Abstract
Diphtheria is under control in industrialized countries. However, single cases and outbreaks still occur and the disease is not completely understood. Forty-three individuals suspected of having diphtheria who were referred to the Infectious Disease Hospital of Arkhangelsk from December 1994 to March 1995 were included in this study. Fifteen patients were diagnosed as having diphtheria and received equine hyperimmune antidiphtheria toxin antiserum, and 28 were diagnosed as carriers, 12 with nondiphtherial tonsillitis or pharyngitis and 16 without symptoms. Serum samples were obtained on admission and during the course of the disease or during follow-up of carrier status. Samples were analyzed for antibodies against diphtheria toxin with both an in vitro neutralization test (NT) and a human-specific enzyme immunoassay. All of the cases but one were confirmed by a positive culture. Twelve patients had pharyngeal diphtheria, and three had combined laryngeal and pharyngeal disease. Half of the patients had life-threatening disease, and one died. On admission, the median antibody titers measured with the NT were 0.085 IU/ml for the patients, 5.12 IU/ml for the symptomatic carriers, and 10.24 IU/ml for the healthy carriers. All of the diphtheria patients but one and nine of the carriers (six symptomatic and three healthy) had increased antibody levels during the first 7 to 10 days after admission. No obvious correlation was revealed between the antibody level or its kinetics and the course of the disease. Antibody levels on admission of >1 IU/ml were associated with a low risk of diphtheria.
Diphtheria is a severe and sometimes fatal disease caused by toxin-producing strains of Corynebacterium diphtheriae. Protection against diphtheria is obtained by the presence of significant levels of antibodies against diphtheria toxin. However, antibodies developing after disease do not usually give full protection against clinical diphtheria on reinfection, which may cause severe and even lethal disease.
After mass vaccination programs against diphtheria were established, the disease became very rare in industrialized countries. Only small outbreaks and isolated imported cases have been reported, despite the fact that seroepidemiological studies have shown insufficient protection, especially among the adult population (6, 11).
However, in the Russian Federation, three epidemics of diphtheria have occurred during the last 50 years involving several regions of the country. The last epidemic, which started in 1990, had 115,000 reported cases and more than 3,000 deaths (15).
Diagnosis of diphtheria is not always easy (3). It is based on clinical symptoms and signs and on the detection of C. diphtheriae. The diagnosis is supported by low levels of diphtheria antibodies in serum. Administration of antidiphtheria antitoxin during the early stage of the disease is often crucial to preventing complications and death. This, however, demands speedy diagnosis, usually before the results of microbiological analyses are available (13). The clinical appearance of the disease is characteristic in severe cases, but in the early phase and in less-severe and mild cases, the diagnosis may be missed. Assessment of the levels of antibodies against diphtheria toxin at the onset of the disease is recommended as a complementary diagnostic criterion (2, 5).
In this study, serum levels of antibodies against diphtheria toxin on hospital admission and the further development of these antibodies were studied with an in vitro neutralization test (NT) and an enzyme immunoassay (EIA) among diphtheria patients and C. diphtheriae carriers.
MATERIALS AND METHODS
Patients and carriers.
According to the guidelines of the Russian Ministry of Health Care, all suspected diphtheria cases in Arkhangelsk and the adjoining parts of the Arkhangelsk region are referred to the Hospital of Infectious Diseases, Arkhangelsk (2). The Arkhangelsk region comprises a population of approximately 1.5 million. According to hospital records, 650 patients and 865 carriers were treated during the last epidemic from 1991 to 1999. The outbreak peaked in 1994 with 261 patients and 266 carriers. According to the national guidelines, pharyngeal and nasopharyngeal swabs for isolation of C. diphtheriae were obtained from all patients with tonsillopharyngitis and from close contacts of diphtheria patients. Everyone with a positive culture was referred to the hospital for eradication of the bacteria.
This study was conducted from December 1994 to March 1995 at the Hospital of Infectious Diseases, Arkhangelsk. Forty-three patients were included in the study and grouped as follows according to clinical and laboratory findings: (i) clinical patients (15 patients; mean age, 35 years; age range, 5 to 58 years), (ii) symptomatic carriers (12 patients; mean age, 21 years; age range, 5 to 46 years), and (iii) healthy carriers (16 individuals; mean age, 14 years [2 unknown age]; age range, 3 to 36 years).
