Abstract
Cross-reactive responses elicited by exposure to nontuberculous mycobacteria often confound the interpretation of antemortem tests for Mycobacterium bovis infection of cattle. The use of specific proteins (e.g., ESAT-6, CFP-10, and MPB83), however, generally enhances the specificity of bovine tuberculosis tests. While genes for these proteins are absent from many nontuberculous mycobacteria, they are present in M. kansasii. Instillation of M. kansasii into the tonsillar crypts of calves elicited delayed-type hypersensitivity and in vitro gamma interferon and nitrite concentration responses of leukocytes to M. avium and M. bovis purified protein derivatives (PPDs). While the responses of M. kansasii-inoculated calves to M. avium and M. bovis PPDs were approximately equivalent, the responses of M. bovis-inoculated calves to M. bovis PPD exceeded their respective responses to M. avium PPD. The gamma interferon and nitrite responses of M. kansasii-inoculated calves to recombinant ESAT-6-CFP-10 (rESAT-6-CFP-10) exceeded corresponding responses of noninoculated calves as early as 15 and 30 days after inoculation, respectively, and persisted throughout the study. The gamma interferon and nitrite responses of M. bovis-inoculated calves to rESAT-6-CFP-10 exceeded the corresponding responses of M. kansasii-inoculated calves beginning 30 days after inoculation. By using a lipoarabinomannan-based enzyme-linked immunosorbent assay, specific serum antibodies were detected as early as 50 days after challenge with M. kansasii. By a multiantigen print immunoassay and immunoblotting, serum antibodies to MPB83, but not ESAT-6 or CFP-10, were detected in M. kansasii-inoculated calves; however, responses to MPB83 were notably weaker than those elicited by M. bovis infection. These findings indicate that M. kansasii infection of calves elicits specific responses that may confound the interpretation of bovine tuberculosis tests.
Mycobacterium bovis, a member of the M. tuberculosis complex, has a wide host range, is infectious to humans, and is the most common cause of tuberculosis (TB) in cattle. The presence of wildlife reservoirs (e.g., various deer species in the United Kingdom and the United States, the Eurasian badger in the United Kingdom, and brush-tailed possums in New Zealand) hinder efforts to eradicate TB within cattle and captive deer herds in developed countries. In Africa, bovine TB is rapidly spreading through Cape buffalo populations as well as other hoofstock, nonhuman primates, and various mammalian predators (23). Most notably, up to 90% of lions in areas of TB endemicity are M. bovis infected, likely due to infection rate amplification from predation on infected prey. In humans, recent TB outbreaks in several U.S. cities are linked with the ingestion of M. bovis-infected, nonpasteurized cheese from Mexico (47). Regardless of the host, the implications of TB diagnosis include regulatory action, public health concerns, movement restriction, isolation of affected individuals, and serious health issues, thus emphasizing the need for accurate diagnosis. The tests most widely used for the detection of TB in humans and cattle include the measurement of delayed-type hypersensitivity (i.e., skin testing) to purified protein derivative (PPD) and an in vitro assay for gamma interferon (IFN-γ) concentrations produced in response to PPD stimulation (Quantiferon; Cellestis, Inc., Valencia, Calif.; and Bovigam; Pfizer Animal Health, Kalamazoo, Mich.). A major limitation of PPD-based tests is cross-reactivity due to responses induced upon exposure to related bacteria, principally other nontuberculous “environmental” mycobacteria.
Mycobacterium kansasii is not included in the M. tuberculosis complex, yet it may cause disease in otherwise healthy individuals, albeit infrequently, that is indistinguishable clinically from M. tuberculosis infection (2, 3, 14, 15). A culture positive for M. kansasii from humans is not indicative of disease, as the organism may be isolated from healthy individuals, and human-to-human transmission is not documented (1). A relatively high rate of human infection has been reported in certain locales within the United States, central Europe, southeast United Kingdom, and southern part of The Netherlands, potentially associated with heavy air pollution (1, 2). As with humans, M. kansasii infection of cattle is exceedingly rare and often associated with lesions of the respiratory tract and associated lymph nodes, diagnosed postmortem (B. Harris, personal observations). Disease management of M. kansasii infection is often complicated by the false interpretation of tests presumptive for M. tuberculosis complex, evoking further diagnostic and epidemiological investigations and action. The use of defined antigens (e.g., early secretory antigenic target-6 [ESAT-6] and culture filtrate protein-10 [CFP-10]) generally enhances the differentiation of immune responses to M. tuberculosis complex organisms from those elicited by nontuberculous mycobacteria (2, 5, 8, 33, 34, 36, 40, 45). The esat-6 and cfp-10 genes are located in region of difference 1, an area of the virulent M. tuberculosis complex genome not present in the vaccine strain M. bovis bacille Calmette-Guérin (BCG) and most other nontuberculous mycobacteria. Unfortunately, the esat-6 and cfp-10 genes as well as the esat-6 family genes TB10.4, TB10.3, and TB12.9 are also present in M. kansasii and are of significant homology (88% to 90% nucleotide homology and >95% amino acid homology) to the respective genes within M. tuberculosis complex organisms (2, 18, 19, 21, 37, 39). Additionally, M. kansasii contains a gene encoding MPB83 (41), a cell surface immunodominant antibody target used for the “specific” diagnosis of M. bovis infection of cattle (28) and several other wildlife reservoirs (e.g., the Eurasian badger [20] and white-tailed deer [46]). It may be anticipated that sensitization and/or infection with M. kansasii would result in antibody and cell-mediated responses that potentially confound the interpretation of TB tests based on either specific antigens (i.e., ESAT-6, CFP-10, or MPB83) or complex antigens (e.g., tuberculins, whole-cell sonicates, or culture filtrates). Indeed, humans with clinical disease resulting from M. kansasii infection have detectable IFN-γ concentration responses to recombinant and peptide mixes of ESAT-6 and CFP-10, albeit at a much lower rate and level than M. tuberculosis-infected individuals (4). The impact of M. kansasii sensitization without the induction of clinical disease on TB diagnostic tests, however, is unclear.
The primary objective of the present study was to evaluate the immune response induced by the inoculation of calves with the subtype of M. kansasii associated with clinical disease in humans, subtype 1 (1, 32), to determine both the virulence in cattle and the potential for interference with the interpretation of bovine TB tests. A large challenge dose was used for M. kansasii inoculation to increase the potential for the generation of a lesion(s) and the induction of an immune response to mycobacterial antigens. In regard to adaptive immunity, the primary goal was the qualitative assessment of the response particularly to Mycobacterium TB-specific antigens, as a cross-reactive response may condemn an individual or a herd of animals. Cell-mediated and antibody responses against defined mycobacterial antigens were compared to those elicited by M. bovis infection. The implications of these studies may be important for the interpretation of new or existing TB tests, where exposure to M. kansasii without elicitation of disease evokes a potentially confounding response.
MATERIALS AND METHODS
Calves, challenge inoculum, and necropsy.
Twenty-three male Holstein calves of approximately 3 months of age were obtained from a TB-free herd in Wisconsin and housed at the National Animal Disease Center in Ames, Iowa, according to institutional guidelines and approved animal care and use protocols. Treatment groups included M. bovis-inoculated (n = 9), M. kansasii-inoculated (n = 4), and noninoculated (n = 10) calves. For experimental infection, the challenge inoculum (diluted in 0.2 ml of 0.15 M phosphate-buffered saline, pH 7.2 [PBS]) was instilled directly into both tonsillar crypts of sedated calves, as described for the inoculation of white-tailed deer (31). Challenge dosages were 4 × 104 CFU for M. bovis and 4 × 108 CFU for M. kansasii. The strain of M. bovis used for the challenge inoculum (95-1315; USDA, Animal Plant and Health Inspection Service [APHIS]) was originally isolated from a white-tailed deer in Michigan (38). The strain of M. kansasii (03-6931; USDA, APHIS) was originally isolated from a pyogranulomatous lymph node detected upon routine TB surveillance at an abattoir in Pennsylvania. Challenge inocula were prepared as previously described (7). Two weeks prior to inoculation, the cattle were moved from an outdoor pen into climate-controlled rooms within either biosafety level 3 (M. bovis-inoculated cattle) or biosafety level 2 (M. kansasii-inoculated and noninoculated cattle, with separate facilities for each of these two groups) confinement facilities. Approximately 4.5 months after inoculation, all cattle were euthanized by intravenous injection of sodium pentobarbital (Fort Dodge Animal Health, Fort Dodge, Iowa) and examined. Various tissues were collected (tonsil, lung, liver, spleen, and lung-associated, head-associated, mesenteric, and prefemoral lymph nodes) for bacteriologic culture and microscopic examination as described previously (7, 45).
