Abstract
Herpes simplex virus type 2 (HSV-2) is a common sexually transmitted infection in sub-Saharan Africa. Glycoprotein G (gG) of HSV-2 elicits a type-specific antibody response and is widely used for serodiagnosis. gG is cleaved into a secreted portion (sgG-2) and a highly O-glycosylated mature portion (mgG-2). The performances of these two native immunosorbent purified antigens were compared in an enzyme-linked immunosorbent assay (ELISA) format with a commercially available assay (FOCUS2) using sera from blood donors (n = 194) and individuals (n = 198) with genital ulcer disease (GUD) from Tanzania. Discordant results were resolved by Western blotting. The HSV-2 seroprevalence for blood donors was estimated as 42%, and that for the GUD cohort was estimated as 78%. The prevalence increased significantly with age for both cohorts and was higher among human immunodeficiency virus (HIV)-positive individuals than among HIV-negative subjects. In the GUD cohort with a high HSV-2 prevalence, all three assays showed statistically similar performances, with sensitivities between 97% and 99% and specificities in the range of 86% to 91%. In contrast, among blood donors with a lower seroprevalence, the mgG-2-based ELISA presented significantly higher specificity (97%) than the sgG-2 ELISA (89%) and FOCUS2 (74%). Overall, the mgG-2 ELISA gave a high performance, with negative and positive predictive values of 96% for blood donors and a negative predictive value of 95% and a positive predictive value of 97% for the GUD cohort. We conclude that native purified mgG-2 showed the highest accuracy for detection of HSV-2 in patient sera from Tanzania and is therefore suitable for seroprevalence studies as well as in clinical settings.
Herpes simplex virus type 2 (HSV-2) infections are common and have spread worldwide, with a reported variation in seroprevalence ranging from less than 1% in children to more than 80% in selected adult populations (13, 23, 27). After primary infection, HSV-2 establishes latency in sensory ganglia, and after reactivation, HSV-2 can be transmitted by clinical lesions or via asymptomatic shedding (33, 35). HSV-2 is sexually transmitted and is the most common cause of genital ulcer disease (GUD) in developing countries (1, 8, 22). The burden of sexually transmitted diseases (STDs) is high in sub-Saharan Africa, and a major problem is that HSV-2 infection facilitates transmission of human immunodeficiency virus (HIV). It has been estimated that the risk of acquiring HIV is doubled for HSV-2-infected individuals (34), and HSV-2-positive patients present higher HIV viral load than HSV-2-negative HIV-infected patients (26). These data emphasize that identification of HSV-2-infected individuals is important not only for control of HSV-2 transmission but also as a strategy for HIV prevention.
The definite diagnosis of HSV-2 infection is achieved by virus isolation (VI) or by the PCR technique using samples from clinical lesions. Both of these methods present high specificity, and PCR is the most sensitive. However, these methods are not always available in developing countries, and neither VI nor PCR can diagnose HSV-2 infection in asymptomatic individuals. In these cases, serological assays detecting type-specific anti-HSV-2 antibodies are the only method that can be used to obtain the correct diagnosis. Furthermore, HSV-2 type-specific serology is the only method available for estimation of seroprevalence in larger populations and for HSV-2 vaccine follow-up programs.
Most of the envelope proteins of HSV-1 and HSV-2 induce cross-reactive antibody responses and are not suitable as antigens to discriminate between HSV-1 and HSV-2 infections. The glycoprotein G of HSV-2 (gG-2) is an exception and elicits exclusively a type-specific antibody response. This protein is translated into a high-mannose precursor protein which is further cleaved into a secreted amino-terminal portion (sgG-2) and a carboxy-terminal highly O-glycosylated membrane-bound portion designated mature gG-2 (mgG-2) (7, 28). Native or recombinant-produced mgG-2 has been widely used for type-specific HSV-2 serology (12, 17, 21, 29). We showed recently that sgG-2 also elicits a type-specific antibody response in HSV-2-infected individuals (11). In that study, sgG-2 presented a high performance in an enzyme-linked immunosorbent assay (ELISA) format comparable with an FDA-approved commercially available type-specific ELISA, the HerpeSelect 2 ELISA (FOCUS2), based on recombinant-produced gG-2 (13).
FOCUS2 and other commercial gG-2-based assays showing high performance for sera from individuals resident in the United States and Europe (2, 4, 9) offer significantly lower specificity in sub-Saharan Africa populations (13, 15, 32). This observation implies that in cohorts where the HSV-2 prevalence is low the positive predictive value (PPV) will also be low. In this situation, misjudgment of the HSV-2 status in clinical settings as well as for larger populations is a major concern.
The reported HSV-2 seroprevalences in Tanzania show considerable variation, dependent on the selected population. The lowest prevalences, ranging from 9% to 35%, are observed in the younger age groups and among healthy blood donors, while patients attending STD clinics show the highest prevalences, ranging from 66% to 87% (22, 24, 25). In general, the HSV-2 prevalence is higher among women than in men and increases with age (16, 22, 24, 25). In this study, we defined the performance of two in-house-produced ELISAs, based on immunosorbent purified sgG-2 and mgG-2, as well as the FOCUS2 assay and estimated the HSV-2 seroprevalence among blood donors and from an STD population presenting with genital ulcers in Tanzania.
MATERIALS AND METHODS
Cells and virus.
African green monkey kidney cells (GMK-AH1) and human epidermoid (HEp-2) cells were cultured in Eagle's minimal essential medium supplemented with 2% calf serum and antibiotics. A local wild-type HSV-2 isolate, B4327UR (14), and a clinical HSV-2 isolate from Tanzania (kindly provided by Lars Haarr, University of Bergen) were used.
Serum samples.
