH673 changed to its bacteriocin-positive state, both immunity genes could be activated and contribute to the broad immunity observed (52, 61). This example illustrates that expression of more than one immunity gene may be a great advantage for the host with respect to self-protection and survival. If single immunity genes such as orfY are widespread among lactic acid bacteria, they may contribute to the apparent sensitivity towards class IIa bacteriocins. By genetic exchange they may spread and be a source for immunity gene-based multiresistance against class IIa bacteriocins. In fact, several other (putative orphan) immunity genes without any clear bacteriocin partner have been reported, i.e., dvnT2 (126), orf-β3 (150), and orf285 (GenBank accession number CAF25009).
MODE OF ACTION OF CLASS IIa BACTERIOCINS
The mode of action of bacteriocins produced by gram-positive bacteria, especially those belonging to classes I and IIa, has been thoroughly studied (83, 133). Initial mode-of-action studies focused on how, from physiological and biochemical points of view, bacteriocins acted at the cellular level. Later, mode-of-action studies that aimed to reveal molecular mechanisms behind the cellular effects were conducted.
Mode of Action at the Cellular Level
Pediocin-PA-1/AcH, one of the first class IIa bacteriocins to be characterized, was used in some of the earliest mode-of-action studies. The bacteriocin was found to induce leakage of K+, amino acids, and other low-molecular-weight molecules from sensitive cells (17, 32). It also dissipated the ΔΨ and the ΔpH from Pediococcus pentosaceus and Listeria monocytogenes (32, 34) and induced rapid depletion of intracellular ATP in these bacteria. When pediocin PA-1/AcH was added to carboxyfluorescein-loaded vesicles derived from membranes from sensitive and insensitive cells, only vesicles made from membranes of pediocin-sensitive cells became leaky (32). The authors proposed that a docking molecule was needed for the pediocin PA-1/AcH to be fully efficient. Subsequently, Chen et al. reported that pediocin PA-1/AcH in fact induced leakage of carboxyfluorescein from artificial liposomes, but possibly at higher concentrations than those needed to induce leakage from membranes of sensitive cells (30, 31). Mode-of-action studies subsequently done with other class IIa bacteriocins such as bavaricin MN (101), enterocin P (84, 85), mesentericin Y105 (120), and mundticin (14) also indicated a membrane-permeabilizing mode of action similar to that of pediocin PA-1/AcH. In addition, mesentericin Y105 has been shown to uncouple mitochondrial respiration (120).
Tryptophan fluorescence spectroscopy studies on pediocin PA-1 and mesentericin Y105 in the presence of liposomes have shown that at least the C-terminal tryptophan-containing parts of class IIa bacteriocins penetrate into liposomes/membranes, but the extent of penetration depends on the charge of the liposomes used and the location of the tryptophan residue in the peptide (30, 32, 134). Taken together, the aforementioned results suggest that class IIa bacteriocins induce permeabilization of the target cell membrane, probably by forming ion-selective pores which cause dissipation of the proton motive force and depletion of intracellular ATP (Fig. 6). However, these results say little about the structure of the suggested bacteriocin pore or whether the peptides act as monomers or oligomers, nor do they reveal the proposed docking molecule. The requirement for a docking molecule became more evident after the discovery that a 15-mer peptide fragment derived from the C-terminal half of pediocin PA-1/AcH inhibited pediocin PA-1/AcH activity and, to a lesser extent, the activities of other class IIa bacteriocins (59). The authors hypothesized that this 15-mer peptide fragment might interact with a docking molecule and thus compete with pediocin PA-1/AcH for recognition/binding to the target molecule. Yan et al. (193) showed that an all-d leucocin A enantiomer was inactive and thereby convincingly demonstrated that permeabilization of target cells by leucocin A involves chiral interactions, possibly with a receptor at the surface of the target cell (193). This receptor could be the mannose permease (see below).
FIG. 6.
Schematic illustration of the two steps involved in the putative mode of action of class IIa bacteriocins.
Mode of Action at the Molecular Level
The mode of action of class IIa bacteriocins has been investigated at the molecular level. An early genetic study, using transposon-induced mutants of L. monocytogenes, showed that the inactivation of the rpoN gene resulted in resistance to mesentericin Y105 (156). According to the same authors, the complementation of this mutant with the wild-type rpoN gene restored the sensitivity. This result indicated that rpoN was involved in the sensitivity of L. monocytogenes. The rpoN gene encodes the σ54 subunit of the bacterial RNA polymerase. This subunit is an alternative sigma factor, responsible for the transcription of a specific set of genes. The σ54-dependent transcription displays three specific features. First, σ54 recognizes specific promoters with a particular consensus sequence, which is different from those recognized by the housekeeping σ70 factor. Second, the promoter sequence is located at position −12/−24. Third, σ54 requires an interaction with an activator protein to initiate the transcription. It has been shown that the interruption of rpoN in another class IIa-sensitive species, Enterococcus faecalis, also leads to resistance (40). Therefore, rpoN might be involved directly or not in a general mechanism of sensitivity to class IIa bacteriocins. It has been hypothesized that rpoN is involved in the expression of a target molecule for class IIa bacteriocins, whose loss of expression leads to resistance.
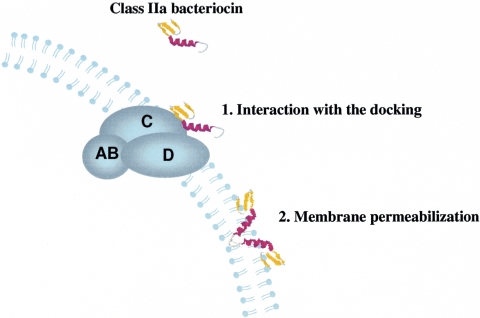
Furthermore, directed mutagenesis of the mpt operons of both L. monocytogenes and E. faecalis led to resistance of these bacteria to class IIa bacteriocins (41, 82). σ54 directs the expression of the mpt operon. High-level resistance to mesentericin Y105 and other class IIa bacteriocins results from the loss of mpt expression either in defined mutants or in spontaneous resistant strains (73). The mpt operon encodes a mannose permease, named EIItMan, which belongs to the phosphotransferase system (PTS). The PTS is responsible for the transport and concomitant phosphorylation of sugars inside both gram-negative and gram-positive bacteria (148). The PTS permeases of the mannose family are composed of four domains, IIA, IIB, IIC, and IID, arranged in two to four subunits. The IIA and IIB cytoplasmic domains are involved in phosphorylation, whereas the IIC and IID membrane domains are involved in transport (Fig. 6). The EIItMan permease of L. monocytogenes is a complex of three subunits, as IIA and IIB are fused. In addition, glucose and mannose induce the expression of mpt, while other sugars, such as cellobiose or fructose, do not. The level of mpt expression is correlated to the level of sensitivity (41, 82). These results suggested that the EIItMan permease might be a target molecule for class IIa bacteriocins. As IIC and IID subunits are probably present in the membrane, they are candidates as targets of class IIa bacteriocins. Finally, the mptACD operon of L. monocytogenes was heterologously expressed in an insensitive species, Lactococcus lactis (153). Upon induction of the mpt operon, the recombinant Lactococcus lactis became sensitive to various class IIa bacteriocins. Furthermore, each gene of the mptACD operon has been expressed independently in Lactococcus lactis. These results showed that the expression of mptC alone is sufficient to confer sensitivity on Lactococcus lactis. Accordingly, it has been proposed that the IIC subunit (Fig. 6) is the target molecule of the class IIa bacteriocins (153).
Other studies have reported a correlation between bacteriocin resistance and cell surface modifications, such as the lipid composition of the membrane, alanine content, and surface charge (177, 178). The authors concluded that membrane adaptation might be part of a resistance mechanism but that several resistance mechanisms may contribute to a resistance phenotype. The levels of resistance may vary according to the type of mechanism present.
SPECTRUM OF ACTIVITY
Despite their high structural similarity, the class IIa bacteriocins differ markedly in their antimicrobial spectrum of activity. In fact, class IIa bacteriocins have a quite limited spectrum compared to other bacteriocins produced by gram-positive bacteria. Early studies suggested that this might be related to the presence of a specific molecule at the surface of the target cell. As discussed above, this target may be the mannose permease. All the class IIa bacteriocins are described as being active against Listeria. They are also active against some other gram-positive target strains belonging to the genera Enterococcus, Lactobacillus, and Clostridium. However, from the literature it is difficult to compare the spectra and potencies of the different bacteriocins, since the target strains, the levels of bacteriocin purification, and the antibacterial assays differ between the studies. Only one study has thoroughly measured the activities of four different bacteriocins, purified to homogeneity, against a large array of indicator strains (52). The results indicated that L. monocytogenes was among the most sensitive indicator strains for all four bacteriocins. Pediocin PA-1/AcH and enterocin A inhibited more strains than sakacin P and curvacin A. Based on dithiothreitol reduction assays, it was hypothesized that the high potencies of pediocin PA-1/AcH and enterocin A may be due to the extra disulfide bond present in the C-terminal regions of these peptides. Another study compared the potencies of various purified class IIa bacteriocins against an L. ivanovii strain. This study strongly indicated that bacteriocins with two disulfide bonds are more potent than those with only one disulfide bond (76). Direct evidence for the importance of the C-terminal disulfide bridge came from a mutational analysis of sakacin P and pediocin PA-1 in which a C-terminal disulfide bridge was inserted in sakacin P and removed in pediocin PA-1 (60). In the case of sakacin P, it was concluded that the extra C-terminal disulfide bridge contributes to widening the antibacterial spectrum as well as to improving the potency at elevated temperatures.
The antimicrobial spectrum is defined as the set of strains that are sensitive to a given bacteriocin. This sensitivity depends on at least two steps of the actual in vivo functional model. In the first step, bacteriocin interacts with cell surface structures, such as the membrane and/or a receptor molecule (Fig. 6). In the second step, bacteriocins permeabilize the membrane via pore formation (Fig. 6). The initial binding might be influenced by envelope composition; the charge of the envelope; and the presence, availability, and structure of a putative target molecule (receptor). The second step is likely to rely on membrane composition, the structure of the C-terminal membrane-permeabilizing part of class IIa bacteriocins, and the presence of immunity proteins (see “Bacteriocin Immunity” above). In fact, using classical antibacterial assays, the effects of bacteriocin modification on cell targeting or on bactericidal potency are indistinguishable. For example, a decrease in bactericidal potency, leading to an undetectable activity of bacteriocin, might be interpreted as a modification of the spectrum, even if the receptor-bacteriocin interaction is unchanged. In addition, one can imagine that the modification of a bacteriocin would have consequences for its interaction with the receptor molecule of one target strain and not with those of another strain, as the receptors are not identical between the strains. The presence of (putative orphan) immunity genes in the target cell also may influence the apparent target cell spectrum of a bacteriocin (see “Bacteriocin Immunity” above for more details).
STRUCTURE-FUNCTION RELATIONSHIP
At least 25 different class IIa bacteriocins have been characterized (Fig. 1). They are all cationic peptides containing 37 to 48 amino acids, have similar primary structures, and can be divided into three or four subgroups on the basis of their primary structure (62, 63, 100, 134). They contain two structural regions: (i) a highly conserved N-terminal region (residues 1 to 16) harboring the consensus YGNGV motif and two cysteines involved in a disulfide bridge and (ii) a less conserved C-terminal region (residues 18 and on). The 3D structures of four class IIa bacteriocins have been determined by NMR spectroscopy (67, 79, 176, 189). Class IIa bacteriocins have no structure in water, but they become structured in the presence of membrane-mimicking environments such as micelles and liposomes (64, 67, 79, 176, 189).
In the presence of a membrane-mimicking environment, the N-terminal half forms a three-stranded antiparallel β-sheet-like structure supported by the conserved disulfide bridge, and the C-terminal half forms one or two amphiphilic α helices followed by a somewhat extended C-terminal tail of variable length. In most of these bacteriocins, the C-terminal tail appears to fold back onto the α-helix region, thereby forming a hairpin-like structure (Fig. 2). The β-sheet-like N-terminal half and the hairpin-like C-terminal half are separated by a flexible hinge, which allows the two halves to move relative to each other.
