Abstract
Mycobacterium bovis-infected white-tailed deer (WTD) in northeast Michigan are a reservoir of mycobacteria that pose a threat to both domestic animals and humans. Relatively little work has been done to characterize the immune response of WTD to M. bovis infection; however, an understanding of the immune response to infection and pathogenesis may be critical to the development of an effective vaccine. Immunological responses to infection were characterized by monitoring cytokine gene expression in M. bovis-infected and uninfected deer. Peripheral blood leukocytes (PBL) from infected WTD expressed more gamma interferon (IFN-γ), interleukin-12p40 (IL-12p40), granulocyte-monocyte colony-stimulating factor, and IL-4 mRNA than did PBL from uninfected deer; however, differences were not detected in expression of IL-10 and transforming growth factor-β mRNA. Infected animals could be divided into two groups based on pathology. Lesions were confined primarily to the lymph nodes of the head in animals with less severe pathology. Animals with more severe pathology had lesions in the lung and associated lymph nodes as well as the lymph nodes of the head. More robust IFN-γ mRNA expression correlated with pathology early in infection. These findings indicate that IFN-γ expression likely plays a role in both protection and pathogenesis.
In 1994, tuberculosis caused by Mycobacterium bovis was detected in free-ranging white-tailed deer (WTD) in northeastern Michigan (33). Subsequent surveys revealed a focus of infected deer (16-18), the first known wildlife reservoir of M. bovis in the United States. This reservoir poses a significant risk to domestic livestock and humans. The experiences of other developed countries with wildlife reservoirs of M. bovis have demonstrated that the eradication of tuberculosis in domestic livestock is nearly impossible, presumably because of the continued transmission of the bacteria from the wildlife reservoir to domestic livestock (2, 4). Therefore, the elimination of M. bovis in the free-ranging WTD population is essential to the U.S. bovine tuberculosis eradication program. An effective vaccine for WTD would be a valuable aid in the elimination of M. bovis from deer populations. Vaccine development often requires the understanding of protective immune responses; however, only limited research has been done characterizing WTD immune responses to M. bovis infection.
Immune responses are generally divided into two general types, T helper type 1 (TH1) and T helper type 2 (TH2), based on the type of cytokines produced upon antigenic stimulation. TH1 responses are characterized primarily by the production of gamma interferon (IFN-γ) and interleukin-12 (IL-12), whereas the production of IL-4 and IL-10 characterize a TH2 response (1).
Studies in mice suggest that immunity to mycobacteria is mediated by a TH1 response dominated by the production of IFN-γ (15). An effective TH1 response limits bacterial growth and pathology; however, it does not eliminate the pathogen (7, 15). Conversely, it has been proposed that the development of a TH2 response results in disease progression. The WTD is a good model for the study of cytokine expression for several reasons; WTD are naturally infected with M. bovis, natural infection can be mimicked by intratonsillar infection (22), and low-dose infection produces variable pathology. Current knowledge of the immune responses of WTD to M. bovis infection is limited. It has been reported that infected WTD produce IFN-γ and develop a delayed-type hypersensitivity reaction and produce M. bovis-specific antibody (23, 25, 38, 40). Cytokines other than IFN-γ may also contribute to the immune responses to and pathogenesis of M. bovis. Using this model of infection, we sought to determine the contributions of TH1 (IFN-γ, IL-12, and granulocyte-monocyte colony-stimulating factor [GM-CSF]) and TH2 (IL-4, IL-10, and transforming growth factor β [TGF-β]) cytokines to the pathogenesis of M. bovis. Cytokine gene expression was assessed in peripheral blood leukocytes (PBL) from infected and uninfected WTD at various time points after infection. PBL were stimulated with either a complex protein mixture of purified protein derivative (PPD) from M. bovis or a recombinant fusion protein from M. bovis, 6-kDa early secretory antigenic target and 10-kDa culture filtrate protein (rESAT6:CFP10). ESAT6:CFP10 is a dominant antigenic protein produced by M. bovis (26) and has been used to increase the specificity of diagnostic assays (36).
In this study, it is reported that infected animals expressed more IFN-γ, IL-12p40, GM-CSF, inducible nitric oxide synthase (iNOS), and IL-4 mRNA than did uninfected animals. More robust IFN-γ expression early in infection correlated with increased pathology.
MATERIALS AND METHODS
Animals.
Ten 12-month-old WTD (castrated males and females) were inoculated with M. bovis, while five uninfected deer served as controls. Deer were experimentally inoculated by intratonsillar instillation of 300 CFU of M. bovis strain 1315 into each tonsil as previously described (22). Strain 1315 was originally isolated from a WTD in Michigan in 1995. Deer were housed two to three per pen; each pen was approximately 16 square meters and located inside a biosafety level 3 building with directional airflow to prevent room-to-room transfer of air. Deer had access to a circulating watering device and were fed deer and elk complete feed 55P3 (Purina Mills, St. Louis, MO). Five naïve, noninoculated control deer were housed similarly but in a separate building with no direct contact with inoculated deer. All animals were observed twice daily by animal care or veterinary staff. A protocol detailing the experimental and animal care procedures was approved by the Institutional Animal Care and Use Committee prior to the experiment.
Inoculum preparation.
Inoculum was prepared as described previously (20). Briefly, mid-log-phase M. bovis was harvested from the culture broth by centrifugation, washed twice with 1 ml of phosphate-buffered saline (PBS) solution (pH 7.2) diluted to the appropriate density and then stored at −80°C until needed. At the time of inoculation, aliquots of inoculum were thawed and diluted to 300 CFU per 100 μl. Plate counts were repeated on the day of inoculation to retrospectively confirm the inoculum dosage.
Leukocyte preparation and culture.
Total PBL were prepared from the buffy coat fraction of peripheral blood collected in 2× acid citrate dextrose. Contaminating red blood cells were removed by hypotonic lysis as described previously (11, 30).
Blastogenesis assay.