Sera were derived from two different cities in Tanzania, Dar es Salaam and Mbeya. Dar es Salaam is the former administrative capital of Tanzania, and Mbeya is situated in the southwest part along the main transportation roads from Zambia. Two cohorts were studied. The first cohort included 196 sera collected from healthy blood donors from Muhimbili National Hospital, Dar es Salaam (10 female, 186 male), with an age range from 16 to 69 years (mean age, 33 years). All sera were negative for HIV antibodies assayed with Enzygnost anti-HIV1/HIV2 (Behring, Marburg, Germany) and Ortho HIV-1/HIV-2 (Ortho-Clinical Diagnostics, Raritan, NJ), as described recently (1). The second cohort included 198 sera from patients with GUD. Sera were obtained during 2001 from patients presenting at sexually transmitted infection clinics in Dar es Salaam and Mbeya. The patients from Dar es Salaam consisted of 24 females (age range, 21 to 45 years; mean age, 30 years) and 33 males (age range, 18 to 55 years; mean age, 29 years). The Mbeya population consisted of 105 females (age range, 16 to 53 years; mean age, 25 years) and 36 males (age range, 18 to 59 years; mean age, 29 years). In total, the GUD cohort consisted of 129 females and 69 males with ages ranging from 15 to 59 years (mean age, 27 years). HIV status in the GUD cohort was determined as described above, and the seroprevalence of HIV infection was 57% (Dar es Salaam, 49%; and Mbeya, 60%). None of the patients was on treatment with antiviral drugs against HIV or HSV. No other clinical data were available. The study was conducted with the approval of the ethics committee in Tanzania.
Purification of HSV-2 gG.
sgG-2 was purified from the medium of virus-infected GMK-AH1 cells by immunoaffinity chromatography using the anti-sgG-2 monoclonal antibody (MAb) 4.A5.A9 as described previously (18). For production of mgG-2, 4 mg of the anti-mgG-2 MAb O1.C5.B2 (20) was coupled to a cyanogen bromide-activated Sepharose 4B (Amersham Pharmacia) column according to the manufacturer's instructions. Virus-infected HEp-2 cells (HSV-2 isolate B4327UR) were harvested and solubilized in Tris-buffered saline (TBS) containing 1% sodium deoxycholate and 1% NP-40, followed by centrifugation at 2,000 × g for 10 min and ultracentrifugation at 100,000 × g for 1 h. The supernatant was added to the column and recirculated for 1 h, followed by washing using TBS and 0.5 M NaCl. The proteins were eluted with 0.1 M glycine-HCl buffer (pH 2.8) and neutralized with Tris-HCl (pH 8). The protein concentration was measured by DCProteinAssay (Bio-Rad).
sgG-2 ELISA.
sgG-2 was used at a coating concentration of 0.7 μg/ml and incubated overnight in carbonate buffer (pH 9.6) at 4°C on Maxisorp microtiter plates (Nalge Nunc International). To avoid protein aggregation, 5% glycerol was added to purified sgG-2 before coating. Plates were blocked with 2% skim milk in phosphate-buffered saline (PBS), and sera were diluted in PBS with 0.6 M NaCl, 1% skim milk, and 0.05% Tween 20; tested in duplicate at a 1:100 dilution; and incubated at 4°C overnight. Peroxidase-conjugated goat anti-human immunoglobulin G (IgG) (Jackson ImmunoResearch Laboratories), at a 1:3,000 dilution, was used as a conjugate, and O-phenylenediamine was used as a substrate. The reaction was stopped with 1 M sulfuric acid after 10 min, and absorbance was measured at 490 nm. Results are given as the mean optical density (OD) value of each duplicate. A positive control serum (PS) and a negative cutoff control serum (CS) were selected from Swedish cohorts of patients as described earlier (11) and included on each plate. The assay was considered valid if the OD value of the PS was ≥2.0 and the OD value of the CS was ≤0.25. A serum sample was considered positive if the OD value was equal to or higher than the OD value for the CS plus 0.35 OD unit and negative if the OD value was less than the OD value for the CS plus 0.35 OD unit.
mgG-2 ELISA.
mgG-2 was used at a coating concentration of 0.7 μg/ml and incubated overnight at 4°C in carbonate buffer (pH 9.6) on microtiter plates (Nunc Nalge International). Plates were washed and blocked with 2% skim milk in PBS for 30 min at 37°C. Sera were tested in duplicate at a 1:100 dilution and incubated at 4°C overnight. Serum samples were diluted in PBS containing 1% skim milk and 0.05% Tween 20. Alkaline phosphate-conjugated goat anti-human IgG (Jackson ImmunoResearch Laboratories) was used as a conjugate at a 1:2,000 dilution, with p-nitrophenylphosphate as a substrate. The absorbance was read at 405 nm every 5 min until PS reached an absorbance value of ≥2.0 OD units. The PS and CS, validation criteria, calculation of cutoff, and judgment of positive and negative samples were determined as described for the sgG-2 ELISA.
Commercially available HSV-2-specific assay.
The HerpeSelect 2 ELISA (FOCUS2; Focus Diagnostics, Inc., Cypress, CA) was performed according to the manufacturer's instructions.
Interpretation of results from the different ELISAs.
Sera from both blood donors and the GUD cohort were derived from individuals with no confirmed HSV-2 infection using VI or PCR. We therefore interpreted concordant ELISA results in all three assays as true negative (TN) or true positive (TP). Discordant results were resolved by Western blotting (WB), which was used as the “gold standard” (3). Results for sera presenting concordant ELISA reactivity as well as results of the reactivity in WB for discordant sera were in this study referred to as the consensus for estimation of the seroprevalences. Receiver-operating characteristic (ROC) plots (36) were created for the sgG-2 and the mgG-2 ELISAs by adding 0.10 to 0.6 OD units to the reactivity of the CS by using 0.02-OD-unit incremental steps. For the mgG-2 ELISA, values representing reactivity of the CS plus 0.7, 0.8, 0.9, and 1.0 OD unit were plotted as well for the GUD cohort. The PPV was calculated as TP/(TP + false positive), and the negative predictive value (NPV) was calculated as TN/(TN + false negative).
