Abstract
Oropharyngeal candidiasis (OPC) remains the most common oral infection in human immunodeficiency virus (HIV) disease. In a high percentage of HIV+ persons with reduced CD4+ T cells, oral lesions with Candida present at the outer epithelium have an accumulation of CD8+ T cells at the epithelium-lamina propria interface associated with reduced expression of the mucosal cell-trafficking adhesion molecule E-cadherin. The purpose of the present study was to characterize the immune status of these CD8+ T cells. Immunohistochemical staining for phenotypic and activation and costimulation markers was performed on frozen biopsy tissue sections from HIV+ OPC+ persons with accumulated CD8+ T cells. CD8+ T cells consisted primarily of central memory cells by virtue of positive CD45RO (memory) and CD27 (central memory) expression. However, concomitant negative expression of CD62L and CCR7 (effector memory) was suggestive of a transitioning memory phenotype within the tissue. Despite this, the cells are considered to be activated on the basis of positive expression of CD69. The CD8+ T cells are not considered to be NK T cells or anti-HIV CD8+ T cells because of negative or low expression of CD161 and vascular cell adhesion molecule, respectively. These results suggest that the accumulated mucosal migratory-challenged CD8+ T cells are otherwise normal memory T cells in an activated state.
Oropharyngeal candidiasis (OPC), caused by Candida albicans, is the most common oral infection in those with human immunodeficiency virus (HIV) (11, 16, 18, 20). C. albicans is a ubiquitous fungal organism that is part of the normal microflora of the gastrointestinal and reproductive tracts. As a result of early exposure, most healthy individuals exhibit Candida-specific immunity that protects against infection. However, under immunocompromised conditions, such as HIV infection, C. albicans is capable of rapid conversion to a pathogen causing symptomatic mucosal infections (4, 8, 16, 17, 20, 23). Clinically, OPC can be observed in lesions as a mixture of hyphae and yeast, normally located in the stratum corneum-keratin layer of the outer epithelium, and can affect the buccal mucosa, gingival cuff, palate, and tongue. The infections can be erythematous, atrophic lesions that appear reddish or pseudomembranous, white, curd-like lesions commonly referred to as thrush (7). OPC can lead to difficulty in chewing, painful swallowing, and ultimately reduced nutritional consumption with significant morbidity (10).
It has been postulated that protection against OPC is multifactorial, albeit primarily dependent on a threshold level of blood CD4+ T cells (usually 200 cells/μl). Below the CD4+ cell threshold, systemic Th1-type cell-mediated immunity is no longer protective and protection becomes dependent on several locally associated immune mechanisms (9), including Th cytokines in saliva, epithelial cell anti-Candida activity, and the local presence of CD8+ T cells. The major lymphocyte population in OPC lesions is αβ T-cell receptor-positive CD8+ T cells that accumulate at the epithelium-lamina propria interface within OPC lesions but at a considerable distance from the site of infection at the outer epithelium (21). The increased levels of CD8+ cells at this junction is associated with higher levels of mucosal addressin cell adhesion molecule-1 in OPC+ tissue, indicating cellular migration from the peripheral circulation rather than proliferation within the tissue. On the other hand, decreased levels of tissue-associated E-cadherin are consistent with the inability of CD8+ organisms to migrate further through the epithelium to Candida organisms at the outer epithelium, resulting in cellular accumulation (21).
The purpose of this study was to explore the immune status of the CD8+ T cells located at the epithelium-lamina propria interface of the oral tissue during episodes of OPC.
MATERIALS AND METHODS
Study population.
Patients were recruited and evaluated at the Louisiana State University Health Sciences Center HIV+ Outpatient Dental Clinic associated with the HIV Outpatient Program of the Medical Center of Louisiana at New Orleans and the Louisiana State University School of Dentistry. Informed consent was obtained from all participants and patients, and all procedures were followed in the conduct of clinical research in accordance with the Institutional Review Board at the Louisiana State University Health Sciences Center. Specimens examined came from a cohort (n = 473) established between 1998 and 2003 comprising 124 HIV-negative persons and 349 HIV-infected persons, including 128 HIV+ OPC+ and 221 HIV+ OPC− persons. Of the remaining banked specimens, 14 biopsy samples were from OPC+ persons. Of those, the 10 OPC+ tissue samples had accumulated CD8+ T cells at the epithelium-lamina propria interface and were used for the present immunohistochemistry analyses. Of this representative subcohort, OPC+ individuals had average blood CD4+ and CD8+ cell counts of 125 and 426 cells/μl, respectively, and the average HIV load was 183,000 copies/ml. Forty-four percent of the HIV+ persons in the subcohort were receiving highly active antiretroviral therapy (HAART). In this cohort, HAART was defined as three or more antiretroviral medications, with at least one being a protease inhibitor. Because of uncertainty of compliance, HAART failures were not able to be effectively identified. Finally, six HIV− healthy volunteers were used as controls for specific assays.