M. kansasii genotypic characterization.
The 16S-23S rRNA gene internal transcribed spacer (ITS) region of strain 03-6931 was amplified by using the primers ITS-F (5′-AAG TCG TAA CAA GGT ARC CG-3′) and ITS-R (5′-YYG CCA AGG CAT CCA CC-3′). For this step, 2 μl of heat-deactivated bacteria (heated to 80°C for 30 min) was added to the PCR mix containing 10× buffer, 5% dimethyl sulfoxide (Sigma, St. Louis, Mo.), 1.5 mM MgCl2, 0.2 mM each deoxynucleoside triphosphate, 0.4 mM of each primer, and 1.25 U Taq polymerase (Invitrogen, Carlsbad, Calif.). Thermocycling conditions were as follows: an initial denaturation step at 94°C for 3 min followed by 30 cycles of denaturation at 94°C for 1 min, annealing at 55°C for 2 min, with extension at 72°C for 2 min, and a final extension step at 72°C for 5 min. Sequencing was performed with a CEQ 8000 cycle sequencer (Beckman-Coulter, Fullerton, Calif.). The complete ITS region was aligned with previously published ITS sequences from the five subtypes of M. kansasii (1) using the MegAlign v. 6.1 program (DNASTAR, Inc., Madison, Wis.).
Tuberculin skin test procedures.
Two months after inoculation, cattle were tested for in vivo responsiveness to mycobacterial antigens using the caudal fold tuberculin skin test (CFT). The thickness of the right caudal fold (the fold of skin on either side of the tail head) was measured using calipers, injected intradermally with 0.1 ml (100 μg) of M. bovis-derived PPD, and measured again 72 h after injection. Four months after inoculation, all inoculated cattle and five of the noninoculated cattle received a comparative cervical skin tuberculin test (CCT). The other five noninoculated calves received a CCT 1 week after the CFT; however, responses (i.e., serological, in vitro cellular, and in vivo cellular) did not differ between the two groups of noninoculated calves, so for the purposes of the present study, responses of the noninoculated calves are included in the same group. For the CCT, 0.1 ml (100 μg) of M. bovis PPD and 0.1 ml (40 μg) of M. avium PPD were administered at separate clipped sites in the midcervical region, according to guidelines described in the USDA, APHIS circular 91-45-01(39a). As with the CFT, skin thickness was measured with calipers prior to PPD administration and 72 h after injection. All PPDs for skin test procedures were obtained from the Brucella and Mycobacterial Reagents section of the National Veterinary Services Laboratory, Ames, Iowa.
Mononuclear cell culture and blastogenesis.
Peripheral blood mononuclear cells (PBMC) were isolated from buffy coat fractions of peripheral blood collected in 2× acid citrate dextrose (9). Wells of 96-well round-bottom microtiter plates (Falcon; Becton-Dickinson, Lincoln Park, N.J.) were seeded with 2 × 105 mononuclear cells in a total volume of 200 μl per well. The medium was RPMI 1640 supplemented with 25 mM HEPES buffer, 100 units/ml penicillin, 0.1 mg/ml streptomycin, 50 μM 2-mercaptoethanol (Sigma), and 10% (vol/vol) fetal bovine serum. Wells contained medium plus 5 μg/ml M. bovis PPD (CSL Limited), 5 μg/ml M. avium PPD (CSL Limited), 1 μg/ml rESAT-6-CFP-10, or medium alone (no stimulation). Leukocyte cultures were incubated for 6 days at 37°C in 5% CO2 in air, as described previously by our group (44, 45) and others (40). After 6 days, 0.5 μCi of [methyl-3H]thymidine (specific activity, 6.7 Ci mmol−1; Amersham Life Science, Arlington Heights, Ill.) in 50 μl of medium was added to each well, and cells were incubated for an additional 20 h. Well contents were harvested onto fiber filters with a 96-well plate harvester (EG & G Wallac, Gaithersburg, Md.), and the incorporated radioactivity (in counts per minute [cpm]) was measured by liquid scintillation counting. Treatments were run in triplicate. Data are presented as the mean (±standard error) cpm for stimulated samples minus the mean cpm for samples receiving medium alone (i.e., Δcpm) for each treatment group (i.e., noninoculated and M. kansasii- and M. bovis-inoculated cattle).
Griess reaction assay.
Culture supernatants (72 h) were harvested from replicate cultures (see the mononuclear cell culture procedure in the preceding paragraph) for analysis of nitrite. The amount of nitrite, the stable oxidation product of nitric oxide (NO), within culture supernatants is a correlate of the amount of NO produced by cells in culture. Nitrite was measured using the Griess reaction assay (35), performed in 96-well microtiter plates (Immunolon 2; Dynatech Laboratories, Inc., Chantilly, VA). Culture supernatant (100 μl) was mixed with 100 μl of Griess reagent (0.5% sulfanilamide; Sigma) in 2.5% phosphoric acid (Mallinckrodt Chemicals, Inc., Paris, Kentucky) and 0.05% N-(1-naphthyl) ethylenediamine dihydrochloride (Sigma). The mixture was incubated at 21°C for 10 min. Absorbencies of test and standard samples at 550 nm were measured using an automated enzyme-linked immunosorbent assay (ELISA) plate reader. Samples were diluted in culture medium (RPMI 1640 medium with 2 mM l-glutamine and 10% [vol/vol] fetal bovine serum). Absorbencies of standards and test samples were converted to ng/ml of nitrite by comparing them to absorbencies of sodium nitrite standards (Fisher Chemicals, Fair Lawn, N.J.) within a linear curve fit. Assays were run on three sets of pooled triplicates for each treatment.
IFN-γ assay.
Heparinized blood samples were dispensed in 1.5-ml aliquots into individual wells of a 24-well plate (Falcon 353047; Becton Dickinson and Company, Franklin Lakes, N.J.). Wells contained whole blood plus 20 μg/ml M. bovis PPD (Pfizer Animal Health), 20 μg/ml M. avium PPD (Pfizer Animal Health), and 1 μg/ml rESAT-6-CFP-10 (29, 45) or medium alone (no stimulation). Blood cultures were incubated for 24 h, and plasma was harvested and stored at −80°C. IFN-γ concentrations in stimulated plasma were determined using a commercial ELISA-based kit (Bovigam; Pfizer Animal Health). Absorbencies of standards and test samples were read at 450 nm using an ELISA plate reader (Molecular Devices, Menlo Park, Calif.). Concentrations (ng/ml) of test samples were quantified by comparing the absorbencies of test samples with the absorbencies of standards within a linear curve fit. Duplicate samples for individual treatments were analyzed.
ELISA of M. bovis LAM.
