Abstract
Meningococcal group C polysaccharide-protein conjugate vaccines (MCV) prime infants and children for memory anticapsular responses upon subsequent exposure to unconjugated polysaccharide. The objective of this study was to determine whether MCV primes vaccine-naïve adults and adults previously vaccinated with meningococcal polysaccharide vaccine (MPSV) for memory antibody responses. Meningococcal vaccine-naïve adults were randomized to receive either MCV (MCV/naïve group) (n = 35) or pneumococcal conjugate vaccine (PCV) (PCV/naïve group) (n = 34). Participants with a history of receiving MPSV were given MCV (MCV/MPSV group) (n = 26). All subjects were challenged 10 months later with one-fifth of the usual dose of MPSV (10 μg of each polysaccharide). Sera were obtained before the conjugate vaccination and before and 7 days after the MPSV challenge and assayed for immunoglobulin G (IgG) anticapsular antibody concentrations and bactericidal titers. The MCV/naïve group had 7- to 10-fold-higher serum IgG and bactericidal responses after the MPSV challenge than the PCV/naïve group (P < 0.001). The increases (n-fold) in anticapsular antibody concentrations in the MCV/naïve group were greatest in subjects with antibody concentrations of ≤2 μg/ml before the challenge (geometric mean increase [n-fold] of 8.3 versus 1.1 in subjects with concentrations of >2 μg/ml before the challenge; P < 0.0001). Only 3 of 11 MCV-vaccinated subjects who had received MPSV before enrollment and who had antibody concentrations of ≤2 μg/ml before the polysaccharide challenge showed more-than-twofold increases in anticapsular antibody concentration or bactericidal titer after the challenge. MCV vaccination of meningococcal vaccine-naïve adults primes for robust memory antibody responses. There was no evidence of induction of memory by MCV in adults previously vaccinated with MPSV.
Meningococcal group C polysaccharide-protein conjugate vaccines (MCV) are immunogenic at all ages and prime for immunoglobulin G (IgG) booster antibody responses in infants and young children (2, 6, 15, 18, 24). In contrast, unconjugated meningococcal group C polysaccharide vaccines (MPSV) are poorly immunogenic in infants and young children (21) and induce antibody hyporesponsiveness at all ages, as evidenced by impaired antibody responses upon exposure to a second injection of MPSV (3, 8, 11, 18, 26). There are conflicting data on whether MCV immunization of adults primes for immunologic memory (10, 25, 28) or whether prior exposure of adults to MPSV interferes with the development of immunologic memory after vaccination with MCV (10, 28). This information is important, since meningococcal conjugate vaccines have been introduced in Europe and North America and are being used extensively in adolescents and young adults, including persons previously vaccinated with MPSV.
The primary purpose of this study was to determine the ability of MCV to prime meningococcal vaccine-naïve adults for group C memory antibody responses. A secondary objective was to determine the possible effect of prior exposure to MPSV on the induction of memory by a dose of MCV.
MATERIALS AND METHODS
Study design.
Healthy adults ages 18 to 50 years were eligible to enroll in a phase 2, partially randomized, controlled, single-center study to evaluate the safety and immunogenicity of a meningococcal group C conjugate vaccine that is licensed in Canada and Europe (Menjugate; Chiron Vaccines). A dose of the vaccine consists of 10 μg of meningococcal group C oligosaccharide conjugated to the nontoxic mutant diphtheria toxin CRM197 protein carrier, which was administered with 1 mg of aluminum hydroxide. Subjects were recruited from the San Francisco Bay area. Individuals who had never received a meningococcal vaccine were randomized to receive either an intramuscular dose of MCV (MCV/naïve group) or, as a control, a U.S.-licensed 7-valent pneumococcal polysaccharide-CRM197 conjugate vaccine (PCV) (PCV/naïve group) (Prevnar; Wyeth Lederle). Individuals who had previously received MPSV at least 6 months prior to enrollment were assigned to a third group and were given a dose of MCV (MCV/MPSV group). Ten months later, all participants were given a subcutaneous challenge consisting of 0.1 ml (one-fifth of the regular dose) of the licensed quadrivalent meningococcal polysaccharide vaccine (Menomune; Sanofi-Pasteur), which is equivalent to 10 μg of each of the four polysaccharides. The lower dose served as an immunologic probe to evaluate memory antibody responses to group C polysaccharide (defined as higher antibody responses in the MCV/naïve group compared with the responses of the control PCV/naïve group) (11). The data reported herein were obtained from serum samples collected upon enrollment (prior to the conjugate vaccination) and 10 months later (before the MPSV challenge), as well as 7 days after the MPSV challenge.