Diphtheria cases.
Patients with diphtheria were defined as those with a respiratory tract infection and clinical signs of a local and/or systemic toxin effect. Patients with local disease had typical faucial pseudomembranes and edema. Pseudomembranes were thick and adherent to the mucosal surface. Systemic complications included neck edema, myocarditis, and peripheral neuropathy. These patients were further grouped as having (i) mild disease with localized tonsillar membranes but without signs of a systemic effect, (ii) moderate disease with extensive membranes and neck edema but no life-threatening symptoms, or (iii) severe disease with life-threatening airway obstruction and/or cardiac complications. Symptomatic carriers were patients with a positive culture for C. diphtheriae and pharyngitis and/or tonsillitis but without signs of localized or general diphtheria toxin effects as described above. Healthy carriers were individuals with a positive culture for C. diphtheriae but without any clinical symptoms or signs.
Clinical and laboratory examinations.
On admission, all of the patients and carriers included in this study were clinically examined regarding their general condition and the presence of pseudomembranes, edema, and possible complications. Neurological examinations were performed when neurological symptoms were suspected. Electrocardiograms were taken on admission and later if necessary. Standard laboratory tests (hemoglobin, white blood cell count, blood cell differential count, platelet count, serum urea and creatinine concentrations, and urine analysis) were performed. Nose and throat swabs for bacterial cultivation were obtained on admission and daily for 3 days from patients and after 1 week from carriers. The samples were cultured, isolates were identified, and toxin production tests were performed according to World Health Organization (WHO) recommendations (1, 7, 8). Except for one patient, sera were obtained for determination of antibodies against diphtheria toxin on the day of admission to the hospital and repeatedly during the hospital stay (see Table 2). Information on previous diphtheria vaccination was collected if available.
TABLE 2.
Development of antibodies against diphtheria toxin in diphtheria patients as measured by NT (on admission) and EIA
| Diphtheria patient no. | Antibody titer (IU/ml) on admission determined by:
|
Antibody titer (IU/ml) determined by EIA
|
||||
|---|---|---|---|---|---|---|
| NT | EIA | Days 5-10 | Days 11-20 | Days 21-30 | After 30 days | |
| 1 | 1.280 | 0.609 | 0.398 | 3.980 | 2.458 | |
| 2 | 0.160 | 0.022 | 0.072 | 0.316 | 0.496 | |
| 3 | 0.640 | 0.176 | 0.076 | 4.011 | 0.672 | 0.029 |
| 4 | 0.160 | 0.044 | 0.201 | 5.240 | 5.240 | |
| 5 | 82a | 5.240 | 5.240 | 2.566 | 0.628 | |
| 6 | 82a | 0.690 | 5.240 | 5.240 | ||
| 7 | 0.00125 | 0.005 | 1.100 | 0.500 | ||
| 8 | 0.00125 | 0.116 | 5.240 | 5.240 | ||
| 9 | Not taken | Not taken | 0.661 | 2.642 | 1.047 | |
| 10 | Late admission | Late admission | 0.789 | 0.529 | 0.833 | 0.886 |
| 11 | 0.010 | 0.077 | 5.240 | 1.279 | 0.516 | 0.219 |
| 12 | 321a | 0.010 | 0.242 | |||
| 13 | 0.010 | 0.005 | 3.675 | 1.758 | ||
| 14 | 0.320 | 0.218 | 5.240 | 5.240 | 5.240 | 5.240 |
| 15 | 0.010 | 0.043 | 0.169 | 0.789 | 0.581 | |
| Median | 0.085 | 0.077 | 0.530b | 3.670 | 0.886 | |
A serum sample was taken after EHAS was injected.
The increase in antibody levels since admission was statistically significant.
This study was approved by the Arkhangelsk Region Department of Public Health.
Serological methods.
For the assessment of the levels of antibodies against diphtheria toxin, two different assays were performed as previously described (17), i.e., (i) an in vitro NT for detecting the total amount of toxin-neutralizing antibodies, both human and equine, and (ii) an EIA for detecting human-specific immunoglobulin G (IgG) antibodies against diphtheria toxoid.