Diagnosis of OPC and detection of oral yeast colonization.
The diagnosis of OPC and detection of oral yeast colonization were described previously (22, 25). Briefly, diagnosis of OPC was made on the basis of the clinical appearance of the oral mucosa, i.e., red, atrophic areas (erythematous) or white, curd-like plaques (pseudomembranous). To confirm the presence of Candida in each biopsy sample taken, the specific site was swabbed and cultured. Oral swabs were cultured on Sabouraud dextrose agar (Becton Dickinson Microbiology Systems, Franklin Lakes, NJ) and CHROMagar (CHROMagar Microbiology, Paris, France). Identification of OPC was further confirmed by Candida hyphae or blastoconidia present on a wet-mount slide preparation with potassium hydroxide (KOH), a positive swab culture with characteristic colony morphology, and a silver stain of the tissue section from the lesions, as previously described (22). Initial identification to the species level was done by screening for a color reaction on CHROMagar. Green colonies, representing probable C. albicans, were processed for germ tube formation, and nongreen colonies were further identified to the species level by API biochemical tests (API ID 32C; BioMérieux, Durham, NC). Only those patients with pseudomembranous OPC were included in the subcohort because of the extremely small number of erythematous OPC cases, as well as the difference in the site of infection that would not allow appropriate comparisons. Of the OPC+ subjects in the subcohort (n = 10), lesions from six patients were identified as having C. albicans exclusively. Of the remaining four patients, lesions from three patients were positive for both C. albicans and C. glabrata and the other patient was positive for C. albicans and C. krusei. These data were comparable to our previous study (22).
Specimen collection and processing. (i) Biopsy.
Collection of buccal mucosa biopy samples was done as described previously (22). For immunohistochemistry analysis, tissue sections (5 μm) were collected on glass slides, fixed in ice-cold acetone (5 min), and stored at −20°C.
(ii) PBLs.
Venous blood (10 ml) was collected, and peripheral blood lymphocytes (PBLs) were isolated by differential centrifugation with Ficoll-Paque (Amersham Biosciences Corp., Piscataway, NJ). Cells were pelleted on slides by cytospin and used for controls in immunohistochemistry analysis.
Antibodies.
The antibodies used for immunohistochemical staining were monoclonal mouse anti-human CD8 (DAKO Cytomation, Carpinteria, CA), CD28 (BD Biosciences Pharmingen, San Diego, CA), CD27 (BD Biosciences Pharmingen), CD161 (natural killer [NK] T-cell marker; BD Biosciences Pharmingen), CD103 (vascular cell adhesion molecule [VCAM]; Serotec Inc., Raleigh, NC), CD45RA (Serotec Inc.), CD40L (Beckman Coulter, Fullerton, CA), CD62L (BD Biosciences Pharmingen), and CCR7 (BD Biosciences Pharmingen) antibodies with the appropriate isotype control antibody (mouse immunoglobulin G [IgG1]; DAKO Cytomation); monoclonal mouse anti-human CD45RO (Serotec Inc.), CD40 (Serotec Inc.), CD69 (Serotec Inc.), and CD152 (CTLA-4; Serotec Inc.) antibodies with the appropriate isotype control antibody (mouse IgG2a; Serotec Inc.); and monoclonal rat anti-human CD8 (Serotec Inc.) antibody with the appropriate isotype control antibody (rat IgG2b; Serotec Inc.).
Immunohistochemistry analysis.
Immunohistochemical staining of buccal mucosa with chromogen and fluorescence has been previously described, as was hematoxylin-and-eosin staining (22).