The protocol was approved by the Institutional Review Board of the Children's Hospital and Research Center at Oakland.
Serology.
All serologic studies were performed blindly on coded serum samples at Chiron Vaccines (Emeryville, CA). IgG group C anticapsular antibody was measured by a modified enzyme-linked immunosorbent assay that incorporated a chaotropic agent in the serum-diluting buffer to favor the detection of higher-avidity anticapsular antibodies, which was performed as previously described (12). Serum bactericidal activity was measured using extrinsic human complement as previously described (18). For statistical analyses, antibody concentrations or titers that were less than the lower limit of detection were assigned values half that of the lower limit (i.e., 0.2 μg/ml for group C anticapsular IgG concentrations <0.4 μg/ml and a bactericidal titer of 1:2 for sera with titers of <1:4). The respective geometric means of the antibody concentrations or titers and associated two-sided 95% confidence intervals (CIs) were computed from the log (base 10) values. Statistical comparisons were all two tailed. Because of heterogeneity in the serum antibody concentrations at the time of enrollment for subjects previously vaccinated with MPSV, the subjects in that group were stratified based on MPSV immunization <3 years or ≥3 years prior to enrollment.
RESULTS
The vaccines were generally well tolerated. No serious adverse events were noted. Table 1 summarizes the demographic information for the subjects in all of the vaccine groups. Although the subjects in the MCV/MPSV group who received MPSV 3 years or more prior to enrollment were slightly younger than the subjects in the other groups, there were no other differences among the vaccine groups.
TABLE 1.
Demographics of the sample
| Group | No. of subjects | Median age (range) | % Females | % Caucasians |
|---|---|---|---|---|
| MCV/naïve | 35 | 29 (20-49) | 66 | 63 |
| PCV/naïve | 34 | 29.5 (19-51) | 68 | 68 |
| MCV/MPSV <3 yrs priora | 14 | 29.5 (19-46) | 36 | 64 |
| MCV/MPSV ≥3 yrs priorb | 12 | 22.5 (19-39) | 75 | 50 |
Median interval between MPSV immunization and enrollment, 1 year (range, 1 to 2 years).
Median interval between MPSV immunization and enrollment, 3.5 years (range, 3 to 11 years).
Table 2 summarizes the IgG anticapsular antibody concentrations as measured by enzyme-linked immunosorbent assay. Ten months after the conjugate vaccine immunization (immediately before the MPSV challenge dose), the serum IgG anticapsular antibody concentrations were higher in the groups immunized with MCV than those of controls given PCV (P < 0.0001). Compared with controls primed with PCV, MCV immunization of the meningococcal vaccine-naïve group primed for memory IgG antibody responses (i.e., geometric mean antibody concentrations postimmunization that were more than eightfold higher than those of controls). The groups immunized with MPSV before enrollment and given a dose of MCV had higher geometric mean antibody concentrations after the MPSV challenge at 10 months than the meningococcal vaccine-naïve control group primed with PCV (P < 0.001). However, as described below, induction of immunologic memory in subjects previously given MPSV was difficult to evaluate based on the geometric mean antibody concentrations.
TABLE 2.
Serum IgG group C anticapsular responsesa
| Group | No. of subjects | Geometric mean IgG [μg/ml (95% CI)]
|
||
|---|---|---|---|---|
| Preconjugate | Pre-MPSV boost | Post-MPSV boost | ||
| MCV/naïve | 35 | 0.4 A (0.2-0.6) | 2.2 B (1.0-4.6) | 6.1 C (3.6-10.2) |
| PCV/naïve | 34 | 0.3 (0.2-0.3) | 0.2 D (0.2-0.3) | 0.5 E (0.3-0.9) |
| MCV/MPSV <3 yr prior | 14 | 4.9 F (1.8-13.4) | 7.7 G (2.8-21.0) | 7.5 H (2.9-19.5) |
| MCV/MPSV ≥3 yr prior | 12 | 0.9 I (0.3-2.3) | 2.3 J (0.6-8.4) | 4.1 K (1.5-11.7) |
For comparison between A and B, P < 0.0001; for comparison between F and G, P = 0.2; for comparison between I and J, P = 0.006; for comparison between B and C, P < 0.0001; for comparison between D and E, P < 0.002; for comparison between G and H, P = 0.9; for comparison between J and K, P < 0.02 (paired t test). For comparison between C and E, P < 0.0001; for comparison between E and H, P < 0.0001; for comparison between E and K, P = 0.0002 (unpaired t test).