In brief, the NT was performed with twofold dilutions of serum mixed with a dose of diphtheria toxin equivalent to four times the minimal cytotoxic dose (Statens Seruminstitut, Copenhagen, Denmark) and incubated at 37°C for 1 h before Vero cells were added. An antitoxin-positive control serum, a toxin control, and a cell control were run for every 17th serum sample. The pH-mediated color change from red to yellow caused by growing Vero cells was recorded after 5 days of incubation at 37°C. The antibody level of each sample was determined by comparing the color change to that of a WHO standard (Statens Seruminstitut) analyzed simultaneously.
The EIA, a three-layer indirect assay, was performed with microtiter plates with diphtheria toxoid (Statens Seruminstitut) coating the wells and to which 100 μl of serum, diluted 1:50 in phosphate-buffered saline (pH 7.4) with 0.05% Tween 20 (PBST), was added in duplicate, and the plates were incubated at 37°C for 2 h and then washed three times with PBST. After a further 2 h of incubation with 100 μl of swine anti-human IgG conjugated to alkaline phosphatase (Orion Diagnostica, Helsinki, Finland) diluted 1:200 in PBST and three more washings, the amount of bound conjugate was measured in a microplate reader by adding a substrate that was allowed to react for 45 min. The test was standardized against selected sera analyzed by the WHO reference laboratory in Copenhagen, Denmark.
The results are presented as international units per milliliter and were interpreted as follows: <0.01 IU/ml, no protection; 0.01 to 0.1 IU/ml, partial protection (i.e., possible protection against severe toxic disease); >0.1 IU/ml, protection (9). A significant increase was defined as a fourfold or greater increase in the amount of antibody from the values measured on admission. The upper cutoff level for the EIA was 5.24 IU/ml.
Statistical analysis.
The Wilcoxon matched-pair signed rank sum test was used to compare antibody levels on different days of disease. Paired and independent t tests were used to compare titers among carriers after log transformation. The odds ratio was used to estimate the risk of disease when antibody levels were ≥0.5 IU/ml or ≥1 IU/ml.
RESULTS
Clinical characteristics.
The main clinical characteristics of the 15 patients with clinical diphtheria are given in Table 1. All patients received specific treatment with equine hyperimmune antidiphtheria toxin antiserum (EHAS) immediately. Two patients had mild disease (patients 1 and 2), six had moderate disease (patients 3 to 8), and seven had severe disease (patients 9 to 15). Twelve patients developed pharyngeal diphtheria, and three patients developed combined pharyngeal and laryngeal disease. Seven patients had life-threatening disease due to airway obstruction or cardiac symptoms, and one died of heart failure.
TABLE 1.
Clinical characteristics of 15 diphtheria patients
| Patient no. | Age (yr) | Sexa | Days from onset to admission | Disease severity | Symptoms | Complication(s) |
|---|---|---|---|---|---|---|
| 1 | 14 | M | 2 | Mild | Pseudomembrane islets localized to tonsils; LN,b 1.5 cm | |
| 2 | 42 | F | 2 | Mild | Pseudomembrane islets localized to tonsils; LN,b 1.5 cm | |
| 3 | 5 | M | 2 | Moderate | Pseudomembranes covering tonsils, edema in upper part of the neck; LN, 2.5 cm | |
| 4 | 11 | F | 1 | Moderate | Pseudomembranes covering tonsils, uvula, and pharyngeal wall; swollen pharynx and upper neck; LN, 2 cm | |
| 5 | 48 | F | 2 | Moderate | Pseudomembranes covering tonsils, uvula, and pharyngeal wall; swollen pharynx and upper neck; LN, 2 cm | PNPc |
| 6 | 47 | F | 3 | Moderate | Pseudomembranes covering tonsils, swollen upper part of neck; LN, 4 cm | PNP |
| 7 | 39 | F | 3 | Moderate | Pseudomembranes extending to pharynx, very swollen pharynx and neck | |
| 8 | 42 | F | 2 | Moderate | Pseudomembranes extending to pharynx, very swollen pharynx and neck | PNP |
| 9 | 52 | F | 5 | Severe | Pseudomembranes covering tonsils, uvula, pharyngeal wall; swollen pharynx and neck; LN, 2.5 cm | PNP |
| 10 | 43 | M | 5 | Severe | Pseudomembranes extending to fauces; swollen fauces, neck; LN, very swollen, 6 cm | PNP |
| 11 | 33 | F | 2 | Severe | Pseudomembranes extending to fauces; swollen fauces, neck; LN, very swollen, 6 cm | MC,d PNP |
| 12 | 21 | M | 2 | Severe | Pseudomembranes extending to fauces; swollen fauces, neck; LN, very swollen, 6 cm | MC, death |
| 13 | 39 | M | 3 | Severe | Pseudomembranes extending to pharynx and larynx; swollen pharynx, larynx, and neck; LN, 3 cm | MC, PNP |
| 14 | 58 | F | 4 | Severe | Pseudomembranes extending to pharynx and larynx, very intensive edema of pharynx and larynx, bull neck; LN, 6 cm | |
| 15 | 40 | M | 3 | Severe | Pseudomembranes extending to pharynx and larynx, very intensive edema of pharynx and larynx, bull neck; LN, 6 cm | MC, PNP |
M, male; F, female.