The conserved YGNGV sequence is part of the N-terminal β-sheet-like structure. It was proposed at an early stage that this conserved sequence was responsible for the antilisterial activity of class IIa bacteriocins, since all these bacteriocins share either this consensus sequence or the very similar YGNGL (2, 174, 182, 192) or YGTNGV (103) sequence (Fig. 1). Altering specifically the residues in the YGNGV sequence of carnobacteriocin B2 revealed that its potency was reduced upon replacing Tyr3 with Phe, indicating the importance of the hydroxyl group on tyrosine in this position (149). Similarly, the activity of pediocin PA-1/AcH was shown to be dramatically reduced by the Asn5-to-Lys mutation within the YGNGV motif (128). A more detailed mutational study of the YYGNGV sequence in sakacin P revealed that Tyr2 and Tyr3 could be replaced by Phe and Trp without great loss of potency. On the other hand, Asn5 was highly sensitive to alterations and did not tolerate even replacement with the similar residues Asp, Gln, and Ser. A large hydrophobic residue is needed in position 7 (G. Fimland, unpublished results). These recent results indicate that some alterations in the YGNGV sequence are tolerated.
The positively charged residues in class IIa bacteriocins are located mostly in the hydrophilic N-terminal region (Fig. 1). The affinity of pediocin PA-1/AcH-derived peptide fragments for target cells suggests that electrostatic interactions, and not the YGNGV sequence, mediate the initial binding of pediocin PA-1/AcH to target cells (30). The results indicated that Lys11 and His12, being part of a cationic patch in the N-terminal β-sheet-like region of pediocin PA-1/AcH and sakacin P, are of special importance for the electrostatic interactions (30, 108, 176). Subsequent mutagenesis studies in which charged residues in pediocin PA-1/AcH and sakacin P were replaced with neutral residues are consistent with this conclusion (108, 128).
The hairpin-like C-terminal region of class IIa bacteriocins is diverse with respect to the numbers of residues and alpha helices and the amino acid sequence and length of the C-terminal extension following the helical segment. Mutational analyses and mode-of-action studies, including tryptophan fluorescence spectroscopy, indicate that the variable C-terminal region penetrates the cell membrane, thereby inducing leakage and cell death (55, 58, 63, 128, 132, 134). It has also been demonstrated that pediocin PA-1/AcH with a large N-terminal maltose-binding protein fusion partner is active (127). This suggests that the N-terminal part of class IIa bacteriocins does not cause membrane permeabilization.
Substitution of Trp residues in sakacin P has been used to determine the orientation of the bacteriocin in cell membranes. It was concluded that Trp18 and Trp41 of sakacin P locates in the membrane-water interface of the target cell, whereas Trp33 locates in the hydrophobic part of the membrane, thereby creating a hairpin-like structure in the C-terminal part (Fig. 5) (62). In this model the hairpin-like C-terminal half orients obliquely in the membrane, and the hydrophilic β-sheet-like N-terminal half is attached to the cell surface (Fig. 5). The NMR structure of a structurally stabilized sakacin P variant indicates that such an orientation is possible (176). More recently, analyses of the role of Trp residues in mesentericin Y105 support this model (27, 134). For a more comprehensive discussion on the role of tryptophan residues in class IIa bacteriocins, see reference 63.
Other mutations within the hairpin-like C-terminal half usually result in decreased potency of the bacteriocins. There are, however, a few exceptions. For instance, when a C-terminal disulfide bridge was inserted into sakacin P, the new structurally stabilized sakacin P variant displayed a broader target cell spectrum and increased potency at elevated temperatures (60). Conserved substitutions in amino acid residues in the C-terminal half may have little if any effect on potency (60, 97). This was utilized to construct a pediocin PA-1/AcH variant in which the Met31 residue, which is prone to detrimental oxidation, was replaced by Leu without significant loss of potency (97). On the other hand, substitution by residues with opposite hydrophobicity or charge is most often detrimental (62, 97, 128, 149). These drastic mutations may disturb important intra- or intermolecular interactions that are necessary for optimal 3D structure of the bacteriocin and/or optimal interaction with the target cell (134).
The C-terminal region is important in determining the target cell specificity for class IIa bacteriocins. This has been shown by using hybrid bacteriocins constructed by combining N- and C-terminal regions from different class IIa bacteriocins. The hybrid bacteriocins displayed target cell specificities similar to that of the bacteriocin from which the C-terminal half was derived (58). Furthermore, results showing that a 15-mer peptide fragment derived from pediocin PA-1/AcH (from residue 20 to residue 34) specifically inhibited the bactericidal activity of pediocin PA-1/AcH also indicate a role for the C-terminal half of class IIa bacteriocins in recognition of target cells (59). All the class IIa bacteriocins whose modes of action have been studied permeabilize the cytoplasmic membrane. Clearly this is achieved by pore formation through insertion of the C-terminal regions of the bacteriocins into the membrane. Moreover, the sensitivity of a target cell is dependent on the presence of the mannose permease, EIItMan, at least in vivo. Forthcoming studies should reveal the involvement of the mannose permease as a target molecule, the specific role of the YGNGV motif, and the potency of the C-terminal region.
PURIFICATION AND HETEROLOGOUS PRODUCTION OF CLASS IIa BACTERIOCINS
The most frequently used methods for isolation, concentration, and purification of class IIa bacteriocins from culture supernatants involve salt precipitation followed by various combinations of gel filtration (64, 123), ion-exchange chromatography (26, 53, 123), hydrophobic-interaction chromatography (12, 26, 52, 123), and reverse-phase high-performance liquid chromatography (18, 52, 123, 126, 163). A review on bacteriocin purification methods by Carolissen-MacKay et al. in 1997 (26) highlighted the disadvantages of the then-existing complex and time-consuming purification protocols, which usually resulted in low proteins yields. These drawbacks also depend on the variation in the physicochemical properties of the many different bacteriocins themselves, perhaps most importantly their net charge (172). To overcome this problem, reliable methods such as cation-exchange chromatography based on the utilization of hydrophobic C-terminal domains of class IIa bacteriocins have been developed (15). Thus, the utilization of the cation-exchange chromatography method to purify pediocin PA-1/AcH resulted in a sevenfold enhancement in the specific activity of this class IIa bacteriocin (70). A requirement for the continued improvement of structure-function models of class IIa bacteriocins through biophysical studies is the production and purification of large quantities (more than 10 mg) of class IIa bacteriocins. For this purpose, an original and efficient system, based on the expression of a synthetic gene coding for recombinant divercin V41 using the Escherichia coli alphabet, was successfully achieved with E. coli Origami, enabling a yield of 23 mg of pure and highly active recombinant divercin V41/liter (155); this yield is currently being increased by using a high-density culture with regulated pH and oxygen levels (D. Drider, unpublished results). Another interesting system allowing large-scale production of pure recombinant piscicolin 126 at 26 mg/liter from a native DNA was reported with E. coli AD494(DE3) (72). Divercin V41 and piscicolin 126 have been expressed as bacteriocin-thioredoxin chimeric soluble proteins requiring, respectively, enzymatic or chemical treatment to release the recombinant bacteriocin (72, 155). Importantly, both systems provide ease of purification and a high yield of bacteriocins with correct disulfide bond formation. Finally, a versatile system allowing cloning and expression of secretable mature and self-cleaving fusion forms of nonmodified class IIa bacteriocins such as enterocin P, pediocin PA-1/AcH, piscicolin 126, and divercin V41 has been developed by Ingham et al. (93). By demonstrating that a large quantity of class IIa bacteriocins could be obtained upon expression of their native or synthetic DNAs, the three above-cited examples are the most significant developments in terms of the heterologous expression of class IIa bacteriocins in E. coli. Despite these efforts, E. coli does not have a GRAS (generally recognized as safe) status and remains a food pathogen. Alternative hosts must be considered when the production of class IIa bacteriocins is required for industrial purposes. The choice of LAB and Saccharomyces cerevisiae as heterologous hosts presents a real opportunity for future applications. Within LAB, Lactococcus lactis may offer advantages due to the availability of tools for its genetic manipulation (121), and continuing academic research on this model organism will undoubtedly increase its utility as a heterologous host for the expression of many class IIa bacteriocins, as recently reported for enterocin P (75, 86) and divercin V41 (Drider, unpublished results). Finally, most class IIa bacteriocins heterologously produced in LAB have been clearly reported elsewhere (158) and will not be discussed in this review.
The development of bactericidal yeast strains by expressing class IIa bacteriocins such as pediocin PA-1/AcH (166) and plantaricin 423 has been successfully carried out with S. cerevisiae (181), which could provide a potential application for bacteriocin-producing yeast in beverage industries. On the other hand, the use of the methylotrophic yeast Pichia pastoris as a heterologous host for production of significant concentrations of class IIa bacteriocins has been assessed for enterocin P (74) and pediocin PA-1/AcH (13). Both recombinant bacteriocins were produced at high levels, but pediocin PA-1/AcH was devoid of biological activity because of a “collagen-like” nature (13).
APPLICATIONS OF CLASS IIa BACTERIOCINS TO FOODS
In recent years, concerns about the safety and quality of foods have increased the attention given to the discovery and development of new methods of preservation of foods. Many class IIa bacteriocins of LAB have been well characterized; however, to date their application to foods has been mainly experimental. The source of bacteriocins for application to foods can be either a purified compound, a crude bacterial fermentate, or the bacteriocin-producing organism (171). No class IIa bacteriocins have been commercialized in a pure form, but pediocin is available from Quest International as ALTA 2341. Much of the research on the application of class IIa bacteriocins has focused on the use of the bacteriocin-producing culture in foods to control the growth of spoilage organisms or food-borne pathogens, such as L. monocytogenes. Table 2 summarizes some of the applications to foods of different class IIa bacteriocins or the bacteriocin-producing strain.
TABLE 2.
Applications of class IIa bacteriocins and their producing organisms to control the growth of food-borne pathogens and LAB
| Bacteriocin | Producer strain(s) | Food treated for targeting of the following organism(s):
|
Reference(s) | |||
|---|---|---|---|---|---|---|
| Listeria monocytogenes | Clostridium botulinum | Staphylococcus aureus | LAB | |||
| Divercin V41 | Carnobacterium divergens V41 | Smoked salmon | 50 | |||
| Enterocin A | Enterococcus faecium CTC 492 | Dry fermented sausage | 6 | |||
| Enterocin CCM 4231 | Enterococcus faecium CCM 4231 | Dry fermented sausage | 115 | |||
| Soy milk | Soy milk | 114 | ||||
| Leucocin A | Leuconostoc gelidum UAL187 | Ground beef | 146 | |||
| Beef slices | 116 | |||||
| Leucocin A-4010 | Leuconostoc carnosum 4010 | Cooked sausage | 24 | |||
| Mundticin | Enterococcus mundtii ATO6 | Mung bean sprouts | 14 | |||
| Pediocin PA-1/AcH | Pediococcus acidilactici | Cheese | Cheese | 157 | ||
| Frankfurters | 28, 45 | |||||
| Chicken summer sausage | 9 | |||||
| Red smear cheese | 119 | |||||
| Chilled soup | 159 | |||||
| Piscicolin 126 | Carnobacterium maltaromaticum JG126 | Ham paste | 95 | |||
| Camembert cheese | 188 | |||||
| Piscicosin CS526 | Carnobacterium pisciola CS526 | Surimi | 191 | |||
| Plantaricin 423 | Lactobacillus plantarum 423 | Ostrich meat salami | 47 | |||
| Sakacin A or K or curvacin A | Lactobacillus sakei 790 and Lactobacillus curvatus LTH 1174 | Chicken cold cuts | 185 | |||
| Lactobacillus sakei CTC 494 | Raw minced pork, poultry, cooked pork | 89 | ||||
| Cooked pork | 7 | |||||
| Sakacin P or bavaricin A | Lactobacillus sakei 790 | Cold smoked salmon | 1, 104 | |||
| Lactobacillus sakei MI401 | Brined shrimp | 54 | ||||
Use of Class IIa Bacteriocins To Extend Food Storage Life
The incorporation of class IIa bacteriocins into foods has the potential to prevent the growth of spoilage bacteria, a phenomenon that results in significant economic loss to the food industry. The efficacy of class IIa bacteriocins as pure or partially purified substances to control spoilage in foods is typically compared to that of nisin or nisin Z. In some cases, the class IIa bacteriocin products are less effective than nisin in controlling spoilage in perishable foods but do provide some protection against the growth of spoilage organisms (54). However, there are examples where class IIa bacteriocins perform better than nisin. Enterocins A and B, produced by Enterococcus faecium CTC 492, were able to inhibit the production of slime by Lactobacillus sakei CTC 746, while nisin and sakacin K had no effect (7).