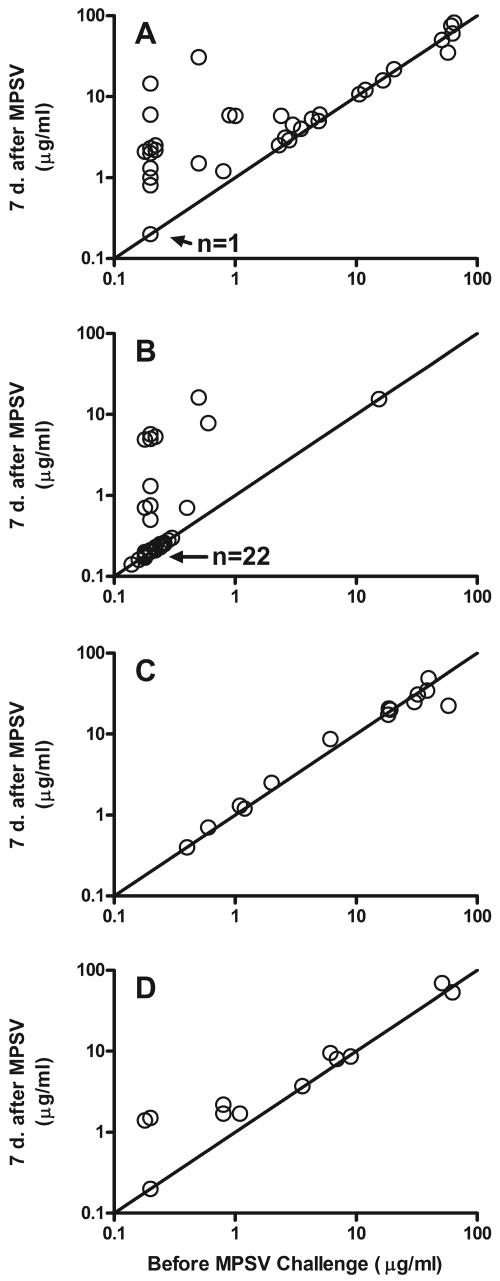
Figure 1 shows the individual antibody concentrations of each subject after the MPSV challenge in relation to the respective antibody concentrations immediately before the challenge. In the meningococcal vaccine-naïve group primed with MCV (Fig. 1A), the increases (n-fold) in anticapsular antibody concentration after the MPSV challenge were greatest in subjects with antibody concentrations of ≤2 μg/ml prior to polysaccharide challenge (geometric mean increase [n-fold] of 8.3 in subjects with ≤2 μg/ml of antibody versus 1.1 in subjects with >2 μg/ml of antibody; P < 0.0001). Thus, evidence of priming by the conjugate vaccine was restricted to subjects with low antibody concentrations before the polysaccharide challenge. In the meningococcal vaccine-naïve control group primed with PCV (Fig. 1B), 7 (21%) of the 33 subjects with ≤2 μg/ml of antibody before the MPSV challenge showed increases in antibody concentrations greater than fourfold after the challenge, and 10 (30%) subjects had increases greater than twofold. These subjects may have been naturally primed by exposure to Neisseria meningitis group C or cross-reacting bacteria. There were 11 subjects given MCV who had received MPSV before enrollment (Fig. 1C and D) and who had ≤2 μg/ml of antibody before the MPSV challenge. Of these subjects, only two (18%) had more-than-fourfold increases in antibody concentration after the challenge, and three (36%) had more-than-twofold increases, which were not different from those observed in the PCV/naïve control group. None of the 15 subjects with >2 μg/ml of serum antibody before the polysaccharide challenge had more-than-twofold increases in antibody after the challenge.
FIG. 1.
Serum group C anticapsular IgG antibody concentrations of individual subjects after MPSV challenge in relation to prechallenge concentrations. (A) MCV/naïve group. (B) PCV/naïve group. (C) MCV/MPSV subset that received MPSV <3 years prior to enrollment. (D) MCV/MPSV subset that received MPSV ≥3 years prior to enrollment. “n” refers to the number of subjects who had antibody concentrations below the limit of detection in sera obtained before and after the MPSV challenge.