LN, lymph nodes.
PNP, polyneuropathy.
MC, myocarditis.
All patients belonged to low socioeconomic groups. Three patients (patients 9, 13, and 15) were proven alcohol abusers. Patient 3 was a neglected child.
Vaccination histories were available only for the children. Patient 3 had not been vaccinated, and patients 1 and 4 had incomplete vaccination according to the national vaccination program, having received three and two primary doses, respectively. Most adults did not know their vaccination status but had not received a booster vaccination after 16 years of age. Patient 8 had received a single vaccination dose 1 year before getting the disease as part of the extraordinary adult vaccination program during the last epidemic. Patient 12 had not been vaccinated because of severe epilepsy.
On admission, all of the patients but one (patient 14) had positive cultures of toxigenic C. diphtheriae, 12 patients had biotype gravis, the dominant biovar during the epidemic, and two patients had biotype mitis. Subsequent cultures were all negative. No other obligate pathogenic bacteria were isolated from the patients. Biotype gravis was isolated from all of the symptomatic and healthy carriers.
Antidiphtheria antibody levels on admission.
Among the clinical patients, five (patients 7, 8, 11, 13, and 15) had antibody levels of ≤0.01 IU/ml measured by the NT on hospital admission, four (patients 2, 3, 4, and 14) had values between 0.1 and 1 IU/ml, and one (patient 1) had a value of ≥1 IU/ml (Table 2). Five (patients 5, 6, 9, 10, and 12) received EHAS before the first serum sample was taken. The median antibody levels were 0.085 IU/ml as measured with the NT and 0.077 IU/ml as measured with the EIA.
Among the 12 symptomatic carriers, only 4 (33%) had antibody levels of <1 IU/ml; the median for the group was 5.12 IU/ml (range, 0.16 to 328 IU/ml) as measured with the NT and 2.10 IU/ml (range, 0.005 to ≥5.24 IU/ml) as measured with the EIA (Table 3). Among the 16 healthy carriers, the median was 10.24 IU/ml (range, 2.56 to 81.9 IU/ml) by the NT and ≥5.24 (range, 0.49 to ≥5.24 IU/ml) by the EIA (Table 4). The geometric mean antibody titer on admission measured with the NT was higher in the healthy carrier group (14.15 IU/ml) than in the symptomatic carrier group (4.81 IU/ml).
TABLE 3.