Western blotting.
Antigen preparations for WB were produced by infecting HEp-2 cells with the HSV-2 isolate B4327UR (20). Antigen preparations were mixed with sample buffer containing sodium dodecyl sulfate and subjected to polyacrylamide gel electrophoresis under reducing conditions using NuPAGE 7% Tris-acetate gels (Novex). The proteins were electrotransferred to an Immobilon-P transfer membrane (Millipore Corp.). Strips were incubated overnight with sera at a 1:100 dilution. An HSV-2-positive serum from Sweden and an anti-mgG-2 MAb (20) were used for correct identification of the carboxy-terminal intermediate portion of gG-2 and the mgG-2 protein. Peroxidase-conjugated rabbit anti-human or rabbit anti-mouse IgG (Dako) was used as a conjugate with 4-chloro-1-naphthol used as the substrate. A positive WB profile was defined as reactivity to mgG-2 (≈120 kDa) alone or in combination with reactivity to the carboxy-terminal intermediate portion of gG-2 (≈70 kDa) (20). As sera were derived from Tanzania, we also produced strips with a local clinical HSV-2 isolate for comparison.
Detection of anti-HSV-2 IgM antibodies.
An indirect immunofluorescence technique was used to detect IgM antibodies as previously described (31). In a recent study of sera from patients with primary HSV-2 infections, confirmed by virus isolation, the assay detected IgM antibodies in 76% of the patients (11).
Statistics.
StatView (version 5.0.1) software (SAS Institute Inc.) was used for statistical analysis, and P values were determined by Fisher's exact test. A P value of ≤0.05 was considered significant.
RESULTS
Purity of sgG-2 and mgG-2 antigens.
The gG-2 precursor protein is localized intracellularly in small amounts, as judged from reactivity in WB of HSV-2-infected cell lysates using MAbs against both sgG-2 and mgG-2 (18). Immunosorbent-purified sgG-2 from cell medium contained no precursor gG-2, because an anti-mgG-2 MAb (O1.C5.B2) was unreactive to the sgG-2 in ELISA. Similarly, an anti-sgG-2 MAb (4.A5.A9) presented no reactivity in the mgG-2 ELISA, indicating that the mgG-2 protein was also devoid of precursor gG-2 (data not shown).
CV.
The intra-assay and interassay coefficients of variation (CVs) of the sgG-2 ELISA have previously been determined as 4.4% and 10.8%, respectively (11). The intra-assay CV of the mgG-2 ELISA was determined by analyzing a Swedish HSV-2-positive serum with a medium high reactivity in 28 positions (wells) in the same microtiter plate. The mean OD value ± standard deviation was 1.98 ± 0.066, giving an intra-assay CV of 3.3%. The interassay CV was determined by testing the same HSV-2-positive sample in duplicate on seven different occasions. The mean ± standard deviation OD value was 2.12 ± 0.2, giving an interassay CV of 9.4%.
Outcome of three different gG-2-based assays.
As all blood donors were HIV negative and mainly males, no stratification was performed regarding HIV status or gender. The GUD cohort consisted of male and female patients from two cities in Tanzania. As no significant epidemiological differences or differences in performance of the assays were observed between individuals residing in Dar es Salaam and those residing in Mbeya (data not shown), these two populations are presented as a single cohort (the GUD cohort).
All sera were analyzed with the in-house-produced sgG-2 ELISA and mgG-2 ELISA as well as the commercially available FOCUS2. Two samples among blood donors were repeatedly equivocal in FOCUS2 and excluded (these two samples were negative in the sgG-2 and mgG-2 ELISAs as well as in WB). The results of the three ELISAs and WB for blood donors (n = 194) and the GUD cohort (n = 198) are presented in Table 1. For blood donors, 74 samples (38%) were positive in all three ELISAs and 76 samples (39%) were concordantly negative, while 44 samples (23%) presented discordant results. In the GUD cohort, 149 samples (75%) showed concordantly positive results and 31 samples (16%) were negative, while 18 samples (9%) showed discordant results.
TABLE 1.
Outcome of assays for sera tested by sgG-2 ELISA, mgG-2 ELISA, FOCUS2, and WBa
| Serum panel (n) | No. of sera with sgG-2 ELISA/mgG-2 ELISA/FOCUS2/WB result (discordant)b:
|
No. of sera with sgG-2 ELISA/mgG-2 ELISA/ FOCUS2 result (concordant):
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| −−+− | +−−− | +−+− | −+++ | −−++ | −++− | +−++ | ++−− | −+−− | −+−+ | +++ | −−− | |
| Blood donors (194) | 23 | 7 | 4 | 3 | 3 | 2 | 1 | 1 | 0 | 0 | 74 | 76 |
| GUD cohort (198) | 3 | 5 | 0 | 3 | 0 | 2 | 2 | 1 | 1 | 1 | 149 | 31 |
Results are denoted as positive (+) or negative (−).
HSV-2 serostatus was resolved by WB.
Discordant sera.