Class IIa-producing cultures can be added to foods to either control the microbial succession during fermentation (162) or inhibit the growth of spoilage organisms during storage (7, 54). In modified-atmosphere-packaged foods, the adventitious microbiota is composed of LAB that will eventually spoil the product. Some LAB are known to cause rapid spoilage of modified-atmosphere-packaged meats. Lactobacillus sakei 1218 causes distinct sulfur odors in vacuum-packaged meat stored at 2°C; however, coinoculation of meat with a leucocin A-producing strain of Leuconostoc gelidum delays the growth of Lactobacillus sakei and inhibits the spoilage of meat for up to 8 weeks of storage (116).
One of the concerns about the addition of a bacteriocin-producing culture to a food is that the initial number of bacteria is higher than that found in naturally contaminated foods and the addition of the bacteriocin-producing culture may cause sensory changes in the product faster than the adventitious population. This is not always the case. When Leuconostoc gelidum UAL187 was added to ground beef at 5 log CFU/g, which is much higher than the level of adventitious lactic microbiota in fresh ground beef, a descriptive sensory panel found that Leuconostoc gelidum UAL187 reduced the incidence of putrid odors in vacuum-packaged ground beef stored at 4°C for up to 21 days, and there was no difference between the flavor profiles of samples inoculated with Leuconostoc gelidum UAL187 and the uninoculated control samples (L. M. McMullen, R. J. Worobo, G. G. Greer, and M. E. Stiles, Abstr. 5th Symp., Lactic Acid Bacteria, abstr. A33, 1996). Although this is an example in which a bacteriocin-producing culture improved the sensory characteristics of a food, care must be taken to ensure that the bacteriocin-producing culture is compatible with the intended food product.
While the use of class IIa bacteriocins to inhibit spoilage processes in foods holds promise to reduce economic losses in the food industry, the research in this area has been limited to a few studies. The focus on food safety and control of pathogens in foods has overshadowed the application for controlling spoilage processes.
Use of Class IIa Bacteriocins To Control Food-Borne Pathogens
One significant potential advantage of the application of class IIa bacteriocins or their producer organisms to foods is their ability to inhibit the growth of or kill food-borne pathogens. This has been the focus of much of the research on the applications of class IIa bacteriocin-producing LAB to foods. Results for the inhibition of Listeria spp. with bacteriocin-producing cultures in fresh and processed meats have been variable. Results obtained with fresh meat products have shown good inhibition of the growth of L. monocytogenes or Listeria innocua (88, 146). However, with cooked processed meats, storage temperature seems to play a role in the ability of some cultures to inhibit the growth of L. monocytogenes. Degnan et al. (45) found that Pediococcus acidilactici JBL1095 was able to inhibit the growth of L. monocytogenes when vacuum-packaged frankfurters were stored at 25°C, but at 4°C the protective culture was ineffective. Katla et al. (105) found that 104 CFU/g of L. sakei LB790, which produces sakacin P, was not able to inhibit the growth of L. monocytogenes on chicken cold cuts stored at 4°C, but inhibition was detected when the product was stored at 10°C. This illustrates the potential for bacteriocin-producing LAB to have a protective effect under conditions of temperature abuse. However, strains of Carnobacterium maltaromaticum that produce at least one class IIa bacteriocin are able to inhibit the growth and significantly reduce the numbers of different strains of L. monocytogenes on vacuum-packaged frankfurters stored at refrigeration temperatures (M. E. Stiles and L. M. McMullen, unpublished data). Carnobacterium divergens, which produces divercin V41, inhibits the growth of L. monocytogenes on vacuum-packaged cold-smoked salmon (7, 20, 38, 50).
Purified or partially purified class IIa bacteriocins also have an inhibitory effect against L. monocytogenes in foods. Addition of 2,000 activity units (AU)/g of piscicolin 126 to ham paste reduced the population of L. monocytogenes to below detectable levels immediately after addition, and numbers of L. monocytogenes did not increase during 14 days of storage at 10°C. In contrast, nisin and ALTA 2341 were not able to suppress L. monocytogenes growth during storage (95). The growth of L. monocytogenes is of major concern for the dairy industry, and some attention has been given to the use of purified bacteriocins as adjuncts to cheese fermentation and in milk. Piscicolin 126 and enterocin CCM 4231 reduced the numbers of L. monocytogenes in Camembert cheese and soy milk, respectively (114, 188). Other class IIa bacteriocins, such as those listed in Table 3, are also able to inhibit the growth of L. monocytogenes in foods.
TABLE 3.
Origins of LAB producing class IIa bacteriocins
| Bacteriocin | Producer
|
Origin | Reference(s) | |
|---|---|---|---|---|
| Species | Strain | |||
| Bavaricin A (same as sakacin P) | Lactobacillus sakei | MI401 | Sourdough | 113 |
| Bifidocin B | Bifidobacterium bifidum | NCFB 1454 | 194, 195 | |
| Carnobacteriocin B2 | Carnobacterium piscicola | LV17 | Fresh pork in modified atmosphere | 2 |
| Carnobacteriocin BM1 | Carnobacterium piscicola | LV17B | Fresh pork in modified atmosphere | 2 |
| Coagulin | Bacillus coagulans | I4 | Cattle feces | 91 |
| Curvacin A | Lactobacillus curvatus | LTH1174 | Fermented sausage | 173 |
| Divercin V41 | Carnobacterium divergens | V41 | Fish viscera | 126 |
| Divergicin M35 | Carnobacterium divergens | M35 | Smoked salmon | 172 |
| Enterocin SE-K4 | Enterococcus faecium | K-4 | Grass silage in Thailand | 51 |
| Enterocin P | Enterococcus faecium | P13 | Spanish dry fermented sausage | 35 |
| Enterocin CRL35 | Enterococcus faecium | CRL35 | Argentinean cheese | 56 |
| Enterocin A | Enterococcus faecium | CTCA92/T136 | Spanish dry fermented sausage | 8 |
| Lactococcin MMFII | Lactococcus lactis | MMFII | Tunisian dairy product | 57 |
| Leucocin A | Leuconostoc gelidum | UAL 187 | Vacuum-packaged meat | 77 |
| Leucocin C | Leuconostoc mesenteroides | TA33a | Vacuum-packaged meat | 147 |
| Mundticin | Enterococcus mundtii | ATO6 | Fresh chicory endive | 14 |
| Mesentericin Y105 | Leuconostoc mesenteroides | Y105 | Goat's milk in France | 81 |
| Plantaricin 423 | Lactobacillus plantarum | 423 | Sorghum beer | 180 |
| Pediocin PA-1 | Pediococcus acidilactici | PAC 1.0 | Sorghum beer | 32 |
| Pediocin AcH | Pediococcus acidilactici | AcH | 19 | |
| Pediocin SJ-1 | Pediococcus acidilactici | SJ-1 | Meat | 167 |
| Piscicolin 126 | Carnobacterium piscicola | JG126 | Spoiled ham | 94 |
| Sakacin P | Lactobacillus sakei | 674 | Meat | 87 |
| Sakacin G | Lactobacillus sakei | 2512 | Rhodia food collection | 168 |
| Sakacin A | Lactobacillus sakei | 706 | Meat | 164 |
| Sakacin 5X | Lactobacillus sakei | 5 | Malt | 145 |
An alternative mechanism for applying class IIa bacteriocins to foods is to deliver them as part of the packaging film. Antimicrobial packaging films have been developed for the delivery of nisin and pediocin (39, 131, 137). An uncharacterized bacteriocin from Lactobacillus curvatus 32Y was applied to polyethylene-oriented polyamide packaging films and found to reduce L. monocytogenes (124), but other known class IIa bacteriocins have not yet been used in this manner. Traditional starter cultures are usually chosen on the basis of their ability to rapidly produce acid to change the nature of the food ingredients. However, many of the bacteria that produce class IIa bacteriocins are not suitable for traditional fermentation processes in foods because they lack the ability to rapidly produce sufficient lactic acid for the process. One approach to incorporate class IIa bacteriocins into food fermentations is to transform the traditional starter cultures with the required genetic material for the production, immunity, and transport of class IIa bacteriocins. This has been successful for in situ production of pediocin in cheese (25, 157). Buyong et al. (25) found that the inclusion of a plasmid containing the operon for pediocin production did not alter the cheese-making properties of the original Lactococcus lactis subsp. lactis culture used for cheese making. The in situ production of pediocin by the genetically modified starter culture reduced the numbers of L. monocytogenes from a starting population of 3.65 log CFU/g to <1.0 log CFU/g after 92 days of ripening. In contrast, L. monocytogenes counts in the control cheese made with the isogenic starter culture increased and decreased during ripening but remained above 4 log CFU/g throughout the 184 days of ripening. Similarly, the inclusion of the pediocin operon in Lactococcus lactis CL1 used for cheese making resulted in a 3-log reduction of L. monocytogenes counts during ripening and reduced populations of Staphylococcus aureus by 1 log after 30 days of ripening (157). The possibilities of this approach are tremendous, and they open the door for a much broader application of class IIa bacteriocins to foods.
Impact of Environmental Conditions and Processing on Inhibition by Protective Cultures
The complex environment found in a food system can affect the production and efficacy of a class IIa bacteriocin. Adsorptions to proteins (1, 136) and interactions with fat (1) have been postulated to reduce the bacteriocinogenic activity of sakacin P. Proteolytic activity may reduce the efficacy of class IIa bacteriocins in foods, but the evidence for this is a decrease in activity during storage compared to that seen in cooked counterparts. No definitive enzymatic activity in foods has been identified for class IIa bacteriocins. It is known that the glutathione present in raw beef and pork can react with the dehydro residues of nisin to inactivate the antimicrobial activity (160, 161). However, this type of approach has never been elucidated for class IIa bacteriocins in meats. One emerging concern related to the application of class IIa bacteriocins or their producer strains in foods is the impact that the formulation might have on the efficacy or the production of bacteriocins. Some common ingredients have a synergistic effect on bacteriocin efficacy or production, while some ingredients can reduce antimicrobial activity. NaCl at the concentration found in a number of processed meat products, including fermented meats, can reduce the growth of protective cultures and thereby reduce the production of bacteriocins, such as sakacin K (118), and it can also protect L. monocytogenes against the antimicrobial effects of sakacin K (89). However, other data suggest that NaCl could improve the activities of sakacin P and curvacin A (68). This discrepancy in the results could be due to different target strains and different experimental conditions or, as suggested by Gänzle et al. (68), to the choice of assay. Many of the studies on the impact of ingredients on bacteriocin activity are done in broth systems that may or may not translate to a food environment. Other ingredients in sausage formulations may help to overcome the impact of NaCl on the efficacy of bacteriocins to inhibit the growth of L. monocytogenes. Black pepper, which contains relatively high levels of manganese, and manganese enhance the antilisterial activity of L. sakei CTC 494 and reduce the growth of L. monocytogenes in sausage (89). Other spices, such as nutmeg and paprika, inhibit the production of curvacin A by Lactobacillus curvatus LTH1174, while the presence of garlic enhances production (185). High concentrations of nitrite can have a negative impact on the growth of cultures producing class IIa bacteriocins and thus reduce the production of bacteriocins (118).
Extending the Hurdle Concept with Class IIa Bacteriocins
The application of class IIa bacteriocins to foods provides only one hurdle to the growth of spoilage or pathogenic organisms. The combination of bacteriocins with other sublethal treatments has proved to provide effective antimicrobial hurdles in food systems. For example, the combination of pediocin with sodium diacetate and sodium lactate had a greater inhibitory effect on the growth of L. monocytogenes on beef frankfurters stored at 4°C for 3 weeks than did pediocin alone (175). Moreover, a combination of ALTA 2341 (containing pediocin) and sodium lactate extended the lag phase of L. monocytogenes on the surface of cooked chicken stored at 3.5°C; however, the antimicrobial effect was not sustained throughout the 35-day storage life (11). In addition to the effects of combinations of chemical inhibitors, different processing steps may affect the efficacy of microbial control by class IIa bacteriocins. The combination of high-pressure processing with enterocins A and B, sakacin K, or pediocin enhanced the antilisterial activity in a meat model system (69). Pediocin (as ALTA 2341; 3,000 AU/g) combined with low-dose irradiation (2.3 kGy) had a greater inhibitory effect on the growth of L. monocytogenes on frankfurters (29) than pediocin (6,000 AU/g) combined with postprocessing thermal pasteurization (96°C for 60 s) (28).