PBL were seeded into 96-well, round-bottom microtiter plates (Falcon; Becton-Dickinson, Lincoln Park, N.J.) at 2 × 105 cells in a total volume of 200 μl of complete RPMI (RPMI 1640 supplemented with 2 mM l-glutamine, 25 mM HEPES buffer, 100 units/ml penicillin, 0.1 mg/ml streptomycin, 1% nonessential amino acids [Sigma, St. Louis, MO], 2% essential amino acids [Sigma], 1% sodium pyruvate [Sigma], 50 μM 2-mercaptoethanol [Sigma], and 10% [vol/vol] fetal bovine serum). Wells contained medium plus 10 μg/ml M. bovis PPD (CSL Animal Health, Parkville, Victoria, Australia), 10 μg/ml Mycobacterium avium PPD (CSL Animal Health), 10 μg/ml rESAT6:CFP10 (kindly provided by F. Chris Minion [37]), and 1 μg/ml pokeweed mitogen (Sigma) or medium alone (no stimulation). Responses to pokeweed mitogen provided an indication of the general responsiveness of PBL to polyclonal stimulation. Leukocyte cultures were incubated for 6 days at 37°C in a 5% CO2 atmosphere. After 6 days, 0.5 μCi of [methyl-3H]thymidine (specific activity, 6.7 Ci mmol−1; Amersham Life Science, Arlington Heights, IL) in 50 μl of medium was added to each well and cells were incubated for an additional 20 h. Well contents were harvested onto glass fiber filters with a 96-well plate harvester (EG&G Wallac, Gaithersburg, MD), and the incorporated radioactivity (counts per minute [cpm]) were measured by liquid scintillation counting. Treatments were run in triplicate.
Enzyme-linked immunosorbent assay.
Lipoarabinomannan-enriched mycobacterial protein was prepared, and the enzyme-linked immunosorbent assay (ELISA) was performed as described previously (38). Briefly, bacilli harvested from 4-week cultures were sonicated and then disrupted with glass beads. The lysate was clarified by filtration using 0.45-μm and 0.22-μm filters. The clarified lysate was digested with proteinase K, and the protein concentrations were determined (Bio-Rad, Hercules, CA). Immulon II 96-well microtiter plates (Dynatech, Chantilly, Virginia) were coated with 100 μl/well (4 μg) antigen diluted in carbonate-bicarbonate coating buffer (pH 9.6). Antigen-coated plates, including control wells containing coating buffer alone, were incubated for 15 h at 4°C. Test and control sera were diluted 1:100 in PBS containing 0.1% gelatin. After incubation for 20 h at 4°C with diluted test sera, wells were washed nine times with 200 μl/well PBS-Tween 20 (PBST) and incubated for 1 h at 37°C with 100 μl/well of biotin-protein G (Sigma) diluted 1:5,000 in PBS plus 0.1% gelatin. Wells were washed nine times with 200 μl/well PBST and incubated for 1 h at 37°C with 100 μl/well of peroxidase-streptavidin (Sigma) diluted 1:2,000 in PBS plus 0.1% gelatin. Wells were washed nine times with 200 μl/well PBST and incubated for 4 min at room temperature with 100 μl/well of SureBlue TMB microwell peroxidase substrate (Kirkegaard and Perry Laboratories). The reaction was stopped by the addition of 100 μl/well of 0.18 M sulfuric acid, and the absorbance (450 nm) of individual wells was measured using an automated ELISA plate reader (Molecular Devices, Menlo Park, CA). Data are presented as change in optical density (OD) calculated by subtracting the mean OD for wells receiving coating buffer alone (two replicates) from the mean OD for antigen-coated wells (two replicates) receiving the same serum sample.
Cells for RNA analysis.
PBL were seeded into wells of 96-well, round-bottom microtiter plates (Falcon) at 1 × 106 in a total volume of 200 μl of complete RPMI. Cells were stimulated as previously described for blastogenesis assays.
Isolation of RNA and reverse transcription.
Cells were pelleted by centrifugation, and the supernatant was removed. Cells were lysed with 200 μl buffer RLT (QIAGEN, Valencia, CA) according to the manufacturer's directions and stored at −80°C. RNA was isolated using an RNeasy mini kit (QIAGEN) according to the manufacturer's directions and eluted from the column with 50 μl RNase-free water (Ambion, Austin, TX). Any contaminating DNA was enzymatically removed by treating with DNA-free (Ambion) as directed by the manufacturer. A 50-μl reverse-transcription reaction was carried out by first adding 1 μg of oligo(dT)12-18 (Invitrogen, Carlsbad, CA) to 20 μl of the RNA preparation. The RNA and primers were then heated to 70°C for 5 min and rapidly cooled to 4°C. A reverse transcriptase master mix (Superscript II; Invitrogen), prepared according to the manufacturer’s directions, was then added. Reverse transcription was then carried out at 42°C for 60 min. Reverse transcriptase was inactivated by heating to 70°C for 5 min. The resulting cDNA was stored at −80°C until used in real-time PCRs.
Real-time PCR for cytokine genes.
Real-time PCR was performed using SYBR green master mix (Applied Biosystems, Foster City, CA) according to the manufacturer's directions. Briefly, 2.5 μl of cDNA was added to a 25-μl reaction with 1 μM of each primer. Primers were designed with Primer3 (31) using sequences from related species Cervus elaphus (red deer), and when red deer sequences were not available, sequences from cattle (Bos taurus) were used (Table 1). PCR products were sequenced to verify the primers. In each case, the product from WTD was ≥98% similar to the sequences used to create the primers for the region sequenced. All reactions were performed in triplicate, and data were analyzed with the 2−ΔΔCT method as described previously (13). β-Actin served as the internal control, and the media-only (no stimulation) sample from each animal was used as the calibrator. Validation of the use of β-actin as the internal control was performed as suggested by Livak and Schmittgen (13).
TABLE 1.