In total, 44 sera among blood donors and 18 sera in the GUD cohort presented discordant results (Table 1) and were tested by WB. No indeterminate or atypical WB profiles were seen (10). Among blood donors, 16% (7/44) were positive by WB. Twenty-three sera were false positive in FOCUS2 alone, and 7 sera showed false-positive results only in the sgG-2 ELISA. Other combinations of discordance were less frequent (Table 1). In the GUD cohort, 18 sera presented discordant results in which WB was positive for 6 sera. Consequently, 12 sera were classified as false positive in one or two assays, where 7 sera were from women and 5 sera were from men. Of the 12 false-positive sera, 9 were HIV negative (data not shown). The sgG-2 ELISA presented the most false-positive results (five sera) compared to FOCUS2 and the mgG-2 ELISA.
WB reactivities were compared for the discordant sera using strips prepared with antigens from a Swedish HSV-2 isolate and a Tanzanian HSV-2 isolate. HSV-2-positive samples showed reactivity against the mgG-2 (≈120 kDa) alone or to both mgG-2 and the intermediate (≈70 kDa), as illustrated in a recent study (18). No differences in WB profile or intensity were seen for the positive samples, and all negative sera were negative irrespective of the antigen used.
Anti-HSV-2 IgM antibodies.
We and others (5, 11, 21) have shown that the FOCUS2 assay is more sensitive at detecting seroconversion than the sgG-2 ELISA and WB in primary HSV-2 infections. Here we investigated whether the classification of false-positive reactivities in FOCUS2 (negative in WB, Table 1) might be explained by a primary HSV-2 infection and early seroconversion. The presence of anti-HSV-2 IgM antibodies was investigated for 29 sera from blood donors and 5 sera from the GUD cohort. All of these 34 sera were negative for IgM antibodies (data not shown), suggesting that these sera were true false positives.
Performance of the ELISAs.
Based on the results shown in Table 1, the sensitivity, specificity, and the PPV and NPV for each assay are summarized in Table 2. Among blood donors, the highest sensitivity (100%) was achieved by FOCUS2 followed by the mgG-2 ELISA (95%), a nonsignificant difference. The sgG-2 ELISA showed a significantly lower sensitivity (93%) than FOCUS2 (P = 0.03). The mgG-2 ELISA showed a significantly higher specificity (97%) than the sgG-2 ELISA (89%; P = 0.03) as well as the FOCUS2 (74%; P < 0.0001). In addition, the FOCUS2 assay showed a significant lower specificity than the sgG-2 ELISA (P = 0.03). As expected from the specificity data, the mgG-2 ELISA presented a higher PPV than both the sgG-2 ELISA and FOCUS2.
TABLE 2.
Performance of three HSV-2-specific assays for sera from blood donors and the GUD cohort in Tanzania
| Assay | Result (%) fora:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Blood donors
|
GUD cohort
|
|||||||||
| Prevalence | Sensitivity | Specificity | NPV | PPV | Prevalence | Sensitivity | Specificity | NPV | PPV | |
| ELISA | ||||||||||
| sgG-2 | 45 A | 93 B | 89 C | 94 | 86 D | 79 | 97 | 86 | 90 | 96 E |
| mgG-2 | 41 F | 95 | 97 G | 96 | 96 | 79 | 99 | 91 | 95 | 97 |
| FOCUS2 | 57 H | 100 I | 74 J | 100 | 74 K | 80 | 99 | 88 | 97 | 97 L |
| Consensusb | 42 | − | − | − | − | 78 | − | − | − | − |
−, not applicable. Statistical significance is as follows: P = 0.03 for A versus H, B versus I, C versus G, and D versus E; P = 0.005 for C versus J; P = 0.003 for F versus H; and P < 0.0001 for G versus J and K versus L.
Based on concordant results from all three ELISAs and reactivity by WB of discordant sera.
In the GUD cohort, both the mgG-2 ELISA and FOCUS2 showed the highest sensitivity (99%) followed by the sgG-2 ELISA (97%). The specificities for the sgG-2 ELISA, the mgG-2 ELISA, and FOCUS2 were 86%, 91%, and 88%, respectively. None of these differences was statistically significant. When the PPVs for each assay were compared between the two cohorts, the sgG-2 ELISA (P = 0.03) as well as FOCUS2 (P < 0.0001) showed significantly lower PPVs for blood donors than for the GUD cohort (Table 2). This difference in performance between the cohorts could not be shown for the mgG-2 ELISA.
ROC plots.
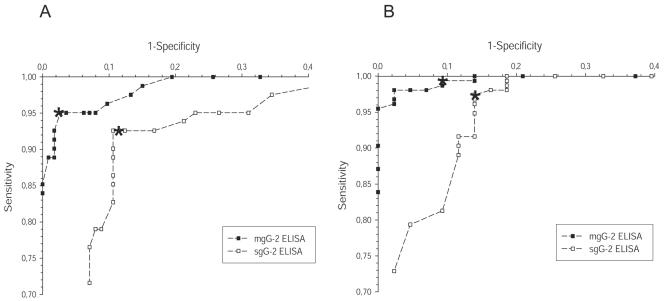
ROC plots were created for the sgG-2 ELISA and the mgG-2 ELISA as described in Materials and Methods (Fig. 1). For blood donors, the sgG-2 ELISA and the mgG-2 ELISA presented lower sensitivity than that of the GUD cohort. For example, a decrease of the cutoff by 0.1 OD unit compared to what was used in the present study decreased the specificity from 89% to 86% for the sgG-2 ELISA and from 97% to 94% for the mgG-2 ELISA with unaltered sensitivity. For the mgG-2 ELISA, the ROC plot, based on the performance in the GUD cohort (Fig. 1), showed that an increment of the cutoff of 0.1 OD unit increased the specificity from 91% to 98% while the sensitivity decreased only from 99% to 97%. The same increase of the cutoff for the sgG-2 ELISA showed that the sensitivity decreased slightly from 97.4% to 96.8% with an identical specificity. As the plotted values for the mgG-2 ELISA were closer to the upper left corner in the ROC plot than for the sgG-2 ELISA (Fig. 1A and B), it is evident that the mgG-2 ELISA presented higher performance than the sgG-2 ELISA for both cohorts.
