Abstract
Tuberculosis (TB) in elephants is a re-emerging zoonotic disease caused primarily by Mycobacterium tuberculosis. Current diagnosis relies on trunk wash culture, the only officially recognized test, which has serious limitations. Innovative and efficient diagnostic methods are urgently needed. Rapid identification of infected animals is a crucial prerequisite for more effective control of TB, as early diagnosis allows timely initiation of chemotherapy. Serology has diagnostic potential, although key antigens have not been identified and optimal immunoassay formats are not established. To characterize the humoral responses in elephant TB, we tested 143 serum samples collected from 15 elephants over time. These included 48 samples from five culture-confirmed TB cases, of which four were in Asian elephants infected with M. tuberculosis and one was in an African elephant with Mycobacterium bovis. Multiantigen print immunoassay (MAPIA) employing a panel of 12 defined antigens was used to identify serologic correlates of active disease. ESAT-6 was the immunodominant antigen recognized in elephant TB. Serum immunoglobulin G antibodies to ESAT-6 and other proteins were detected up to 3.5 years prior to culture of M. tuberculosis from trunk washes. Antibody levels to certain antigens gradually decreased in response to antitubercular therapy, suggesting the possibility of treatment monitoring. In addition to MAPIA, serum samples were evaluated with a recently developed rapid test (RT) based on lateral flow technology (ElephantTB STAT-PAK). Similarly to MAPIA, infected elephants were identified using the RT up to 4 years prior to positive culture. These findings demonstrate the potential for TB surveillance and treatment monitoring using the RT and MAPIA, respectively.
Tuberculosis (TB) caused by Mycobacterium tuberculosis or Mycobacterium bovis has recently re-emerged as an important infectious disease in exotic animals, particularly captive elephants (22, 24, 27). While TB has been recognized as a disease of elephants for over 20 centuries, increasing numbers of elephant TB cases have been reported in the past decade (13, 23). During 2001 to 2003, an outbreak of TB in a Swedish zoo involved five elephants and several other species (giraffes, rhinoceroses, and buffaloes), which were found to have been infected by four different strains of M. tuberculosis (13). In zoos and circuses, TB is more frequently detected in Asian elephants (Elephas maximus) than in African elephants (Loxodonta africana) (22, 27). Circumstantial evidence indicates that infection can be transmitted to elephants from humans and that it may spread between elephants and other susceptible species (13, 21, 23). Due to the zoonotic nature of the disease and close proximity of zoo/circus elephants to humans, the rise in elephant TB cases has potential public health ramifications (26), in addition to creating concern for the health and well-being of the animals.
The immunobiology of elephant TB is poorly understood. This lack of knowledge makes it difficult to develop and implement effective methods of testing, treatment, management, and control. In contrast to traditional (e.g., cattle) and nontraditional (e.g., various cervid species) livestock, infected elephants are often treated with antibiotics. Effective therapeutic regimens are more easily implemented if animals are diagnosed early in the course of infection. Early diagnosis also reduces the risk of exposure to other zoo animals, elephant handlers, and visitors to exhibits. Thus, it is critical to develop improved control strategies based upon early identification of infected animals.
After two Asian elephants were diagnosed with M. tuberculosis in 1996, the National Tuberculosis Working Group for Zoo and Wildlife Species was established to develop the best strategy of disease control for a variety of exotic animals. As a result, through the concerted effort of this group in conjunction with the United States Department of Agriculture (USDA), the Guidelines for the Control of Tuberculosis in Elephants were developed in 1997 (updated in 2000 and 2003). The guidelines include TB screening protocols and recommendations for antimicrobial therapy (25). The only USDA-recommended diagnostic test for TB in elephants is mycobacterial culture of trunk wash samples. However, there is a growing body of evidence indicating that this method of diagnosis has poor sensitivity, as it can only identify animals with extensive shedding of the organism that typically occurs late in the course of disease. Recent attempts to identify satisfactory alternatives for antemortem detection of TB in elephants using traditional techniques (i.e., tuberculin skin test) have been unsuccessful (13, 23). In contrast, antibody-based tests appear promising, but improved platforms and antigenic targets are needed (12, 31).