Development of antibodies against diphtheria toxin among symptomatic carriers of C. diphtheriae as measured by NT and EIA
| Symptomatic carrier no. | Age (yr) | Antibody titer (IU/ml) on:
|
|||
|---|---|---|---|---|---|
| Admission
|
Days 5-20
|
||||
| NT | EIA | NT | EIA | ||
| 1 | 5 | 0.64 | 0.373 | 644.0 | ≥5.24 |
| 2 | 10 | 328.0 | ≥5.24 | 328.0 | ≥5.24 |
| 3 | 11 | 0.64 | 0.005 | 40.96 | ≥5.24 |
| 4 | 14 | 81.92 | ≥5.24 | 81.92 | ≥5.24 |
| 5 | 15 | 20.48 | ≥5.24 | 40.96 | ≥5.24 |
| 6 | 19 | 1.28 | 1.155 | 20.48 | ≥5.24 |
| 7 | 19 | 5.12 | ≥5.24 | 20.48 | ≥5.24 |
| 8 | 22 | 40.96 | ≥5.24 | 40.96 | ≥5.24 |
| 9 | 29 | 10.24 | 1.589 | 20.48 | ≥5.24 |
| 10 | 34 | 0.32 | 0.223 | 1.28 | 0.966 |
| 11 | 33 | 0.16 | 0.307 | 20.48 | ≥5.24 |
| 12 | 46 | 5.12 | 2.617 | 10.24 | ≥5.24 |
| Median | 5.12 | 2.103 | 30.72 | ≥5.24 | |
TABLE 4.
Development of antibodies against diphtheria toxin among healthy carriers of C. diphtheriae as measured by NT and EIA
| Healthy carrier no. | Age (yr) | Antibody titer (IU/ml) on:
|
|||
|---|---|---|---|---|---|
| Admission
|
Days 5-10
|
||||
| NT | EIA | NT | EIA | ||
| 1 | 3 | 20.48 | ≥5.24 | 81.92 | ≥5.24 |
| 2 | 7 | 10.24 | ≥5.24 | 10.24 | ≥5.24 |
| 3 | 7 | 5.12 | 4.063 | 40.96 | ≥5.24 |
| 5 | 7 | 5.12 | 2.608 | 2.56 | 2.439 |
| 4 | 8 | 5.12 | 2.964 | 5.12 | 3.694 |
| 6 | 8 | 5.12 | 1.292 | 20.48 | ≥5.24 |
| 7 | 8 | NDb | ND | 40.96 | ≥5.24 |
| 8 | 10 | 40.96 | ≥5.24 | 40.96 | ≥5.24 |
| 9 | 11 | 40.96 | ≥5.24 | 40.96 | ≥5.24 |
| 10 | 11 | 40.96 | ≥5.24 | 40.96 | ≥5.24 |
| 11 | 19 | 81.92 | ≥5.24 | 81.92 | ≥5.24 |
| 12 | 25 | 20.48 | ≥5.24 | 40.96 | ≥5.24 |
| 13 | 30 | 40.96 | ≥5.24 | 40.96 | ≥5.24 |
| 14 | 39 | 10.24 | ≥5.24 | 10.24 | ≥5.24 |
| 15 | —a | 2.56 | 0.486 | 5.12 | 0.702 |
| 16 | —a | 10.24 | 2.005 | 10.24 | 1.955 |
| Median | 10.24 | ≥5.24 | 40.96 | ≥5.24 | |
—, age not known.
ND, not done.
Follow-up of diphtheria patients.
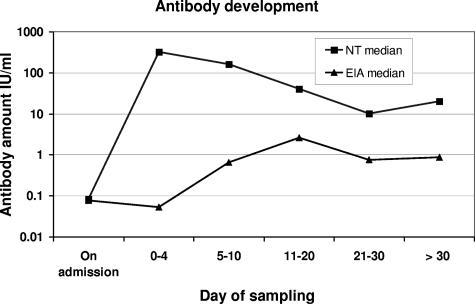
We observed a significant increase (fourfold or greater) in antibody levels within 11 to 20 days for 11 of the 12 diphtheria patients (92%) whose antibody levels had been measured with the EIA and for whom samples were available during the first 5 days of the disease. The only patient not showing an increase (patient 5) had the highest value of all on admission (5.24 IU/ml). The two patients with the lowest peak values were the one who died (patient 12) and one with mild disease (patient 2). The increase in antibody levels was followed by a decrease in eight of the patients after 2 to 3 weeks (Table 2). The difference in dynamics of serum neutralizing activity and antibody levels measured with the human-specific EIA is shown in Fig. 1.
FIG. 1.
Development of antibodies against diphtheria toxin in 15 diphtheria patients (median values) before and after administration of EHAS as measured with an NT and a human-specific EIA.