FIG. 1.
Performance of sgG-2 and mgG-2 ELISAs presented as ROC plots for blood donors (A) and the GUD cohort (B). Cutoff values of control serum (CS) plus 0.1 to 0.6 OD units by incremental steps of 0.02 are plotted for both cohorts. For mgG-2 ELISA in the GUD cohort, plots representing CS plus 0.7, 0.8, 0.9, and 1.0 OD unit are plotted as well. The plots corresponding to CS plus 0.35 OD unit used as cutoff values are indicated with asterisks.
For the sgG-2 ELISA, 18 sera were classified as false positive. Eight of these sera (five sera from blood donors and three sera from the GUD cohort) showed high reactivities (OD values of ≥1.0 unit), indicating that these sera could not be correctly classified as negative by adjusting the cutoff value. For the FOCUS2 assay, 6 (all from blood donors) of 35 false-positive sera showed index values of ≥3.4. For the mgG-2 ELISA, only 1 of 7 false-positive sera presented a high reactivity (OD value of 0.9). The remaining sera showed reactivities close to the cutoff (data not shown).
Seroprevalence.
The HSV-2 seroprevalences for both cohorts estimated from the individual assays are presented in Table 2. Using the consensus definition as described, the HSV-2 prevalence for blood donors was 42%, compared to 78% for the GUD cohort. Using FOCUS2 as a single assay, the seroprevalence for blood donors was estimated to be 57%, compared to 45% (P = 0.03) for the sgG-2 ELISA and 41% (P = 0.003) for the mgG-2 ELISA. In the GUD cohort, all assays estimated the seroprevalences similarly (79% and 80%) and close to the consensus (78%).
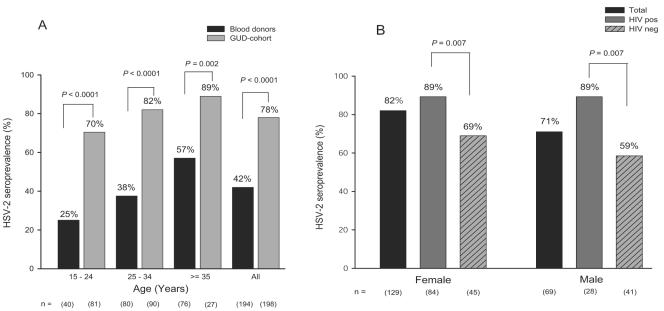
The distribution of HSV-2 infection in relation to age is shown in Fig. 2A for blood donors and for the GUD cohort. For all age-stratified groups, the HSV-2 prevalence was significantly higher for individuals belonging to the GUD cohort than for blood donors and for both cohorts there was an increasing HSV-2 prevalence with age. The seroprevalence in the youngest age group (15 to 24 years) was 25% among blood donors, increasing to 57% in the age group of ≥35 years. Similarly, the seroprevalence in the GUD cohort increased from 70% in the youngest age group (15 to 24 years) to 89% for individuals in the age group of ≥35 years.
FIG. 2.
Age-stratified HSV-2 seroprevalences for blood donors and patients with GUD (A) and HSV-2 prevalences related to gender and HIV status in the GUD cohort (B). pos, positive; neg, negative.
In the GUD cohort, the association between HSV-2 status and gender as well as HIV status is illustrated in Fig. 2B. Such analysis was not possible for blood donors as all were HIV negative and predominantly men. HSV-2 infection was found to be associated with HIV status for both males and females. The HSV-2 seroprevalence was significantly higher among HIV-positive females (89%) than among HIV-negative females (69%; P = 0.007). Similarly, the HSV-2 prevalence among HIV-positive men was 89% compared to 59% for HIV-negative men (P = 0.007). The overall higher HSV-2 prevalence among females (82%) than among males (71%; not significant, P = 0.07) was explained by the fact that the HSV-2 prevalence was higher among HIV-negative females (69%) than among HIV-negative males (59%).
DISCUSSION
Recent data concerning detection of type-specific antibodies against HSV-2 indicate that the performance of an assay may vary considerably between different populations. An important problem has been identified in that assays which present reliable results in Western world populations present significantly lower performance in developing countries (6, 13, 15, 32). In this study, we evaluated two in-house assays based on immunosorbent purified sgG-2 and mgG-2 and compared the results with FOCUS2 by using sera from two selected populations in Tanzania.
Here we show that the levels of performance of the sgG-2 ELISA and FOCUS2 differ significantly between blood donors and an STD population within the same country. Such difference was not observed for the mgG-2 ELISA (Table 2). In the GUD cohort, the levels of performance were equally good for all three assays, with no significant differences, indicating that all assays can be used with good reliability and accuracy. In contrast, among blood donors, both the sgG-2 ELISA and especially FOCUS2 presented significantly lower specificity than the mgG-2 ELISA and overestimated the seroprevalence to be 57%, compared to the consensus prevalence of 42%. The resulting PPV of this assay was 74%, which means that 26 individuals of 100 HSV-2-positive blood donors would be given an incorrect HSV-2 serological diagnosis if FOCUS2 was used as a single assay.
The low specificity for FOCUS2 among blood donors was due to the fact that 23 sera of 194 (12%) were classified as false positive (negative in the sgG-2 and mgG-2 ELISAs, as well as in WB). An explanation of this discordance might be that the FOCUS2 assay is more sensitive than the other ELISAs and WB and therefore detects HSV-2 seroconversion earlier, findings which have been described recently (5, 21). In this study, all 34 sera with positive reactivity in the FOCUS2 assay but giving negative results in WB were negative for anti-HSV-2 IgM antibodies. This finding suggests that primary HSV-2 infection was not a common event among blood donors and that at least the majority of the sera presented false-positive reactivity.