Cytokine primer sequences and their origin
| Gene | Primer sequence | Speciesa | Referenceb |
|---|---|---|---|
| IFN-γ forward | TTCTTGAATGGCAGCTCTGA | Cervus elaphus | L07502.1 |
| IFN-γ reverse | GATTTTGGCGACAGGTCATT | Cervus elaphus | L07502.1 |
| IL-12p40 forward | CTGCCCATTGAGGTCGTAGT | Cervus elaphus | U57752.1 |
| IL-12p40 reverse | TGAAGAAGCTGCTGGTGTAGTT | Cervus elaphus | U57752.1 |
| IL-4 forward | CCACACGTGCTTGAACAGAT | Cervus elaphus | L07081.1 |
| IL-4 reverse | TCGTCTTGGCTTCATTCACA | Cervus elaphus | L07081.1 |
| IL-10 forward | CAGGATGGTGACTCGACAGA | Cervus elaphus | U11767.1 |
| IL-10 reverse | AGGGAGCTGGTTCTGCTCTT | Cervus elaphus | U11767.1 |
| Beta-actin forward | CGCCATGGATGATGATATTGC | Bos taurus | BRTPLc |
| Beta-actin reverse | AAGCCGGCCTTGCACAT | Bos taurus | BRTPLc |
| GM-CSF forward | CAGAAGTGGAAGCTTACCTCACAGA | Bos taurus | BRTPLc |
| GM-CSF reverse | CCTCCAGTGTGAAGATCCTGAGTT | Bos taurus | BRTPLc |
| TGF-β1 forward | CTGAGCCAGAGG CGGACTAC | Bos taurus | 5 |
| TGF-β1-reverse | TGCCGTATTCCACCATTAGCA | Bos taurus | 5 |
| iNOS-forward | ATCTGCAGACACGTGCGTTA | Bos taurus | AJ699400.1 |
| iNOS-reverse | GTTCCAGACCCGGAAGTCAT | Bos taurus | AJ699400.1 |
Species used to generate the primer sequence.
GenBank accession number of the sequence used to generate the primer or the reference to a published sequence.
Bovine real-time primer list [version 8/6/2004]. Michigan State University Center for Animal Functional Genomics. (http://gowhite.ans.msu.edu/primers/realtimePRIMERlist.xls).
Necropsy.
Seven months after inoculation, all deer were euthanized by intravenous administration of sodium pentobarbital. A thorough postmortem examination was done. Lungs and lymph nodes were subjected to semiquantitative scoring of gross lesions adapted from the method of Vordermeier et al. (35). Lung lobes (left cranial, left caudal, right cranial, right caudal, middle, and accessory) were subjected to the following scoring system: 0, no visible lesions; 1, no external gross lesions, but lesions seen upon slicing; 2, <5 gross lesions of <10 mm in diameter; 3, >5 gross lesions of <10 mm in diameter; 4, >1 distinct gross lesion of >10 mm in diameter; and 5, coalescing gross lesions. The scoring of lymph node gross lesions was based on the following scoring system: 0, no visible lesions; 1, small focal lesions of 1 to 2 mm in diameter; 2, several small foci; and 3, extensive lesions. Specimens collected for bacteriologic culture and microscopic examination included tonsil, lung, liver, and mandibular, parotid, medial retropharyngeal, tracheobronchial, mediastinal, mesenteric, hepatic, and prefemoral lymph nodes. Specimens for bacteriologic culture were placed individually in sterile bags and stored at −80°C until processing. Specimens were processed as previously described (22). Mycobacterial isolates were identified using standard growth and biochemical characteristics. Isolates were confirmed to belong to the Mycobacterium tuberculosis complex by genetic probe analysis using AccuProbe (Gen-Probe, Inc., San Diego, CA). Results were considered positive if M. bovis was isolated.
Samples for microscopic examination were fixed in neutral buffered 10% formalin and processed by routine paraffin-embedment techniques. Sections (3-μm thick) were stained with hematoxylin and eosin and examined by light microscopy. Adjacent 3-μm sections were cut from specimens with lesions suggestive of tuberculosis (caseonecrotic granulomata) and stained by the Ziehl-Neelsen technique for the visualization of acid-fast bacteria. Microscopic findings were considered positive when lesions consistent with tuberculosis contained acid-fast bacilli.
Statistical analysis.
To account for repeated measurements on the same experimental unit and therefore correlation of observations with an experimental unit, a mixed linear model using the method of restricted maximum likelihood was used to fit the data. The correlation structure specified for observations within an experimental unit was first-order autoregressive (12). The model was implemented in PROC MIXED SAS 9.1.3 (SAS Institute, Inc., Cary, NC). For each stimulus and gene, the outcome variable was log (base 2) transformed. The explanatory variables used to explain the outcome were infection, a categorical variable with two levels (infected or uninfected), and pathology, a categorical variable with three levels (high, low, and none); the day postinfection (p.i.) was also a categorical variable, with eight levels. Changes in the associations between the outcome and the explanatory variable over time were examined by including in interaction term between pathology or infection and time. If the interaction term was not significant, this term was dropped from the model. The fit of the models was assessed by using standard residual plots and examining the influence statistics for each experimental unit using PRESS, Cook's D, and restricted likelihood distance. For data presentation purposes, graphs reporting the level of the outcome for each group at each time point were plotted using nontransformed data. A P value of less than 0.05 was considered significant. Correlation was calculated using the PROC CORR function. The Spearman rank correlation was calculated for correlations between pathology and gene expression, while a Pearson product-moment correlation was calculated for cytokine-to-cytokine correlations.
RESULTS
Blastogenic responses and antibody production.
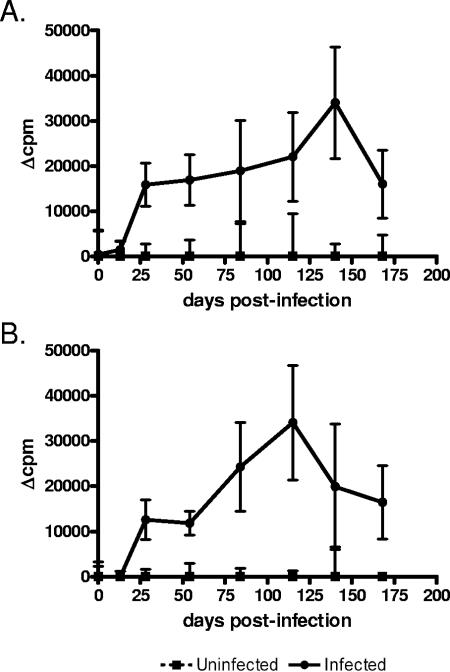
Ten WTD were inoculated with M. bovis strain 1315. Tritiated thymidine uptake assays were performed with PBL from infected and uninfected animals as an indicator of lymphocyte blastogenesis (Fig. 1). Stimulation with either PPD or rESAT6:CFP10 resulted in lymphocyte blastogenesis, indicating that WTD developed antigen-specific responses. Antigen-specific proliferation was clearly evident by 28 days after infection and was sustained for the remainder of the experiment.
FIG. 1.