Lipoarabinomannan (LAM)-enriched mycobacterial antigen was prepared from M. bovis strain 95-1315 as described previously (42). Briefly, bacilli harvested from 4-week-old cultures were sonicated in PBS, further disrupted with 0.1- to 0.15-mm glass beads (Biospec Products, Bartlesville, Okla.) in a bead beater (Biospec Products), centrifuged, filtered (0.22 μm), digested in a 1-mg/ml proteinase K (Roche Molecular Biochemicals, Indianapolis, Ind.) solution (50 mM Tris, 1 mM CaCl2 buffer, pH 8.0) for 1 h at 50°C, and stored at −20°C. Immulon II 96-well microtiter plates (Dynatech, Chantilly, Va.) were coated with 100 μl/well (8 μg/well) antigen diluted in a carbonate/bicarbonate coating buffer (pH 9.6). Antigen-coated plates, including control wells containing coating buffer alone, were incubated for 20 h at 4°C. Plates were washed three times with 200 μl/well PBS containing 0.05% Tween 20 (PBST; Sigma) and blocked with 200 μl/well of a commercial milk diluent/blocking solution (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, Md.). Wells were washed nine times with 200 μl/well PBST and incubated for 1 h at 37°C with 100 μl/well of peroxidase-labeled antibovine immunoglobulin G heavy and light chains (goat origin; Kirkegaard & Perry Laboratories, Inc.) diluted 1:10,000 in PBS plus 0.1% gelatin. Wells were washed nine times with 200 μl/well PBST and incubated for 4.5 min at room temperature with 100 μl/well of SureBlue Microwell peroxidase substrate (Kirkegaard & Perry Laboratories, Inc.). The reaction was stopped by the addition of 100 μl/well of 0.18 M sulfuric acid, and the absorbency (at 450 nm) of individual wells was measured using an automated ELISA plate reader (Molecular Devices, Menlo Park, Calif.). The sample/positive (S/P) ratios of test samples were calculated from absorbency values using the following formulai sample − negative control/positive control − negative control.
Immunoblot assay.
Electrophoresis and immunoblot assays were performed using previously described procedures (6) with the following modifications. Comparisons of the reactivities of serial serum samples against rMPB83 antigen were conducted using a slot blotting device (Bio-Rad, Richmond, Calif.). Antigen was electrophoresed through preparative 12% (wt/vol) polyacrylamide gels and transferred to nitrocellulose filters. These filters were placed in a blocking solution consisting of PBST and 2% (wt/vol) bovine serum albumin (PBST-BSA). After being blocked, the filters were placed into the slot blot device, and individual sera, diluted 1:400 in PBST-BSA, were added to independent slots. After a 2-h incubation with gentle rocking, blots were washed three times with PBST and incubated with peroxidase-conjugated antibovine immunoglobulin G (Kirkegaard & Perry Laboratories) diluted 1:40,000 in PBST-BSA for 1.5 h. Blots were again washed three times with PBST and developed for chemiluminescence in SuperSignal detection reagent (Pierce Chemical Co.).
MAPIA.
The following recombinant antigens of M. bovis were purified to near homogeneity as polyhistidine-tagged proteins (Rv designations in parentheses are according to the classification of Cole et al. [12]): ESAT-6 (Rv3875) and CFP-10 (Rv3874) (produced at the Statens Serum Institut, Copenhagen, Denmark) and MPB59 (Rv1886c), MPB64 (Rv1980c), MPB70 (Rv2875), and MPB83 (Rv2873) (produced at the Veterinary Sciences Division, Stormont, Belfast, United Kingdom) (25). Alpha-crystallin (Acr1 [Rv3391]) and the 38-kDa protein PstS1 (Rv0934) were purchased from Standard Diagnostics, Seoul, Republic of Korea. The polyprotein fusions CFP-10-ESAT-6 and Acr1-MPB83 were constructed by overlapping PCR, using gene-specific oligonucleotides to amplify the genes from M. tuberculosis H37Rv chromosomal DNA. The fused polygene PCR products were cloned into the pMCT6 Escherichia coli expression vector using SmaI/BamHI restriction enzymes. The polyproteins were purified to near homogeneity by exploiting the polyhistidine tag encoded by the vector. The M. bovis culture filtrate was obtained from a field strain of M. bovis (T/91/1378; Veterinary Sciences Division, Belfast, United Kingdom) cultured in synthetic Sauton's medium. The multiantigen print immunoassay (MAPIA) was performed as described previously (26). Briefly, antigens were immobilized on a nitrocellulose membrane (Schleicher & Schuell, Keene, N.H.) at a protein concentration of 0.05 mg/ml by using a semiautomated airbrush printing device (Linomat IV, Camag Scientific, Inc., Wilmington, Del.). The membrane was cut perpendicular to the antigen bands into 4-mm-wide strips. Strips were blocked for 1 h with 1% nonfat skimmed milk in PBS with 0.05% Tween 20 and then incubated for 1 h, with serum samples diluted 1:40 in blocking solution. After they were washed, the strips were incubated overnight with peroxidase-conjugated protein G (Sigma) diluted 1:1,000, followed by another washing step. Cattle antibodies bound to printed antigens were visualized with 3,3′,5,5′-tetramethyl benzidine (Kirkegaard & Perry Laboratories). Results were also evaluated by semiquantitative densitometry using Scion Image (version Beta 4.0.2).
Statistics.
Data were analyzed by one-way analysis of variance followed by a Tukey-Kramer multiple-comparison test using a commercially available statistics program (InStat 2.00; GraphPAD Software, San Diego, Calif.). Pearson's product-moment correlations were computed between IFN-γ concentrations in M. avium and M. bovis PPD-stimulated plasma samples.
RESULTS
Mycobacterium kansasii genotyping and infection status.
The ITS sequence obtained for the field strain was a 100% match to the ITS region from M. kansasii subtype 1 (1), confirming that the isolate used in these studies belongs to the subgroup most commonly associated with clinical disease in humans. Despite these associations (i.e., between the subtype 1 group and the original isolation from a pyogranuloma), neither gross or microscopic lesions nor mycobacteria were detected in tissues from 3-month-old calves whose tonsillar crypts had been inoculated with 4 × 108 CFU of M. kansasii. In sharp contrast, M. bovis inoculation resulted in tuberculous lesions in tonsils, lungs, liver, and medial retropharyngeal, mandibular, parotid, mediastinal, tracheobronchial, and mesenteric lymph nodes (all M. bovis-inoculated animals had lesions, but each animal did not have lesions in each of these organs). Lesions and mycobacteria were not detected in tissues from noninoculated (control) animals.
Delayed-type-hypersensitivity response.
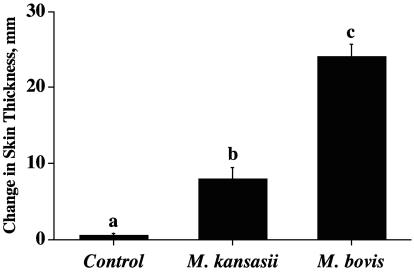
Exposure of cattle to nontuberculous mycobacteria may result in false-positive tuberculin skin test reactions (22). As expected, the injection of M. bovis PPD for the CFT resulted in palpable reactions in all M. kansasii-inoculated cattle, indicating sensitization without persistent infection (i.e., no lesions were detected upon necropsy). All M. bovis-inoculated and 1/10 noninoculated animals also had palpable reactions to M. bovis PPD upon CFT. Ranking of the mean responses by each of the groups resulted in M. bovis-inoculated cattle having stronger reactions than M. kansasii-inoculated cattle and M. kansasii cattle having stronger reactions than noninoculated cattle (Fig. 1A). Within the United States, cattle with CFT reactions are considered “TB suspects” and retested using either the CCT or Bovigam assay (Pfizer Animal Health). All M. bovis-infected cattle in the present study were determined to be “reactors” by CCT (Table 1). All M. kansasii-inoculated and noninoculated cattle were considered negative by CCT, as determined by plotting responses on the USDA-approved scattergram for the interpretation of the CCT results (i.e., APHIS, Veterinary Services form 6-22D [39b]). Responses (i.e., by CCT) to both M. avium and M. bovis PPDs by M. kansasii-inoculated cattle exceeded respective responses by noninoculated cattle (Table 1). Skin test responses by M. kansasii-inoculated cattle to M. bovis PPD did not differ (P > 0.05) from respective responses to M. avium PPD (Fig. 1), whereas responses by M. bovis-inoculated calves to M. bovis PPD greatly exceeded (P < 0.0001) respective responses to M. avium PPD (Table 1).