Multiantigen print immunoassay (MAPIA) was developed as an efficient tool for the identification of seroreactive antigens in human TB (17). More recently, this method was adopted to determine antigen recognition patterns in cattle, white-tailed deer, reindeer, and European badgers infected with M. bovis (9, 18, 33, 34). In the present study, we used MAPIA to characterize antigen recognition profiles and kinetics of immunoglobulin G (IgG) antibody responses to a panel of 12 defined antigens of M. tuberculosis and M. bovis in sera obtained from elephants with culture-confirmed TB. Serial serum samples collected before, during, and after treatment were tested. In addition, a rapid test (RT) for TB in elephants (ElephantTB STAT-PAK kit) was developed using lateral-flow technology and selected recombinant antigens. With the limited number of elephants tested, the RT showed the potential to be a simple and useful screening assay for early detection of TB in elephants, while MAPIA exhibited an added potential for monitoring of treatment and prediction of relapse.
MATERIALS AND METHODS
Animals and disease status.
Five female elephants (L, C, K, N, and I) ranging from 30 to 47 years of age from different locations in the United States were diagnosed with TB by mycobacterial culture between 1997 and 2000. Table 1 summarizes epidemiologic, diagnostic, and treatment data for these animals. Two of them (L and C) had a history of exposure to M. tuberculosis-infected elephants. Four Asian elephants were culture positive for M. tuberculosis on the basis of trunk washes, and one African elephant (N) was culture positive for M. bovis at necropsy. The four Asian elephants were treated with antimycobacterial compounds for 12 to 20 months, whereas the African elephant did not receive therapy, as the diagnosis was made postmortem. Two of the treated animals, K and L, were given therapy repeatedly after the initial course, as they produced positive cultures of M. tuberculosis in posttreatment follow-up trunk wash testing. Disease was confirmed postmortem by culture and histopathology examination in the untreated African elephant infected with M. bovis and two of the four treated Asian elephants infected with M. tuberculosis. In one of the other two, which was euthanized for a severe joint disease, no evidence of TB was found 6 years after treatment. The second one remains alive and well at the time of this writing. Additional clinical and diagnostic findings are presented in Results. Forty-eight serial serum samples (6 to 12 from each animal) collected from the five elephants before diagnosis as well as during and after treatment (the total observation period was 1.5 to 8.5 years) were used to measure the antibody responses by MAPIA and the RT. Ninety-five sera were also collected over time (for up to 10 years and 7 months) from 10 Asian females which had never been trunk wash culture positive, although they lived during 1986 to 2006 in the same five locations where the elephants with TB were identified. These samples were used as negative controls for the serological studies. Eight of the culture-negative elephants were exposed to the TB cases. All serum samples were stored frozen at −20°C or −70°C until testing.
TABLE 1.
Diagnosis, treatment, and outcomes for culture-positive elephantsa
| Elephant | Age (yr),b date of diagnosis, and species | TB sign | MAPIA and RT (n) | Treatment | Status |
|---|---|---|---|---|---|
| L | 46; October 1996, M. tuberculosis | Weight loss | Reactive (6) | INH/PZA/RIF over food for 12 mo; might be compromised by use of expired medications; restarted in 2001, but soon discontinued due to weakness | Relapse 3-4 yr after initial treatment; died of TB in February 2005, confirmed by positive culture and histopathology at necropsy |
| C | 30; March 1997, M. tuberculosis | None | Reactive (12) | INH/PZA/RIF orally for 6 wk and then rectally for 17 mo | No positive trunk wash cultures after treatment; euthanized March 2004 for deteriorating health (degenerative joint disease); no evidence of TB at necropsy |
| K | 31; May 2000, M. tuberculosis | None | Reactive (12) | INH/PZA rectally for 8 mo, followed by same plus RIF orally for 12 mo; multiple breaks due to intolerance; restarted in March 2003 with PZA; EMB; ENRO, AMK for 14 mo | Relapse and development of MDR strain after frequently interrupted treatment; euthanized June 2004 due to medication intolerance and risk of exposure to MDR TB; disease confirmed by culture and histopathology at necropsy |
| N | 47; August 2000, M. bovis (postmortem) | None | Reactive (6) | None | Never trunk wash culture positive; euthanized August 2000 for chronic osteomyelitis; gross lesions and positive culture from tissues at necropsy |
| I | 30; October 2000, M. tuberculosis | None | Reactive (12) | INH and PZA rectally for 12 mo | Cured (pending confirmation); monthly trunk washes negative for culture after treatment to date |
n, number of serum samples tested; RT, rapid test; MAPIA, multiantigen print immunoassay; INH, isoniazid; PZA, pyrazinamide; RIF, rifampin; EMB, ethambutol; ENRO, enrofloxacin; AMK, amikacin; MDR, multidrug resistant.