Blastogenic responses of leukocytes from infected and uninfected WTD to M. bovis antigens. PBL were stimulated with PPD (A) or rESAT6:CFP10 (B). The changes in cpm (Δcpm) were calculated by subtracting the cpm of the unstimulated control cultures from the cultures with specific antigen. Data were standardized by subtracting the values for the uninfected animals from the values for the infected animals at each time point. Data are presented as the means ± SEMs (error bars). Proliferative responses of infected animals were greater than response of uninfected animals (P < 0.01).
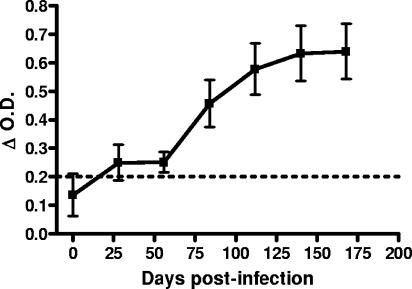
Antibody to M. bovis was detected over the course of infection by ELISA (Fig. 2). Five animals had antibodies to M. bovis-lipoarabinomannan by 28 days p.i. (data not shown). All animals produced M. bovis-lipoarabinomannan antibody by 56 days postinfection and antibody levels continued to increase through 140 days p.i., at which time antibody concentration appeared to stabilize.
FIG. 2.
M. bovis-specific antibodies in sera from infected WTD. M. bovis-specific antibody in sera from experimental animals collected at the indicated time points was measured by ELISA. The change in OD (ΔOD) was calculated by subtracting the mean OD of the control from the sample OD. Data from infected animals are represented as the mean ODs ± SEMs (error bars). An OD greater than 0.2 (dotted line) was considered positive. After 56 days postinfection values were different from preinfection (P < 0.03).
Cytokine gene expression.
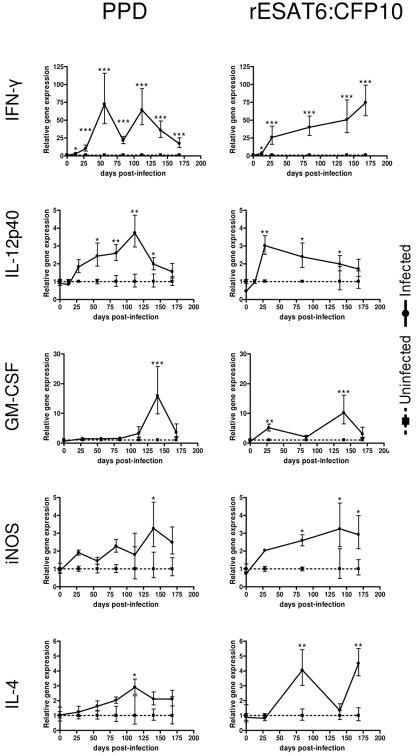
TH1 and TH2 responses were characterized by analyzing cytokine mRNA expression in PBL stimulated with either PPD or rESAT6:CFP10. In general, PBL from infected animals produced significantly more IFN-γ, IL-12p40, GM-CSF, iNOS, and IL-4 mRNA over the course of infection than did PBL from uninfected animals (Fig. 3). IL-10 and TGF-β were not expressed differently between the infected and uninfected animals.
FIG. 3.
Cytokine gene expression. Gene expression was measured in PBL from infected and uninfected animals stimulated with PPD or rESAT6:CFP10. Data were normalized by subtracting the control animals from the infected animals. Data are presented as the means ± SEMs (error bars). Data show infected versus uninfected animals at each time point. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Expression of IFN-γ in PBL from infected animals stimulated with PPD was detected as early as 14 days p.i. At this time point, 4 out of the 10 infected animals expressed levels of IFN-γ in response to PPD at least twice those expressed by the uninfected controls. Peak expression in response to PPD was at 56 days p.i. and at 112 days p.i., after which time IFN-γ expression decreased (Fig. 3). In contrast, IFN-γ expression by PBL stimulated with rESAT6:CFP10 continued to increase throughout the experiment, reaching a 74-fold increase in expression over that of controls.
Expression of IL-12p40 in PPD-stimulated cells increased from day 13 until day 112 p.i., at which time the average expression was 3.9-fold greater than that of controls (Fig. 3). After day 112 p.i., IL-12p40 expression decreased to a level 1.5-fold greater than that of controls. IL-12p40 expression in PBL stimulated with rESAT6:CFP10 was greatest at 28 days and then declined through the conclusion of the experiment. In this system, IL-12p40 and IFN-γ expression in PBL from infected animals was positively correlated whether stimulated with PPD (r = 0.77; P < 0.0001) or rESAT6:CFP10 (r = 0.62; P < 0.0001).
GM-CSF expression was greater in PBL from infected animals compared to that of uninfected controls at some time points (Fig. 3). GM-CSF expression in PPD-stimulated PBL was not appreciable until after 84 days p.i., with peak expression being 15-fold greater than that of controls. The level of GMCSF expression in PBL stimulated with rESAT6:CFP10 ranged from 3- to 10-fold greater than that in controls.
iNOS, although not a cytokine, is an indicator of macrophage activation and is induced by IFN-γ (6). The expression of the iNOS gene was greater in PBL from infected animals than in the controls (Fig. 3). The expression of iNOS in PBL from infected animals was approximately twofold greater than that in controls at 28 days p.i. and increased to approximately threefold over that in controls by day 140 p.i. Similar results were obtained when PBL were stimulated with rESAT6:CFP10. GM-CSF and iNOS expression were positively correlated in both PPD (r = 0.88; P < 0.0001) and rESAT6:CFP10 (r = 0.83; P < 0.0001).
IL-4 expression was induced by M. bovis antigen in infected animals (Fig. 3). In PPD-stimulated PBL from infected animals, expression was not significantly different from PBL from uninfected animals until day 112 p.i., at which time the expression was 2.8-fold greater than that of controls. Stimulation with rESAT6:CFP10 resulted in oscillating expression of IL-4.
Pathology.
All inoculated animals developed lesions (Table 2). Infected animals were divided into two groups based on their overall pathology score. Animals with low pathologies had a total pathology score that was less than or equal to eight. Their lesions tended to be confined to the lymph nodes of the head. Only two out of the five low-pathology animals had lesions in the lungs. In contrast, animals with total pathology scores greater than or equal to 15 had more severe lesions, with greater dissemination in the lungs. Pathology of the medial retropharyngeal lymph node was positively correlated with overall pathology (r = 0.82; P = 0.0002).