Briefly, for chromogen staining, serial sections were rehydrated, blocked with various blocking reagents, and incubated with primary antibodies (1 μg/ml to 50 μg/ml), followed by the appropriate anti-mouse (R&D Systems, Minneapolis, MN) or anti-rat (Vector Laboratories, Inc., Burlingame, CA) biotinylated IgG secondary antibody (5 μg/ml). Finally, washed slides were incubated with streptavidin-horseradish peroxidase conjugate (R&D Systems), washed, and developed with the substrate 3-amino-9-ethylcarbazole chromogen (R&D Systems). Mayer's hematoxylin (Fisher Diagnostics, Fair Lawn, NJ) was used to counterstain. Slides were preserved by using Crystal Mount aqueous mounting medium solution (Biomedia, Foster City, CA). For dual-color fluorescence, serial sections were treated in ice-cold acetone, blocked with blocking solution, and incubated with the first primary antibody (2 μg/ml to 20 μg/ml), followed by the appropriate biotinylated IgG secondary antibody (5 μg/ml; Vector Laboratories, Inc.), and then incubated with streptavidin-Alexa Fluor 488 (green; 20 μg/ml; Invitrogen, Carlsbad, CA). For the second label, slides were incubated with the second primary antibody (2 μg/ml to 20 μg/ml), followed by secondary antibody labels as described above and stained with streptavidin-Alexa Fluor 555 (red; 20 μg/ml; Invitrogen). Washed slides were rinsed with water, dried at 4°C, and preserved with Vectashield Hard Set mounting medium for fluorescence assay (Vector Laboratories). Slides were viewed on an E600 microscope (Nikon, Melville, NY) with Nikon B-2E/C and G-2E/C filter blocks. Colocalization was determined by image overlay.
RESULTS
Expression of memory and effector markers by CD8+ T cells in oral tissue of OPC+ lesions.
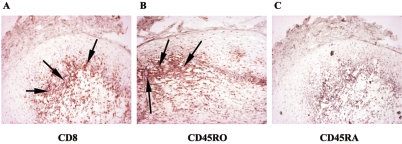
To characterize the CD8+ T cells present in OPC+ tissue, serial sectioned biopsy samples of the buccal mucosa from OPC+ patients with accumulated CD8+ T cells were labeled with anti-CD45RO (memory) and -CD45RA (naive) antibodies, and cells were detected by chromophore. Figure 1 shows a representative result from OPC+ tissue. Isotype-matched controls (not shown) showed no significant staining. The majority of CD8+ cells accumulated at the epithelium (panel A) stained positive for CD45RO (panel B) and negative for CD45RA (panel C).
FIG. 1.
Analysis of the memory phenotype of CD8+ cells in OPC lesions. Representative images of tissues from six OPC+ HIV+ persons are shown. A, CD8; B, CD45RO; C, CD45RA. Magnification, ×100. Arrows point to positively labeled cells.
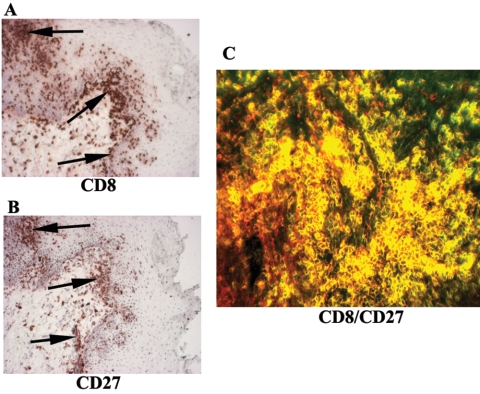
To identify the type of memory T cells present in the OPC+ tissues, serial sections were labeled with anti-CD8 and -CD27 (central memory) antibodies, and cells were detected by chromophore (Fig. 2). Cells staining positive for CD8 (panel A) and CD27 (panel B) were present at similar sites within the tissue. To identify whether both CD8 and CD27 were indeed present on the same cells, tissue sections were dually labeled with anti-CD8 antibody conjugated to streptavidin-Alexa Fluor 488 (green) and anti-CD27 antibody conjugated to streptavidin-Alexa Fluor 555 (red). Figure 2C shows a representative result for OPC+ tissue. Most of the cells positive for CD8 were also positive for CD27 (yellow cells).
FIG. 2.
Identification of memory T-cell subtype in OPC lesions. CD27 expression on CD8+ T cells was evaluated in OPC+ tissue with cell accumulation. Representative images of tissues from seven OPC+ HIV+ persons are shown. Panels A and B are images of chromogen-stained CD8+ and CD27+ cells in OPC+ persons, respectively. Original magnification, ×100. Arrows point to positively labeled cells. Panel C shows CD8+ cells labeled with Alexa Fluor 488 (green) and CD27 receptors labeled with Alexa Fluor 555 (red). Original magnification, ×200.