At time of diagnosis.
Culture and drug susceptibility testing.
Culture for M. tuberculosis and other mycobacteria was performed at the National Veterinary Services Laboratories (Ames, IA) in accordance with Guidelines for the Control of Tuberculosis in Elephants (25). The procedure for collecting triple trunk wash samples was described previously (23). In addition, specimens of feces, urine, vaginal discharge, and various tissues obtained at necropsy were processed for culture. The following solid media were used: Middlebrook 7H10 with glycerol, Middlebrook 7H11 with glycerol, Stonebrinks, and BBL Mycobactosel L-J; also, BACTEC 12B vials were inoculated with 0.5 ml of sample supplemented with PANTA (polymixin B, amphotericin B, nalidixic acid, trimethoprim, and azlocillin) and erythromycin (32 μg/ml). Processed specimens were inoculated on media and incubated at 37°C and 10% CO2 for up to 8 weeks. All isolates and BACTEC bottles with a growth indicator value of >300 were confirmed with a GenProbe AccuProbe Mycobacterium tuberculosis complex culture identification test. If the DNA probe test was positive, the niacin-nitrate reduction test was performed to confirm M. tuberculosis infection. Antimicrobial susceptibility testing was performed using BACTEC 460 and the following antibiotics: isoniazid (INH) at 0.1 μg/ml, rifampin (RIF) at 2 μg/ml, ethambutol (EMB) at 2.5 μg/ml, streptomycin (STR) at 2 μg/ml, and pyrazinamide (PZA) at 100 μg/ml.
Treatment.
Culture-positive elephants were treated with first-line anti-TB drugs as recommended in Guidelines for the Control of Tuberculosis in Elephants in accordance with the protocol mentioned previously (23). Combinations of INH, RIF, and/or PZA were given daily or every 48 h, orally or rectally. Daily drug doses were 2.5 to 7.5 mg/kg for INH, 8 to 10 mg/kg for RIF, and 25 to 35 mg/kg for PZA. To treat multidrug-resistant (MDR) TB, a combination of PZA, EMB, enrofloxacin (ENRO), and amikacin (AMK) was used. Treatment duration varied from elephant to elephant, as indicated in Table 1.
Antigens.
A panel of 12 defined proteins of M. tuberculosis was used in MAPIA, as described below. The following recombinant antigens were purified to near homogeneity as polyhistidine-tagged proteins: ESAT-6 and CFP-10 produced at the Statens Serum Institut (Copenhagen, Denmark); Mtb8 and Mtb48 supplied by Corixa Corp. (Seattle, WA); and MPB59, MPB64, MPB70, and MPB83 produced at the Veterinary Sciences Division (Stormont, United Kingdom). α-Crystallin (Acr1) and the 38-kDa protein were purchased from Standard Diagnostics (Seoul, South Korea). Native MPB83 protein was kindly supplied by Mark Chambers of the Veterinary Laboratories Agency (Weybridge, United Kingdom). Polyprotein fusion TBF10 was developed by Corixa Corp. and purified as described earlier (10). This construct included Mtb8, CFP10, and the 38-kDa protein. The hybrids CFP-10/ESAT-6 and Acr1/MPB83 were constructed by overlapping PCR using gene-specific oligonucleotides to amplify the genes from M. tuberculosis H37Rv chromosomal DNA. The fused polygene PCR products were cloned into the pMCT6 Escherichia coli expression vector using SmaI and BamHI restriction enzymes. The hybrids were purified to near homogeneity by exploiting the polyhistidine tag encoded by the vector.