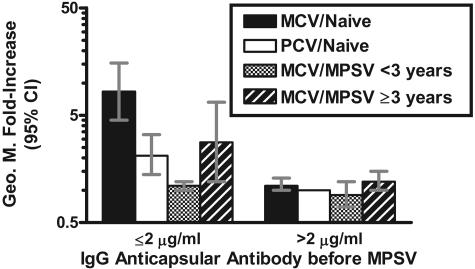
Figure 2 summarizes the geometric mean increases (n-fold) in antibody concentrations of the different groups after the MPSV challenge. The data are stratified based on serum antibody concentrations of ≤2 μg/ml and >2 μg/ml prior to MPSV challenge. Among subjects with concentrations of ≤2 μg/ml prior to challenge, those in the MCV/naïve group had a greater geometric mean increase (n-fold) after the polysaccharide challenge than in the other three groups (P < 0.05 for each comparison). Interestingly, the geometric mean increase (n-fold) after the MPSV challenge of subjects who had received MPSV ≥3 years before enrollment was higher than that of subjects who had received MPSV <3 years before enrollment (geometric mean increase [n-fold] of 2.8 versus 1.1, respectively; P = 0.03).
FIG. 2.
Geometric mean severalfold increases (postchallenge/prechallenge) in relation to serum IgG anticapsular antibody concentrations prior to challenge with MPSV. Among subjects with ≤2 μg/ml of antibody immediately before the challenge, the statistical significance of the differences between the geometric mean (Geo. M.) severalfold increase of the MCV/naïve group versus the PCV/naïve group was a P value of <0.001, that versus the MCV/MPSV group that received MPSV <3 years prior to enrollment was a P value of <0.01, and that versus the MCV/MPSV group that received MPSV ≥3 years prior to enrollment group was a P value of <0.05. The MCV/MPSV group that received MPSV ≥3 years prior to enrollment also had higher responses to the challenge dose than the group that received MPSV <3 years before enrollment (P = 0.03). All other respective differences are not significant (P > 0.1).
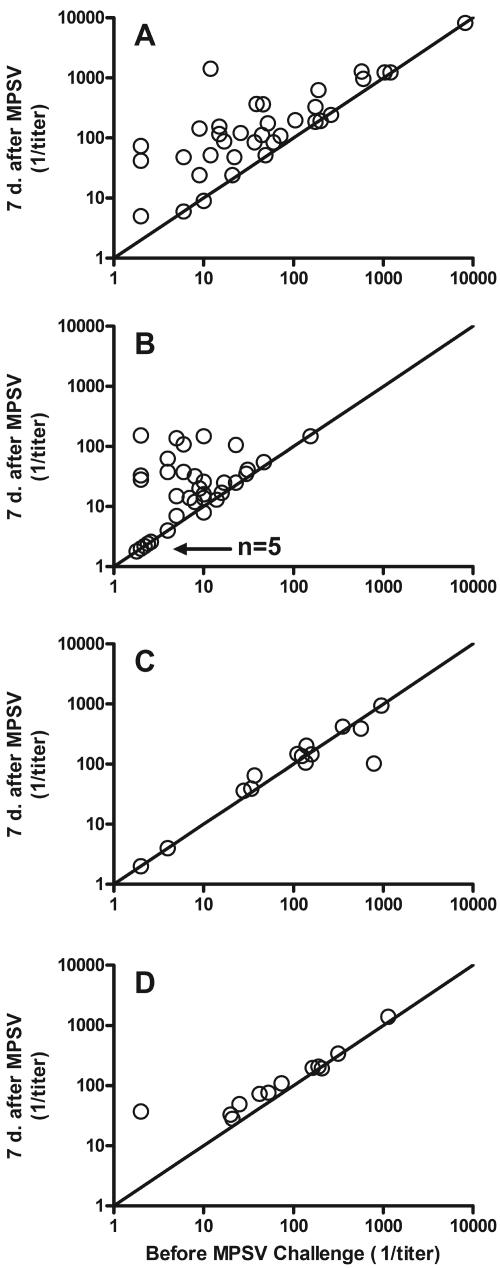
Table 3 summarizes the geometric mean serum bactericidal titers of the groups. Figure 3 shows the bactericidal responses of the individual subjects in relation to the respective bactericidal titers present immediately before the MPSV challenge. The bactericidal antibody responses of the different groups paralleled the respective anticapsular antibody responses.
TABLE 3.