Potential and Challenges for the Future
The use of class IIa bacteriocins has the potential to allow the food industry to better predict the storage lives of its products and provides an additional barrier to the growth of food-borne pathogens. However, along with other processing hurdles, the use of bacteriocins or bacteriocin-producing cultures should be considered only as an additional process that complements good manufacturing practices. There are some challenges ahead for expanded use of class IIa bacteriocins in foods. Regulatory restrictions and requirements will slow the widespread application to foods. A better understanding of the development of bacteriocin-resistant pathogens in foods and strategies to mitigate their emergence need to be developed. A lack of consumer acceptance of genetically modified organisms in foods could be a barrier to the expanded possibilities of genetically improved starter cultures for the production of class IIa bacteriocins. However, as consumers continue to demand products that are minimally processed and preserved, the use of class IIa bacteriocins may become more common as a means of “naturally” preserving foods.
CONCLUDING REMARKS AND FUTURE PROSPECTS
Undoubtedly, the class IIa bacteriocins constitute one of the most promising groups of antimicrobial peptides. Despite the efforts made to understand their mode of action, biosynthesis, and heterologous production, several points remain to be elucidated. As summarized, the overall mechanisms behind bacteriocin secretion and regulation of bacteriocin production are fairly well described, but the molecular details involved in these mechanisms are only partly understood, and several fundamental questions remain unsolved. Extensive structural and genetic analyses of both the transporter proteins and the regulatory proteins, including the inducer peptide, are necessary to unravel their molecular mechanisms and how they interact in the cell.
In the future, detailed structural information obtained by NMR spectroscopy, X-ray crystallography, and site-directed mutagenesis of more class IIa immunity proteins will be required to get in-depth understanding on how class IIa immunity proteins recognize various class IIa bacteriocins, how they differentiate between them, and how they physically inhibit their antimicrobial activity. Moreover, the functionality of class IIa immunity proteins in a cellular context has to be investigated to see if immunity depends on additional factors present in the cell and whether the molecular mechanism of bacteriocin immunity may be linked to bacteriocin resistance and/or the suggested bacteriocin receptor.
Do bacteriocins interact directly with the IIC subunit of the mannose permease? Which biological function is linked to the conservation of the YGNGV motif? What specific modifications or structures render the C-terminal region more potent? Finally, an essential issue is to understand the link between the two steps of bacteriocin action, interaction with the permease and pore formation.
Food application of class IIa bacteriocins is appearing as a good alternative to protect foods against pathogenic microorganisms. Nevertheless, we also expect more applications of class IIa bacteriocins in the medical area as antibiotic complements, since encouraging examples have been reported for nisin (a class I bacteriocin) in the treatment of infections caused by Pseudomonas aeruginosa (71) and methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci (21).
Acknowledgments
We thank Carol Robins for improvement of the English of the manuscript. We thank Pablo Hernandez (Madrid, Spain) for comments and suggestions.
D.D. and H.P. thank the French Ministry of Agriculture, La Région des Pays de la Loire, and the European Union (SEAFOODplus) for their financial support for antimicrobial peptide projects carried out at ENITIAA. Y.H. thanks Rhodia-Food Company, la Région Poitou-Charentes, and the French Ministry of Education for supporting his research projects. Research at the laboratory of L.M.M. was funded by the Natural Sciences and Engineering Research Council of Canada. G.F. was supported by the Norwegian Research Council.
REFERENCES
- 1.Aasen, I. M., S. Markussen, T. Moretro, T. Katla, L. Axelsson, and K. Naterstad. 2003. Interactions of the bacteriocins sakacin P and nisin with food constituents. Int. J. Food Microbiol. 87:35-43. [DOI] [PubMed] [Google Scholar]
- 2.Ahn, C., and M. E. Stiles. 1990. Plasmid-associated bacteriocin production by a strain of Carnobacterium piscicola from meat. Appl. Environ. Microbiol. 56:2503-2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderssen, E. L., D. B. Diep, I. F. Nes, V. G. Eijsink, and J. Nissen-Meyer. 1998. Antagonistic activity of Lactobacillus plantarum C11: two new two-peptide bacteriocins, plantaricins EF and JK, and the induction factor plantaricin A. Appl. Environ. Microbiol. 64:2269-2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aucher, W., C. Lacombe, A. Héquet, J. Frère, and J.-M. Berjeaud. 2005. Influence of amino acid substitutions in the leader peptide on maturation and secretion of mesentericin Y105 by Leuconostoc mesenteroides. J. Bacteriol. 187:2218-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Axelsson, L., T. Katla, M. Bjørnslett, V. G. H. Eijsink, and A. Holck. 1998. A system for expression of bacteriocins in Lactobacillus sakei. FEMS Microbiol. Lett. 168:137-143. [DOI] [PubMed] [Google Scholar]
- 6.Aymerich, M. T., M. G. Artigas, M. Garriga, J. M. Monfort, and M. Hugas. 2000. Effect of sausage ingredients and additives on the production of enterocins A and B by Enterococcus faecium CTC492. Optimization of in vitro production and anti-listeria effect in dry fermented sausages. J. Appl. Microbiol. 88:686-694. [DOI] [PubMed] [Google Scholar]
- 7.Aymerich, M. T., M. Garriga, S. Costa, J. M. Monfort, and M. Hugas. 2002. Prevention of ropiness in cooked pork by bacteriocinogenic cultures. Int. Dairy J. 12:239-246. [Google Scholar]
- 8.Aymerich, T., H. Holo, L. S. Håvarstein, M. Hugas, M. Gariga, and I. F. Nes. 1996. Biochemical and genetic characterization of enterocin A from Enterococcus faecium, a new antilisterial bacteriocin in the pediocin family of bacteriocins. Appl. Environ. Microbiol. 62:1676-1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baccustaylor, G. K, A. Glass, J. B. Luchansky, and A. J. Maurer. 1993. Fate of Listeria monocytogenes and pediococcal starter cultures during the manufacture of chicken summer sausage. Poult. Sci. 72:1772-1778. [DOI] [PubMed] [Google Scholar]
- 10.Banerjee, S., and J. N. Hansen. 1988. Structure and expression of a gene encoding the precursor of subtilin, a small protein antibiotic. J. Biol. Chem. 263:9508-9514. [PubMed] [Google Scholar]
- 11.Barakat, R. K., and L. J. Harris. 1999. Growth of Listeria monocytogenes and Yersinia enterocolitica on cooked modified-atmosphere-packaged poultry in the presence and absence of a naturally occurring microbiota. Appl. Environ. Microbiol. 65:342-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beaulieu, L., H. Aomari, D. Groleau, and M. Subirade. 2006. An improved and simplified method for the large-scale purification of pediocin PA-1 produced by Pediococcus acidilactici. Biotechnol. Appl. Biochem. 43:77-84. [DOI] [PubMed] [Google Scholar]
- 13.Beaulieu, L., D. Groleau, C. B. Miguez, J. F. Jetté, H. Aomari, and M. Subirade. 2005. Production of pediocin PA-1 in the methylotrophic yeast Pichia pastoris reveals unexpected inhibition of its biological activity due to the presence of collagen-like material. Protein Expr. Purif. 43:111-125. [DOI] [PubMed] [Google Scholar]
- 14.Bennik, M. H. J., B. Vanloo, R. Brasseur, L. G. M. Gorris, and E. J. Smid. 1998. A novel bacteriocin with a YGNGV motif from vegetable-associated Enterococcus mundtii: full characterization and interaction with target organisms. Biochim. Biophys. Acta 1373:47-58. [DOI] [PubMed] [Google Scholar]
- 15.Berjeaud, J. M., and Y. Cenatiempo. 2004. Purification of antilisterial bacteriocins. Methods Mol. Biol. 268:225-233. [DOI] [PubMed] [Google Scholar]
- 16.Beukes, M., and J. W. Hastings. 2001. Self-protection against cell wall hydrolysis in Streptococcus milleri NMSCC 061 and analysis of the millericin B operon. Appl. Environ. Microbiol. 67:3888-3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhunia, A. K., M. C. Johnson, B. Ray, and N. Kalchayanand. 1991. Mode of action of pediocin AcH from Pediococcus acidilactici H on sensitive bacterial strains. J. Appl. Bacteriol. 70:25-33. [Google Scholar]
- 18.Biet, F., J. M. Berjeaud, R. W. Worobo, Y. Cenatiempo, and C. Frémaux. 1998. Heterologous expression of the expression of the bacteriocin mesentericin Y105 using dedicated transport system and the general secretion pathway. Microbiology 144:2845-2854. [DOI] [PubMed] [Google Scholar]
- 19.Biswas, S. R., P. Ray, M. C. Johnson, and B. Ray. 1991. Influence of growth conditions on the production of a bacteriocin, pediocin AcH, by Pediococcus acidilactici H. Appl. Environ. Microbiol. 57:1265-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brillet, A., M. F. Pilet, H. Prévost, A. Bouttefroy, and F. Leroi. 2004. Biodiversity of Listeria monocytogenes sensitivity to bacteriocin-producing Carnobacterium strains and application in sterile cold-smoked salmon. J. Appl. Microbiol. 97:1029-1037. [DOI] [PubMed] [Google Scholar]
- 21.Brumfitt, W., M. R. J. Salton, and J. M. T. Hamilton-Miller. 2002. Nisin, alone and combined with peptidoglycan-moduling antibiotics: activity against methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci. J. Antimicrobiol. Chemother. 50:731-734. [DOI] [PubMed] [Google Scholar]
- 22.Brurberg, M. B., I. F. Nes, and V. G. H. Eijsink. 1997. Pheromone-induced production of antimicrobial peptides in Lactobacillus. Mol. Microbiol. 26:347-360. [DOI] [PubMed] [Google Scholar]
- 23.Buchman, G. W., S. Banerjee, and J. N. Hansen. 1988. Structure, expression, and evolution of a gene encoding the precursor of nisin, a small protein antibiotic. J. Biol. Chem. 263:16260-16266. [PubMed] [Google Scholar]
- 24.Budde, B. B., T. Hornbæk, T. Jacobsen, V. Barkholt, and A. G. Koch. 2003. Leuconostoc carnosum 4010 has the potential for use as a protective culture for vacuum-packed meats: culture isolation, bacteriocin identification, and meat application experiments. Int. J. Food Microbiol. 83:171-184. [DOI] [PubMed] [Google Scholar]
- 25.Buyong, N., J. Kok, and J. B. Luchansky. 1998. Use of a genetically enhanced, pediocin-producing starter culture, Lactococcus lactis subsp. lactis MM217, to control Listeria monocytogenes in cheddar cheese. Appl. Environ. Microbiol. 64:4842-4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carolissen-MacKay, V., G. Arendse, and J. W. Hastings. 1997. Purification of bacteriocins of lactic acid bacteria: problems and pointers. Int. J. Food Microbiol. 34:1-16. [DOI] [PubMed] [Google Scholar]