MAPIA.
MAPIA was performed as previously described (17). Briefly, purified antigens were immobilized on nitrocellulose membranes (Schleicher & Schuell, Keene, N.H.) at a protein concentration of 0.05 mg/ml using a semiautomatic microaerosolization device (Linomat IV, Camag Scientific Inc, Wilmington, DE) to generate invisible parallel bands. After antigen printing, the membrane was cut into strips 3.5 mm wide, perpendicular to the antigen bands, so that each strip carried all antigens. Next, strips were blocked for 1 h with 1% nonfat milk in phosphate-buffered saline containing 0.05% Tween 20 and then incubated with individual serum samples diluted 1:50 in blocking solution for 1 h at room temperature. After washing, strips were incubated for 1 h with peroxidase-conjugated protein G (Sigma) diluted 1:1,000 (Kirkegaard & Perry Laboratories), followed by another washing step. Elephant IgG antibodies bound to immobilized antigens were visualized with 3,3′,5,5′-tetramethyl benzidine (Kirkegaard & Perry Laboratories). MAPIA results were scored by two independent operators who did not know the true status of elephant samples, with a band of any intensity being read as a positive reaction. Results were also evaluated semiquantitatively by scanning and densitometry using Scion Image software (version Beta 4.0.2).
RT.
Chembio Diagnostic Systems, Inc. (Medford, NY), developed an RT for TB in elephants (ElephantTB STAT-PAK kit) using lateral-flow technology. The antibody detection test employs a unique cocktail of several selected M. tuberculosis and/or M. bovis antigens and a blue latex bead-based signal detection system. The ready-to-use disposable device consists of a plastic cassette containing a strip of nitrocellulose membrane impregnated with test antigen and laminated with several pads made of glass fiber and cellulose. The test requires 30 μl of serum sample (plasma or whole blood) and 3 drops of sample diluent buffer (included in the kit) that are added sequentially to the sample pad. As the diluted test sample migrates to the conjugate pad, the latex particles conjugated to antigen bind antibody, if present in the sample, thus creating a colored immune complex. Driven by capillary forces, the complex flows laterally across the nitrocellulose membrane impregnated with specific antigen and binds to the immobilized antigen, thus producing a blue band in the test area of the device. In the absence of specific antibody, no band develops in the test window. The liquid continues to migrate along the membrane, producing a similar blue band in the control area of the device, irrespective of the presence of specific antibody in the test sample, demonstrating that the test reagents are functioning properly. Results are read 20 min after addition of the sample and diluent buffer either visually or by using a portable optical reader. Any visible band in the test area, in addition to the control line, was considered an antibody-positive result, whereas no band was considered a negative result. Semiquantitative evaluation of test band intensity in this study was performed by an in-house-developed computer-assisted optical reader designed to measure reflectance at 624 nm.
RESULTS
Clinical and diagnostic findings, treatment and pathology.
The disease status and medical histories of five culture-positive and ten culture-negative elephants are presented below and summarized in Tables 1 and 2.
TABLE 2.
Disease status of culture-negative elephantsa
| Elephant | Age (yr)b and source of TB (exposure) | Other diagnosis | MAPIA and RT (n) | Treatment | Status |
|---|---|---|---|---|---|
| B | 30; unknown | Chronic wasting disease | NR (1) | None | Died May 2004; no evidence of TB at necropsy |
| D1 | 60; elephant L | Osteomyelitis | NR (7) | None | Annual trunk washes negative for culture to date |
| T1 | 31; elephant C | Foot disease | NR (20) | INH orally for 5 mo; discontinued May 1998 due to poor acceptance | Euthanized March 2005 for foot problems; no evidence of TB at necropsy |
| P | 41; unknown | Chronic arthritis | NR (7) | Anti-inflammatory drugs | Euthanized November 1992 for chronic arthritis; M. intracellulare isolated from lungs; no evidence of TB at necropsy |
| S1 | 36; elephant K | Glomerulonephritis | NR (15) | Antibiotics, aspirin, nutritional supplements | Died February 2003; no evidence of TB at necropsy |
| T2 | 31; elephant K | None | NR (22) | None | Quarterly trunk washes negative for culture to date |
| S2 | 25; elephant N | None | NR (1) | None | Annual trunk washes negative for culture to date |
| A | 30; elephant I | None | NR (8) | None | Semiannual trunk washes negative for culture to date |
| D2 | 16; elephant I | None | NR (6) | None | Semiannual trunk washes negative for culture to date |
| R | 12; elephant I | None | NR (8) | None | Semiannual trunk washes negative for culture to date |
n, number of serum samples tested; RT, rapid test; MAPIA, multiantigen print immunoassay; INH, isoniazid; NR, nonreactive.