Serum bactericidal antibody responsesa
| Group | No. of subjects | Bactericidal antibody titer [1/GMT (95% CI)]
|
||
|---|---|---|---|---|
| Preconjugate | Pre-MPSV boost | Post-MPSV boost | ||
| MCV/naïve | 35 | 7.2 A (4.2-12.3) | 44.7 B (23.1-86.7) | 140.2 C (80.8-243.2) |
| PCV/naïve | 34 | 8.2 (5.8-11.6) | 7.4 D (5.1-10.7) | 20.4 E (12.8-32.4) |
| MCV/MPSV <3 yrs prior | 14 | 37.9 F (16.8-85.3) | 87.7 G (30.5-252.2) | 83.0 H (31.3-220.4) |
| MCV/MPSV ≥3 yrs prior | 12 | 22.3 I (8.8-56.7) | 67.1 J (23.5-191.9) | 111.7 K (54.2-230.3) |
For comparison between A and B, P < 0.0001; for comparison between F and G, P = 0.07; for comparison between I and J, P = 0.02; for comparison between B and C, P < 0.0001; for comparison between D and E, P < 0.0001; for comparison between G and H, P = 0.7; for comparison between J and K, P < 0.05 (paired t test). For comparison between C and E, P < 0.0001; for comparison between E and H, P < 0.004; for comparison between E and K, P = 0.0003 (unpaired t test). GMT, geometric mean titer.
FIG. 3.
Serum bactericidal titers of individual subjects after MPSV challenge in relation to prechallenge titers. (A) MCV/naïve group. (B) PCV/naïve group. (C) MCV/MPSV subset that received MPSV <3 years prior to enrollment. (D) MCV/MPSV subset that received MPSV ≥3 years prior to enrollment. “n” refers to the number of subjects who had bactericidal titers below the limit of detection in sera obtained before and after the MPSV challenge.
DISCUSSION
The antibody responses to unconjugated MPSV are thought to be relatively T-cell independent, and immunization does not prime for memory antibody responses. In contrast, meningococcal polysaccharide-protein conjugate vaccines are immunogenic for all ages. In infants (2, 6, 15, 20) and young children (18, 23), group C meningococcal conjugate vaccines also prime for the ability to respond with serum IgG memory antibody responses upon subsequent exposure to unconjugated MPSV.
In previous studies, it has been difficult to determine whether adults immunized with polysaccharide-protein conjugate vaccines are primed for memory responses, in part because antibody responses of vaccine-naïve adults to a first dose of polysaccharide vaccine are characteristic of memory antibody responses (rapid rises in serum antibody and class switched to IgG with hypermutation of variable regions) (1, 16), probably as a result of natural priming. For example, pneumococcal conjugate vaccine primes infants and young children for memory, yet several studies of adults did not demonstrate an induction of immunologic memory after pneumococcal conjugate vaccination (22, 27).
There are conflicting data on the ability of meningococcal conjugate vaccines to prime adults for meningococcal group C immunologic memory. Two studies of MCV-immunized adults attempted to infer priming for memory by measurement of group C antibody avidity maturation, a surrogate that has been useful in investigations of immunologic memory in infants and young children (4, 23). One study observed a significant increase in the anticapsular avidity index (i.e., avidity maturation) at 6 months (25), which was consistent with a memory response, while the second study reported a significant decrease (10). The lack of avidity maturation in some MCV-immunized adults may reflect maximal stimulation of memory B cells that had already undergone extensive hypermutation at 1 month after MCV vaccination. In a third study, adults immunized with an investigational group A plus group C meningococcal conjugate vaccine had memory group C antibody responses to a 1-μg challenge dose of MPSV given 4 to 5 years later (11). However, the sample sizes in this study were small, the results were of borderline statistical significance, the adults in the conjugate group had received different doses 4 to 5 years earlier, and the control group of vaccine-naïve adults was recruited at the time of the MPSV challenge and therefore may have differed from the group of adults who received the conjugate vaccine 4 to 5 years earlier. Thus, there were confounding factors that could have affected the assessment of memory.
Keyserling et al. previously reported that group C antibody responses to a second dose of quadrivalent MCV in adolescents who had been immunized 3 years earlier were higher than responses in control adolescents who were given a first dose of MCV (14). Although boostable anticapsular antibody responses to repeated injections of MCV are consistent with an induction of immunologic memory, the data do not distinguish between the possible role of memory B cells induced by the polysaccharide component of the conjugate vaccine and that of T cells elicited by the diphtheria toxoid carrier on the booster anticapsular antibody responses observed after the second dose of MCV.
The present study was designed prospectively to assess the induction of memory to group C polysaccharide directly by measuring group C antibody responses to one-fifth (10 μg) of the usual dose of MPSV, which was given 10 months after a dose of MCV or PCV. Meningococcal vaccine-naïve adults randomized to receive MCV showed much higher IgG anticapsular and bactericidal antibody responses to the polysaccharide challenge than those of meningococcal vaccine-naïve adults randomized to receive the control polysaccharide conjugate vaccine that employed the same carrier protein (CRM197) as MCV. In both priming groups, nearly all subjects with >2 μg/ml of antibody before the polysaccharide challenge showed minimal increases in serum antibody levels after the challenge. Therefore, evidence of priming by MCV was observed only in subjects with ≤2 μg/ml of antibody.