In six (50%) symptomatic and three (19%) healthy carriers, the antibody level increased significantly. For the group of symptomatic carriers as a whole, this increase was significant, the median value (NT) increasing from 5.12 to 30.72 IU/ml within 3 weeks, and for the healthy carrier group an increase from 10.24 to 40.96 IU/ml was observed (Tables 3 and 4).
The risk of developing clinical diphtheria correlated inversely with the concentration of antibodies against diphtheria toxin on admission. The odds ratio was 0.05 (95% confidence interval, 0.02 to 0.12) when the cutoff point for antibody levels was set to 0.5 IU/ml, and it was even lower when the cutoff point was 1 IU/ml, i.e., 0.01 (95% confidence interval, 0.0036 to 0.037). No significant correlation between the magnitude of the immune response and the course and outcome of the disease was revealed.
DISCUSSION
Diphtheria is a disease that can be prevented by vaccination, and measuring the amount of serum antibodies against diphtheria toxin in individuals is the only way to survey the level of protection in a community. This raises the question of what the adequate protective levels of antibodies for an individual and for a community are. The understanding of the individual protective level of diphtheria antibodies comes from a study performed in the 1940s (10). No prospective trials evaluating the protective threshold have been performed. The antibody level that provides high individual protection may be very different from the level that provides protection in the community, where herd immunity has to be taken into account.
Any threshold of protection established should be based on a careful diagnosis of suspected cases considering the degree of clinical disease. Doctors have a tendency on the one hand to delay making a diagnosis or to fail to make the correct diagnosis in societies in which there are no or few indigenous cases (14, 16) and on the other hand to overdiagnose cases during an epidemic (15). During the recent epidemic in the former Union of Soviet Socialist Republics, as many as 25% of the patients who were classified as having mild cases could in fact be classified as carriers according to the WHO classification (12). Most of them were probably symptomatic carriers suffering from viral or bacterial nondiphtherial upper respiratory tract infections. The most difficult diagnostic challenge is to distinguish true diphtheria patients from patients with confluent exudative (purulent) tonsillopharyngitis of nondiphtherial origin, which is a highly prevalent disease. A positive culture usually helps to make a diagnosis in suspected cases. However, positive cultures for toxigenic C. diphtheriae could also confuse the physician if the patient is a carrier of C. diphtheriae and at the same time has throat symptoms due to another bacterial or viral infection.
In our study, we classified the patients according to signs of local and systemic effects of diphtheria toxin. Locally, toxin induces tissue necrosis and an inflammatory reaction resulting in tough, adherent membranes. The membrane islets tend to spread outside the tonsils and to be confluent in most cases. The membrane size usually corresponds to the spread of local edema. The first and most specific severe, systemic sign is neck edema of various magnitudes. The presence of typical cardiac and neurological complications clearly supports the diagnosis. Persons who did not meet these criteria were considered not to have diphtheria but rather to be symptomatic carriers with nondiphtherial tonsillitis.
This approach could have resulted in some very mild cases not being identified. However, these patients never needed specific EHAS treatment, and further spread of C. diphtheriae was stopped by antimicrobial treatment similar to the treatment given to healthy carriers. We find that the tendency to classify carriers as patients is more problematic, since they would unnecessarily have become eligible for aggressive equine serum treatment, which can have serious side effects. Most of these individuals have been vaccinated, which prevents them from developing serious clinical disease. Hence, including them as diphtheria patients in epidemiological studies would give the impression that vaccination is less effective than it actually is.
On admission, all but two patients had neutralizing antibody titers that were higher than those considered to be relatively protective. The incubation period, in which bacteria are present, plus the period from the onset of the disease until admission (2 to 4 days) may have resulted in an increase in antibody levels before the first serum sample was collected. This is supported by the fact that antibody levels varied considerably on admission among patients with severe disease. However, all of the patients but one showed a significant increase in antidiphtheria antibodies during the course of the disease. The increased level was maintained for more than a month, although the level started to fall after approximately 3 weeks. Groundstroem et al. studied antibody levels in 133 diphtheria patients (K. Groundstroem, H. Huhtala, J. Lumio, O. Melnick, R.-M. Ölander, A. Rakhmanova, and J. Vuopio-Varkila, Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. 575, 2003). In contrast to our findings, they found no clear tendency to increasing antibody levels over time. A possible explanation for this difference may be a longer delay (median, 11 days) between the onset of disease and hospital admission when sera were collected in the Finnish-Russian study. Many patients could already have had the increase prior to admission.