The antigen used is crucial for the performance of an assay. The mgG-2 ELISA which presented the best performance overall is based on immunosorbent purified antigen. The hybridoma has been subcloned several times, and the MAb was confirmed to be a single clone by isoelectric focusing (20). The MAb has been epitope mapped to a linear stretch of the immunodominant region of mgG-2 and is unreactive to HSV-1 antigen and HSV-1-infected cells (20). In addition, the MAb binds with high affinity to mgG-2 (KD = 2.2 × 10−8 M) (19). Optimal conditions are therefore fulfilled to purify mgG-2 from virus-infected cells. The sgG-2 MAb has also been characterized in detail (18). Despite that, native purified sgG-2 presented a lower level of performance than mgG-2. However, a problem with the present study is that no confirmatory assay is available for sgG-2 as human sera are unreactive in WB. As sgG-2 is secreted, we cannot exclude that an individual can be immunized with sgG-2 but not infected with a productive HSV-2 infection. Sera which only harbor anti-sgG-2 antibodies will therefore be classified incorrectly as false positive in the present study.
The higher specificity for the mgG-2 ELISA among blood donors may partly be explained by the structure of the antigen. The FOCUS2 assay uses a recombinant antigen expressed in a baculovirus system (13), and therefore the antigen is not fully glycosylated. Such modifications are known to conserve antigenic properties of a protein. The type-specific antibody response to gG-1 and mgG-2 is maintained by single (mgG-2) or dual (gG-1) type-specific amino acids in a region with high similarity between the homologous proteins (30). As the seroprevalence of HSV-1 is very high in sub-Saharan Africa (6, 8, 13), correct structure of the gG-2 antigen is important to avoid cross-reactivity from anti-gG-1 antibodies. Golden et al. (10) showed recently that false-positive samples presenting reactivities with index values in the lower range (1.1 to 3.0) in the FOCUS2 assay were derived more often from HSV-1-infected individuals. These results may be explained by cross-reactivity of gG-1 antibodies. A possible explanation for the lower performance for FOCUS2 among blood donors might be that individuals in this population have higher titers of cross-reactive HSV-1 antibodies. In a survey of commercially available gG-2-based assays, Van Dyck et al. (32) showed marked differences in the specificity of the assays in the estimation of the HSV-2 seroprevalence in sub-Saharan Africa. Although it was concluded that there were no clear differences in performance between native-produced mgG-2 antigens compared to those produced recombinantly, four of the five assays presenting the highest specificity were based on native purified mgG-2.
A ROC plot is an important implement to evaluate and define the cutoff value to optimize the performance of an assay. We used ROC plots to define the optimal cutoff values for the sgG-2 and mgG-2 ELISAs. In an earlier study, the cutoff for the sgG-2 ELISA was defined (control serum reactivity + 0.35 OD unit) using well-characterized serum panels from individuals residing in Sweden (11). For these cohorts, the sensitivity and specificity were significantly higher than those described here. From the created ROC plot, it is evident that the same cutoff value we used earlier was optimal as well for the two Tanzanian cohorts. Thus, the lower performance of the sgG-2 ELISA cannot be explained by the cutoff setting. A ROC plot has recently been performed for the FOCUS2 assay used in a population from Uganda (15). The optimal performance was achieved when the cutoff index value was raised from 1.1 to 3.4. Those results are in agreement with the results described here, as 22 of 29 (76%) false-positive sera among blood donors presented index values below 3.4. The specificity of the FOCUS2 assay could therefore increase by adjusting the cutoff value. The cutoff selected for the mgG-2 ELISA presented the best diagnostic accuracy for blood donors and close-to-optimal diagnostic accuracy for the GUD cohort. In a comparison between the mgG-2 ELISA and an ELISA based on Helix pomatia lectin-purified mgG-2 (29) for sera from the western part of Sweden, the same cutoff was optimal for the mgG-2 ELISA as defined here (unpublished data). These findings suggest that for the mgG-2 ELISA, the same protocol can be used for populations in both Western world and sub-Saharan Africa populations.
We confirmed what has been shown in other studies: that the HSV-2 prevalence increases with increasing age for both men and women (Fig. 2A) and that HSV-2 infections are more common for both HIV-positive women and men than for HIV-negative individuals (Fig. 2B). There was also a statistical trend (P = 0.07) that women overall had a higher seroprevalence of HSV-2 (82%) than men (71%). A major concern with the HSV-2 infection is the facilitation of HIV transmission. Identification of HSV-2 infections is not performed on a regular basis in Tanzania. An essential advance to launch preventive health care programs to reduce HSV-2 transmission is to identify correctly HSV-2-infected individuals. In clinical settings, the performance of the assay is even more critical than in seroprevalence studies. For the determination of HSV-2 status for a single individual, the PPV and NPV of the assay are the most relevant parameters. Information on HSV-2 infection can be used, e.g., to educate and advise the patient on sexual behavior and condom usage and to initiate antiviral treatment.
In summary, our data emphasize that the performance of a single HSV-2-specific gG-based assay may vary significantly between different cohorts within the same country. To accurately identify HSV-2-infected individuals or to estimate seroprevalences, the accuracy of the assay has to be defined carefully for each cohort. Our findings indicate that immunosorbent affinity-purified mgG-2 is the best antigen for detection of type-specific IgG antibodies against HSV-2, presenting similar levels of performance among blood donors and in the GUD cohort in Tanzania. This antigen has now to be evaluated in other populations in sub-Saharan Africa and Asia as well as in countries of the Western world. Especially in developing countries, there is a need for accurate and inexpensive HSV-2 antigen detection immunoassays. The anti-mgG-2 MAb used for purification of the mgG-2 antigen can be used in the development of such assays.