- 27.Castano, S., B. Desbat, A. Delfour, J. M. Dumas, A. da Silva, and J. Dufourcq. 2005. Study of structure and orientation of mesentericin Y105, a bacteriocin from Gram-positive Leuconostoc mesenteroides, and its Trp-substituted analogues in phospholipid environments. Biochim. Biophys. Acta 1668:87-98. [DOI] [PubMed] [Google Scholar]
- 28.Chen, C.-M., J. G. Sebranek, J. S. Dickson, and A. F. Mendonca. 2004. Combining pediocin (ALTA 2341) with post-packaging pasteurization for control of Listeria monocytogenes on frankfurters. J. Food Prot. 67:1855-1865. [DOI] [PubMed] [Google Scholar]
- 29.Chen, C.-M., J. G. Sebranek, J. S. Dickson, and A. F. Mendonca. 2004. Combining pediocin with post-packaging irradiation for control of Listeria monocytogenes on frankfurters. J. Food Prot. 67:1866-1875. [DOI] [PubMed] [Google Scholar]
- 30.Chen, Y., R. D. Ludescher, and T. J. Montville. 1997. Electrostatic interactions, but not the YGNGV consensus motif, govern the binding of pediocin PA-1 and its fragments to phospholipid vesicles. Appl. Environ. Microbiol. 63:4770-4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen, Y., R. Shapira, M. Eisenstein, and T. J. Montville. 1997. Functional characterization of pediocin PA-1 binding to liposomes in the absence of a protein receptor and its relationship to a predicted tertiary structure. Appl. Environ. Microbiol. 63:524-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chikindas, M. L., M. J. Garcia-Garcera, A. J. Driessen, A. M. Ledeboer, J. Nissen-Meyer, I. F. Nes, T. Abee, W. N. Konings, and G. Venema. 1993. Pediocin PA-1, a bacteriocin from Pediococcus acidilactici PAC1.0, forms hydrophilic pores in the cytoplasmic membrane of target cells. Appl. Environ. Microbiol. 59:3577-3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cho, H. S., J. G. Pelton, D. Yan, S. Kustu, and D. E. Wemmer. 2001. Phosphoaspartates in bacterial signal transduction. Curr. Opin. Struct. Biol. 11:679-684. [DOI] [PubMed] [Google Scholar]
- 34.Christensen, D. P., and R. W. Hutkins. 1992. Collapse of the proton motive force in Listeria monocytogenes caused by a bacteriocin produced by Pediococcus acidilactici. Appl. Environ. Microbiol. 58:3312-3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cintas, L. M., P. Casaus, L. S. Håvarstein, P. E. Hernández, and I. F. Nes. 1997. Biochemical and genetical characterization of enterocin P, a novel sec-dependent bacteriocin from Enterococcus faecium P13 with a broad antimicrobial spectrum. Appl. Environ. Microbiol. 63:4321-4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cleveland, J., T. J., Montville, I. F. Nes, and M. L. Chikindas. 2001. Bacteriocins: safe natural antimicrobials for food preservation. Int. J. Food Microbiol. 71:1-20. [DOI] [PubMed] [Google Scholar]
- 37.Cole, A. M., P. Weiss, and G. Diamond. 1997. Isolation and characterization of pleurocidin, an antimicrobial peptide in the skin secretions of winter flounder. J. Biol. Chem. 272:12008-12013. [DOI] [PubMed] [Google Scholar]
- 38.Connil, N., H. Prévost, and X. Dousset. 2002. Production of biogenic amines and divercin V41 in cold smoked salmon inoculated with Carnobacterium divergens V41, and specific detection of this strain by multiplex PCR. J. Appl. Microbiol. 92:611-617. [DOI] [PubMed] [Google Scholar]
- 39.Cutter, C. N., J. L. Willett, and G. R. Siragusa. 2001. Improved antimicrobial activity of nisin-incorporated polymer films by formulation change and addition of food grade chelator. Lett. Appl. Microbiol. 33:325-328. [DOI] [PubMed] [Google Scholar]
- 40.Dalet, K., C. Briand, Y. Cenatiempo, and Y. Héchard. 2000. The rpoN gene of Enterococcus faecalis directs sensitivity to subclass IIa bacteriocins. Curr. Microbiol. 41:441-443. [DOI] [PubMed] [Google Scholar]
- 41.Dalet, K., Y. Cenatiempo, P. Cossart, the European Listeria Genome Consortium, and Y. Héchard. 2001. A σ54-dependent PTS permease of the mannose family is responsible for sensitivity of Listeria monocytogenes to mesentericin Y105. Microbiology 147:3263-3269. [DOI] [PubMed] [Google Scholar]
- 42.Dalhus, B., L. Johnsen, and J. Nissen-Meyer. 2003. Crystallization and prelimnary X-ray data investigation of the bacterial enterocin A immunity protein at 1.65 Å resolution. Acta Crystallogr. 59:1291-1293. [DOI] [PubMed] [Google Scholar]
- 43.Davidson, A. L., and J. Chen. 2004. ATP-binding cassette transporters in bacteria. Annu. Rev. Biochem. 73:241-268. [DOI] [PubMed] [Google Scholar]
- 44.Dayem, M. A., Y. Fleury, G. Devilliers, E. Chaboisseau, R. Girard, P. Nicolas, and A. Delfour. 1996. The putative immunity protein of the Gram-positive bacteria Leuconostoc mesenteroides is preferentially located in the cytoplasm compartment. FEMS Microbiol. Lett. 138:251-259. [DOI] [PubMed] [Google Scholar]
- 45.Degnan, A. J., A. E. Yousef, and J. B. Luchansky. 1992. Use of Pediococcus acidilactici to control Listeria monocytogenes in temperature abused vacuum-packaged wieners. J. Food Prot. 55:98-103. [DOI] [PubMed] [Google Scholar]
- 46.De la Cruz, I., and I. Davies. 2000. Horizontal gene transfer and the origin of species: lessons from bacteria. Trends Microbiol. 8:128-133. [DOI] [PubMed] [Google Scholar]
- 47.Dicks, L. M. T., F. D. Mellett, and L. C. Hoffman. 2004. Use of bacteriocin-producing starter cultures of Lactobacillus plantarum and Lactobacillus curvatus in production of ostrich meat salami. Meat Sci. 66:703-708. [DOI] [PubMed] [Google Scholar]
- 48.Diep, D. B., L. S. Havarstein, and I. F. Nes. 1996. Characterization of the locus responsible for the bacteriocin production in Lactobacillus plantarum C11. J. Bacteriol. 178:4472-4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Diep, D. B., L. Axelsson, C. Grefsli, and I. F. Nes. 2000. The synthesis of the bacteriocin sakacin A is a temperature-sensitive process regulated by a pheromone peptide through a three-component regulatory system. Microbiology 146:2155-2160. [DOI] [PubMed] [Google Scholar]
- 50.Duffes, F., C. Corre, F. Leroi, X. Dousset, and P. Boyaval. 1999. Inhibition of Listeria monocytogenes by in situ produced and semi purified bacteriocins of Carnobacterium spp. on vacuum-packed, refrigerated cold-smoked salmon. J. Food Prot. 62:1394-1403. [DOI] [PubMed] [Google Scholar]
- 51.Eguchi, T., K. Kaminaka, J. Shima, S. Kawamoto, K. Mori, S. H. Choi, K. Doi, S. Ohomomo, and S. Ogata. 2001. Isolation and characterization of enterocin SE-K4 produced by thermophilic enterococci, Enterococcus faecalis K-14. Biosci. Biotechnol. Biochem. 65:247-253. [DOI] [PubMed] [Google Scholar]
- 52.Eijsink, V. G., M. Skeie, P. H. Middelhoven, M. B. Brurberg, and I. F. Nes. 1998. Comparative studies of class IIa bacteriocins of lactic acid bacteria. Appl. Environ. Microbiol. 64:3275-3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eijsink, V. G. H., L. Axelsson, D., B. Diep, L. S. Håvarstein, H. Holo, and I. F. Nes. 2002. Production of bacteriocins by lactic acid bacteria; an example of biological warfare and communication. Antonie Leeuwenhoek 81:639-654. [DOI] [PubMed] [Google Scholar]
- 54.Einarsson, J., and J. L. Lauzon. 1995. Biopreservation of brined shrimp (Pandalus borealis) by bacteriocins from lactic acid bacteria. Appl. Environ. Microbiol. 61:669-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ennahar, S., T. Sashihara, K. Sonomoto, and A. Ishizaki. 2000. Class IIa bacteriocins: biosynthesis, structure and activity. FEMS Microbiol. Rev. 24:85-106. [DOI] [PubMed] [Google Scholar]
- 56.Farías, M. E., A. Ruiz Holgado, and F. Sesma. 1994. Bacteriocin production by lactic acid bacteria isolated from regional cheeses: inhibition of foodborne pathogens. J. Food Prot. 57:1013-1015. [DOI] [PubMed] [Google Scholar]
- 57.Ferchichi, M., M. Fathallah, P. Mansuelle, H. Rochat, J. M. Sabatier, M. Manai, and K. Mabrouk. 2001. Chemical synthesis, molecular modeling, and antimicrobial activity of a novel bacteriocin, lactococcin MMFII. Biochem. Biophys. Res. Commun. 289:13-18. [DOI] [PubMed] [Google Scholar]
- 58.Fimland, G., O. R. Blingsmo, K. Sletten, G. Jung, I. F. Nes, and J. Nissen-Meyer. 1996. New biologically active hybrid bacteriocins constructed by combining regions from various pediocin-like bacteriocins: the C-terminal region is important for determining specificity. Appl. Environ. Microbiol. 62:3313-3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fimland, G., R. Jack, G. Jung, I. F. Nes, and J. Nissen-Meyer. 1998. The bactericidal activity of pediocin PA-1 is specifically inhibited by a 15-mer fragment that spans the bacteriocin from the center toward the C terminus. Appl. Environ. Microbiol. 64:5057-5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fimland, G., L. Johnsen, L. Axelsson, M. B. Brurberg, I. F. Nes, V. G. H. Eijsink, and J. Nissen-Meyer. 2000. A C-terminal disulfide bridge in pediocin-like bacteriocins renders bacteriocin activity less temperature dependent and is a major determinant of the antimicrobial spectrum. J. Bacteriol. 182:2643-2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fimland, G., V. G. Eijsink, and J. Nissen-Meyer. 2002. Comparative studies of immunity proteins of pediocin-like bacteriocins. Microbiology 148:3661-3670. [DOI] [PubMed] [Google Scholar]
- 62.Fimland, G., V. G. H. Eijsink, and J. Nissen-Meyer. 2002. Mutational analysis of the role of tryptophan residues in an antimicrobial peptide. Biochemistry 41:9508-9515. [DOI] [PubMed] [Google Scholar]
- 63.Fimland, G., L. Johnsen, B. Dalhus, and J. Nissen-Meyer. 2005. Pediocin-like antimicrobial peptides (class IIa bacteriocins) and their immunity proteins: biosynthesis, structure and mode of action. J. Pept. Sci. 11:688-696. [DOI] [PubMed] [Google Scholar]
- 64.Fleury, Y., M. A. Dayem, J. J. Montagne, E. Chaboisseau, J. P. Le Caer, P. Nicolas, and A. Delfour. 1996. Covalent structure, synthesis, and structure-function studies of mesentericin Y 105(37), a defensive peptide from gram-positive bacteria Leuconostoc mesenteroides. J. Biol. Chem. 271:14421-14429. [DOI] [PubMed] [Google Scholar]
- 65.Franke, C. M., J. Tiemersma, G. Venema, and J. Kok. 1999. Membrane topology of the lactococcal bacteriocin ATP cassette transporter protein LcnC. Involvement of LcnC in lactococcin A maturation. J. Biol. Chem. 274:8484-8490. [DOI] [PubMed] [Google Scholar]
- 66.Franz, C. M. A. P., M. J. van Belkum, R. W. Worobo, J. C. Vederas, and M. E. Stiles. 2000. Characterization of the genetic locus responsible for production and immunity of carnobacteriocin A: the immunity gene confers cross-protection to enterocin B. Microbiology 146:621-631. [DOI] [PubMed] [Google Scholar]
- 67.Fregeau Gallagher, N. L., M. Sailer, W. P. Niemczura, T. T. Nakashima, M. E. Stiles, and J. C. Vederas. 1997. Three-dimensional structure of leucocin A in trifluoroethanol and dodecylphosphocholine micelles: spatial location of residues critical for biological activity in type IIa bacteriocins from lactic acid bacteria. Biochemistry 36:15062-15072. [DOI] [PubMed] [Google Scholar]
- 68.Gänzle, M. G., S. Weber, and W. P. Hammes. 1999. Effect of ecological factors on the inhibitory spectrum and activity of bacteriocins. Int. J. Food Microbiol. 46:207-217. [DOI] [PubMed] [Google Scholar]