At time of TB diagnosis in same zoo.
Elephant L.
A 46-year-old Asian female elephant resided in a herd that had a history of TB. In late 1996, elephant L was diagnosed with M. tuberculosis by positive trunk wash culture. At the time, she was noticed to have lost a significant amount of weight. Several elephant handlers had tested positive for TB exposure a few months before elephant L was diagnosed (21). Treatment with INH, RIF, and PZA given with food was initiated in December 1996 and continued for 12 months. Although trunk washes were culture negative during follow-up testing, the quality of treatment might have been compromised, as the amounts of anti-TB drug administered were insufficient, based on current recommendations. In the following years, elephant L remained “excessively thin” and became emaciated by 2001, when her trunk washes again produced positive culture results. She was treated repeatedly for a short period in the second half of 2001 before the intervention was discontinued due to her weakness and poor acceptance of medications. Although follow-up testing of monthly trunk washes or feces did not yield positive findings, elephant L died of TB in February 2005. Weakness, depressed appetite, and dyspnea were noted several days prior to her death. Postmortem examination revealed multiple gross lesions compatible with TB in the lungs and lymph nodes. M. tuberculosis was cultured from the lungs, lymph nodes, liver, and uterus. The isolates obtained from elephant L in 1996, 2001, and 2005 were identical, indicating disease reactivation rather than reinfection. Since it was believed that she had long-standing disease combined with an inadequate treatment history, the increased likelihood of MDR TB was considered; however, drug susceptibility testing of the M. tuberculosis isolates failed to confirm this suspicion.
Elephant C.
A 30-year-old Asian female elephant was originally a performer in a traveling circus before spending her last several years in zoos. Elephant C had no symptoms suggestive of TB when she tested positive for M. tuberculosis from trunk wash cultures collected in April 1997. The diagnosis occurred weeks after she was transported from another zoo where an elephant had just died of Salmonella enterica infection but necropsy revealed evidence of pulmonary TB (M. tuberculosis was isolated from a mesenteric lymph node) and where three elephant handlers reportedly tested positive for TB exposure. The M. tuberculosis isolates were susceptible to all five antibiotics in vitro. Treatment with INH, RIF, and PZA given over food was initiated in May 1997. The treatment was changed 3 months later to drug administration via rectal suppository and completed in December 1998. Follow-up trunk wash cultures remained negative from July 1997 (2 months after initiation of oral treatment). In March 2004, elephant C was euthanized due to severe joint disease. No evidence of active TB was found at necropsy.
Elephant K.