Acknowledgments
This work was supported by grants from the Local Research and Development Council of Göteborg and Southern Bohuslän, the LUA Foundation at Sahlgrenska University Hospital, the Sahlgrenska University Hospital Foundations, and the Swedish Society of Medicine.
We thank Mona Brantefjord for excellent and skillful technical assistance.
REFERENCES
- 1.Ahmed, H. J., J. Mbwana, E. Gunnarsson, K. Ahlman, C. Guerino, L. A. Svensson, F. Mhalu, and T. Lagergard. 2003. Etiology of genital ulcer disease and association with human immunodeficiency virus infection in two Tanzanian cities. Sex. Transm. Dis. 30:114-119. [DOI] [PubMed] [Google Scholar]
- 2.Ashley, R. L. 2002. Performance and use of HSV type-specific serology test kits. Herpes 9:38-45. [PubMed] [Google Scholar]
- 3.Ashley, R. L., J. Militoni, F. Lee, A. Nahmias, and L. Corey. 1988. Comparison of Western blot (immunoblot) and glycoprotein G-specific immunodot enzyme assay for detecting antibodies to herpes simplex virus types 1 and 2 in human sera. J. Clin. Microbiol. 26:662-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashley, R. L., L. Wu, J. W. Pickering, M.-C. Tu, and L. Schnorenberg. 1998. Premarket evaluation of a commercial glycoprotein G-based enzyme immunoassay for herpes simplex virus type-specific antibodies. J. Clin. Microbiol. 36:294-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ashley-Morrow, R., E. Krantz, and A. Wald. 2003. Time course of seroconversion by HerpeSelect ELISA after acquisition of genital herpes simplex virus type 1 (HSV-1) or HSV-2. Sex. Transm. Dis. 30:310-314. [DOI] [PubMed] [Google Scholar]
- 6.Ashley-Morrow, R., J. Nollkamper, N. J. Robinson, N. Bishop, and J. Smith. 2004. Performance of focus ELISA tests for herpes simplex virus type 1 (HSV-1) and HSV-2 antibodies among women in ten diverse geographical locations. Clin. Microbiol. Infect. 10:530-536. [DOI] [PubMed] [Google Scholar]
- 7.Balachandran, N., and L. M. Hutt-Fletcher. 1985. Synthesis and processing of glycoprotein gG of herpes simplex virus type 2. J. Virol. 54:825-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, C. Y., R. C. Ballard, C. M. Beck-Sague, Y. Dangor, F. Radebe, S. Schmid, J. B. Weiss, V. Tshabalala, G. Fehler, Y. Htun, and S. A. Morse. 2000. Human immunodeficiency virus infection and genital ulcer disease in South Africa: the herpetic connection. Sex. Transm. Dis. 27:21-29. [DOI] [PubMed] [Google Scholar]
- 9.Eis-Hübinger, A. M., M. Däumer, B. Matz, and K. E. Schneweis. 1999. Evaluation of three glycoprotein G2-based enzyme immunoassays for detection of antibodies to herpes simplex virus type 2 in human sera. J. Clin. Microbiol. 37:1242-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Golden, M. R., R. Ashley-Morrow, P. Swenson, W. R. Hogrefe, H. H. Handsfield, and A. Wald. 2005. Herpes simplex virus type 2 (HSV-2) Western blot confirmatory testing among men testing positive for HSV-2 using the focus enzyme-linked immunosorbent assay in a sexually transmitted disease clinic. Sex. Transm. Dis. 32:771-777. [DOI] [PubMed] [Google Scholar]
- 11.Görander, S., B. Svennerholm, and J. Å. Liljeqvist. 2003. Secreted portion of glycoprotein G of herpes simplex virus type 2 is a novel antigen for type-discriminating serology. J. Clin. Microbiol. 41:3681-3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ho, D. W., P. R. Field, E. Sjogren-Jansson, S. Jeansson, and A. L. Cunningham. 1992. Indirect ELISA for the detection of HSV-2 specific IgG and IgM antibodies with glycoprotein G (gG-2). J. Virol. Methods 36:249-264. [DOI] [PubMed] [Google Scholar]
- 13.Hogrefe, W., X. Su, J. Song, R. Ashley, and L. Kong. 2002. Detection of herpes simplex virus type 2-specific immunoglobulin G antibodies in African sera by using recombinant gG2, Western blotting, and gG2 inhibition. J. Clin. Microbiol. 40:3635-3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeansson, S., and L. Molin. 1974. On the occurrence of genital herpes simplex virus infection. Clinical and virological findings and relation to gonorrhoea. Acta Dermato-Venereol. 54:479-485. [PubMed] [Google Scholar]
- 15.Laeyendecker, O., C. Henson, R. H. Gray, R. H.-N. Nguyen, B. J. Horne, M. J. Wawer, D. Serwadda, N. Kiwanuka, R. A. Morrow, W. Hogrefe, and T. C. Quinn. 2004. Performance of a commercial, type-specific enzyme-linked immunosorbent assay for detection of herpes simplex virus type 2-specific antibodies in Ugandans. J. Clin. Microbiol. 42:1794-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Langeland, N., L. Haarr, and F. Mhalu. 1998. Prevalence of HSV-2 antibodies among STD clinic patients in Tanzania. Int. J. STD AIDS 9:104-107. [DOI] [PubMed] [Google Scholar]
- 17.Lee, F. K., R. M. Coleman, L. Pereira, P. D. Bailey, M. Tatsuno, and A. J. Nahmias. 1985. Detection of herpes simplex virus type 2-specific antibody with glycoprotein G. J. Clin. Microbiol. 22:641-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liljeqvist, J. A., E. Trybala, J. Hoebeke, B. Svennerholm, and T. Bergstrom. 2002. Monoclonal antibodies and human sera directed to the secreted glycoprotein G of herpes simplex virus type 2 recognize type-specific antigenic determinants. J. Gen. Virol. 83:157-165. [DOI] [PubMed] [Google Scholar]
- 19.Liljeqvist, J.-Å. 2000. Thesis. Göteborg University, Göteborg, Sweden.