TABLE 2.
Pathology scoresa
| Tissue type | Animal no.b
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 601 | 561 | 607 | 571 | 604 | 574 | 563 | 528 | 729 | 576 | |
| Lymph nodes | ||||||||||
| Tonsil | 0 | 0 | 1 | 1 | 0 | 2 | 0 | 0 | 1 | 3 |
| Mandibular | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 2 | 2 |
| Parotid | 0 | 1 | 0 | 0 | 3 | 0 | 0 | 0 | 2 | 1 |
| Medial retropharyngeal | 2 | 3 | 2 | 2 | 3 | 3 | 3 | 1 | 3 | 3 |
| Tracheobronchial | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 3 | 2 | 3 |
| Mediastinal | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Hepatic | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Mesenteric | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Prefemoral | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Lung lobes | ||||||||||
| Right cranial | 0 | 0 | 2 | 0 | 0 | 3 | 4 | 3 | 3 | 5 |
| Right/middle caudal | 0 | 0 | 0 | 0 | 0 | 3 | 3 | 3 | 3 | 5 |
| Left cranial | 0 | 0 | 0 | 2 | 0 | 0 | 3 | 3 | 3 | 5 |
| Left caudal | 0 | 0 | 0 | 2 | 0 | 2 | 3 | 3 | 3 | 5 |
| Accessory | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 3 | 0 | 5 |
| Total score | 2 | 4 | 5 | 7 | 8 | 15 | 19 | 19 | 22 | 45 |
Tissues were scored as described in Materials and Methods.
Lesions were not present in uninfected controls.
Cytokine expression and pathology.
The expression of IFN-γ, IL-12p40, GM-CSF, and iNOS was compared between the high- and low-pathology groups to assess associations between TH1 cytokine gene expression and pathology. Expressions of IL-12p40, GM-CSF, and iNOS were not different between the pathology groups.
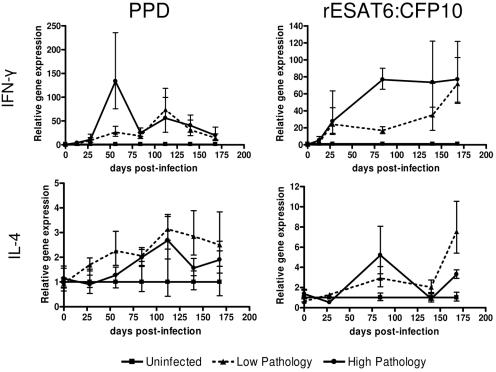
Animals in the high-pathology group expressed more IFN-γ by 56 days p.i. than did animals in the low-pathology group (Fig. 4). At 56 days p.i., IFN-γ expression positively correlated with pathology (r = 0.87; P = 0.0003). IFN-γ expression measured on days 28 and 84 p.i. also correlated with pathology, although the correlations were weaker (r = 0.719 and 0.721, respectively). As infection progressed, gene expression of the two pathology groups became similar.
FIG. 4.
Cytokine expression in animals with differing pathologies. Animals were divided into groups based upon their total pathology score. Animals with total pathology scores of greater than 15 were placed in the high-pathology group. Infected animals with pathology scores of less than eight were placed in the low-pathology group. Uninfected animals are included for comparison. Data were normalized by subtracting the control animals from the infected animals at each time point. Data are represented as the means ± SEMs (error bars).
Recombinant ESAT6:CFP10 stimulation resulted in IFN-γ expression with a different expression pattern between the high- and low-pathology groups. PBL from animals with low pathologies produced less IFN-γ than did PBL from animals in the high-pathology group (Fig. 4). At 84 days p.i., the high-pathology group expressed 5.6-fold more IFN-γ than did the animals in the low-pathology group. IFN-γ gene expression strongly correlated with pathology (r = 0.96; P < 0.0001) at this time point. As the infection progressed, expression of IFN-γ in the low-pathology group increased to equal that of the high-pathology group.
In addition to TH1 cytokines, TH2 cytokines may contribute to pathogenesis. The expressions of IL-4 and IL-10 between the high- and low-pathology groups were compared. IL-10 expressions between the high- and low-pathology groups were not different. IL-4 expression in response to PPD was greater in the low-pathology group than that of the high-pathology group (Fig. 4). Although the differences were not statistically significant, the animals in the low-pathology group consistently produced more IL-4 than those in the high-pathology group. In contrast to PPD, stimulation with rESAT6:CFP10 did not result in a statistically significant or consistent pattern of expression between the pathology groups.
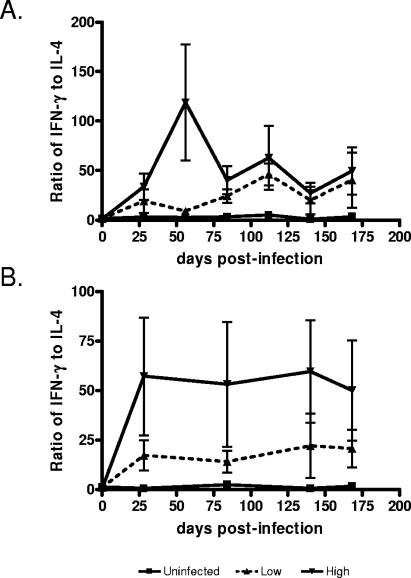
In vivo and in vitro responses to M. bovis may elicit both TH1 and TH2 T cells. The ratio of IFN-γ to IL-4 was used as an indicator of the TH1/TH2 polarization of the response (Fig. 5). The ratio of IFN-γ to IL-4 was greater in animals in the high-pathology group than in the uninfected and low- pathology groups. The time point with the most striking difference in the PPD-stimulated cells was 56 days p.i., at which time the mean ratio (± standard error of the mean [SEM]) in the high-pathology group was 118.8 ± 58.4, while the low-pathology group averaged 9.2 ± 2.0 (Fig. 5A). These data correlated with the total pathology score (r = 0.90; P = 0.0002).
FIG. 5.
Ratio of IFN-γ to IL-4 expression. To represent the relative TH1 to TH2 response, the ratio of IFN-γ to IL-4 was calculated from cultures stimulated with (A) PPD or (B) rESAT6:CFP10. Data are the means ± SEMs (error bars).