FIG. 1.
Delayed-type-hypersensitivity responses to M. bovis PPD as determined by the caudal fold test. Caudal fold skin test responses (means ± standard errors) by control (n = 10), M. kansasii-infected (n = 4), and M. bovis-infected (n = 9) cattle to M. bovis PPD. Responses represent the change in skin thickness relative to preinjection CFT values. The letters a to c indicate responses that differ (P < 0.05).
TABLE 1.
Comparative cervical skin tuberculin test responses
| Infection status (no. of cattle) | Mean ± SE change in skin thickness (mm)a
|
Statusb | |
|---|---|---|---|
| M. avium PPD | M. bovis PPD | ||
| Noninfected (5) | 1.5 ± 0.53 A | 0.5 ± 0.15 A | All negative |
| M. kansasii infected (4) | 5.4 ± 0.52 B | 4.6 ± 0.28 B | All negative |
| M. bovis infected (9) | 4.3 ± 0.5 B | 26.3 ± 3.65 C | All reactors |
Animals received 0.1 ml (100 μg) of M. bovis PPD and 0.1 ml (40 μg) of M. avium PPD in the midcervical region according to guidelines described in USDA, APHIS circular 91-45-01 (39a). Skin thickness changes (mm) in response to PPD stimulation (mycobacterial origin indicated) are presented as the means ± standard errors at 72 h after injection minus the skin thickness prior to injection. The letters A through C indicate responses that differ (P < 0.01) for a specific stimulation (i.e., vertical comparisons). The same letter indicates that the responses did not differ.
Change in skin thickness values for individual animals were plotted on a scattergram for the interpretation of CCT results (USDA, APHIS, Veterinary Services form 6-22D, cattle bison interpretation [39b]) and characterized as negative or suspect for TB or reactive to tuberculosis exposure.
Lymphocyte blastogenesis.
As with skin test responses, the ranking of blastogenic responses (Δcpm) resulted in M. bovis-inoculated cattle having stronger responses than M. kansasii-inoculated cattle and M. kansasii-inoculated cattle having stronger responses than noninoculated cattle (Table 2). Mean blastogenic responses to M. bovis PPD by M. kansasii-inoculated cattle did not differ (P > 0.05) from respective responses to M. avium PPD, whereas responses to M. bovis PPD by M. bovis-inoculated calves greatly exceeded (P < 0.001) respective responses to M. avium PPD (data not shown). At 129 days postchallenge, the mean (±standard error of the mean) blastogenic responses by M. bovis-inoculated calves to rESAT-6-CFP-10 (32,577 ± 4,978) exceeded (P < 0.05) respective responses by M. kansasii-inoculated (2,140 ± 815) and noninoculated (662 ± 697) calves, with similar differences at 50 days postchallenge.
TABLE 2.
Lymphocyte blastogenesis responses to M. bovis PPD stimulation
| Infection status | Lymphocyte blastogenesis Δcpm (mean ± SE) ata:
|
||
|---|---|---|---|
| Preinoculation | Day 50 postinoculation | Day 124 postinoculation | |
| Noninfected | 33.3 ± 80 A | −425 ± 812 A | −284 ± 980 A |
| M. kansasii infected | −127 ± 171 A | 4,676 ± 2,014 B | 9,123 ± 4,067 B |
| M. bovis infected | 52 ± 105 A | 17,836 ± 3,378 C | 48,741 ± 5,409 C |
Shown are mean (±standard error) lymphocyte blastogenic responses (Δcpm = M. bovis PPD stimulation − no stimulation) from noninfected (n = 10), M. kansasii-infected (n = 4), and M. bovis-infected (n = 9) cattle. The letters A through C indicate responses that differ (P < 0.05) for a specific time after inoculation (i.e., vertical comparisons). The same letter indicates that the responses did not differ.
Nitrite response.
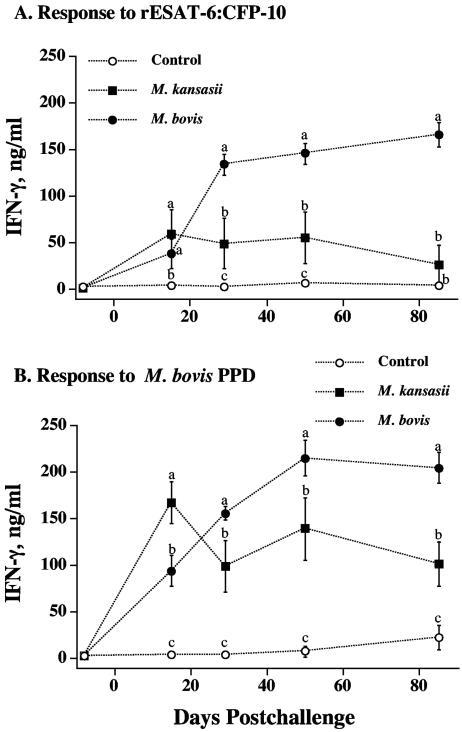
The stimulation of inducible nitric oxide synthase in macrophages and the subsequent generation of reactive nitrogen intermediates are potent mechanisms of mycobacterial killing (10, 11, 13, 17, 27). As previously demonstrated (43), robust recall nitrite responses, a correlate to NO production, are induced upon M. bovis infection (Fig. 2). At 15 days postchallenge, the in vitro production of nitrite by PBMC from M. kansasii-inoculated calves in response to M. bovis PPD exceeded (P < 0.05) respective responses by M. bovis-inoculated and noninoculated calves (Fig. 2B). At other time points after challenge (with one exception), nitrite responses by PBMC from M. bovis-inoculated calves exceeded (P < 0.05) respective responses by M. kansasii-inoculated and noninoculated calves (Fig. 2). The nitrite responses by M. kansasii-inoculated calves to rESAT-6-CFP-10 and to M. bovis PPD exceeded (P < 0.05) respective responses by noninoculated calves at 29, 50, and 85 days postchallenge. Mean nitrite responses by M. kansasii-inoculated calves to M. bovis PPD did not differ (P > 0.05) from respective responses to M. avium PPD, whereas responses by M. bovis-inoculated calves to M. bovis PPD exceeded (P < 0.05) respective responses to M. avium PPD (data not shown).
FIG. 2.
Longitudinal nitrite production to mycobacterial antigens. Blood mononuclear cells were isolated from control (n = 10), M. kansasii-infected (n = 4), and M. bovis-infected (n = 9) cattle at the indicated time points after challenge. Isolates were cultured for 72 h with medium alone, 1 μg/ml rESAT-6-CFP-10, or 5 μg/ml PPDs, and supernatants were harvested for analysis of nitrite (Griess reaction) as an indication of NO production. Values are presented as mean (±standard error) responses to rESAT-6-CFP-10 (A) or M. bovis PPD (B) stimulation minus the response to medium alone. The letters a to c indicate responses that differ (P < 0.05) for each respective time point after challenge.
IFN-γ response.