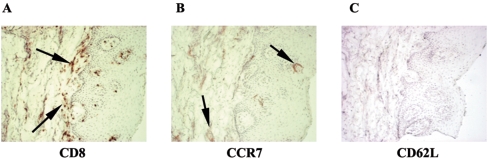
To confirm the type of memory cells present within the lesions, serial sections were labeled with the chemokine receptor CCR7 and the activation marker CD62L, both of which are markers for central memory. The CD8+ T cells (Fig. 3A) lacked both CCR7 (panel B) and CD62L (panel C). However, CCR7 was present in the tissue (panel B) and both markers were present on phytohemagglutinin (PHA)-stimulated PBLs from HIV− persons (data not shown).
FIG. 3.
Further analysis of central memory CD8+ T cells in OPC lesions from HIV-positive persons. OPC+ tissue with accumulated CD8+ T cells was labeled with CCR7 and CD62L. Representative images of tissues from five HIV+ OPC+ persons are shown. A, CD8; B, CCR7; C, CD62L. Magnification, ×100. Arrows point to positive staining.
Expression of tissue and CD8+ cell activation markers in OPC+ tissue.
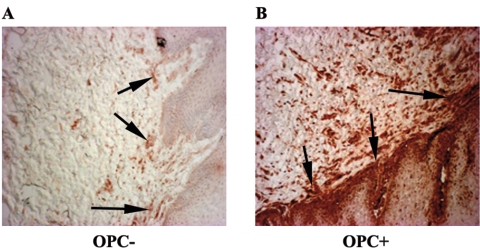
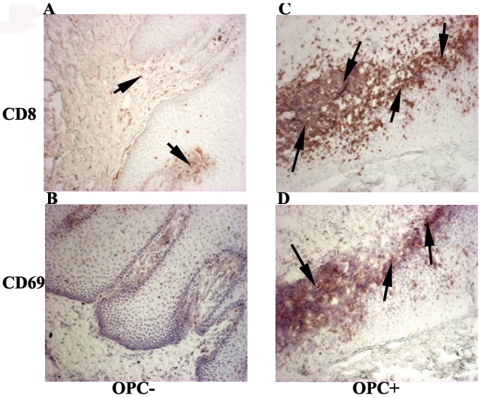
To evaluate the state of immune activation within the OPC+ tissues, biopsy tissues from those with and without OPC were labeled with anti-CD40 antibodies. Figure 4 shows representative results from serial buccal mucosa sections. Results show little CD40-positive staining in OPC− tissue (panel A) and high levels of CD40-positive staining through both the lamina propria and epithelium of OPC+ tissue (panel B). In addition, we examined CD8+ cell activation on the same OPC+ and OPC− tissues by CD69 expression. Representative images in Fig. 5 show that OPC− tissue with no accumulated CD8+ T cells (panel A) had little to no positive staining for CD69 (panel B), whereas high levels of cell-associated CD69 expression are evident in OPC+ tissue (panel D), matching the sites of accumulated presence of CD8+ cells (panel C).
FIG. 4.
Analysis of immune activation in tissue. Tissue was evaluated for CD40 in both OPC− and OPC+ tissues of HIV-positive patients. Representative images of OPC− tissue (panel A) from four HIV+ persons and OPC+ tissue with accumulated CD8+ T cells (panel B) from seven HIV+ patients are shown. Original magnification, ×100. Arrows point to positively stained tissue.
FIG. 5.
Analysis of CD8+ T-cell activation in OPC+ tissue from HIV-positive patients. CD69 expression was evaluated in tissue from three HIV+ OPC− patients and in tissue with accumulated CD8+ T cells from three HIV+ OPC+ patients. Representative images of OPC− (panels A and B) and OPC+ (panels C and D) tissues stained for CD8 (panels A and C) and CD69 (panels B and D) expression are shown. Original magnification, ×100. Arrows point to areas of positively stained cells.
As additional evidence of cellular activation, serial sections of buccal mucosa with OPC lesions were labeled with anti-CD40L antibodies. Staining was predominant negative (data not shown). To examine this further, dual fluorescent labeling and confocal microscopy were used to identify whether both CD8 and CD40L were coexpressed to any degree on PHA-stimulated PBLs from HIV− persons. Results showed little (∼20%) colocalization of CD8 and CD40L (data not shown).
NK T cells in OPC+ tissue.