- 69.Garriga, M., M. T. Aymerich, S. Costa, J. M. Monfort, and M. Hugas. 2002. Bactericidal synergism through bacteriocins and high pressure in a meat model system during storage. Food Microbiol. 19:509-518. [Google Scholar]
- 70.Gaussier, H., H. Morency, M. Lavoie, and M. Subirade. 2002. Replacement of trifluoroacetic acid with HCl in the hydrophobic purification steps of pediocin PA-1: a structural effect. Appl. Environ. Microbiol. 68:4803-4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Giacometti, A., O. Cirioni, F. Barchiesi, M. Fortuna, and G. Scalise. 1999. In-vitro activity of cationic peptides alone and in combination with clinically used antimicrobial agents against Pseudomonas aeruginosa. J. Antimicrob. Chemother. 44:641-645. [DOI] [PubMed] [Google Scholar]
- 72.Gibbs, G. M., B. E. Davidson, and A. J. Hillier. 2004. Novel expression system for large-scale production and purification of recombinant class IIa bacteriocins and its application to piscicolin 126. Appl. Environ. Microbiol. 70:3292-3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gravesen, A., M. Ramnath, K. B. Rechinger, N. Andersen, L. Jansch, Y. Héchard, J. W. Hastings, and S. Knochel. 2002. High-level resistance to class IIa bacteriocins is associated with one general mechanism in Listeria monocytogenes. Microbiology 148:2361-2369. [DOI] [PubMed] [Google Scholar]
- 74.Gutiérrez, J., R. Criado, M. Martín, C. Herranz, L. M. Cintas, and P. E. Hernández. 2005. Production of enterocin P, an antilisterial pediocin-like bacteriocin from Enterococcus faecium P13, in Pichia pastoris. Antimicrob. Agents Chemother. 49:3004-3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gutiérrez, J., R. Larsen, L. M. Cintas, J. Kok, and P. E. Hernández. 2006. High-level heterologous production of the sec-dependent enterocin P from Enterococcus faecium P13 in Lactococcus lactis. Appl. Microbiol. Biotechnol. 17:1-11. [DOI] [PubMed] [Google Scholar]
- 76.Guyonnet, D., C. Frémaux, Y. Cenatiempo, and J. M. Berjeaud. 2000. Method for rapid purification of class IIa bacteriocins and comparison of their activities. Appl. Environ. Microbiol. 66:1744-1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hastings, J. W., M. Sailer, K. Johnson, K. L. Roy, J. C. Vederas, and M. E. Stiles. 1991. Characterization of leucocin A-UAL 187 and cloning of the bacteriocin gene from Leuconostoc gelidum. J. Bacteriol. 173:7491-7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hauge, H. H., D. Mantzilas, G. N. Moll, W. N. Konings, A. J. M. Driessen, V. G. H. Eijsink, and J. Nissen-Meyer. 1998. Plantaricin A is an amphiphilic α-helical bacteriocin-like pheromone which exerts antimicrobial and pheromone activities through different mechanisms. Biochemistry 37:16026-16032. [DOI] [PubMed] [Google Scholar]
- 79.Haugen, H. S., G. Fimland, J. Nissen-Meyer, and P. E. Kristiansen. 2005. Three-dimensional structure in lipid micelles of the pediocin-like antimicrobial peptide curvacin A. Biochemistry 44:16149-16157. [DOI] [PubMed] [Google Scholar]
- 80.Håvarstein, L. S., D. B. Diep, and I. F. Nes. 1995. A family of bacteriocin ABC transporters carry out proteolytic processing of their substrates concomitant with export. Mol. Microbiol. 16:229-240. [DOI] [PubMed] [Google Scholar]
- 81.Héchard, Y., B. Dérijard, F. Letellier, and Y. Cenatiempo. 1992. Characterization and purification of mesentericin Y105, an anti-Listeria bacteriocin from Leuconostoc mesenteroides. J. Gen. Microbiol. 138:2725-2731. [DOI] [PubMed] [Google Scholar]
- 82.Héchard, Y., C. Pelletier, Y. Cenatiempo, and J. Frère. 2001. Analysis of sigma(54)-dependent genes in Enterococcus faecalis: a mannose PTS permease (EII(Man)) is involved in sensitivity to a bacteriocin, mesentericin Y105. Microbiology 147:1575-1580. [DOI] [PubMed] [Google Scholar]
- 83.Héchard, Y., and H. G. Sahl. 2002. Mode of action of modified and unmodified bacteriocins from Gram-positive bacteria. Biochimie 84:545-557. [DOI] [PubMed] [Google Scholar]
- 84.Herranz, C., Y. Chen, H. J. Chung, L. M. Cintas, P. E. Hernandez, T. J. Montville, and M. L. Chikindas. 2001. Enterocin P selectively dissipates the membrane potential of Enterococcus faecium T136. Appl. Environ. Microbiol. 67:1689-1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Herranz, C., L. M. Cintas, P. E. Hernández, G. N. Moll, and A. J. Driessen. 2001. Enterocin P causes potassium ion efflux from Enterococcus faecium T136 cells. Antimicrob. Agents Chemother. 45:901-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Herranz, C., and J. M. Driessen. 2005. Sec-mediated secretion of bacteriocin enterocin P by Lactococcus lactis. Appl. Environ. Microbiol. 71:1959-1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Holck, A. L., L. Axelsson, K. Huhne, and L. Kröckel. 1994. Purification and cloning of sakacin 674, a bacteriocin from Lactobacillus sake Lb674. FEMS Microbiol. Lett. 115:143-150. [DOI] [PubMed] [Google Scholar]
- 88.Hugas, M., F. Pages, M. Garriga, and J. M. Monfort. 1998. Application of the bacteriocinogenic Lactobacillus sakei CTC494 to prevent growth of Listeria in fresh and cooked meat products packaged with different atmospheres. Food Microbiol. 15:639-650. [Google Scholar]
- 89.Hugas, M., M. Garriga, M. Pascual, M. T. Aymerich, and J. M. Monfort. 2002. Enhancement of sakacin K activity against Listeria monocytogenes in fermented sausages with pepper or manganese as ingredients. Food Microbiol. 19:519-528. [Google Scholar]
- 90.Hühne, K., L. Axelsson, A. Holck, and L. Kröckel. 1996. Analysis of the sakacin P gene cluster from Lactobacillus sake Lb674 and its expression in sakacin-negative Lb. sake strains. Microbiology 142:1437-1448. [DOI] [PubMed] [Google Scholar]
- 91.Hyronimus, B., C., C. Le Marrec, and M. C. Urdaci. 1996. Coagulin, a bacteriocin-like inhibitory substance produced by Bacillus coagulans I4. J. Appl. Microbiol. 85:42-50. [DOI] [PubMed] [Google Scholar]
- 92.Ingham, A. B., M. Ford, R. J. Moore, and M. Tizard. 2003. The bacteriocin piscicolin 126 retains antilisterial activity in vivo. J. Antimicrob. Chemother. 51:1365-1371. [DOI] [PubMed] [Google Scholar]
- 93.Ingham, A. B., K. W. Sproat, M. L. V. Tizard, and R. J. Moore. 2005. A versatile system for the expression of non modified bacteriocins in Escherichia coli. J. Appl. Microbiol. 98:67-683. [DOI] [PubMed] [Google Scholar]
- 94.Jack, R., J. R. Tagg, and B. Ray. 1995. Bacteriocins of gram-positive bacteria. Microbiol. Rev. 59:171-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jack, R. W., J. Wan, J. Gordon, K. Harmark, B. E. Davidson, A. J. Hillier, R. E. J. Wettenhall, M. W. Hickey, and M. J. Coventry. 1996. Characterization of the chemical and antimicrobial properties of piscicolin 126, a bacteriocin produced by Carnobacterium piscicola JG126. Appl. Environ. Microbiol. 62:2897-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Joerger, M. C., and T. R. Klaenhammer. 1990. Cloning, expression, and nucleotide sequence of the Lactobacillus helveticus 481 gene encoding the bacteriocin helveticin J. J. Bacteriol. 172:6339-6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Johnsen, L., G. Fimland, V. Eijsink, and J. Nissen-Meyer. 2000. Engineering increased stability in the antimicrobial peptide pediocin PA-1. Appl. Environ. Microbiol. 66:4798-4802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Johnsen, L., G. Fimland, D. Mantzilas, and J. Nissen-Meyer. 2004. Structure-function analysis of immunity proteins of pediocin-like bacteriocins: the C-terminal parts of immunity proteins are involved in specific recognition of cognate bacteriocins. Appl. Environ. Microbiol. 70:2647-2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Johnsen, L., B. Dalhus, I. Leiros, and J. Nissen-Meyer. 2005. 1.56 Å crystal structure of entA-im: a bacterial immunity protein conferring immunity to the antimicrobial activity of the pediocin-like bacteriocin enterocin A. J. Biol. Chem. 280:19045-19050. [DOI] [PubMed] [Google Scholar]
- 100.Johnsen, L., G. Fimland, and J. Nissen-Meyer. 2005. The C-terminal domain of pediocin-like antimicrobial peptides (class IIa bacteriocins) is involved in specific recognition of the C-terminal part of cognate immunity proteins and in determining the antimicrobial spectrum. J. Biol. Chem. 280:9243-9250. [DOI] [PubMed] [Google Scholar]
- 101.Kaiser, A. L., and T. J. Montville. 1996. Purification of the bacteriocin bavaricin MN and characterization of its mode of action against Listeria monocytogenes Scott A cells and lipid vesicles. Appl. Environ. Microbiol. 62:4529-4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kalmokoff, M. L., S. K. Banerjee, T. Cyr, M. A. Hefford, and T. Gleeson. 2001. Identification of a new plasmid-encoded sec-dependent bacteriocin produced by Listeria innocua 743. Appl. Environ. Microbiol. 67:4041-4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kanatani, K., Tahara, T. Oshimura, M. Sano, and K. Umezawa, C. 1995. Cloning and nucleotide sequence of the gene for acidocin 8912, a bacteriocin from Lactobacillus acidophilus TK8912. Lett. Appl. Microbiol. 21:384-386. [DOI] [PubMed] [Google Scholar]
- 104.Katla, T., T. Møretrø, I. M. Aasen, A. Holck, L. Axelsson, and K. Naterstad. 2001. Inhibition of Listeria monocytogenes in cold smoked salmon by addition of sakacin P and/or live Lactobacillus sakei cultures. Food Microbiol. 18:431-439. [Google Scholar]
- 105.Katla, T., T. Møretrø, I. Sveen, I. M. Aasen, L. Axelsson, L. M. Rorvik, and K. Naterstad. 2002. Inhibition of Listeria monocytogenes in chicken cold cuts by addition of sakacin P and sakacin P-producing Lactobacillus sakei. J. Appl. Microbiol. 93:191-196. [DOI] [PubMed] [Google Scholar]
- 106.Kaur, K., L. C. Andrew, D. S. Wishart, and J. C. Vederas. 2004. Dynamic relationships among type IIa bacteriocins: temperature effects on antimicrobial activity and on structure of the C-terminal amphipathic α helix as a receptor binding region. Biochemistry 43:9009-9020. [DOI] [PubMed] [Google Scholar]
- 107.Kawamoto, S., J. Shima, R. Sato, T. Eguchi, S. Ohmomo, J. Shibato, N. Horikoshi, K. Takeshita, and T. Sameshima. 2002. Biochemical and genetic characterization of mundticin KS, an antilisterial peptide produced by Enterococcus mundtii NFRI 7393. Appl. Environ. Microbiol. 68:3830-3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kazazic, M., Nissen-Meyer, J., and G. Fimland. 2002. Mutational analysis of the role of charged residues in target-cell binding, potency and specificity of the pediocin-like bacteriocin sakacin P. Microbiology 148:2019-2027. [DOI] [PubMed] [Google Scholar]
- 109.Kettenring, J. K., A. Malabarba, K. Vekey, and B. Cavalleri. 1990. Sequence determination of actagardin, a novel lantibiotic, by homonuclear 2D NMR spectroscopy. J. Antibiot. (Tokyo) 43:1082-1088. [DOI] [PubMed] [Google Scholar]
- 110.Klaenhammer, T. R. 1993. Genetics of bacteriocins produced by lactic acid bacteria. FEMS. Microbiol. Rev. 12:39-86. [DOI] [PubMed] [Google Scholar]
- 111.Kogler, H., M. Bauch, H. Fehlhaber, C. Griesinger, W. Schubert, and V. Teetz. 1991. NMR spectroscopic investigations on mersacidin, p. 113-122. In G. Jung and H.-G. Sahl (ed.), Nisin and novel lantibiotics. ESCOM, Leiden, The Netherlands.
