Abstract
Passive agglutination (PA) and immunoglobulin M (IgM), IgA, and IgG enzyme-linked immunosorbent assays (ELISAs) for the diagnosis of Mycoplasma pneumoniae were compared with PCR testing of sputum samples obtained from children with lower respiratory tract infections. The sensitivity and specificity of PA were 80.3% and 92.3% at a titer of 1:80. ELISA was found to be less sensitive than PA.
Mycoplasma pneumoniae is an important respiratory pathogen in children as well as adults (6, 9, 13, 14). Laboratory diagnosis of M. pneumoniae infection is usually performed by serological methods, such as passive agglutination (PA), complement fixation, and enzyme-linked immunosorbent assay (ELISA). The diagnostic value of these methods has been discussed previously (1-5, 8, 12, 18, 19, 21). Although appropriate respiratory specimens are more difficult to obtain from children than from adults, sputum has been successfully obtained from infants and young children with respiratory infections by inducing coughing (11, 20). The aim of this study was to clarify the diagnostic value of serological methods for the diagnosis of M. pneumoniae infection in comparison with PCR using sputum from children.
Enrolled in the study were 339 children (181 males, 158 females; mean age, 2.9 ± 2.6 years; median age, 2 years) who were seen consecutively at Saitama Medical School between January 2000 and August 2004. All patients had respiratory symptoms, such as productive cough, and were clinically diagnosed as having lower respiratory tract infection (LRTI); 263 cases had X-ray-confirmed pneumonia, and 76 had bronchitis. The duration of fever (≥38°C) was 3.6 ± 2.6 days. Sputum was obtained by induced coughing from all patients on their initial visit, as described previously (11, 20). After aerobic culture of sputum was performed, the remainder of the sputum was frozen at −80°C. Sputum was thawed and centrifuged at 2,000 rpm for 15 min, and DNA was then extracted using a QIAamp DNA mini kit (QIAGEN, Hilden, Germany) according to the manufacturer's instructions. M. pneumoniae DNA was detected by nested PCR with primer sets for the P1 gene, as described previously (17). The first primer set was ADH2F (5′-GGC AGT GGC AGT CAA CAA ACC ACG TAT-3′) and ADH2R (5′-GAA CTT AGC GCC AGC AAC TGC CAT-3′). The second primer set was ADH3F (5′-GAA CCG AAG CGG CTT TGA CCG CAT-3′) and ADH3R (5′-GTT GAC CAT GCC TGA GAA CAG TAA-3′).
Serum was also obtained from all children. Serum anti-M. pneumoniae antibody was assayed using Serodia-MYCO II (Fuji Rebio Ltd., Tokyo, Japan), which is a PA assay (2). PA titers were determined according to the manufacturer's instructions, and the diagnostic criteria of M. pneumoniae infection were evaluated for each antibody titer. When paired sera were available, titer increases of at least fourfold were evaluated as indicating M. pneumoniae infection. Immunoglobulin G (IgG)-, IgA-, and IgM-specific anti-M. pneumoniae antibodies in serum samples from patients with positive PCR results were assayed using Mycoplasma pneumoniae IgG, IgA, and IgM ELISA kits (Medac GmbH, Wedel, Germany). IgG, IgA, and IgM ELISAs were performed according to the manufacturer's instructions. Briefly, serum diluted 1:100 was incubated on an ELISA plate coated with M. pneumoniae antigens. The optical density was converted to an antibody value using a standard curve. The cutoff values for IgG and IgA were 10 arbitrary units/ml. The cutoff value of the optical density for IgM was the value for the negative control plus 0.380.
Informed consent was obtained from the parents of all children.
Statistical analysis was performed using Epi Info 6, version 6.04d (Centers for Disease Control and Prevention, Atlanta, Ga.) for exact 95% confidence intervals (CI).
PCR gave positive results in 66 (19.5%) of 339 sputum specimens. PA titers of ≥1:40 were seen in 106 of 339 serum samples (positivity, 31.3%). A comparison of PCR results and PA titer assay results is shown in Table 1. When cutoff values for PA titers were 1:40, 1:80, 1:160, 1:320, and 1:640 or higher, the sensitivities and specificities of PA serology relative to PCR results were as follows: 89.4 and 82.8%; 80.3 and 92.3%; 71.2 and 96.0%; 56.1 and 97.4%; and 50.0 and 99.3%, respectively.