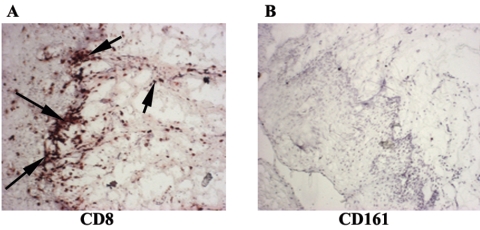
To examine whether the accumulated CD8+ T cells were NK T cells that expressed CD8 (3), serial sections of OPC+ tissue were labeled with anti-CD161 antibodies. Results in Fig. 6 show little to no positive staining for CD161 (panel B), despite the presence of numerous CD8+ cells (panel A).
FIG. 6.
Analysis of NK T cells in OPC+ tissue from HIV-positive persons. CD161 expression was evaluated in tissue with accumulated CD8+ T cells from six HIV+ OPC+ patients. Representative images of tissue stained for CD8 (panel A) and CD161 (panel B) are shown. Original magnification, ×100. Arrows point to positively stained cells.
Evidence for anti-HIV CD8+ T cells.
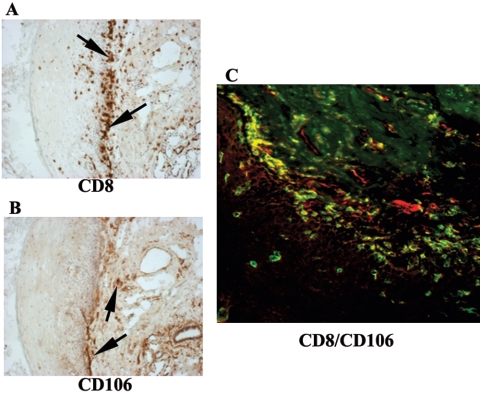
To evaluate whether the accumulated CD8+ cells in OPC+ lesions were similar to anti-HIV CD8+ T cells that express VCAM-1 (6), OPC+ tissue was labeled with anti-CD106 antibodies (VCAM-1) for both single-chromophore and dual-fluorescent confocal detection. Figure 7 shows representative results from serial buccal mucosa sections of OPC+ persons. Positive staining for CD106 is present throughout the tissue (panel B), but it does not appear to match the sites where CD8+ cells are present (panel A). This was confirmed by confocal microscopy, where the majority of cells positive for CD8 were not positive for CD106 (predominately only red and green staining) (panel C).
FIG. 7.
Evaluation of VCAM-1 expression on CD8+ T cells in OPC lesions. CD106 expression was evaluated in tissue with CD8+ T-cell accumulation from seven HIV+ OPC+ patients. Representative images are shown. Panels A and B are images of tissue chromogen stained for CD8 and CD106, respectively. Original magnification, ×100. Arrows point to positively stained areas. (C) CD8+ cells were labeled with Alexa Fluor 488 (green), and CD106 receptors were labeled with Alexa Fluor 555 (red). Original magnification, ×200.
DISCUSSION
The increased presence of CD8+ T cells in OPC+ lesions suggests a role for CD8+ T cells against OPC. However, inasmuch as the presence of the cells suggests some role, the distance of the accumulated T cells from Candida organisms at the outer epithelium indicates a potential dysfunction in either the T cells or the microenvironment that inhibits the migration of the T cells to the organism. A previous study from our group examined this and demonstrated that the accumulated CD8+ cells in OPC+ tissue correlated with a significant reduction in the adhesion molecule E-cadherin, suggesting a dysfunction in the local microenvironment of this subset of OPC+ patients (21). However, there have been no in-depth studies to date investigating the immune status of the accumulated CD8+ cells.