A 31-year-old Asian female elephant lived in a zoo that had no history of TB. Screening elephants for mycobacterial diseases included triple trunk swabs in 1997, replaced by triple trunk washes starting in 1998. In May 2000, testing of mucous discharge from the trunk yielded positive results for acid-fast bacilli (AFB) and culture of M. tuberculosis. During the following weeks, M. tuberculosis was also isolated from vaginal discharge and feces. No symptoms suggestive of TB were observed. Daily rectal therapy with INH and PZA was immediately initiated. After 2 months, treatment was changed to 48-h medication intervals for another 9 months, in accordance with the 2000 Guidelines for the Control of Tuberculosis in Elephants. Treatment was then changed to oral administration, and RIF was added to the protocol. Therapy continued for another 12 months, but on multiple occasions it was discontinued for several days to a few weeks due to poor appetite and depression. Follow-up trunk wash and vaginal discharge samples repeatedly tested culture negative for 1 year after treatment, until M. tuberculosis was detected in vaginal discharge and urine samples in February 2003. The isolate was found to be an MDR strain (resistant to INH and RIF). Treatment with PZA, EMB, and ENRO orally and AMK intramuscularly was initiated and continued for another year, but again there were frequent interruptions because of drug intolerance causing illness, poor appetite, and depression. Elephant K was euthanized in June 2004 due to recurring intolerance to medication and risk of exposure of staff and animal collection to MDR TB. A postmortem examination confirmed active disease with moderate to severe, chronic, diffuse granulomatous lymphadenitis with mineralization and moderate to severe, chronic, multifocal and coalescing granulomatous pneumonia with mineralization and rare intralesional AFB. Granulomatous lesions were also found in the uterus and abdomen. M. tuberculosis was cultured from the lungs and lymph nodes.
Elephant N.
A 46-year-old female African bush elephant (Loxodonta africana knochenhaueri) resided in a zoo that had a history of M. bovis infection in other species. Elephant N had no signs suggestive of TB but suffered from a chronic nonresponsive osteomyelitis and was euthanized in August 2000. Other medical concerns included recurrent episodes of ventral edema and hyperprolactinemia. Screening for mycobacterial diseases by triple trunk swabs in 1997 or by annual triple trunk washes starting in 1998 had produced negative results. At necropsy, in addition to chronic osteomyelitis, an extensive granulomatous pneumonia was found, and M. bovis was cultured from several tissue samples. The isolate was resistant to PZA, which was expected for M. bovis. Also, Mycobacterium avium was isolated from the frontal sinus.
Elephant I.
A 30-year-old female Asian elephant was transported from a circus to a zoo in August 1997. Trunk wash cultures were performed nine times in 1998 with no growth of M. tuberculosis. Elephant I did not have any clinical signs of disease. In late 2000, three separate trunk washes collected in October and November produced positive cultures of M. tuberculosis. Treatment with INH and PZA rectally was initiated in late December 2000. Six weeks later, a follow-up trunk wash culture yielded a positive result. This culture contained two M. tuberculosis isolates, both of which were different from the original isolate. Of the three isolates, two were pansensitive to antitubercular drugs, while the third (one of those found during treatment) was monoresistant to RIF. Administration of INH and PZA continued for 1 year until January 2002 without any further positive cultures. Monthly trunk washes have continued to be culture negative to date.
Culture-negative elephants.
Ten elephants from the same herds where the five TB cases had been found were included in this study as a control (TB-negative) group (Table 2). All were Asian females with a history of multiple “no isolation” trunk wash cultures. The average age in this group was similar to that of the elephants with TB (31.2 and 36.8 years, respectively). Of the 10 control elephants, eight had been exposed to TB, five had other maladies (i.e., chronic wasting disease, osteomyelitis, glomerulonephritis, and chronic arthritis), and two received antibiotic treatment (Table 2). Four elephants were confirmed as negative for TB, as no evidence of TB was found at necropsy when they died or were euthanized for other reasons.
Antibody responses and antigen recognition in MAPIA.