TABLE 1.
Comparison of PCR assay results and titers of passive agglutinin antibody in single serum samples
| Cutoff PA titer | No. of serum samples (n = 339)a
|
% Sensitivity of PA assay (95% CI) | % Specificity of PA assay (95% CI) | |||
|---|---|---|---|---|---|---|
| PCR positive (n = 66)
|
PCR negative (n = 273)
|
|||||
| PA positive | PA negative | PA positive | PA negative | |||
| 1:40 | 59 | 7 | 47 | 226 | 89.4 (78.8-95.3) | 82.8 (77.7-87.0) |
| 1:80 | 53 | 13 | 21 | 252 | 80.3 (68.3-88.7) | 92.3 (88.3-95.1) |
| 1:160 | 47 | 19 | 11 | 262 | 71.2 (58.6-81.4) | 96.0 (92.7-97.9) |
| 1:320 | 37 | 29 | 7 | 266 | 56.1 (43.3-68.1) | 97.4 (94.6-98.9) |
| ≥1:640 | 33 | 33 | 2 | 271 | 50.0 (37.6-62.4) | 99.3 (97.1-99.9) |
PCR positivity, 19.5% (66 PCR-positive samples/339); PA positivity (≥1:40), 31.3% (106 PA antibody-positive samples/339).
Among patients positive by PCR, ≥4-fold increases in PA titers of paired sera were seen in 30 of 36 samples (83.3%) (Table 2). The interval of serum sampling was related to the geometric mean of the increased antibody titers.
TABLE 2.
Passive agglutinin titers in paired serum samples from PCR-positive patients
| Interval (days) | No. of serum samples with an increase in PA antibody titer ofa:
|
GMTb | |||||
|---|---|---|---|---|---|---|---|
| 1× | 2× | 4× | 8× | 16× | ≥32× | ||
| 2 | 1 | 0 | 2 | 2 | 1 | 0 | 9.5 |
| 3 | 0 | 2 | 2 | 2 | 2 | 1 | 10.2 |
| 4 | 0 | 0 | 0 | 1 | 1 | 1 | 18.6 |
| 5-8 | 0 | 1 | 1 | 1 | 0 | 4 | 20.2 |
| 9-12 | 0 | 1 | 2 | 0 | 0 | 3 | 17.6 |
| 13-16 | 0 | 1 | 0 | 0 | 0 | 4 | 26.0 |
≥4-fold increase in PA titers was seen in 30 of 36 samples (83.3%).
GMT, geometric mean titer.
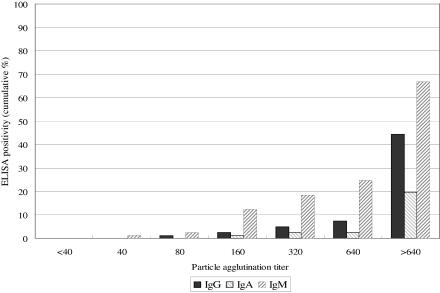
A comparison of PA titers and cumulative percentages of positivity of the ELISAs is shown in Fig. 1. Percentages of positivity for IgG, IgA, and IgM as determined by ELISA of serum samples from patients with positive PCR results were 44.4% (36/81), 19.8% (16/81), and 66.7% (54/81), respectively, even with PA titers of 1:640 or higher.
FIG. 1.
Comparison of passive agglutinin titer and Mycoplasma pneumoniae IgG, IgA, and IgM ELISA results among PCR-positive samples from children.
The clinical significance of the PCR assay for the diagnosis of M. pneumoniae respiratory infections has been discussed both for adults (7, 15, 16, 21) and for children (4, 8, 10, 18, 19). Michelow et al. (10) compared the results of PCR assays for M. pneumoniae between nasopharyngeal and oropharyngeal samples. They found discrepancies in three of nine positive samples and combined test results from more than one site in order to improve diagnostic accuracy. In addition, using throat swabs, Dorigo-Zetsma et al. (4) compared PCR and culture with serological tests for M. pneumoniae diagnosis among children with respiratory infections. Although they found the same results for culture and for PCR, the sensitivity of PCR using throat swabs was low. They recommended combining PCR and serology for reliable diagnosis of M. pneumoniae. On the other hand, Räty et al. (15) compared PCR results for sputum, nasopharyngeal aspirates, and throat swabs obtained from young adults with pneumonia. Of these, sputum was found to be superior for a diagnosis of M. pneumoniae. Because sputum can be obtained less invasively, we believe that PCR assay using sputum is both rapid and useful for the diagnosis of M. pneumoniae LRTI in children.