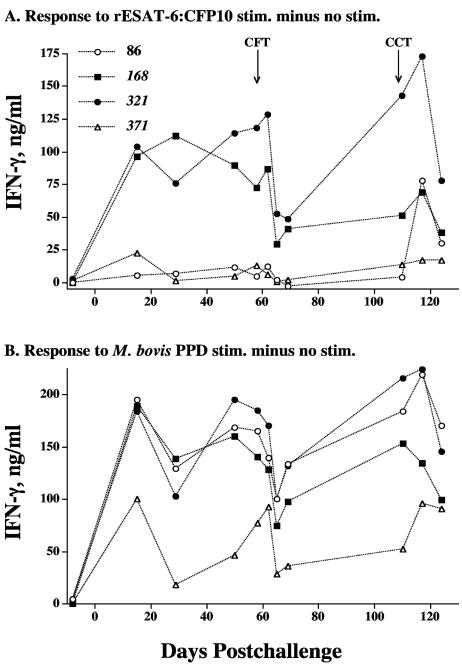
The evaluation of antigen-specific IFN-γ production is widely accepted as an immunodiagnostic tool for the detection of tuberculous cattle (48, 49) and humans (30). At 15 days postchallenge, as with the nitrite responses, IFN-γ responses to M. bovis PPD by PBMC from M. kansasii-inoculated calves exceeded (P < 0.05) respective responses by M. bovis-inoculated and noninoculated calves (Fig. 3B). At 15 days postchallenge, IFN-γ responses to rESAT-6-CFP-10 by M. kansasii- and M. bovis-inoculated calves exceeded (P < 0.05) respective responses by noninoculated calves. At all other time points after challenge (with one exception), IFN-γ responses by PBMC from M. bovis-inoculated calves exceeded (P < 0.05) respective responses by M. kansasii-inoculated and noninoculated calves (Fig. 3). In general, the mean nitrite (Fig. 2) and IFN-γ (Fig. 3) responses by M. kansasii-inoculated calves were intermediate in magnitude between those of M. bovis-inoculated and noninoculated calves. The evaluation of individual responses (Fig. 4) revealed that two of the four M. kansasii-inoculated cattle (animals 168 and 321) had robust IFN-γ responses to rESAT-6-CFP-10 throughout the study, whereas the other two animals had poor to no responses to rESAT-6-CFP-10. These findings, while from a limited number of cattle (n = 4), demonstrate the potential for interference that exists in the interpretation of results from improved TB tests that use defined antigens. Responses to M. bovis PPD, however, were detected for each of the four M. kansasii-inoculated animals (Fig. 4). Linear regression analysis revealed that individual IFN-γ responses by M. kansasii-infected calves to M. bovis PPD correlated to respective M. avium PPD responses (Pearson's product-moment correlations, r = 0.93; P < 0.0001). However, 16/48 tests were considered positive (including positive responses by each of the four animals) using a Bovigam kit M. bovis PPD minus M. avium PPD cutoff value of >0.1 optical density unit, demonstrating the potential for falsely characterizing the animal as a TB reactor.
FIG. 3.
Longitudinal IFN-γ production to mycobacterial antigens. Blood mononuclear cells were isolated from control (n = 10), M. kansasii-infected (n = 4), and M. bovis-infected (n = 9) cattle at the indicated time points after the challenge. Isolates were cultured for 48 h with medium alone, 1 μg/ml rESAT-6-CFP-10, or 5 μg/ml PPDs, and supernatants were harvested for the analysis of IFN-γ by ELISA. Values are presented as mean (±standard error) responses to rESAT-6-CFP-10 (A) or M. bovis PPD (B) stimulation minus the response to medium alone. Letters a to c indicate responses that differ (P < 0.05) for each respective time point after challenge. The same letter indicates that the responses did not differ.
FIG. 4.
Effects of the skin test on in vitro IFN-γ production by individual M. kansasii-sensitized cattle in response to mycobacterial antigens. Blood mononuclear cells were isolated from M. kansasii-infected cattle at the indicated time points after challenge (x axis) and cultured for 48 h with medium alone, 1 μg/ml rESAT-6-CFP-10, or 5 μg/ml PPDs, and supernatants were harvested for analysis of IFN-γ by ELISA. Values are presented as individual responses (in the key, numbers next to symbols refer to the animals' numbers) to rESAT-6-CFP-10 (A) or M. bovis PPD (B) stimulation (stim.) minus the response to the medium alone. Arrows below “CFT” and “CCT” indicate time points of injection of PPDs for the caudal fold and comparative cervical skin tests, respectively.
The magnitude of IFN-γ responses to both M. bovis PPD and rESAT-6-CFP-10 was reduced following the injection of PPDs for skin tests (Fig. 4). The reduction in the response to rESAT-6-CFP-10 followed an initial increase 3 days after the injection of PPDs. The administration of PPDs for the second skin test (i.e., CCT) elicited a response to rESAT-6-CFP-10 by a previously nonresponding M. kansasii-inoculated animal (Fig. 4, animal 86).
Antibody response.
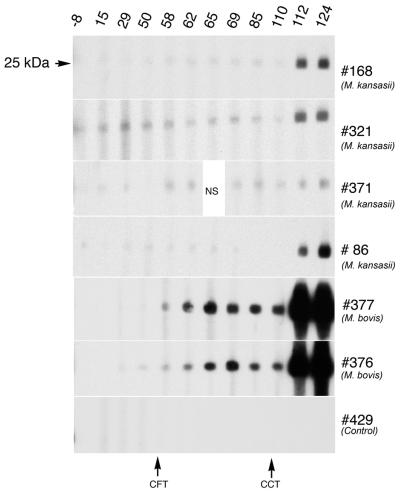
Recent studies have indicated that serum antibody to mycobacterial infection, either natural or experimental, is detectable relatively early after infection (24, 44). The use of an ELISA-based assay for LAM demonstrated that Mycobacterium-specific antibody was detected as early as 50 days after challenge for M. kansasii-infected cattle and 124 days after challenge for M. bovis-infected cattle (Table 3). Responses by M. bovis-infected cattle exceeded (P < 0.05) respective responses by M. kansasii-infected cattle at 124 days after the challenge but not at 50 days after the challenge. The early LAM response by M. kansasii relative to M. bovis infection may be due to the larger challenge dose (i.e., 4 × 108 CFU for M. kansasii versus 4 × 104 CFU for M. bovis). By immunoblotting (Fig. 5), the specific antibody to MPB83 was detectable at 112 and 124 days after M. kansasii challenge. It should be noted that significant responses to MPB83 by M. kansasii-infected cattle were detected only after the injection of PPDs for the comparative cervical skin test. A low level of background, nonspecific reactivity was detected in each of the M. kansasii-inoculated animals prior to skin testing. Antibody to MPB83 was elicited as early as 58 days after M. bovis inoculation (Fig. 5), and responses were more robust than those elicited upon M. kansasii infection (Fig. 6). In contrast to cell-mediated responses to rESAT-6-CFP-10, responses to ESAT-6 and/or CFP-10 were not detected in sera from M. kansasii-sensitized cattle, as determined by MAPIA and immunoblotting (data not shown). However, these antigens elicited antibody responses in cattle infected with M. bovis. Using MAPIA, antibodies to M. bovis PPD were detected in sera from three of four M. kansasii-infected cattle and all M. bovis-infected cattle. Other antigens included in the MAPIA did not react with sera from M. kansasii-infected cattle (data not shown).
TABLE 3.
Response kinetics of serum antibody to mycobacterial LAM-enriched antigen
| Infection status | S/P ratio (mean ± SE) of serum responsesa
|
||
|---|---|---|---|
| Preinoculation | Day 50 postinoculation | Day 124 postinoculation | |
| Noninfected | −0.06 ± 0.03 A | 0.00 ± 0.05 A | 0.04 ± 0.14 A |
| M. kansasii infected | 0.05 ± 0.06 A | 0.55 ± 0.26 B | 0.76 ± 0.38 B |
| M. bovis infected | −0.10 ± 0.11 A | 0.01 ± 0.03 A | 1.64 ± 0.19 C |
Shown are S/P ratio means (±standard errors) of serum ELISA responses from noninfected (n = 10), M. kansasii-infected (n = 4), and M. bovis-infected (n = 9) cattle. The S/P ratios of test samples were calculated from absorbency values using the following formulai sample − negative control/positive control − negative control. The letters A through C indicate responses that differ (P < 0.05) for a specific time after inoculation (i.e., vertical comparisons). The same letter indicates that the antibody responses did not differ.