- 112.Kristiansen, P. E., G. Fimland, D. Mantzilas, and J. Nissen-Meyer. 2005. Structure and mode of action of the membrane permeabilizing antimicrobial peptide pheromone plantaricin A. J. Biol. Chem. 280:22945-22950. [DOI] [PubMed] [Google Scholar]
- 113.Larsen, A. G., Vogensen, F. K., and J. Josephsen. 1993. Antimicrobial activity of lactic acid bacteria isolated from sour doughs: purification and characterization of bavaricin A, a bacteriocin produced by Lactobacillus bavaricus MI401. J. Appl. Bacteriol. 75:113-122. [DOI] [PubMed] [Google Scholar]
- 114.Lauková, A., and S. Czikková. 1999. The use of enterocin CCM 4231 in soy milk to control the growth of Listeria monocytogenes and Staphylococcus aureus. J. Appl. Microbiol. 87:182-186. [DOI] [PubMed] [Google Scholar]
- 115.Lauková, A., S. Czikková, S. Laczková, and P. Turek. 1999. Use of enterocin CCM 4231 to control Listeria monocytogenes in experimentally contaminated dry fermented Hornad salami. Int. J. Food Microbiol. 52:115-119. [DOI] [PubMed] [Google Scholar]
- 116.Leisner, J. J., G. G. Greer, and M. E. Stiles. 1996. Control of beef spoilage by a sulfide-producing Lactobacillus sake strain with bacteriocinogenic Leuconostoc gelidum UAL187 during anaerobic storage at 2°C. Appl. Environ. Microbiol. 62:2610-2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Le Marrec, C., B. Hyronimus, P. Bressollier, B. Verneuil, and M. C. Urdaci. 2000. Biochemical and genetic characterization of coagulin, a new antilisterial bacteriocin in the pediocin family of bacteriocins, produced by Bacillus coagulans. Appl. Environ. Microbiol. 66:5213-5220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Leroy, F., and L. De Vuyst. 1999. The presence of salt and a curing agent reduces bacteriocin production by Lactobacillus sakei CTC 494, a potential starter culture for sausage fermentation. Appl. Environ. Microbiol. 65:5350-5356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Loessner, M., S. Guenther, S. Steffan, and S. Scherer. 2003. A pediocin-producing Lactobacillus plantarum strain inhibits Listeria monocytogenes in a multispecies cheese surface microbial ripening consortium. Appl. Environ. Microbiol. 69:1854-1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Maftah, A., D. Renault, C. Vignoles, Y. Héchard, P. Bressollier, M. H. Ratinaud, Y. Cenatiempo, and R. Julien. 1993. Membrane permeabilization of Listeria monocytogenes and mitochondria by the bacteriocin mesentericin Y105. J. Bacteriol. 175:3232-3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Maguin, E., H. Prévost, S. D. Ehrlich, and A. Gruss. 1996. Efficient insertional mutagenesis in lactococci and other gram-positive bacteria. J. Bacteriol. 178:931-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Martinez, B., M. Fernandez, J. E. Suarez, and A. Rodríguez. 1999. Synthesis of lactococcin 972, a bacteriocin produced by Lactococcus lactis IPLA 972, depends on the expression of a plasmid-encoded bicistronic operon. Microbiology. 145:3155-3161. [DOI] [PubMed] [Google Scholar]
- 123.Martinez, J. M., M. I. Martinez, A. M. Suarez, C. Herranz, P. Casaus, L. M. Cintas, J. M. Rodriguez, and P. E. Hernandez. 1998. Generation of polyclonal antibodies of predetermined specificity against pediocin PA-1. Appl. Environ. Microbiol. 64:4536-4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mauriello, G., D. Ercolini, A. La Storia, A. Casaburi, and F. Villani. 2004. Development of polythene films for food packaging activated with an antilisterial bacteriocin from Lactobacillus curvatus 32Y. J. Appl. Microbiol. 97:314-322. [DOI] [PubMed] [Google Scholar]
- 125.McCormick, J. K., R. W. Worobo, and M. E. Stiles. 1996. Expression of the antimicrobial peptide carnobacteriocin B2 by a signal peptide-dependent general secretory pathway. Appl. Environ. Microbiol. 62:4095-4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Métivier, A., M. F. Pilet, X. Dousset, O. Sorokine, P. Anglade, M. Zagorec, J. C. Piard, D. Marion, Y. Cenatiempo, and C. Frémaux. 1998. Divercin V41, a new bacteriocin with two disulphide bonds produced by Carnobacterium divergens V41: primary structure and genomic organization. Microbiology 144:2837-2844. [DOI] [PubMed] [Google Scholar]
- 127.Miller, K. W., R. Schamber, Y. Chen, and B. Ray. 1998. Production of active chimeric pediocin AcH in Escherichia coli in the absence of processing and secretion genes from the Pediococcus pap operon. Appl. Environ. Microbiol. 64:14-20. (Erratum, 64:1587.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Miller, K. W., R. Schamber, O. Osmanagaoglu, and B. Ray. 1998. Isolation and characterization of pediocin AcH chimeric protein mutants with altered bactericidal activity. Appl. Environ. Microbiol. 64:1997-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Miller, K. W., P. Ray, T. Steinmetz, T. Hanekamp, and B. Ray. 2005. Gene organization and sequences of pediocin AcH/PA-1 production operons in Pediococcus and Lactobacillus plasmids. Lett. Appl. Microbiol. 40:56-62. [DOI] [PubMed] [Google Scholar]
- 130.Miller, M. B., and B. L. Bassler. 2001. Quorum sensing in bacteria. Annu. Rev. Microbiol. 55:165-199. [DOI] [PubMed] [Google Scholar]
- 131.Ming, X., G. Weber, J. Ayres, and W. Sandine. 1997. Bacteriocins applied to food packaging materials to inhibit Listeria monocytogenes on meats. J. Food Sci. 62:413-415. [Google Scholar]
- 132.Moll, G. N., S., Brul, W. N. Konings, and A. J. Driessen. 2000. Comparison of the membrane interaction and permeabilization by the designed peptide Ac-MB21-NH2 and truncated dermaseptin S3. Biochemistry 39:11907-11912. [DOI] [PubMed] [Google Scholar]
- 133.Montville, T. J., and Y. Chen. 1998. Mechanistic action of pediocin and nisin: recent progress and unresolved questions. Appl. Microbiol. Biotechnol. 50:511-519. [DOI] [PubMed] [Google Scholar]
- 134.Morisset, D., J. M. Berjeaud, D. Marion, C. Lacombe, and J. Frère. 2004. Mutational analysis of mesentericin Y105, an anti-Listeria bacteriocin, for determination of impact on bactericidal activity, in vitro secondary structure, and membrane interaction. Appl. Environ. Microbiol. 70:4672-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mulders, J. W., I. J. Boerrigter, H. S. Rollema, R. J. Siezen, and W. M. de Vos. 1991. Identification and characterization of the lantibiotic nisin Z, a natural nisin variant. Eur. J. Biochem. 201:581-584. [DOI] [PubMed] [Google Scholar]
- 136.Murry, M., and J. A. Richard. 1997. Comparative study of the antilisterial activity of nisin A and pediocin AcH in fresh ground pork stored aerobically at 5°C. J. Food Prot. 60:1534-1540. [DOI] [PubMed] [Google Scholar]
- 137.Natrajan, N., and B. Sheldon. 2000. Efficacy of nisin-coated polymer films to inactivate Salmonella typhimurium on fresh broiler skin. J. Food Prot. 63:1189-1196. [DOI] [PubMed] [Google Scholar]
- 138.Nes, I. F., D. B. Diep, L. S. Håvarstein, M. B. Brurberg, V. Eijsink, and H. Holo. 1996. Biosynthesis of bacteriocins in lactic acid bacteria. Antonie Leeuwenhoek 70:113-128. [DOI] [PubMed] [Google Scholar]
- 139.Nes, I. F., and V. G. H. Eijsink. 1999. Regulation of group II peptide bacteriocin synthesis by quorum-sensing mechanisms, p. 175-192. In G. M. Dunny and S. C. Winans (ed.), Cell-cell signalling in bacteria. American Society for Microbiology, Washington, D.C.
- 140.Nes, I. F., H. Holo, G. Fimland, H. H. Hauge, and J. Nissen-Meyer. 2002. Unmodified peptide-bacteriocins (class II) produced by lactic acid bacteria, p. 81-115. In C. J. Dutton, M. A. Haxell, H. A. I. McArthur, and R. G. Wax (ed.), Peptide antibiotics: discovery, modes of action and application. Marcel Dekker, New York, N.Y.
- 141.Nieto Lozano, J. C., J. Nissen-Meyer, K. Sletten, C. Pelaz, and I. F. Nes. 1992. Purification and amino acid sequence of a bacteriocin produced by Pediococcus acidilactici. J. Gen. Microbiol. 138:1985-1990. [DOI] [PubMed] [Google Scholar]
- 142.Nissen-Meyer, J., L. S. Håvarstein, H. Holo, K. Sletten, and I. F. Nes. 1993. Association of the lactococcin A immunity factor with the cell membrane: purification and characterization of the immunity factor. J. Gen. Microbiol. 139:1503-1509. [DOI] [PubMed] [Google Scholar]
- 143.Nissen-Meyer, J., H. H. Hauge, G. Fimland, V. G. H. Eijsink, and I. F. Nes. 2001. Ribosomally synthesized antimicrobial peptides by lactic acid bacteria: their function, structure, biogenesis, and their mechanism of action. Recent Res. Dev. Microbiol. 1:141-154. [Google Scholar]
- 144.O'Keeffe, T., C. Hill, and R. Ross. 1999. Characterization and heterologous expression of the genes encoding enterocin A production, immunity, and regulation in Enterococcus faecium DPC1146. Appl. Environ. Microbiol. 65:1506-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.O'Mahony, A. T., F. O'Sullivan, Y. Walsh, A. Vaughan, M. Maher, G. F. Fitzerald, and D. van Sindersen. 2000. Characterization and heterologous of antimicrobial producing lactic acid bacteria from malted barley. J. Inst. Brew. 106:403-410. [Google Scholar]
- 146.Panayach, R. 1998. Listeria monocytogenes: growth and control in vacuum-packaged ground beef. M.Sc. thesis. University of Alberta, Edmonton, Canada.