Stimulation of the PBL with rESAT6:CFP10, the proposed major TH1 antigen of M. bovis, produced consistent ratios of IFN-γ to IL-4 gene expression. The animals in the high-pathology group expressed an average ratio of 55.0 ± 12.7, while animals in the low-pathology group expressed 18.9 ± 5.7 (Fig. 5B). The ratio of IFN-γ to IL-4 gene expression correlated with pathology (r = 0.81; P = 0.0001) at 84 days p.i. Similar results were obtained when the ratio of IFN-γ to IL-10 or TGF-β was compared (data not shown).
DISCUSSION
Since the discovery of M. bovis infection in white-tailed deer, a number of studies have been undertaken to describe transmission and pathogenesis. Palmer et al. reported that intratonsillar inoculation of WTD more closely mimics natural infection than does aerosol inoculation (19). In this study, intratonsillar inoculation resulted in lesion distribution similar to that reported for naturally infected deer (16, 24, 33), with primary involvement of lymph nodes of the head and particularly the medial retropharyngeal lymph node. With more-disseminated disease, lesions were found in the lungs and lung-associated lymph nodes. The severity of lesions in the medial retropharyngeal lymph node was indicative of general lesion development within the animal. As the lesion score in the medial retropharyngeal lymph node increased, more lesions were reported in other tissues. The variable pathological outcome provides an excellent model to investigate the immunological processes that may contribute to disease in infected WTD.
Little is known about the immune responses of WTD to M. bovis infection. It has been reported that M. bovis-infected WTD develop cell-mediated responses to M. bovis infection, as evidenced by a delayed-type hypersensitivity response to the intradermal injection of PPD (14, 25) and the in vitro production of IFN-γ and nitric oxide upon restimulation with PPD or recombinant proteins (21, 39). In addition to cell-mediated immunity responses, infected WTD generate antibodies to a number of M. bovis proteins and lipids (23, 38, 40). Each of these processes represents an attempt of the host to control M. bovis infection. In the present study, the relative gene expressions of TH1 and TH2 cytokine genes were determined. Although the relative quantity of mRNA may not reflect the relative levels of protein, this analysis does provide a sensitive method of assessing responses to the pathogen. Infected WTD expressed more TH1-type cytokine mRNAs, IFN-γ, IL-12p40, and GM-CSF, than did uninfected animals. In other models, each of these has been reported to be vital for control of mycobacterial infections (7, 8, 15). The expression of IFN-γ in response to infection is consistent with reports from other species. In the present study, increased expression of IFN-γ was associated with more severe pathology which was characterized by widespread lesion distribution. These data are consistent with a report that IFN-γ production in response to rESAT6 positively correlates with pathology in cattle (35). Others have suggested that, in cattle (3, 27, 29, 35, 41) and humans (34), IFN-γ expression is positively correlated with disease severity.
The relationship between IFN-γ expression and pathology may reflect a failure to control infection or be the result of an exaggerated immune response. In an animal unable to control bacterial growth, continual antigen stimulation may result in chronic production of IFN-γ. If however, the immune response is effective at reducing bacterial growth, there will be less immune stimulation, allowing the M. bovis-specific lymphocyte population to contract. Conversely, increased IFN-γ expression may be reflective of an exaggerated immune response to M. bovis, resulting in increased immunopathology. This immunopathology may be beneficial to the mycobacteria by providing an environment that favors bacterial growth and enhances dissemination through lesion liquefaction and cavity formation in the lung.
IL-4 expression is indicative of a TH2 response. It has been suggested that a switch from a TH1- to a TH2-dominated response is responsible for the failure of the host to control M. bovis infection because IFN-γ production is inhibited (9, 28, 32). In WTD, IL-4 may have a moderating effect since animals with low pathologies consistently expressed lower ratios of IFN-γ to IL-4. This finding suggests that IL-4 does not compromise the protective response but rather reduces IFN-γ-induced pathology. Rhodes et al. reached a similar conclusion after measuring IFN-γ and IL-4 expression in experimentally and naturally infected cattle (29). Experiments with gene knockout mice have also led to similar conclusions (10). When mice which are unable to generate a TH2 response (IL-4/IL-13 knockout) are infected with M. tuberculosis, they produce more IFN-γ mRNA than do immune-competent controls, while the mycobacterial burden was approximately equal between the two groups. These data confirm that TH2 responses do not enhance disease and may moderate IFN-γ-mediated immunopathology. This is particularly evident in response to rESAT6:CFP10, where increased IFN-γ expression was matched by increased IL-4 expression (Fig. 4 and 5). Even in the cultures stimulated with PPD, the increases in IFN-γ production were complemented by increases in IL-4 production, which resulted in a consistent lower ratio of IFN-γ to IL-4 (Fig. 5).
The pathological outcome in the WTD may be established early during infection. The difference in expression of IFN-γ and IL-4 between the pathology groups was detected in the first three months. In addition, correlation of gene expression and pathology was significant during only this same time period, suggesting that the pathological outcome is established early. In susceptible and resistant mice, the clinical outcome is established in the first 20 days after infection (for a review, see reference 15). At 20 days postinfection, resistant mice inhibit the logarithmic growth of M. tuberculosis, while susceptible mice are unable to inhibit bacterial growth. This same model may be applicable here since animals with high pathologies had more disseminated disease, suggesting the inability of the immune response to limit bacterial growth.
M. bovis elicited both TH1 and TH2 cytokine-expressing cells in WTD. The immunological key to protection remains as elusive in deer as it is in other species studied to date. Understanding the interplay of cytokine expression may be critical in developing a vaccine strategy that will result in protection. From this and other studies, it is clear that IFN-γ expression and the absence of IL-4/IL-10 expression are not adequate markers for vaccine efficacy. IFN-γ expression is likely to play a significant role in both protection and pathogenesis. Additional studies on the interaction between M. bovis and WTD are needed to elucidate the role of cytokines in protection and pathogenesis.
Acknowledgments
We thank Brian Nonnecke and Rachel Huegel for their help in preparing the manuscript and Debra Palmquist and Annette M. O'Connor for their help with the statistical analysis. We also thank Jessica Pollock, Michael Howard, Peter Lasley, Rebecca Lyon, and Shelly Zimmerman for excellent technical support and Richard Auwerda, Doug Ewing, Todd Holtz, Terry Krausman, and Jay Steffen for excellent animal care.
Funding for this research was provided by the U.S. Department of Agriculture, Agricultural Research Service and Animal and Plant Health Inspection Service (APHIS).