The clear differences between the results obtained with the NT and the EIA are due to the administration of EHAS, which gives an immediate increase in serum neutralizing activity without any effect on the level of human-specific IgG against the diphtheria toxin (Fig. 1). This indicates that there was no significant cross-reactivity from equine antibodies in the EIA. This is further supported by the observation that while the equine antibody amount gradually decreased, the human IgG response to diphtheria toxoid gradually increased. Our results show that specific antibodies develop in patients despite the fact that EHAS is given. Whether administration of EHAS to some degree suppresses the patient's own development of antibodies against diphtheria toxin is not known, but it certainly would neutralize the toxin effects.
In our study, neither the initial antibody level nor the pattern of antibody development was able to predict the severity of disease or its complications. However, the larger study by Groundstroem et al. showed that antibody levels of <1 IU/ml during the first week of the disease indicated a much higher risk of developing extensive and toxic disease (Groundstroem et al., 43rd ICAAC).
In both the symptomatic and asymptomatic carrier groups, antibody levels varied greatly on admission and the increase observed during the following 10 days also varied. The reason for this could also be that serum samples were collected at different stages during the immune response that was observed among carriers (4). Symptomatic carriers had lower antibody levels than healthy carriers, but this difference was not significant. However, the median antibody titer was 60 times higher than among diphtheria patients. High antibody levels were evidently protective against developing clinical diphtheria.
Our results do not explain the differences in antibody level between symptomatic and asymptomatic carriers. However, patients sought medical help because of symptoms and were subsequently screened for C. diphtheriae while contacts were selected and screened only when an index patient was diagnosed.
There was an increasing mean age from the healthy carrier group (15 years) through the symptomatic carrier group (21 years) to the diphtheria patient group (35 years). This finding gives an indication that children and young people generally were better protected against clinical disease when infected with toxigenic strains of C. diphtheriae. This is supported by epidemiological data from the epidemic in Russia showing the highest fatality rate among adults more than 40 years old and children less than 2 years old (incomplete vaccination) (15).
In our study, patients had a very low risk (5%) of developing clinical diphtheria if antibody levels were ≥0.5 IU/ml on admission and an even lower risk (1%) if the level was ≥1 IU/ml. In the study by Groundstroem et al., more than half of the diphtheria patients had antibody levels of ≥1 IU/ml on admission (Groundstroem et al., 43rd ICAAC). However, the proportion of patients with such high levels was much smaller (8 of 32 patients) when the analysis was restricted to those who had the first sample taken during the first week of the disease. In our study, the median period between the onset of disease and hospital admission was only 2 days. It seems clear that the levels of antibody against diphtheria toxin are very low in almost all clinical cases of diphtheria when the symptoms first appear and that antibody levels usually increase rapidly during the following 2 to 3 weeks. The possible preventive effect of the antibody level on the seriousness of the symptoms must always be related to the time elapsed from symptom onset to the time when the serum sample was taken.
It seems wise to use an antibody titer of 0.1 IU/ml as measured by the EIA as a lower threshold for basic individual protection in a population (9). However, when faced with a patient who is strongly suspected of having diphtheria, the decision to give equine antiserum must primarily be based on a combination of the clinical symptoms present and information about the duration of symptoms. For a patient with a sore throat and a positive diphtheria culture together with no typical membranes, no signs of toxicity, and an antibody level higher than 1 IU/ml, one should suspect carrier status rather than disease.
Acknowledgments
We are grateful to T. M. Veselova, V. N. Koroleva, and all of the doctors on duty at the Infectious Disease Hospital of Arkhangelsk for excellent clinical work and for assistance and help during the study period.
REFERENCES
- 1.Anonymous. 1998. Guidance: laboratory diagnosis of diphtheria infection, vol. 4.2.698-98. Head State Sanitary Doctor of the Russian Federation, Moscow, Russia.
- 2.Anonymous. 1986. Order no. 450, p. 39-90. Ministry of Health of the Union of Soviet Socialist Republics, Moscow, Russia.
- 3.Anonymous. 2003. Resolution 139. Head State Sanitary Doctor of the Russian Federation, Moscow, Russia.