Most of the CD8+ T cells in OPC+ tissue with accumulated CD8+ cells expressed the CD45RO memory T-cell marker rather than the CD45RA “naive” T-cell marker, consistent with memory T cells being present from prior exposure to Candida as a commensal organism. Further characterization of the memory phenotype for the CD8+ cells demonstrated a high percentage of accumulated CD8+ cells expressing CD27. These results suggest that the accumulated CD8+ cells in tissue from OPC lesions are “central” memory cells usually restricted to secondary lymphoid tissue that were recruited to the mucosa in response to the candidal infection. Humphreys et al., who conducted a similar characterization of CD8+ T cells in Haemophilus ducreyi skin lesions, showed a predominance of CD27− (effector memory) CD8+ T cells more appropriately found at mucosal sites (13). Interestingly, additional analyses of OPC+ tissue with two additional markers for central memory cells (CCR7 and CD62L) revealed that the same majority of CD8+ cells lacked CCR7 and CD62L, suggestive of an apparent mixed phenotype with properties of both central and effector memory cells. Given this, several explanations are possible. One is that the cells are indeed effector memory cells, despite the surface expression of CD27, but cannot function because of their reduced migration from the decreased E-cadherin within the tissue. Another is that since the CD8+ T-cell response to Candida infection is not a classic cytotoxic T-lymphocyte response (Candida is not an intracellular pathogen), the cells may represent central memory cells with an atypical mixed phenotype that similarly cannot migrate to the outer epithelium because of reduced tissue E-cadherin. Yet a third explanation is that the cells are effector memory cells in a transitioning phase and are reverting back to central memory cells (regaining CD27) because they have not been able to conduct any effector function because of the lack of an ability to migrate to Candida at the outer epithelium. We favor the latter explanation, but a longitudinal study where these memory cell markers, as well as E-cadherin expression, can be evaluated before, during, and after infection will be important to determine which, if any, of these explanations is correct. Evaluation of additional marker expression may also help clarify the cellular phenotype. Killer cell lectin-like receptor G1 (KLRG1) represents another effector memory marker, while CD57 and CD127 are markers for replicative senescence and longevity, respectively, on effector memory cells (14). If the effector phenotype is dominating, the evaluation of these markers will be useful and possibly provide clues regarding function.
The accumulated CD8+ T cells present within OPC lesions appear to be immune activated. Evidence for this comes from the upregulation of CD40 within the tissue and CD69 expression on the CD8+ T cells. The CD40 expression in the tissue likely comes from antigen-presenting cells (12). We also attempted to evaluate CD40L and CTLA-4 on the CD8+ cells in these studies, but results demonstrated little to no expression. This may be a function of the cellular phenotype where CD40L and CTLA-4 are expressed more on CD4+ than on CD8+ T cells (26), which was confirmed by analyzing PHA-activated PBLs. The costimulatory molecule CD28 is also a useful marker for activation, but similar to CD40L and CTLA-4 in tissue, the CD8+ cells had little to no detectable expression of CD28. This is expected, though, since activated CD8+ T cells in HIV-infected persons have been shown to lose CD28 expression during progression of disease (2, 24).
Taken together, the evidence indicates that, despite these nuances, CD8+ T cells are activated as one would expect of memory T cells recruited in response to infection. Although the increased expression of the adhesion molecule mucosal addressin cell adhesion molecule-1 (21) suggests that, indeed, the CD8+ T cells were recruited to the tissue from the peripheral circulation, it is also possible, on the basis of the atypical phenotype, that the cells are resident mucosal cells expanded within the tissue. In relation to this concept, studies have shown that some CD8+ T cells in mucosal tissues are actually NK T cells on the basis of their expression of CD161 (3, 15). Such cells provide innate surveillance at mucosal sites to protect against infectious breeches. Thus, despite the memory phenotype, but taking into account a lack of evidence for Candida antigen specificity, it was reasonable to investigate for the presence of NK T cells. However, the lack of CD161 expression on the cells significantly reduces this possibility. This also provides additional evidence for cellular recruitment rather than resident mucosal cellular expansion.
Finally, on the basis of a study by Diaz et al. showing that peripheral anti-HIV CD8+ T cells present in HIV-infected persons could be identified by the unlikely expression of surface VCAM-1, a cell adhesion molecule found on endothelial cells (6), it was reasonable to evaluate this phenotype in CD8+ cells in OPC. If so, CD8+ T cells could be present because of HIV infection and not Candida infection. However, it appears that the cells are not part of the HIV response repertoire on the basis of the absence of cellular VCAM-1, although tissue expression of VCAM-1 served as a representative positive control.
On the basis of our present data, as well as our prior data (21, 22), the lesion-associated CD8+ T cells appear to be normal relative to their activation and presence within the tissue. The issue of a central versus an effector memory phenotype remains unclear, but this interesting finding may be a function of the response to Candida and/or the cells being inhibited from migrating further in the tissue to function against Candida. The evidence for activation status is somewhat limited or atypical, but this is not uncommon for CD8+ T cells. Future studies will attempt to evaluate the effector function of CD8+ T cells. We may identify an interesting scenario with atypical memory cells having an atypical or nonclassical effector function against Candida. Indeed, Beno et al. have shown that interleukin-2 (IL-2)-activated CD8+ mouse splenocytes are capable of inhibiting the growth of Candida in a non-major histocompatibility complex-restricted manner (1) and a similar population of cells was identified in PBLs of HIV+ persons with a recent episode of OPC (5). This is entirely possible given that IL-2 is increased within OPC lesions along with IL-15 and gamma interferon, among other cytokines and chemokines (19).