Our results indicate that the sensitivity and the specificity of PA with single serum samples varied with the titer cutoff values investigated. However, we believe that a titer of 1:80 or 1:160 is useful for the diagnosis of M. pneumoniae infection in children. PA titer increases of at least fourfold were seen in 30 of 36 paired serum samples from children with positive PCR results. In addition, none of the paired serum samples from patients with negative PCR results showed PA antibody titer increases of fourfold or more. This suggests that PA using paired sera is clinically useful for the diagnosis of M. pneumoniae infection when sputum specimens are not available.
The present results show that the sensitivity of the Mycoplasma pneumoniae IgM ELISA is high and that it can be applied to serological diagnosis using unpaired serum. However, the specificity of IgM ELISA should be examined further using sera from patients with negative PCR results. Discrepancies between IgA and IgG ELISA results and PA titers indicate that the PA titer reflects IgM class antibodies. This discrepancy is thus indicative of the patterns of production of immunoglobulin subclasses in the present group of children.
In conclusion, our results demonstrate that PA serology using paired sera shows good agreement with PCR results. In unpaired sera, a PA antibody titer of 1:80 or 1:160 is useful for the serological diagnosis of M. pneumoniae infection among children with LRTI.
Acknowledgments
This work was supported in part by a grant for studies of emerging and reemerging infectious diseases (H15-Shinko-24) from the Ministry of Health, Labor, and Welfare of Japan.
We also thank Satowa Suzuki of the National Institute of Infectious Diseases, Tokyo, Japan, for suggestions regarding statistical analysis.
REFERENCES
- 1.Alexander, T. S., L. D. Gray, J. A. Kraft, D. S. Leland, M. T. Nikaido, and D. H. Willis. 1996. Performance of Meridian ImmunoCard Mycoplasma test in a multicenter clinical trial. J. Clin. Microbiol. 34:1180-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barker, C. E., M. Sillis, and T. G. Wreghitt. 1990. Evaluation of Serodia Myco II particle agglutination test for detecting Mycoplasma pneumoniae antibody: comparison with μ-capture ELISA and indirect immunofluorescence. J. Clin. Pathol. 43:163-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beersma, M. F. C., K. Dirven, A. P. van Dam, K. E. Templeton, E. C. J. Claas, and H. Goossens. 2005. Evaluation of 12 commercial tests and the complement fixation test for Mycoplasma pneumoniae-specific immunoglobulin G (IgG) and IgM antibodies, with PCR used as the “gold standard”. J. Clin. Microbiol. 43:2277-2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dorigo-Zetsma, J. W., S. A. J. Zaat, P. M. E. Wertheim-van Dillen, L. Spanjaard, J. Rijntjes, G. van Waveren, J. S. Jensen, A. F. Angulo, and J. Dankert. 1999. Comparison of PCR, culture, and serological tests for diagnosis of Mycoplasma pneumoniae respiratory tract infection in children. J. Clin. Microbiol. 37:14-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunn, J. J., A. K. Malan, J. Evans, and C. M. Litwin. 2004. Rapid detection of Mycoplasma pneumoniae IgM antibodies in pediatric patients using ImmunoCard Mycoplasma compared to conventional enzyme immunoassays. Eur. J. Clin. Microbiol. Infect. Dis. 23:412-414. [DOI] [PubMed] [Google Scholar]
- 6.Foy, H. M., G. E. Kenny, R. McMaban, A. M. Mansy, and J. T. Grayston. 1970. Mycoplasma pneumoniae pneumonia in an urban area. JAMA 214:1666-1672. [PubMed] [Google Scholar]
- 7.Gnarpe, J., A Lundbäck, H. Gnarpe, and B. Sundelöf. 1997. Comparison of nasopharyngeal and throat swabs for the detection of Chlamydia pneumoniae and Mycoplasma pneumoniae by polymerase chain reaction. Scand. J. Infect. Dis. Suppl. 104:11-12. [PubMed] [Google Scholar]