- 4.Casadevall, A., and L. A. Pirofski. 2000. Host-pathogen interactions: basic concepts of microbial commensalism, colonization, infection, and disease. Infect. Immun. 68:6511-6518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clarridge, J. E., T. Popovic, and T. Inzana. 1998. Diphtheria and other corynebacterial and coryneform infections, p. 347-371. In L. H. Collier, A. Balows, and M. Sussman (ed.), Topley and Wilson's microbiology and microbial infections, 9th ed., vol. 3. Edward Arnold, London, United Kingdom. [Google Scholar]
- 6.Edmunds, W. J., R. G. Pebody, H. Aggerback, S. Baron, G. Berbers, M. A. Conyn-van Spaendonck, H. O. Hallander, R. Olander, P. A. Maple, H. E. Melker, P. Olin, F. Fievret-Groyne, C. Rota, S. Salmaso, A. Tischer, C. von Hunolstein, and E. Miller. 2000. The sero-epidemiology of diphtheria in Western Europe. ESEN Project. European Sero-Epidemiology Network. Epidemiol. Infect. 125:113-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Efstratiou, A., K. H. Engler, I. K. Mazurova, T. Glushkevich, J. Vuopio-Varkila, and T. Popovic. 2000. Current approaches to the laboratory diagnosis of diphtheria. J. Infect. Dis. 181(Suppl. 1):S138-S145. [DOI] [PubMed] [Google Scholar]
- 8.Efstratiou, A., and R. C. George. 1999. Laboratory guidelines for the diagnosis of infections caused by Corynebacterium diphtheriae and C. ulcerans. World Health Organization. Commun. Dis. Public Health 2:250-257. [PubMed] [Google Scholar]
- 9.Galazka, A. 1993. Diphtheria, p. 1-12. In The immunological basis for immunization series, module 2. World Health Organization, Geneva, Switzerland.
- 10.Ipsen, J., Jr. 1946. Circulating antitoxin at the onset of diphtheria in 425 patients. J. Immunol. 54:325-347. [PubMed] [Google Scholar]
- 11.Jenum, P. A., V. Skogen, E. Danilova, A. Eskild, and H. Sjursen. 1995. Immunity to diphtheria in northern Norway and northwestern Russia. Eur. J. Clin. Microbiol. Infect. Dis. 14:794-798. [DOI] [PubMed] [Google Scholar]
- 12.Kadirova, R., H. U. Kartoglu, and P. M. Strebel. 2000. Clinical characteristics and management of 676 hospitalized diphtheria cases, Kyrgyz Republic, 1995. J. Infect. Dis. 181(Suppl. 1):S110-S115. [DOI] [PubMed] [Google Scholar]
- 13.Logina, I., and M. Donaghy. 1999. Diphtheritic polyneuropathy: a clinical study and comparison with Guillain-Barre syndrome. J. Neurol. Neurosurg. Psychiatry 67:433-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lumio, J., R. M. Olander, K. Groundstroem, P. Suomalainen, T. Honkanen, and J. Vuopio-Varkila. 2001. Epidemiology of three cases of severe diphtheria in Finnish patients with low antitoxin antibody levels. Eur. J. Clin. Microbiol. Infect. Dis. 20:705-710. [DOI] [PubMed] [Google Scholar]
- 15.Markina, S. S., N. M. Maksimova, C. R. Vitek, E. Y. Bogatyreva, and A. A. Monisov. 2000. Diphtheria in the Russian Federation in the 1990s. J. Infect. Dis. 181(Suppl. 1):S27-S34. [DOI] [PubMed] [Google Scholar]
- 16.Perles, Z., A. Nir, E. Cohen, A. Bashary, and D. Engelhard. 2000. Atrioventricular block in a toxic child: do not forget diphtheria. Pediatr. Cardiol. 21:282-283. [DOI] [PubMed] [Google Scholar]
- 17.Skogen, V., P. A. Jenum, V. N. Koroleva, E. Danilova, D. S. Halvorsen, N. Maksimova, and H. Sjursen. 1999. Detection of diphtheria antitoxin by four different methods. Clin. Microbiol. Infect. 5:628-633. [DOI] [PubMed] [Google Scholar]