REFERENCES
- 1.Abbas, A. K., K. M. Murphy, and A. Sher. 1996. Functional diversity of helper T lymphocytes. Nature 383:787-793. [DOI] [PubMed] [Google Scholar]
- 2.Barrow, P. A., and J. Gallagher. 1981. Aspects of the epidemiology of bovine tuberculosis in badgers and cattle. I. The prevalence of infection in two wild animal populations in south-west England. J. Hyg. 86:237-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buddle, B. M., D. N. Wedlock, M. Denis, and M. A. Skinner. 2005. Identification of immune response correlates for protection against bovine tuberculosis. Vet. Immunol. Immunopathol. 108:45. [DOI] [PubMed] [Google Scholar]
- 4.Coleman, J. D., and M. M. Cooke. 2001. Mycobacterium bovis infection in wildlife in New Zealand. Tuberculosis 81:191-202. [DOI] [PubMed] [Google Scholar]
- 5.Coussens, P. M., N. Verman, M. A. Coussens, M. D. Elftman, and A. M. McNulty. 2004. Cytokine gene expression in peripheral blood mononuclear cells and tissues of cattle infected with Mycobacterium avium subsp. paratuberculosis: evidence for an inherent proinflammatory gene expression pattern. Infect. Immun. 72:1409-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ehrt, S., D. Schnappinger, S. Bekiranov, J. Drenkow, S. Shi, T. R. Gingeras, T. Gaasterland, G. Schoolnik, and C. Nathan. 2001. Reprogramming of the macrophage transcriptome in response to interferon-gamma and Mycobacterium tuberculosis: signaling roles of nitric oxide synthase-2 and phagocyte oxidase. J. Exp. Med. 194:1123-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flynn, J. L., and J. Chan. 2001. Immunology of tuberculosis. Annu. Rev. Immunol. 19:93-129. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez-Juarrero, M., J. M. Hattle, A. Izzo, A. P. Junqueira-Kipnis, T. S. Shim, B. C. Trapnell, A. M. Cooper, and I. M. Orme. 2005. Disruption of granulocyte macrophage-colony stimulating factor production in the lungs severely affects the ability of mice to control Mycobacterium tuberculosis infection. J. Leukoc. Biol. 77:914-922. [DOI] [PubMed] [Google Scholar]
- 9.Hernandez-Pando, R., H. Orozcoe, A. Sampieri, L. Pavon, C. Velasquillo, J. Larriva-Sahd, J. M. Alcocer, and M. V. Madrid. 1996. Correlation between the kinetics of Th1, Th2 cells and pathology in a murine model of experimental pulmonary tuberculosis. Immunology 89:26-33. [PMC free article] [PubMed] [Google Scholar]
- 10.Jung, Y. J., R. LaCourse, L. Ryan, and R. J. North. 2002. Evidence inconsistent with a negative influence of T helper 2 cells on protection afforded by a dominant T helper 1 response against Mycobacterium tuberculosis lung infection in mice. Infect. Immun. 70:6436-6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kehrli, M. E., Jr., B. J. Nonnecke, and J. A. Roth. 1989. Alterations in bovine lymphocyte function during the periparturient period. Am. J. Vet. Res. 50:215-220. [PubMed] [Google Scholar]
- 12.Littell, R. C., G. A. Milliken, W. W. Stroup, and R. D. Wolfinger. 1996. SAS system for mixed models. SAS Institute, Inc., Cary, N.C.
- 13.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402-408. [DOI] [PubMed] [Google Scholar]
- 14.Norden, D., M. A. Essey, and R. Meyer. 1996. Evaluation of tuberculin testing in Cervidae. In Veterinary Services, Centers for Epidemiology and Animal Health (ed.), CADIA technical report 02-96. APHIS, USDA, Fort Collins, Colo.
- 15.North, R. J., and Y. J. Jung. 2004. Immunity to tuberculosis. Annu. Rev. Immunol. 22:599-623. [DOI] [PubMed] [Google Scholar]
- 16.O'Brien, D. J., S. D. Fitzgerald, T. J. Lyon, K. L. Butler, J. S. Fierke, K. R. Clarke, S. M. Schmitt, T. M. Cooley, and D. E. Derry. 2001. Tuberculous lesions in free-ranging white-tailed deer in Michigan. J. Wildl. Dis. 37:608-613. [DOI] [PubMed] [Google Scholar]
- 17.O'Brien, D. J., S. M. Schmitt, D. E. Berry, S. D. Fitzgerald, J. R. Vanneste, T. J. Lyon, D. Magsig, J. S. Fierke, T. M. Cooley, L. S. Zwick, and B. V. Thomsen. 2004. Estimating the true prevalence of Mycobacterium bovis in hunter-harvested white-tailed deer in Michigan. J. Wildl. Dis. 40:42-52. [DOI] [PubMed] [Google Scholar]
- 18.O'Brien, D. J., S. M. Schmitt, J. S. Fierke, S. A. Hogle, S. R. Winterstein, T. M. Cooley, W. E. Moritz, K. L. Diegel, S. D. Fitzgerald, D. E. Berry, and J. B. Kaneene. 2002. Epidemiology of Mycobacterium bovis in free-ranging white-tailed deer, Michigan, USA, 1995-2000. Prev. Vet. Med. 54:47-63. [DOI] [PubMed] [Google Scholar]
- 19.Palmer, M. V., W. R. Waters, and D. L. Whipple. 2003. Aerosol exposure of white-tailed deer (Odocoileus virginianus) to Mycobacterium bovis. J. Wildl. Dis. 39:817-823. [DOI] [PubMed] [Google Scholar]
- 20.Palmer, M. V., W. R. Waters, and D. L. Whipple. 2004. Shared feed as a means of deer-to-deer transmission of Mycobacterium bovis. J. Wildl. Dis. 40:87. [DOI] [PubMed] [Google Scholar]
- 21.Palmer, M. V., W. R. Waters, D. L. Whipple, R. E. Slaughter, and S. L. Jones. 2004. Evaluation of an in vitro blood-based assay to detect production of interferon-gamma by Mycobacterium bovis-infected white-tailed deer (Odocoileus virginianus). J. Vet. Diagn. Investig. 16:17-21. [DOI] [PubMed] [Google Scholar]
- 22.Palmer, M. V., D. L. Whipple, and S. C. Olsen. 1999. Development of a model of natural infection with Mycobacterium bovis in white-tailed deer. J. Wildl. Dis. 35:450-457. [DOI] [PubMed] [Google Scholar]