FIG. 5.
Western blot analysis of antibody responses to MPB83. Preparative immunoblots of M. bovis MPB83 antigen probed with sera from cattle experimentally infected with either M. kansasii, M. bovis, or serum from a noninfected animal (control). Molecular mass is indicated to the left of the top blot (uniform for each blot), days after infection are shown along the top, animal number and infection status are shown on the right, and time points of injection of PPD(s) for skin tests are shown at the bottom. NS, no sample.
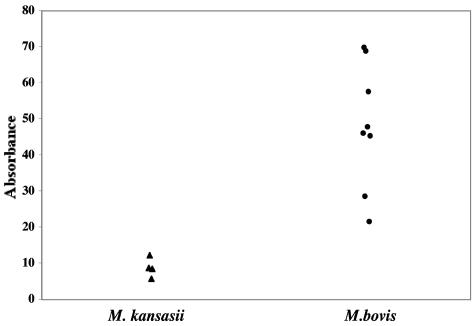
FIG. 6.
Relative intensities of responses to MPB83 as measured by MAPIA. Sera collected from M. bovis-infected (circles; n = 9) and M. kansasii-infected (triangles; n = 4) cattle 112 days after inoculation were evaluated by MAPIA for reactivity to mycobacterial antigens. For comparison, the intensities of bands of reactivity to MPB83 were determined by densitometry and are presented as absorbency values.
DISCUSSION
The present findings demonstrate that while the experimental challenge of cattle with M. kansasii failed to cause clinical disease, it did induce detectable immune responses that potentially confound the interpretation of traditional (i.e., skin test) and modern (i.e., IFN-γ-based and antibody-based) TB tests. The Mantoux test for humans, as with the CFT for cattle, is based on in vivo reactivity to a single tuberculin produced from an M. tuberculosis complex mycobacterium. Unlike with many TB testing strategies for cattle, the Mantoux test is not followed up with a secondary skin test (e.g., CCT) designed to differentiate between M. tuberculosis complex responses and responses induced by exposure to M. avium and/or other nontuberculous mycobacteria. Based on the single tuberculin test (i.e., CFT), all cattle inoculated with M. kansasii in this study would be considered TB responders. Nonspecific reactions are not uncommon with complex antigens such as PPDs. A recently approved IFN-γ-based test for use with whole-blood samples from humans (Quantiferon Gold; Cellestis) appears promising for clinical use and relies on reactivity to ESAT-6 and CFP-10 (16). Despite a proven record of the improved specificity of these antigens over those of tuberculin-based tests, the present findings indicate that caution may be warranted in the interpretation of responses to ESAT-6 and CFP-10 antigens, at least in certain (presumably rare) instances, as demonstrated with M. kansasii sensitization.
Mycobacterium kansasii is genetically heterogenous with certain genotypes associated with clinical disease in humans (1, 32). Currently, there are five distinct groups defined by sequences in the 16S-23S rRNA intergenic spacer region of the bacterial DNA. Specific genotypes of bovine isolates, however, have not been characterized accordingly. A relatively large challenge dose was used to induce a response to M. kansasii. Challenge doses achieved under field conditions are not clear; however, cattle with lesions associated with M. kansasii are occasionally detected. Fortunately, an immune response was induced with this challenge dose, thereby meeting a primary goal of this study. If responses had not been induced, as may have occurred with lower doses, then the potential for interference with TB tests would not have been recognized. The M. kansasii strain used in the present study was isolated from a tuberculous lesion (i.e., pyogranuloma) detected upon routine slaughter surveillance. The isolate had 100% identity with the subtype 1 genotype, most commonly associated with disease in immunocompetent humans. Other mycobacteria were not isolated from this lesion, and the animal of origin was considered negative for TB. Despite its associations with clinical disease, the intratonsillar inoculation of four calves in the present study did not result in the clinical manifestation of disease, gross or microscopic lesions, or reisolation of the mycobacterium from the animals, all of which are indicative of effective clearance of the organism. Thus, immune responses were elicited and maintained throughout the study with an apparent absence of on-going, persistent infection. In contrast, humans with clinical disease due to M. kansasii infection maintain a state of constant or increasing antigen stimulation, as occurs with chronic TB infection. With ongoing clinical disease, mycobacterial antigens such as ESAT-6, CFP-10, and MPB83 are continually produced and available for immune stimulation of the host. In our studies with samples from humans, we have found strong antibody responses against MPB83 in immunocompetent patients with M. kansasii-positive pulmonary disease (data not shown). The present findings for cattle demonstrate that immune responses to ESAT-6, CFP-10, and MPB83 may be evoked by M. kansasii sensitization even without persistent infection and clinical disease. While only a limited number of animals were used for M. kansasii inoculation (n = 4), the number was adequate to demonstrate that cattle exposed to M. kansasii may respond to M. bovis antigens. Also, as evidenced by serum reactivity to MPB83 following skin tests, initially weak antibody responses in M. kansasii-inoculated cattle are further stimulated by injection of PPDs, although antibody levels remained significantly lower than those detected in sera from cattle infected with M. bovis. These findings further substantiate the stealthy nature of preexisting mycobacterial exposure events that elicit low-level and potentially undetectable responses to epitopes and/or antigens contained within tuberculous mycobacteria. These low-level responses may then be boosted to detectable levels by the injection of tuberculin for skin testing.
Molecular and traditional microbiological methods are readily available to distinguish between M. bovis and M. kansasii infection upon collection of tissues at necropsy. The isolation of nontuberculous mycobacteria (e.g., M. kansasii) does not result in the condemnation of carcasses from human consumption. More importantly, the detection of M. bovis in cattle results in serious regulatory action, including costly epidemiologic studies, increased testing, and potentially the loss of TB-free status for states or regions and the loss of trading partners. Thus, it is critical that M. bovis infection be differentiated from infection/sensitization resulting from exposure to nontuberculous mycobacteria. Over the past 4.25 years, 26,480 (16,506 from cattle) diagnostic specimens have been cultured by the National Veterinary Services Laboratories for the recovery of mycobacterial pathogens. Of these, M. kansasii was isolated from only 29 cases, 14 of these from cattle. These findings demonstrate that disease resulting from M. kansasii infection is exceedingly rare. However, the number of animals that are exposed in the field to M. kansasii and do not develop disease associated with exposure to the organism is not known. As demonstrated from the present study, M. kansasii exposure of cattle may induce sensitization without disease. More importantly, this exposure may elicit specific responses that confound the interpretation of antemortem bovine TB tests. In particular, this exposure may interfere with the interpretation of improved TB tests that use “TB-specific” antigens (i.e., ESAT-6, CFP-10, and MPB83). These findings demonstrate one explanation for false-positive responses to TB tests that rely on responses to purportedly M. tuberculosis complex-specific antigens.
Acknowledgments
We thank Mike Howard, Peter Lasley, Rebecca Lyon, Jessica Pollock, Todd Stuber, and Shelly Zimmerman for excellent technical support. We also thank Richard Auwerda, Doug Ewing, Andrew Gibson, Don Hackbarth, Todd Holtz, Terry Krausman, David Panthen, Brian Pottebaum, Don Robinson, Jay Steffen, Johann Thiel, Wayne Varland, and Larry Wright for excellent animal care.