- 20.Liljeqvist, J.-Å., E. Trybala, B. Svennerholm, S. Jeansson, E. Sjögren-Jansson, and T. Bergström. 1998. Localization of type-specific epitopes of herpes simplex virus type 2 glycoprotein G recognized by human and mouse antibodies. J. Gen. Virol. 79:1215-1224. [DOI] [PubMed] [Google Scholar]
- 21.Morrow, R. A., D. Friedrich, and E. Krantz. 2003. Performance of the focus and Kalon enzyme-linked immunosorbent assays for antibodies to herpes simplex virus type 2 glycoprotein G in culture-documented cases of genital herpes. J. Clin. Microbiol. 41:5212-5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mwansasu, A., D. Mwakagile, L. Haarr, and N. Langeland. 2002. Detection of HSV-2 in genital ulcers from STD patients in Dar es Salaam, Tanzania. J. Clin. Virol. 24:183-192. [DOI] [PubMed] [Google Scholar]
- 23.Nahmias, A. J., F. K. Lee, and S. Beckman-Nahmias. 1990. Sero-epidemiological and -sociological patterns of herpes simplex virus infection in the world. Scand. J. Infect. Dis. Suppl. 69:19-36. [PubMed] [Google Scholar]
- 24.Nilsen, A., D. Mwakagile, H. Marsden, N. Langeland, R. Matre, and L. Haarr. 2005. Prevalence of, and risk factors for, HSV-2 antibodies in sexually transmitted disease patients, healthy pregnant females, blood donors and medical students in Tanzania and Norway. Epidemiol. Infect. 133:915-925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riedner, G., M. Rusizoka, O. Hoffmann, F. Nichombe, E. Lyamuya, D. Mmbando, L. Maboko, P. Hay, J. Todd, R. Hayes, M. Hoelscher, and H. Grosskurth. 2003. Baseline survey of sexually transmitted infections in a cohort of female bar workers in Mbeya Region, Tanzania. Sex. Transm. Infect. 79:382-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Serwadda, D., R. H. Gray, N. K. Sewankambo, F. Wabwire-Mangen, M. Z. Chen, T. C. Quinn, T. Lutalo, N. Kiwanuka, G. Kigozi, F. Nalugoda, M. P. Meehan, R. Ashley Morrow, and M. J. Wawer. 2003. Human immunodeficiency virus acquisition associated with genital ulcer disease and herpes simplex virus type 2 infection: a nested case-control study in Rakai, Uganda. J. Infect. Dis. 188:1492-1497. [DOI] [PubMed] [Google Scholar]
- 27.Smith, J. S., and N. J. Robinson. 2002. Age-specific prevalence of infection with herpes simplex virus types 2 and 1: a global review. J. Infect. Dis. 186(Suppl. 1):S3-S28. [DOI] [PubMed] [Google Scholar]
- 28.Su, H. K., J. D. Fetherston, M. E. Smith, and R. J. Courtney. 1993. Orientation of the cleavage site of the herpes simplex virus glycoprotein G-2. J. Virol. 67:2954-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Svennerholm, B., S. Olofsson, S. Jeansson, A. Vahlne, and E. Lycke. 1984. Herpes simplex virus type-selective enzyme-linked immunosorbent assay with Helix pomatia lectin-purified antigens. J. Clin. Microbiol. 19:235-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tunback, P., T. Bergstrom, G. B. Lowhagen, J. Hoebeke, and J. A. Liljeqvist. 2005. Type-specific reactivity of anti-glycoprotein G antibodies from herpes simplex virus-infected individuals is maintained by single or dual type-specific residues. J. Gen. Virol. 86:247-251. [DOI] [PubMed] [Google Scholar]
- 31.Tunback, P., J. A. Liljeqvist, G. B. Lowhagen, and T. Bergstrom. 2000. Glycoprotein G of herpes simplex virus type 1: identification of type-specific epitopes by human antibodies. J. Gen. Virol. 81:1033-1040. [DOI] [PubMed] [Google Scholar]
- 32.Van Dyck, E., A. Buvé, H. A. Weiss, J. R. Glynn, D. W. G. Brown, B. De Deken, J. Parry, and R. J. Hayes. 2004. Performance of commercially available enzyme immunoassays for detection of antibodies against herpes simplex virus type 2 in African populations. J. Clin. Microbiol. 42:2961-2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wald, A. 2004. Herpes simplex virus type 2 transmission: risk factors and virus shedding. Herpes 11(Suppl. 3):130A-137A. [PubMed] [Google Scholar]
- 34.Wald, A., and K. Link. 2002. Risk of human immunodeficiency virus infection in herpes simplex virus type 2-seropositive persons: a meta-analysis. J. Infect. Dis. 185:45-52. [DOI] [PubMed] [Google Scholar]
- 35.Wald, A., J. Zeh, S. Selke, T. Warren, A. J. Ryncarz, R. Ashley, J. N. Krieger, and L. Corey. 2000. Reactivation of genital herpes simplex virus type 2 infection in asymptomatic seropositive persons. N. Engl. J. Med. 342:844-850. [DOI] [PubMed] [Google Scholar]
- 36.Zweig, M. H., and G. Campbell. 1993. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin. Chem. 39:561-577. [PubMed] [Google Scholar]