Taken together, the evidence indicates that tissue adhesion molecules (E-cadherin), and not CD8+ T cells, appear to be a major factor in susceptibility to OPC with reduced tissue-associated E-cadherin as a dysfunction contributing to OPC in those HIV+ persons with low CD4+ T-cell levels. Current studies are evaluating tissue-associated E-cadherin and the memory phenotype of CD8+ T cells in a longitudinal analysis. These and future studies will shed considerable light on the function of CD8+ T cells against Candida and the immune factors and mechanisms associated with susceptibility to OPC when CD4+ T cells are not available.
Acknowledgments
This work was supported by a National Institutes of Health Public Health Service grant (DE-12178) from the National Institute of Dental and Craniofacial Research.
REFERENCES
- 1.Beno, D. W. A., A. G. Stover, and H. L. Mathews. 1995. Growth inhibition of Candida albicans hyphae by CD8+ lymphocytes. J. Immunol. 154:5273-5281. [PubMed] [Google Scholar]
- 2.Burgisser, P., C. Hammann, D. Kaufmann, M. Battegay, O. Rutschmann, and The Swiss HIV Cohort Study. 1999. Expression of CD28 and CD38 by CD8+ T lymphocytes in HIV-1 infection correlates with markers of disease severity and changes towards normalization under treatment. Clin. Exp. Immunol. 115:458-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cameron, A., B. Kirby, W. Fei, and C. Griffiths. 2002. Natural killer and natural killer-T cells in psoriasis. Arch. Dermatol. Res. 294:363-369. [DOI] [PubMed] [Google Scholar]
- 4.Clift, R. A. 1984. Candidiasis in the transplant patient. Am. J. Med. 77(Suppl. 4D):34-38. [PubMed] [Google Scholar]
- 5.Colon, M., N. Toledo, C. L. Valiente, N. Rodriquez, N. Yano, H. L. Mathews, and Y. Yamamura. 1998. Anti-fungal and cytokine producing activities of CD8+ T lymphocytes from HIV-1 infected individuals. Bol. Asoc. Med. Puerto Rico 90:21-26. [PubMed] [Google Scholar]
- 6.Diaz, L., H. Foster, M. Stone, S. Fujimura, D. Relman, and J. Levy. 2005. VCAM-1 expression on CD8+ cells correlates with enhanced anti-HIV suppressing activity. J. Immunol. 174:1574-1579. [DOI] [PubMed] [Google Scholar]
- 7.Dodd, C. L., D. Greenspan, M. H. Katz, J. L. Westenhouse, D. W. Feigal, and J. S. Greenspan. 1991. Oral candidiasis in HIV infection: pseudomembranous and erythematous candidiasis show similar rates of progression to AIDS. AIDS 5:1339-1343. [PubMed] [Google Scholar]
- 8.Fichtenbaum, C. J., S. L. Koletar, C. Yiannoutsos, F. Holland, J. Pottage, S. E. Cohn, A. Walanwander, P. Frame, J. Feinberg, M. S. Saag, C. Van der Horst, and W. G. Powderly. 2000. Refractory mucosal candidiasis in advanced human immunodeficiency virus infection. Clin. Infect. Dis. 30:749-756. [DOI] [PubMed] [Google Scholar]
- 9.Fidel, P. L., Jr. 2002. Distinct protective host defenses against oral and vaginal candidiasis. Med. Mycol. 40:359-375. [PubMed] [Google Scholar]
- 10.Fisher-Hoch, S. P., and L. Hutwagner. 1995. Opportunistic candidiasis: an epidemic of the 1980s. Clin. Infect. Dis. 21:897-904. [DOI] [PubMed] [Google Scholar]
- 11.Greenspan, J. S., C. E. Barr, J. J. Sciubba, and J. R. Winkler. 1992. Oral manifestations of HIV infection: definitions, diagnostic criteria and principles of therapy. Oral Surg. Oral Med. Oral Pathol. 73:142-144. [DOI] [PubMed] [Google Scholar]
- 12.Hajishengallis, G., H. Sojar, R. Genco, and E. DeNardin. 2004. Intracellular signaling and cytokine induction upon interactions of Porphyromonas gingivalis fimbriae with pattern-recognition receptors. Immunol. Investig. 33:157-172. [DOI] [PubMed] [Google Scholar]