REFERENCES
- 1.Alcaide, F., I. Richter, C. Bernasconi, B. Springer, C. Hagenau, R. Schulze-Robbecke, E. Tortoli, R. Martin, E. C. Böttger, and A. Telenti. 1997. Heterogeneity and clonality among isolates of Mycobacterium kansasii: implications for epidemiological and pathogenicity studies. J. Clin. Microbiol. 35:1959-1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arend, S. M., P. de Haas, E. Leyten, I. Rosenkrands, L. Rigouts, P. Andersen, W. Mijs, J. T. van Dissel, and D. van Soolingen. 2005. ESAT-6 and CFP-10 in clinical versus environmental isolates of Mycobacterium kansasii. J. Infect. Dis. 191:1301-1310. [DOI] [PubMed] [Google Scholar]
- 3.Arend, S. M., E. Cerda de Palou, P. de Haas, R. Janssen, M. A. Hoeve, E. M. Verhard, T. H. Ottenhoff, D. van Soolingen, and J. T. van Dissel. 2004. Pneumonia caused by Mycobacterium kansasii in a series of patients without recognised immune defect. Clin. Microbiol. Infect. 10:738-748. [DOI] [PubMed] [Google Scholar]
- 4.Arend, S. M., K. E. van Meijgaarden, K. de Boer, E. C. de Palou, D. van Soolingen, T. H. Ottenhoff, and J. T. van Dissel. 2002. Tuberculin skin testing and in vitro T cell responses to ESAT-6 and culture filtrate protein 10 after infection with Mycobacterium marinum or M. kansasii. J. Infect. Dis. 186:1797-1807. [DOI] [PubMed] [Google Scholar]
- 5.Arend, S. M., T. H. Ottenhoff, P. Andersen, and J. T. van Dissel. 2001. Uncommon presentations of tuberculosis: the potential value of a novel diagnostic assay based on the Mycobacterium tuberculosis-specific antigens ESAT-6 and CFP-10. Int. J. Tuberc. Lung Dis. 5:680-686. [PubMed] [Google Scholar]
- 6.Bannantine, J., and J. R. Stabel. 2000. HspX is present within Mycobacterium paratuberculosis-infected macrophages and is recognized by sera from some infected cattle. Vet. Microbiol. 76:343-358. [DOI] [PubMed] [Google Scholar]
- 7.Bolin, C. A., D. L. Whipple, K. V. Khanna, J. M. Risdahl, P. K. Peterson, and T. W. Molitor. 1997. Infection of swine with Mycobacterium bovis as a model of human tuberculosis. J. Infect. Dis. 176:1559-1566. [DOI] [PubMed] [Google Scholar]
- 8.Buddle, B. M., A. R. McCarthy, T. J. Ryan, J. M. Pollock, H. M. Vordermeier, R. G. Hewinson, P. Andersen, and G. W. de Lisle. 2003. Use of mycobacterial peptides and recombinant proteins for the diagnosis of bovine tuberculosis in skin test-positive cattle. Vet. Rec. 153:615-620. [DOI] [PubMed] [Google Scholar]
- 9.Burton, J. L., and M. E. Kehrli. 1996. Effects of dexamethasone on bovine circulating T lymphocyte populations. J. Leukoc. Biol. 59:90-99. [DOI] [PubMed] [Google Scholar]
- 10.Chan, J., K. Tanaka, D. Carroll, J. Flynn, and B. R. Bloom. 1995. Effects of nitric oxide synthase inhibitors on murine infection with Mycobacterium tuberculosis. Infect. Immun. 63:736-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan, J., Y. Xing, R. Magliozzo, and B. R. Bloom. 1992. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J. Exp. Med. 175:1111-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, et al. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. [DOI] [PubMed] [Google Scholar]
- 13.Denis, M. 1991. Interferon-gamma-treated murine macrophages inhibit growth of tubercle bacilli via the generation of reactive nitrogen intermediates. Cell. Immunol. 132:150-157. [DOI] [PubMed] [Google Scholar]
- 14.Evans, A. J., A. J. Crisp, R. B. Hubbard, A. Colville, S. A. Evans, and I. D. Johnston. 1996. Pulmonary Mycobacterium kansasii infection: comparison of radiological appearances with pulmonary tuberculosis. Thorax 51:1243-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans, S. A., A. Colville, A. J. Evans, A. J. Crisp, and I. D. Johnston. 1996. Pulmonary Mycobacterium kansasii infection: comparison of the clinical features, treatment and outcome with pulmonary tuberculosis. Thorax 51:1248-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferrara, G., M. Losi, M. Meacci, B. Meccugni, R. Piro, P. Roversi, B. M. Bergamini, R. D'Amico, P. Marchegiano, F. Rumpianesi, L. M. Fabbri, and L. Richeldi. 2005. Routine hospital use of a commercial whole blood interferon-gamma assay for tuberculosis infection. Am. J. Respir. Crit. Care Med. 172:631-635. [DOI] [PubMed] [Google Scholar]
- 17.Flesch, I. E., and S. H. Kaufmann. 1991. Mechanisms involved in mycobacterial growth inhibition by gamma interferon-activated bone marrow macrophages: role of reactive nitrogen intermediates. Infect. Immun. 59:3213-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gey van Pittius, N. C., R. M. Warren, and P. D. van Helden. 2002. ESAT-6 and CFP-10: what is the diagnosis? Infect. Immun. 70:6509-6511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gey Van Pittius, N. C., J. Gamieldien, W. Hide, G. D. Brown, R. J. Siezen, and A. D. Beyers. 2001. The ESAT-6 gene cluster of Mycobacterium tuberculosis and other high G+C Gram-positive bacteria. Genome Biol. 2:0044-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greenwald, R., J. Esfandiari, S. Lesellier, R. Houghton, J. Pollock, C. Aagaard, P. Andersen, R. G. Hewinson, M. Chambers, and K. Lyashchenko. 2003. Improved serodetection of Mycobacterium bovis infection in badgers (Meles meles) using multiantigen test formats. Diagn. Microbiol. Infect. Dis. 46:197-203. [DOI] [PubMed] [Google Scholar]
- 21.Hughes, M. S., N. W. Ball, J. McCarroll, M. Erskine, M. J. Taylor, J. M. Pollock, R. A. Skuce, and S. D. Neill. 2005. Molecular analyses of mycobacteria other than the M. tuberculosis complex isolated from Northern Ireland cattle. Vet. Microbiol. 108:101-112. [DOI] [PubMed] [Google Scholar]
- 22.Karlson, A. G. 1962. Non-specific or cross sensitivity reactions to tuberculin in cattle. Adv. Vet. Sci. 7:147-181. [Google Scholar]
- 23.Keet, D. F., N. P. Kriek, R. G. Bengis, and A. L. Michel. 2001. Tuberculosis in kudus (Tragelaphus strepsiceros) in the Kruger National Park. Onderstepoort J. Vet. Res. 68:225-230. [PubMed] [Google Scholar]
- 24.Koets, A. P., V. P. Rutten, M. de Boer, D. Bakker, P. Valentin-Weigand, and W. van Eden. 2001. Differential changes in heat shock protein-, lipoarabinomannan-, and purified protein derivative-specific immunoglobulin G1 and G2 isotype responses during bovine Mycobacterium avium subsp. paratuberculosis infection. Infect. Immun. 69:1492-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lightbody, K. A., R. A. Skuce, S. D. Neill, and J. M. Pollock. 1998. Mycobacterial antigen-specific antibody responses in bovine tuberculosis: an ELISA with potential to confirm disease status. Vet. Rec. 142:295-300. [DOI] [PubMed] [Google Scholar]
- 26.Lyashchenko, K. P., M. Singh, R. Colangeli, and M. L. Gennaro. 2000. A multiantigen print immunoassay for the development of serological diagnosis of infectious diseases. J. Immunol. Methods 242:91-100. [DOI] [PubMed] [Google Scholar]