In previous studies, immunization with MPSV was associated with immune refractoriness to a second dose of MPSV (decreased group C antibody responses compared with the responses to a first dose of vaccine). This phenomenon has been observed in immunized infants, toddlers, older children (7-9, 13, 17, 18), and adults (11, 26). Several studies have also shown that MCV is immunogenic in children and adults who were previously immunized with MPSV (3, 5, 17, 28), although in general, their serum antibody responses to the conjugate vaccine (particularly bactericidal antibody) were lower than those of vaccine-naïve subjects (3, 17, 26).
Less information is available on the possible influence of previous exposure to MPSV on priming by MCV for memory responses. In a study in The Gambia, memory group C antibody responses to MPSV were observed in 5-year-old children who had been immunized with two doses of MPSV as infants and one dose of a group A plus group C conjugate vaccine given in the second year of life (19). In a second study in the United Kingdom, there was evidence of avidity maturation in serum samples obtained at 1 and 6 months after a dose of MCV in children previously immunized with MPSV (3). However, in two studies of adults who were immunized with MPSV and who were given a subsequent dose of MCV, there was no evidence of avidity maturation (10, 28).
In the present study, the adults who had been given MPSV before enrollment and who were immunized with MCV had IgG anticapsular antibody concentrations and bactericidal titers after the polysaccharide challenge that were higher than those of the vaccine-naïve control group given PCV. However, there was no evidence of induction of memory by MCV in subjects previously exposed to unconjugated polysaccharide vaccine. Even among the 11 subjects who had been given MPSV prior to enrollment and who had ≤2 μg/ml of antibody before the MPSV challenge, only two subjects showed evidence of immunologic priming, which was not different than the natural priming observed in the meningococcal vaccine-naïve control PCV group. Thus, prior exposure to unconjugated polysaccharide vaccine not only induces group C antibody hyporesponsiveness to a subsequent challenge dose of polysaccharide but also interferes with the ability of MCV to prime for memory antibody responses. Since the severalfold increase in antibody concentrations after the MPSV challenge were greater in the group given MPSV ≥3 years before enrollment than in the group given MPSV <3 years before enrollment, the effect of prior exposure to unconjugated polysaccharide on interference with induction of memory by the conjugate vaccine appears to decline over time.
Acknowledgments
This study was funded in part by a grant from the Chiron Corporation. Additional funding was from grants RO1 AI46464 and AI58122 from the National Institutes of Allergy and Infectious Disease of the NIH. David Vu is supported by Ruth L. Kirschstein National Research Service Award F32 AI056828 from the National Institutes of Allergy and Infectious Disease, NIH. This work was also supported in part by NIH grant M01-RR01271, the Pediatric Clinical Research Center, and Research Facilities Improvement Program grant number CO6 RR-16226 from the National Center for Research Resources, NIH.
REFERENCES
- 1.Baxendale, H. E., Z. Davis, H. N. White, M. B. Spellerberg, F. K. Stevenson, and D. Goldblatt. 2000. Immunogenetic analysis of the immune response to pneumococcal polysaccharide. Eur. J. Immunol. 30:1214-1223. [DOI] [PubMed] [Google Scholar]
- 2.Borrow, R., A. J. Fox, P. C. Richmond, S. Clark, F. Sadler, J. Findlow, R. Morris, N. T. Begg, and K. A. Cartwright. 2000. Induction of immunological memory in UK infants by a meningococcal A/C conjugate vaccine. Epidemiol. Infect. 124:427-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borrow, R., D. Goldblatt, N. Andrews, P. Richmond, J. Southern, and E. Miller. 2001. Influence of prior meningococcal C polysaccharide vaccination on the response and generation of memory after meningococcal C conjugate vaccination in young children. J. Infect. Dis. 184:377-380. [DOI] [PubMed] [Google Scholar]