- 8.Maltezou, H. C., B. La-Scola, H. Astra, I. Constantopoulou, V. Vlahou, D. A. Kafetzis, A. G. Constantopoulos, and D. Raoult. 2004. Mycoplasma pneumoniae and Legionella pneumophila in community-acquired lower respiratory tract infections among hospitalized children: diagnosis by real time PCR. Scand. J. Infect. Dis. 36:639-642. [DOI] [PubMed] [Google Scholar]
- 9.McCracken, G. H., Jr. 2000. Etiology and treatment of pneumonia. Pediatr. Infect. Dis. J. 19:373-377. [DOI] [PubMed] [Google Scholar]
- 10.Michelow, I. C., K. Olsen, J. Lozano, L. B. Duffy, G. H. McCracken, and R. D. Hardy. 2004. Diagnostic utility and clinical significance of naso- and oropharyngeal samples used in a PCR assay to diagnose Mycoplasma pneumoniae infection in children with community-acquired pneumonia. J. Clin. Microbiol. 42:3339-3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murayama, K., T. Yamazaki, A. Ito, S. Uehara, and N. Sasaki. 2005. Simplified semi-quantitative culture using washed sputum from children with lower respiratory tract infections. J. Clin. Pathol. 58:896. [PMC free article] [PubMed] [Google Scholar]
- 12.Petitjean, J., A. Vabret, S. Gouarin, and F. Freymuth. 2002. Evaluation of four commercial immunoglobulin G (IgG)- and IgM-specific enzyme immunoassays for diagnosis of Mycoplasma pneumoniae infections. J. Clin. Microbiol. 40:165-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Principi, N., and S. Esposito. 2001. Emerging role of Mycoplasma pneumoniae and Chlamydia pneumoniae in paediatric respiratory-tract infections. Lancet Infect. Dis. 1:334-344. [DOI] [PubMed] [Google Scholar]
- 14.Principi, N., S. Esposito, F. Blasi, L. Allegra, and the Mowgli Study Group. 2001. Role of Mycoplasma pneumoniae and Chlamydia pneumoniae in children with community-acquired lower respiratory tract infections. Clin. Infect. Dis. 32:1281-1289. [DOI] [PubMed] [Google Scholar]
- 15.Räty, R., E. Rönkkö, and M. Kleemola. 2005. Sample type is crucial to the diagnosis of Mycoplasma pneumoniae pneumonia by PCR. J. Med. Microbiol. 54:287-291. [DOI] [PubMed] [Google Scholar]
- 16.Reznikov, M., T. K. Blackmore, J. J. Finlay-Jones, and D. L. Gordon. 1995. Comparison of nasopharyngeal aspirates and throat swab specimens in a polymerase chain reaction-based test for Mycoplasma pneumoniae. Eur. J. Clin. Microbiol. Infect. Dis. 14:58-61. [DOI] [PubMed] [Google Scholar]
- 17.Sasaki, T., T. Kenri, N. Okazaki, M. Iseki, R. Yamashita, M. Shintani, Y. Sasaki, and M. Yayoshi. 1996. Epidemiological study of Mycoplasma pneumoniae infections in Japan based on PCR-restriction fragment length polymorphism of the P1 cytadhesin gene. J. Clin. Microbiol. 34:447-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skakni, L., A. Sardet, J. Just, J. Landman-Parker, J. Costil, N. Moniot-Ville, F. Bricout, and A. Garbarg-Chenon. 1992. Detection of Mycoplasma pneumoniae in clinical samples from pediatric patients by polymerase chain reaction. J. Clin. Microbiol. 30:2638-2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Templeton, K. E., S. A. Scheltinga, A. W. Graffelman, J. M. van Schie, J. W. Crielaard, P. Sillekens, P. J. van den Broek, H. Goossens, M. F. C. Beersma, and E. C. J. Claas. 2003. Comparison and evaluation of real-time PCR, real-time nucleic acid sequence-based amplification, conventional PCR, and serology for diagnosis of Mycoplasma pneumoniae. J. Clin. Microbiol. 41:4366-4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uehara, S. 1988. A method of bacteriological examination of washed sputum in infants and children. Acta Paediatr. Jpn. 30:253-260. [PubMed] [Google Scholar]
- 21.Waris, M., P. Toikka, T. Saarinen, S. Nikkari, O. Meurman, R. Vainionpää, J. Mertsola, and O. Ruuskanen. 1998. Diagnosis of Mycoplasma pneumoniae pneumonia in children. J. Clin. Microbiol. 36:3155-3159. [DOI] [PMC free article] [PubMed] [Google Scholar]