- 27.MacMicking, J. D., R. J. North, R. LaCourse, J. S. Mudgett, S. K. Shah, and C. F. Nathan. 1997. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc. Natl. Acad. Sci. USA 94:5243-5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McNair, J., D. M. Corbett, R. M. Girvin, D. P. Mackie, and J. M. Pollock. 2001. Characterization of the early antibody response in bovine tuberculosis: MPB83 is an early target with diagnostic potential. Scand. J. Immunol. 53:365-371. [DOI] [PubMed] [Google Scholar]
- 29.Menon, S. A., M. J. Wannemuehler, G. G. Mahairas, and F. C. Minion. 2002. Mycobacterial ESAT-6 protein enhances mouse IFN-γ responses to Mycoplasma hyopneumoniae P71 protein. J. Interferon Cytokine Res. 22:807-813. [DOI] [PubMed] [Google Scholar]
- 30.Pai, M., L. W. Riley, and J. M. Colford. 2004. Interferon-γ assays in the immunodiagnosis of tuberculosis: a systematic review. Lancet Infect. Dis. 4:761-776. [DOI] [PubMed] [Google Scholar]
- 31.Palmer, M. V., D. L. Whipple, and S. C. Olsen. 1999. Development of a model of natural infection with Mycobacterium bovis in white-tailed deer. J. Wildl. Dis. 35:450-457. [DOI] [PubMed] [Google Scholar]
- 32.Picardeau, M., G. Prod'Hom, L. Raskine, M. P. LePennec, and V. Vincent. 1997. Genotypic characterization of five subspecies of Mycobacterium kansasii. J. Clin. Microbiol. 35:25-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pollock, J. M., and P. Andersen. 1997. The potential of the ESAT-6 antigen secreted by virulent mycobacteria for specific diagnosis of tuberculosis. J. Infect. Dis. 175:1251-1254. [DOI] [PubMed] [Google Scholar]
- 34.Pollock, J. M., R. M. Girvin, K. A. Lightbody, R. A. Clements, S. D. Neill, B. M. Buddle, and P. Andersen. 2000. Assessment of defined antigens for the diagnosis of bovine tuberculosis in skin test-reactor cattle. Vet. Rec. 146:659-665. [DOI] [PubMed] [Google Scholar]
- 35.Rajaraman, V., B. J. Nonnecke, S. T. Franklin, D. C. Hammell, and R. L. Horst. 1998. Effect of vitamins A and E on nitric oxide production by blood mononuclear leukocytes from neonatal calves fed milk replacer. J. Dairy Sci. 81:3278-3285. [DOI] [PubMed] [Google Scholar]
- 36.Ravn, P., M. E. Munk, Å. B. Andersen, B. Lundgren, J. D. Lundgren, L. N. Nielsen, A. Kok-Jensen, P. Andersen, and K. Weldingh. 2005. Prospective evaluation of a whole-blood test using Mycobacterium tuberculosis-specific antigens ESAT-6 and CFP-10 for diagnosis of active tuberculosis. Clin. Diagn. Lab. Immunol. 12:491-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roth, A., M. Fischer, M. E. Hamid, S. Michalke, W. Ludwig, and H. Mauch. 1998. Differentiation of phylogenetically related slowly growing mycobacteria based on 16S-23S rRNA gene internal transcribed spacer sequences. J. Clin. Microbiol. 36:139-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmitt, S. M., S. D. Fitzgerald, T. M. Cooley, C. S. Bruning-Fann, L. Sullivan, D. Berry, T. Carlson, R. B. Minnis, J. B. Payeur, and J. Sikarskie. 1997. Bovine tuberculosis in free-ranging white-tailed deer from Michigan. J. Wildl. Dis. 33:749-758. [DOI] [PubMed] [Google Scholar]
- 39.Skjøt, R. L. V., I. Brock, S. M. Arend, M. E. Munk, M. Theisen, T. H. M. Ottenhoff, and P. Andersen. 2002. Epitope mapping of the immunodominant antigen TB10.4 and the two homologous proteins TB10.3 and TB12.9, which constitute a subfamily of the esat-6 gene family. Infect. Immun. 70:5446-5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39a.USDA, APHIS. 1999. Bovine tuberculosis eradication uniform methods and rules, p. 1-34. U.S. Government Printing Office, Washington, D.C.
- 39b.USDA, APHIS, Veterinary Services. 1999. Scattergram for interpretation of the comparative cervical test. Veterinary Services form 6-22D. USDA, APHIS, Ames, Iowa.
- 40.Vordermeier, H. M., A. Whelan, P. J. Cockle, L. Farrant, N. Palmer, and R. G. Hewinson. 2001. Use of synthetic peptides derived from the antigens ESAT-6 and CFP-10 for differential diagnosis of bovine tuberculosis in cattle. Clin. Diagn. Lab. Immunol. 8:571-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vosloo, W., P. Tippoo, J. E. Hughes, N. Harriman, M. Emms, D. W. Beatty, H. Zappe, and L. M. Steyn. 1997. Characterisation of a lipoprotein in Mycobacterium bovis (BCG) with sequence similarity to the secreted protein MPB70. Gene 188:123-128. [DOI] [PubMed] [Google Scholar]
- 42.Waters, W. R., M. V. Palmer, and D. L. Whipple. 2002. Mycobacterium bovis-infected white-tailed deer (Odocoileus virginianus): detection of immunoglobulin specific to crude mycobacterial antigens by ELISA. J. Vet. Diagn. Investig. 14:470-475. [DOI] [PubMed] [Google Scholar]
- 43.Waters, W. R., M. V. Palmer, D. L. Whipple, M. P. Carlson, and B. J. Nonnecke. 2003. Diagnostic implications of antigen-induced gamma interferon, nitric oxide, and tumor necrosis factor alpha production by peripheral blood mononuclear cells from Mycobacterium bovis-infected cattle. Clin. Diagn. Lab. Immunol. 10:960-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Waters, W. R., J. M. Miller, M. V. Palmer, J. R. Stabel, D. E. Jones, K. A. Koistinen, E. M. Steadham, M. J. Hamilton, W. C. Davis, and J. P. Bannantine. 2003. Early induction of humoral and cellular immune responses during experimental Mycobacterium avium subsp. paratuberculosis infection of calves. Infect. Immun. 71:5130-5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Waters, W. R., B. J. Nonnecke, M. V. Palmer, S. Robbe-Austermann, J. P. Bannantine, J. R. Stabel, D. L. Whipple, J. B. Payeur, D. M. Estes, J. E. Pitzer, and F. C. Minion. 2004. Use of recombinant ESAT-6:CFP-10 fusion protein for differentiation of infections of cattle by Mycobacterium bovis and by M. avium subsp. avium and M. avium subsp. paratuberculosis. Clin. Diagn. Lab. Immunol. 11:729-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Waters, W. R., M. V. Palmer, J. P. Bannantine, D. L. Whipple, R. Greenwald, J. Esfandiari, P. Andersen, J. McNair, J. M. Pollock, and K. P. Lyashchenko. 2004. Antigen recognition by serum antibodies in white-tailed deer (Odocoileus virginianus) experimentally infected with Mycobacterium bovis. Clin. Diagn. Lab. Immunol. 11:849-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Winters, A., C. Driver, M. Macaraig, C. Clark, S. S. Munsiff, C. Pichardo, J. Driscoll, M. Salfinger, B. Kreiswirth, J. Jereb, P. LoBue, and M. Lynch. 2005. Human tuberculosis caused by Mycobacterium bovis—New York City, 2001-2004. Morb. Mortal. Wkly. Rep. 54:605-608. [PubMed] [Google Scholar]
- 48.Wood, P. R., L. A. Corner, J. S. Rothel, C. Baldock, S. L. Jones, D. B. Cousins, B. S. McCormick, B. R. Francis, J. Creepeer, and N. E. Tweddle. 1991. Field comparison of the interferon-gamma assay and the intradermal tuberculin test for the diagnosis of bovine tuberculosis. Aust. Vet. J. 68:286-290. [DOI] [PubMed] [Google Scholar]
- 49.Wood, P. R., and J. S. Rothel. 1994. In vitro immunodiagnostic assays for bovine tuberculosis. Vet. Microbiol. 40:125-135. [DOI] [PubMed] [Google Scholar]