- 4.Borrow, R., D. Goldblatt, N. Andrews, J. Southern, L. Ashton, S. Deane, R. Morris, K. Cartwright, and E. Miller. 2002. Antibody persistence and immunological memory at age 4 years after meningococcal group C conjugate vaccination in children in the United Kingdom. J. Infect. Dis. 186:1353-1357. [DOI] [PubMed] [Google Scholar]
- 5.Borrow, R., P. Richmond, E. B. Kaczmarski, A. Iverson, S. L. Martin, J. Findlow, M. Acuna, E. Longworth, R. O'Connor, J. Paul, and E. Miller. 2000. Meningococcal serogroup C-specific IgG antibody responses and serum bactericidal titres in children following vaccination with a meningococcal A/C polysaccharide vaccine. FEMS Immunol. Med. Microbiol. 28:79-85. [DOI] [PubMed] [Google Scholar]
- 6.Campagne, G., A. Garba, P. Fabre, A. Schuchat, R. Ryall, D. Boulanger, M. Bybel, G. Carlone, P. Briantais, B. Ivanoff, B. Xerri, and J. P. Chippaux. 2000. Safety and immunogenicity of three doses of a Neisseria meningitidis A + C diphtheria conjugate vaccine in infants from Niger. Pediatr. Infect. Dis. J. 19:144-150. [DOI] [PubMed] [Google Scholar]
- 7.Gold, R., M. L. Lepow, I. Goldschneider, T. F. Draper, and E. C. Gotshlich. 1979. Kinetics of antibody production to group A and group C meningococcal polysaccharide vaccines administered during the first six years of life: prospects for routine immunization of infants and children. J. Infect. Dis. 140:690-697. [DOI] [PubMed] [Google Scholar]
- 8.Gold, R., M. L. Lepow, I. Goldschneider, T. L. Draper, and E. C. Gotschlich. 1975. Clinical evaluation of group A and group C meningococcal polysaccharide vaccines in infants. J. Clin. Investig. 56:1536-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gold, R., M. L. Lepow, I. Goldschneider, and E. C. Gotschlich. 1977. Immune response of human infants of polysaccharide vaccines of group A and C Neisseria meningitidis. J. Infect. Dis. 136(Suppl.):S31-S35. [DOI] [PubMed] [Google Scholar]
- 10.Goldblatt, D., R. Borrow, and E. Miller. 2002. Natural and vaccine-induced immunity and immunologic memory to Neisseria meningitidis serogroup C in young adults. J. Infect. Dis. 185:397-400. [DOI] [PubMed] [Google Scholar]
- 11.Granoff, D. M., R. K. Gupta, R. B. Belshe, and E. L. Anderson. 1998. Induction of immunologic refractoriness in adults by meningococcal C polysaccharide vaccination. J. Infect. Dis. 178:870-874. [DOI] [PubMed] [Google Scholar]
- 12.Granoff, D. M., S. E. Maslanka, G. M. Carlone, B. D. Plikaytis, G. F. Santos, A. Mokatrin, and H. V. Raff. 1998. A modified enzyme-linked immunosorbent assay for measurement of antibody responses to meningococcal C polysaccharide that correlate with bactericidal responses. Clin. Diagn. Lab. Immunol. 5:479-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jokhdar, H., R. Borrow, A. Sultan, M. Adi, C. Riley, E. Fuller, and D. Baxter. 2004. Immunologic hyporesponsiveness to serogroup C but not serogroup A following repeated meningococcal A/C polysaccharide vaccination in Saudi Arabia. Clin. Diagn. Lab. Immunol. 11:83-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keyserling, H., T. Papa, K. Koranyi, R. Ryall, E. Bassily, M. J. Bybel, K. Sullivan, G. Gilmet, and A. Reinhardt. 2005. Safety, immunogenicity, and immune memory of a novel meningococcal (groups A, C, Y, and W-135) polysaccharide diphtheria toxoid conjugate vaccine (MCV-4) in healthy adolescents. Arch. Pediatr. Adolesc. Med. 159:907-913. [DOI] [PubMed] [Google Scholar]
- 15.Leach, A., P. A. Twumasi, S. Kumah, W. S. Banya, S. Jaffar, B. D. Forrest, D. M. Granoff, D. E. LiButti, G. M. Carlone, L. B. Pais, C. V. Broome, and B. M. Greenwood. 1997. Induction of immunologic memory in Gambian children by vaccination in infancy with a group A plus group C meningococcal polysaccharide-protein conjugate vaccine. J. Infect. Dis. 175:200-204. [DOI] [PubMed] [Google Scholar]