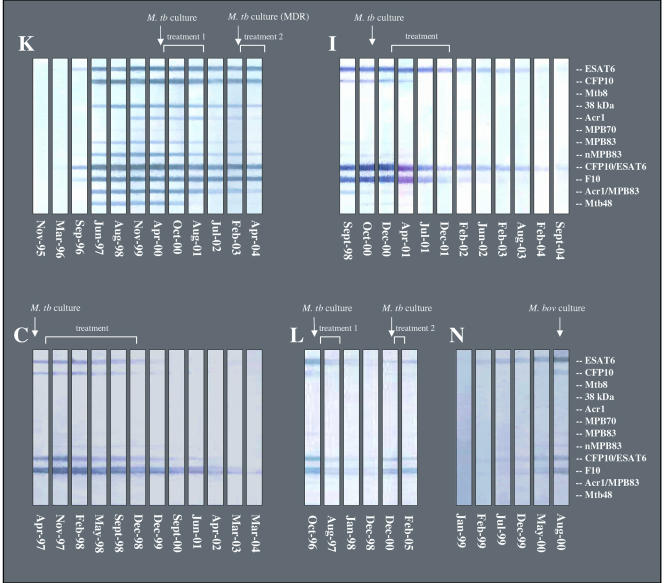
Humoral immune responses in five elephants with TB were characterized by retrospective serological testing of sequential samples collected over time using MAPIA (Fig. 1). In elephant K, the IgG antibody responses were first detected in November 1996, 42 months before the diagnosis was made by trunk wash culture in May 2000. The earliest antigen recognized was ESAT-6, either alone or as a fusion protein with CFP10. The magnitude of the antibody response increased dramatically over time and involved multiple antigens consistently recognized during 1997 to 2000. In addition to ESAT-6, seroreactive proteins were CFP10, the 38-kDa protein, Acr1, MPB83, Mtb48, and the Acr1/MPB83 fusion protein. After treatment was initiated in May 2000, levels of antibodies to certain antigens declined while others remained unchanged in the following years.
FIG. 1.
Serum IgG antibody responses in culture-positive elephants to individual antigens of M. tuberculosis. MAPIA was performed as described in Materials and Methods. Each panel of strips represents a set of serum samples collected over time from one elephant, as shown (K, I, C, L, and N). Each strip represents one serum sample collected on the date indicated. Bands on MAPIA strips show the presence of antibodies to antigens printed (listed on the right). Time of diagnosis by positive culture is shown by arrows, and treatment duration is indicated for each elephant.
Asian elephants C, L, and I, which were infected with M. tuberculosis, and African elephant N, which was infected with M. bovis, produced strong antibody responses to several antigens at the time of culture-based diagnosis. Their sera showed similar patterns of antigen reactivity in MAPIA, including ESAT-6, CFP10, and fusions containing these molecules. An anti-ESAT-6 IgG response in elephant N was first detected in February 1999, 18 months before M. bovis was isolated from tissues at necropsy. In elephant I, the only prediagnosis sample collected in September 1998 (over 2 years prior to positive trunk wash culture) was already reactive in MAPIA, showing a similar magnitude of antibody response with an almost identical antigen recognition pattern to that found at the time of culture-based diagnosis. No samples taken prior to diagnosis by culture were available from elephants C and L to establish the time of seroconversion. As observed in elephant K, the antibody levels in elephants C, L, and I gradually declined for several months after initiation of treatment and remained low or undetectable for many years thereafter. No antibody was detected by MAPIA in any serum sample from the consistently culture-negative elephants (Table 2).
Antibody detection by the RT.
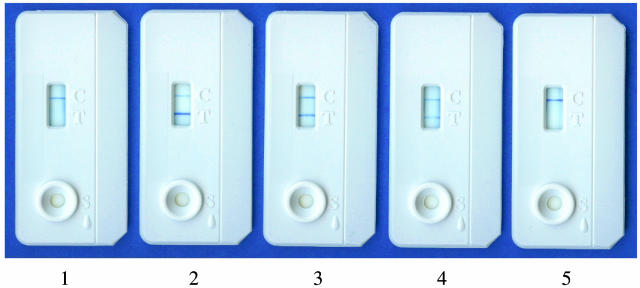
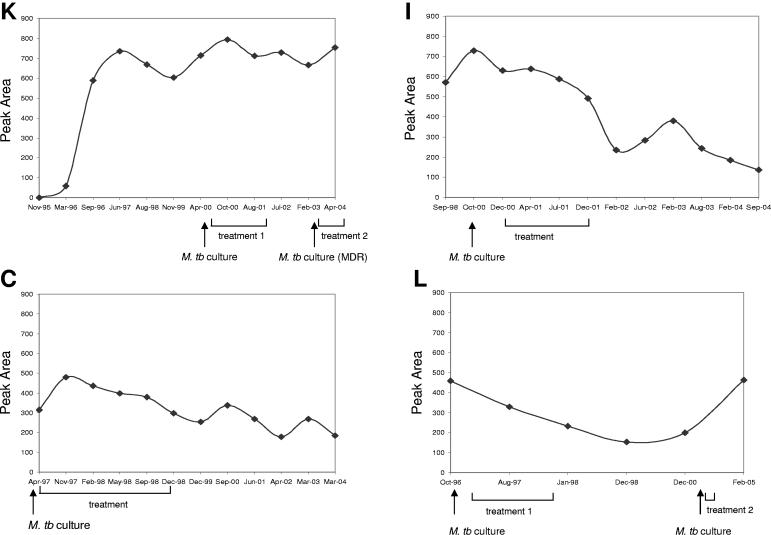
Lateral-flow technology offers a very simple and rapid way to detect antibody of interest in a diagnostic sample. Qualitative “yes-or-no” read-outs can be obtained within 20 min by visual evaluation. Also, if necessary, the intensity of test bands can be measured by an optical reader. Figure 2 shows examples of RT images obtained with selected samples from three elephants (K, N, and I) collected before seroconversion (nonreactive) and at the time of culture-based diagnosis (reactive). Reader-generated data (Fig. 3) demonstrated that antibody