- 13.Humphreys, T., L. Baldridge, S. Billings, J. Campbell, and S. Spinola. 2005. Trafficking pathways and characterization of CD4 and CD8 cells recruited to the skin of human experimentally infected with Haemophilus ducreyi. Infect. Immun. 73:3896-3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ibegbu, C., Y. Xu, W. Harris, D. Maggio, J. Miller, and A. Kourtis. 2005. Expression of killer cell lectin-like receptor G1 on antigen-specific human CD8+ T lymphocytes during active, latent, and resolved infection and its relation with CD57. J. Immunol. 174:6088-6094. [DOI] [PubMed] [Google Scholar]
- 15.Iiai, T., H. Watanabe, T. Suda, H. Okamoto, T. Abo, and K. Hatakeyama. 2002. CD161+ T (NT) cells exist predominately in human intestinal epithelium as well as liver. Clin. Exp. Immunol. 129:92-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klein, R. S., C. A. Harris, C. B. Small, B. Moll, M. Lesser, and G. H. Friedland. 1984. Oral candidiasis in high-risk patients as the initial manifestation of the acquired immunodeficiency syndrome. N. Engl. J. Med. 311:354-357. [DOI] [PubMed] [Google Scholar]
- 17.Knight, L., and J. Fletcher. 1971. Growth of Candida albicans in saliva: stimulation by glucose associated with antibiotics, corticosteroids and diabetes mellitus. J. Infect. Dis. 123:371-377. [DOI] [PubMed] [Google Scholar]
- 18.Laskaris, G., M. Hadjivassiliou, and J. Stratigos. 1992. Oral signs and symptoms in 160 Greek HIV-infected patients. J. Oral Pathol. 21:120-123. [DOI] [PubMed] [Google Scholar]
- 19.Lilly, E., D. J. Hart, J. E. Leigh, S. Hager, K. M. McNulty, D. E. Mercante, and P. L. Fidel, Jr. 2004. Tissue-associated cytokine expression in HIV-positive persons with oropharyngeal candidiasis. J. Infect. Dis. 190:605-612. [DOI] [PubMed] [Google Scholar]
- 20.Macher, A. M. 1988. The pathology of AIDS. Public Health Rep. 103:246-254. [PMC free article] [PubMed] [Google Scholar]
- 21.McNulty, K. M., J. Plianrungsi, J. E. Leigh, D. E. Mercante, and P. L. Fidel, Jr. 2005. Characterization of CD8+ T cells and microenvironment in oral lesions of human immunodefiency virus-infected persons with oropharyngeal candidiasis. Infect. Immun. 73:3659-3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Myers, T. A., J. E. Leigh, A. Arribas, S. Hager, R. A. Clark, E. Lilly, and P. L. Fidel, Jr. 2003. Immunohistochemical evaluation of T cells in oral lesions from human immunodeficiency virus-positive persons with oropharyngeal candidiasis. Infect. Immun. 71:956-963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Odds, F. C. 1988. Candida and candidosis, p. 104-110. University Park Press, Baltimore, Md.
- 24.Paul, M., W. Shearer, C. Kozinetz, and D. Lewis. 2001. Comparison of CD8+ T-cell subsets in HIV-infected rapid progressor children versus non-rapid progressor children. J. Allergy Clin. Immunol. 108:258-264. [DOI] [PubMed] [Google Scholar]
- 25.Slavinsky, J., III, T. Myers, R. K. Swoboda, J. E. Leigh, S. Hager, and P. L. Fidel, Jr. 2002. Th1/Th2 cytokine profiles in saliva of HIV-positive smokers with oropharyngeal candidiasis. Oral Microbiol. Immunol. 17:38-43. [DOI] [PubMed] [Google Scholar]
- 26.Steiner, K., I. Waase, T. Rau, M. Dietrich, B. Fleischer, and B. M. Broker. 1999. Enhanced expression of CTLA-4 (CD152) on CD4+ T cells in HIV infection. Clin. Exp. Immunol. 115:451-457. [DOI] [PMC free article] [PubMed] [Google Scholar]