- 16.Lucas, A. H., K. D. Moulton, V. R. Tang, and D. C. Reason. 2001. Combinatorial library cloning of human antibodies to Streptococcus pneumoniae capsular polysaccharides: variable region primary structures and evidence for somatic mutation of Fab fragments specific for capsular serotypes 6B, 14, and 23F. Infect. Immun. 69:853-864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacDonald, N. E., S. A. Halperin, B. J. Law, L. E. Danzig, and D. M. Granoff. 2000. Can meningococcal C conjugate vaccine overcome immune hyporesponsiveness induced by previous administration of plain polysaccharide vaccine? JAMA 283:1826-1827. [DOI] [PubMed] [Google Scholar]
- 18.MacDonald, N. E., S. A. Halperin, B. J. Law, B. Forrest, L. E. Danzig, and D. M. Granoff. 1998. Induction of immunologic memory by conjugated vs plain meningococcal C polysaccharide vaccine in toddlers: a randomized controlled trial. JAMA 280:1685-1689. [DOI] [PubMed] [Google Scholar]
- 19.MacLennan, J., S. Obaro, J. Deeks, D. Williams, L. Pais, G. Carlone, R. Moxon, and B. Greenwood. 1999. Immune response to revaccination with meningococcal A and C polysaccharides in Gambian children following repeated immunisation during early childhood. Vaccine 17:3086-3093. [DOI] [PubMed] [Google Scholar]
- 20.MacLennan, J. M., F. Shackley, P. T. Heath, J. J. Deeks, C. Flamank, M. Herbert, H. Griffiths, E. Hatzmann, C. Goilav, and E. R. Moxon. 2000. Safety, immunogenicity, and induction of immunologic memory by a serogroup C meningococcal conjugate vaccine in infants: a randomized controlled trial. JAMA 283:2795-2801. [DOI] [PubMed] [Google Scholar]
- 21.Maslanka, S. E., J. W. Tappero, B. D. Plikaytis, R. S. Brumberg, J. K. Dykes, L. L. Gheesling, K. B. Donaldson, A. Schuchat, J. Pullman, M. Jones, J. Bushmaker, and G. M. Carlone. 1998. Age-dependent Neisseria meningitidis serogroup C class-specific antibody concentrations and bactericidal titers in sera from young children from Montana immunized with a licensed polysaccharide vaccine. Infect. Immun. 66:2453-2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Powers, D. C., E. L. Anderson, K. Lottenbach, and C. M. Mink. 1996. Reactogenicity and immunogenicity of a protein-conjugated pneumococcal oligosaccharide vaccine in older adults. J. Infect. Dis. 173:1014-1018. [DOI] [PubMed] [Google Scholar]
- 23.Richmond, P., R. Borrow, D. Goldblatt, J. Findlow, S. Martin, R. Morris, K. Cartwright, and E. Miller. 2001. Ability of 3 different meningococcal C conjugate vaccines to induce immunologic memory after a single dose in UK toddlers. J. Infect. Dis. 183:160-163. [DOI] [PubMed] [Google Scholar]
- 24.Richmond, P., R. Borrow, E. Miller, S. Clark, F. Sadler, A. Fox, N. Begg, R. Morris, and K. Cartwright. 1999. Meningococcal serogroup C conjugate vaccine is immunogenic in infancy and primes for memory. J. Infect. Dis. 179:1569-1572. [DOI] [PubMed] [Google Scholar]
- 25.Richmond, P., D. Goldblatt, P. C. Fusco, J. D. Fusco, I. Heron, S. Clark, R. Borrow, and F. Michon. 1999. Safety and immunogenicity of a new Neisseria meningitidis serogroup C-tetanus toxoid conjugate vaccine in healthy adults. Vaccine 18:641-646. [DOI] [PubMed] [Google Scholar]
- 26.Richmond, P., E. Kaczmarski, R. Borrow, J. Findlow, S. Clark, R. McCann, J. Hill, M. Barker, and E. Miller. 2000. Meningococcal C polysaccharide vaccine induces immunologic hyporesponsiveness in adults that is overcome by meningococcal C conjugate vaccine. J. Infect. Dis. 181:761-764. [DOI] [PubMed] [Google Scholar]
- 27.Shelly, M. A., H. Jacoby, G. J. Riley, B. T. Graves, M. Pichichero, and J. J. Treanor. 1997. Comparison of pneumococcal polysaccharide and CRM197-conjugated pneumococcal oligosaccharide vaccines in young and elderly adults. Infect. Immun. 65:242-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Southern, J., S. Deane, L. Ashton, R. Borrow, D. Goldblatt, N. Andrews, P. Balmer, R. Morris, J. S. Kroll, and E. Miller. 2004. Effects of prior polysaccharide vaccination on magnitude, duration, and quality of immune responses to and safety profile of a meningococcal serogroup C tetanus toxoid conjugate vaccination in adults. Clin. Diagn. Lab. Immunol. 11:1100-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]