- 147.Papathanasopoulos, M. A., G. A. Dykes, A. M. Revol-Junelles, A. Delfour, A. von Holy, and J. W. Hastings. 1998. Sequence and structural relationships of leucocins A-, B-, and C-TA33a from Leuconostoc mesenteroides TA33a. Microbiology 144:1343-1348. [DOI] [PubMed] [Google Scholar]
- 148.Postma, P. W., J. W. Langeler, and G. R. Jacobson. 1993. Phosphoenolpyruvate:carbohydrate phosphotransferase systems of bacteria. Microbiol. Rev. 57:543-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Quadri, L. E., L. Z. Yan, M. E. Stiles, and J. C. Vederas. 1997. Effect of amino acid substitutions on the activity of carnobacteriocin B2. Overproduction of the antimicrobial peptide, its engineered variants, and its precursor in Escherichia coli. J. Biol. Chem. 272:3384-3388. [DOI] [PubMed] [Google Scholar]
- 150.Quadri, L. E. N., M. Sailer, K. L. Roy, J. C. Vederas, and M. E. Stiles. 1994. Chemical and genetic characterization of bacteriocins produced by Carnobacterium piscicola LV17B. J. Biol. Chem. 269:12204-12211. [PubMed] [Google Scholar]
- 151.Quadri, L. E. N., M. Sailer, M. R. Terebiznik, K. L. Roy, J. C. Vederas, and M. E. Stiles. 1995. Characterization of the protein conferring immunity to the antimicrobial peptide carnobacteriocin B2 and expression of carnobacteriocins B2 and BM1. J. Bacteriol. 177:1144-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Quadri, L. E. N., M. Kleerebezem, O. P. Kuipers, W. M. de Vos, K. L. Roy, J. C. Vederas, and M. E. Stiles. 1997. Characterization of a locus from Carnobacterium piscicola LV17B involved in bacteriocin production and immunity: evidence for global inducer-mediated transcriptional regulation. J. Bacteriol. 179:6163-6171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ramnath, M., S. Arous, A. Gravesen, J. W. Hastings, and Y. Héchard. 2004. Expression of mptC of Listeria monocytogenes induces sensitivity to class IIa bacteriocins in Lactococcus lactis. Microbiology 150:2663-2668. [DOI] [PubMed] [Google Scholar]
- 154.Ray, B., R. Schamber, and K. W. Miller. 1999. The pediocin AcH precursor is biologically active. Appl. Environ. Microbiol. 65:2281-2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Richard, C., D. Drider, K. Elmorjani, D. Marion, and H. Prévost. 2004. Heterologous expression and purification of active divercin V41, a class IIa bacteriocin encoded by a synthetic gene in Escherichia coli. J. Bacteriol. 186:4276-4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Robichon, D., E. Gouin, M. Débarbouillé, P. Cossart, Y. Cenatiempo, and Y. Héchard. 1997. The rpoN (σ54) gene from Listeria monocytogenes is involved in resistance to mesentericin Y105, an antibacterial peptide from Leuconostoc mesenteroides. J. Bacteriol. 179:7591-7594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Rodríguez, E., J. Calzada, J. L. Arqués, J. M. Rodríguez, M. Nuñez, and M. Medina. 2005. Antimicrobial activity of pediocin-producing Lactococcus lactis on Listeria monocytogenes, Staphylococcus aureus and Escherichia coli O157:H7 in cheese. Int. Dairy J. 15:51-57. [Google Scholar]
- 158.Rodriguez, J. M., M. I. Martinez, N. Horn, and H. M. Dodd. 2003. Heterologous production of bacteriocins by lactic acid bacteria. Int. J. Food Microbiol. 80:101-116. [DOI] [PubMed] [Google Scholar]
- 159.Rogers, S., P. Peiris, K. Kailasapathy, and J. Cox. 2003. Inhibition of non-proteolytic Clostridium botulinum with lactic acid bacteria in extended shelf-life cook-chill soups. Food Biotechnol. 17:39-52. [Google Scholar]
- 160.Rose, N. L., P. Sporns, M. E. Stiles, and L. M. McMullen. 1999. Inactivation of nisin by glutathione in fresh meat. J. Food Sci. 64:759-762. [Google Scholar]
- 161.Rose, N. L., P. Sporns, H. M. Dodd, M. J. Gasson, F. A. Mellon, and L. M. McMullen. 2003. Involvement of dehydroalanine and dehyrobutyrine in the addition of glutathione to nisin. J. Agric. Food Chem. 51:3174-3178. [DOI] [PubMed] [Google Scholar]
- 162.Ruiz-Barba, J. L., D. P. Cathcar, P. J. Warner, and R. Jimenez-Diaz. 1994. Use of Lactobacillus plantarum LPC01, a bacteriocin producer, as a starter culture in Spanish-style green olive fermentations. Appl. Environ. Microbiol. 60:2059-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Saavedra, L., P. Castellano, and F. Sesma. 2004. Purification of bacteriocins produced by lactic acid bacteria. Methods Mol. Biol. 268:331-336. [DOI] [PubMed] [Google Scholar]
- 164.Schillinger, U., and F. K. Lüke. 1989. Antibacterial activity of Lactobacillus sake isolated from meat. Appl. Environ. Microbiol. 55:1901-1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Schnell, N., K. D. Entian, F. Gotz, T. Horner, R. Kellner, and G. Jung. 1989. Structural gene isolation and prepeptide sequence of gallidermin, a new lanthionine containing antibiotic. FEMS Microbiol. Lett. 49:263-267. [DOI] [PubMed] [Google Scholar]
- 166.Schoeman, H., M. A. Vivier, M. Du Toit, L. M. T. Dicks, and I. S. Pretorius. 1999. The development of bactericidal yeast strains by expressing the Pediococcus acidilactici pediocin gene (pedA) in Saccharomyces cerevisiae. Yeast 15:647-656. [DOI] [PubMed] [Google Scholar]
- 167.Schved, F., A. Lalazar, Y. Henis, and B. J. Juven. 1993. Purification, partial characterization and plasmid-linkage of pediocin SJ-1, a bacteriocin produced by Pediococcus acidilactici. J. Appl. Bacteriol. 74:67-77. [DOI] [PubMed] [Google Scholar]
- 168.Simon, L., C. Frémaux, Y. Cenatiempo, and J. M. Berjeaud. 2002. Sakacin G, a new type of antilisterial bacteriocin. Appl. Environ. Microbiol. 12:6416-6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sprules, T., K. E. Kawulka, A. C. Gibbs, D. S. Wishart, and J. C. Vederas. 2004. NMR solution structure of the precursor for carnobacteriocin B2, an antimicrobial peptide from Carnobacterium piscicola. Eur. J. Biochem. 271:1748-1756. [DOI] [PubMed] [Google Scholar]
- 170.Sprules, T., K. E. Kawulka, and J. C. Vederas. 2004. NMR solution structure of ImB2, a protein conferring immunity to antimicrobial activity of the type IIa bacteriocin, carnobacteriocin B2. Biochemistry 43:11740-11749. [DOI] [PubMed] [Google Scholar]
- 171.Stiles, M. E. 1996. Biopreservation by lactic acid bacteria. Antonie Leeuwenhoek 70:331-345. [DOI] [PubMed] [Google Scholar]
- 172.Tahiri, I., M. Desbiens, R. Benech, E. Kheadr, C. Lacroix, S. Thibault, D. Ouellet, and I. Fliss. 2004. Purification, characterization and amino acid sequencing of divergicine M35: a novel class IIa bacteriocin produced by Carnobacterium divergens M35. Int. J. Food Microbiol. 97:123-136. [DOI] [PubMed] [Google Scholar]
- 173.Tichaczek, P. S., R. F. Vogel, and W. P. Hammes. 1993. Cloning and sequencing of curA encoding curvacin A, the bacteriocin produced by Lactobacillus curvatus LTH1174. Arch. Microbiol. 160:279-283. [DOI] [PubMed] [Google Scholar]
- 174.Tomita, H., S. Fujimoto, K. Tanimoto, and Y. Ike. 1996. Cloning and genetic organization of the bacteriocin 31 determinant encoded on the Enterococcus faecalis pheromone-responsive conjugative plasmid pYI17. J. Bacteriol. 178:3585-3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Uhart, M., S. Ravishankar, and N. D. Maks. 2004. Control of Listeria monocytogenes with combined antimicrobials on beef franks stored at 4°C. J. Food Prot. 67:2296-2301. [DOI] [PubMed] [Google Scholar]
- 176.Uteng, M., H. H. Hauge, P. R. Markwick, G. Fimland, D. Mantzilas, J. Nissen-Meyer, and C. Muhle-Goll. 2003. Three-dimensional structure in lipid micelles of the pediocin-like antimicrobial peptide sakacin P and a sakacin P variant that is structurally stabilized by an inserted C-terminal disulfide bridge. Biochemistry 42:11417-11426. [DOI] [PubMed] [Google Scholar]
- 177.Vadyvaloo, V., S. Arous, A. Gravesen, Y. Héchard, R. Chauhan-Haubrock, J. W. Hastings, and M. Rautenbach. 2004. Cell-surface alterations in class IIa bacteriocin-resistant Listeria monocytogenes strains. Microbiology 150:3025-3033. [DOI] [PubMed] [Google Scholar]
- 178.Vadyvaloo, V., J. W. Hastings, M. J. van der Merwe, and M. Rautenbach. 2002. Membranes of class IIa bacteriocin-resistant Listeria monocytogenes cells contain increased levels of desaturated and short-acyl-chain phosphatidylglycerols. Appl. Environ. Microbiol. 68:5223-5230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Van Belkum, M. J., R. W. Worobo, and M. E. Stiles. 1997. Double-glycine-type leader peptides direct secretion of bacteriocins by ABC transporters: colicin V secretion in Lactococcus lactis. Mol. Microbiol. 23:1293-1301. [DOI] [PubMed] [Google Scholar]
- 180.Van Reenen, C. A., L. M. T. Dicks, and M. L. Chikindas. 1998. Isolation, and purification and partial characterization of plantaricin 423, a bacteriocin produced by Lactobacillus plantarum. J. Appl. Microbiol. 84:1131-1137. [DOI] [PubMed] [Google Scholar]
- 181.Van Reenen, C. A., M. L. Chikindas, W. H. Van Zyl, and L. M. T. Dicks. 2003. Characterization and heterologous expression of class IIa bacteriocin, plantaricin 423 from Lactobacillus plantarum in Saccharomyces cerevisiae. Int. J. Food Microbiol. 81:29-40. [DOI] [PubMed] [Google Scholar]
- 182.Vaughan, A., V. G. H. Eijsink, T. F. O'Sullivan, K. O'Hanlon, and D. van Sindersen. 2001. An analysis of bacteriocins produced by lactic acid bacteria isolated from malted barley. J. Appl. Microbiol. 91:131-138. [DOI] [PubMed] [Google Scholar]
- 183.Vaughan, A., V. G. Eijsink, and D. van Sinderen. 2003. Functional characterization of a composite bacteriocin locus from malt isolates of Lactobacillus sakei 5. Appl. Environ. Microbiol. 69:7194-7203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Venema, K., R. E. Haverkort, T. Abee, A. J. Haandrikman, K. J. Leenhouts, L. de Leij, G. Venema, and J. Kok. 1994. Mode of action of LciA, the lactococcin A immunity protein. Mol. Microbiol. 14:521-532. [DOI] [PubMed] [Google Scholar]
- 185.Verluyten, J., R. Leroy, and L. de Vuyst. 2004. Effects of different spices used in production of fermented sausages on growth of and curvacin A production by Lactobacillus curvatus LTH 1174. Appl. Environ. Microbiol. 70:4807-4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wachsman, M. B., M. E. Farias, E. Takeda F. Sesma, A. P. de Ruiz Holgado, R. A. de Torres, and C. E. Coto. 1999. Antiviral activity of enterocin CRL35 against herpes viruses. Int. J. Antimicrob. Agents 12:293-299. [DOI] [PubMed] [Google Scholar]
- 187.Wachsman, M. B., V. Castilla, A. P. de Ruiz Holgado, R. A. de Torres, F. Sesma, and C. E. Coto. 2003. Enterocin CRL35 inhibits late stages of HSV-1 and HSV-2 replication in vitro. Antivir. Res. 58:17-24. [DOI] [PubMed] [Google Scholar]
- 188.Wan, J., K. Harmark, B. E. Davidson, A. J. Hillier, J. B. Gordon, A. Wilcock, M. W. Hickey, and M. J. Coventry. 1997. Inhibition of Listeria monocytogenes by piscicolin 126 in milk and Camembert cheese manufactured with a thermophilic starter. J. Appl. Microbiol. 82:273-280. [DOI] [PubMed] [Google Scholar]
- 189.Wang, Y., M. E. Henz, N. L. Gallagher, S. Chai, A. C. Gibbs, L. Z. Yan, M. E. Stiles, D. S. Wishart, and J. C. Vederas. 1999. Solution structure of carnobacteriocin B2 and implications for structure-activity relationships among type IIa bacteriocins from lactic acid bacteria. Biochemistry 38:15438-15447. [DOI] [PubMed] [Google Scholar]
- 190.Woodruff, W. A., J. Novak, and P. W. Caufield. 1998. Sequence analysis of mutA and mutM genes involved in the biosynthesis of the lantibiotic mutacin II in Streptococcus mutans. Gene 206:37-43. [DOI] [PubMed] [Google Scholar]
- 191.Yamazaki, K., M. Suzuki, Y. Kawai, N. Inoue, and T. J. Montville. 2003. Inhibition of Listeria monocytogenes in cold-smoked salmon by Carnobacterium piscicola CS526 isolated from frozen surimi. J. Food Prot. 66:1420-1425. [DOI] [PubMed] [Google Scholar]
- 192.Yamazaki, K., M. Suzuki, Y. Kawai, N. Inoue, and T. J. Montville. 2005. Purification and characterization of a novel class IIa bacteriocin, piscicolin CS526, from surimi-assoociated Carnobacterium piscicola CS526. Appl. Environ. Microbiol. 71:554-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Yan, L. Z., A. C. Gibbs, M. E. Stiles, D. S. Wishart, and J. C. Vederas. 2000. Analogues of bacteriocins: antimicrobial specificity and interactions of leucocin A with its enantiomer, carnobacteriocin B2, and truncated derivatives. J. Med. Chem. 43:4579-8451. [DOI] [PubMed] [Google Scholar]
- 194.Yildrim, Z., and M. G. Johnson. 1998. Characterization and antimicrobial spectrum of bifidocin B, a bacteriocin produced by Bifidobacterium bifidum NCFB 1454. J. Food Prot. 61:47-51. [DOI] [PubMed] [Google Scholar]
- 195.Yildrim, Z., and M. G. Johnson. 1999. Purification, amino acid sequence and mode of action of bifidocin B produced by Bifidobacterium bifidum NCFB 1454. J. Appl. Microbiol. 86:45-54. [DOI] [PubMed] [Google Scholar]