- 23.Palmer, M. V., D. L. Whipple, S. C. Olsen, and R. H. Jacobson. 2000. Cell mediated and humoral immune responses of white-tailed deer experimentally infected with Mycobacterium bovis. Res. Vet. Sci. 68:95-98. [DOI] [PubMed] [Google Scholar]
- 24.Palmer, M. V., D. L. Whipple, J. B. Payeur, D. P. Alt, K. J. Esch, C. S. Bruning-Fann, and J. B. Kaneene. 2000. Naturally occurring tuberculosis in white-tailed deer. J. Am. Vet. Med. Assoc. 216:1921-1924. [DOI] [PubMed] [Google Scholar]
- 25.Palmer, M. V., D. L. Whipple, and W. R. Waters. 2001. Tuberculin skin testing in white-tailed deer (Odocoileus virginianus). J. Vet. Diagn. Investig. 13:530-533. [DOI] [PubMed] [Google Scholar]
- 26.Pollock, J. M., and P. Andersen. 1997. Predominant recognition of the ESAT-6 protein in the first phase of interferon with Mycobacterium bovis in cattle. Infect. Immun. 65:2587-2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pollock, J. M., M. D. Welsh, and J. McNair. 2005. Immune responses in bovine tuberculosis: towards new strategies for the diagnosis and control of disease. Vet. Immunol. Immunopathol. 108:37-43. [DOI] [PubMed] [Google Scholar]
- 28.Power, C. A., G. Wei, and P. A. Bretscher. 1998. Mycobacterial dose defines the Th1/Th2 nature of the immune response independently of whether immunization is administered by the intravenous, subcutaneous, or intradermal route. Infect. Immun. 66:5743-5750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rhodes, S. G., N. Palmer, S. P. Graham, A. E. Bianco, R. G. Hewinson, and H. M. Vordermeier. 2000. Distinct response kinetics of gamma interferon and interleukin-4 in bovine tuberculosis. Infect. Immun. 68:5393-5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roth, J. A., and M. L. Kaeberle. 1981. Evaluation of bovine polymorphonuclear leukocyte function. Vet. Immunol. Immunopathol. 2:157-174. [DOI] [PubMed] [Google Scholar]
- 31.Rozen, S., and H. J. Skaletsky. 2000. Primer3 on the WWW for general users and for biologist programmers, p. 365-386. In S. Krawetz and S. Misener (ed.), Bioinformatics methods and protocols: methods in molecular biology. Humana Press, Totowa, N.J. [DOI] [PubMed]
- 32.Sander, B., U. Skansen-Saphir, O. Damm, L. Hakansson, J. Andersson, and U. Andersson. 1995. Sequential production of Th1 and Th2 cytokines in response to live bacillus Calmette-Guerin. Immunology 86:512-518. [PMC free article] [PubMed] [Google Scholar]
- 33.Schmitt, S. M., S. D. Fitzgerald, T. M. Cooley, C. S. Bruning-Fann, L. Sullivan, D. Berry, T. Carlson, R. B. Minnis, J. B. Payeur, and J. Sikarskie. 1997. Bovine tuberculosis in free-ranging white-tailed deer from Michigan. J. Wildl. Dis. 33:749. [DOI] [PubMed] [Google Scholar]
- 34.Verbon, A., N. Juffermans, S. J. Van Deventer, P. Speelman, H. Van Deutekom, and T. Van Der Poll. 1999. Serum concentrations of cytokines in patients with active tuberculosis (TB) and after treatment. Clin. Exp. Immunol. 115:110-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vordermeier, H. M., M. A. Chambers, P. J. Cockle, A. O. Whelan, J. Simmons, and R. G. Hewinson. 2002. Correlation of ESAT-6-specific gamma interferon production with pathology in cattle following Mycobacterium bovis BCG vaccination against experimental bovine tuberculosis. Infect. Immun. 70:3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vordermeier, H. M., A. Whelan, P. J. Cockle, L. Farrant, N. Palmer, and R. G. Hewinson. 2001. Use of synthetic peptides derived from the antigens ESAT-6 and CFP-10 for differential diagnosis of bovine tuberculosis in cattle. Clin. Diagn. Lab. Immunol. 8:571-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Waters, W. R., B. J. Nonnecke, M. V. Palmer, S. Robbe-Austermann, J. P. Bannantine, J. R. Stabel, D. L. Whipple, J. B. Payeur, D. M. Estes, J. E. Pitzer, and F. C. Minion. 2004. Use of recombinant ESAT-6:CFP-10 fusion protein for differentiation of infections of cattle by Mycobacterium bovis and by M. avium subsp. avium and M. avium subsp. paratuberculosis. Clin. Diagn. Lab. Immunol. 11:729-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waters, W. R., M. V. Palmer, J. P. Bannantine, D. L. Whipple, R. Greenwald, J. Esfandiari, P. Andersen, J. McNair, J. M. Pollock, and K. P. Lyashchenko. 2004. Antigen recognition by serum antibodies in white-tailed deer (Odocoileus virginianus) experimentally infected with Mycobacterium bovis. Clin. Diagn. Lab. Immunol. 11:849-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Waters, W. R., M. V. Palmer, R. E. Sacco, and D. L. Whipple. 2002. Nitric oxide production as an indication of Mycobacterium bovis infection in white-tailed deer (Odocoileus virginianus). J. Wildl. Dis. 38:338-343. [DOI] [PubMed] [Google Scholar]
- 40.Waters, W. R., M. V. Palmer, and D. L. Whipple. 2002. Mycobacterium bovis-infected white-tailed deer (Odocoileus virginianus): detection of immunoglobulin specific to crude mycobacterial antigens by ELISA. J. Vet. Diagn. Investig. 14:470-475. [DOI] [PubMed] [Google Scholar]
- 41.Welsh, M. D., R. T. Cunningham, D. M. Corbett, R. M. Girvin, J. McNair, R. A. Skuce, D. G. Bryson, and J. M. Pollock. 2005. Influence of pathological progression on the balance between cellular and humoral immune responses in bovine tuberculosis. Immunology 114:101. [DOI] [PMC free article] [PubMed] [Google Scholar]