detection by the RT was generally in agreement with MAPIA results (Fig. 1). The RT detected antibody as early during infection as it was measurable by MAPIA or even several months earlier in one TB case. In elephant K, the March 1996 sample was reactive by the RT but still nonreactive by MAPIA (presumably due to the ability of the RT to detect IgM in addition to IgG). Strong RT bands were observed with sera from all five culture-positive elephants (Fig. 2 and 3), with optical reflectance readouts ranging from 257 units for elephant N to 728 units for elephant I obtained at the time of diagnosis. No antibody was detected in any of the consistently culture-negative elephants (Table 2). Optical reader values for all 95 sera in this group were 0.
FIG. 2.
Rapid detection of serum antibodies by the ElephantTB STAT-PAK kit. The RT was performed as described in Materials and Methods. In the test window, the upper line is the control band and the lower line is the test band. Results were obtained with selected serum samples from elephant K (1, November 1995; 2, April 2000), elephant L (3, October 1996), and elephant N (4, August 2000; 5, January 1999). RT images 1 and 5 represent nonreactive samples collected from elephants K and N, respectively, before seroconversions. RT images 2 and 4 represent reactive samples from the same two elephants collected at the time of diagnosis by positive cultures of M. tuberculosis and M. bovis, respectively. Semiquantitative results obtained with an optical reader (intensity of test band measured as peak area) were as follows: 1, 0 units; 2, 714 units; 3, 458 units; 4, 257 units; and 5, 0 units.
FIG. 3.
Detection of serum antibodies by ElephantTB STAT-PAK kit in elephant TB before, during, and after treatment. The RT was performed as described in Materials and Methods. Results were measured with an optical reader and expressed in reflectance units (peak area). Each panel shows kinetics of antibody responses found by the RT with a set of serum samples collected over time from one elephant, as indicated (K, I, C, and L). The time of diagnosis by positive culture is shown by arrows, and treatment duration is indicated for each elephant.
Seroconversion and shedding.
Availability of prediagnosis samples from three of the infected elephants provided an opportunity to compare the timing of antibody response onset to that of culture-based diagnosis. In elephant K, serum collected in March 1996 was positive by the RT, over 4 years earlier than the diagnosis made by culture in May 2000 (Fig. 3). By MAPIA, an IgG antibody response to ESAT-6 was first detected in September 1996, or 3.5 years earlier than M. tuberculosis was isolated from trunk washes (Fig. 1). For elephant I, a high-level antibody response was detected by both MAPIA and the RT in a sample collected in September 1998 (no earlier sample was available), over 2 years before the culture-based diagnosis (Fig. 1 and 3). Sera from elephant N were reactive by the RT and MAPIA 1.5 years prior to M. bovis isolation at necropsy. The remaining two culture-positive elephants had IgG responses already well established by the time of diagnosis (no prediagnosis serum samples were available). Thus, our observations strongly suggest that antibody-based assays have the potential to identify infected elephants long before shedding stages of the disease.
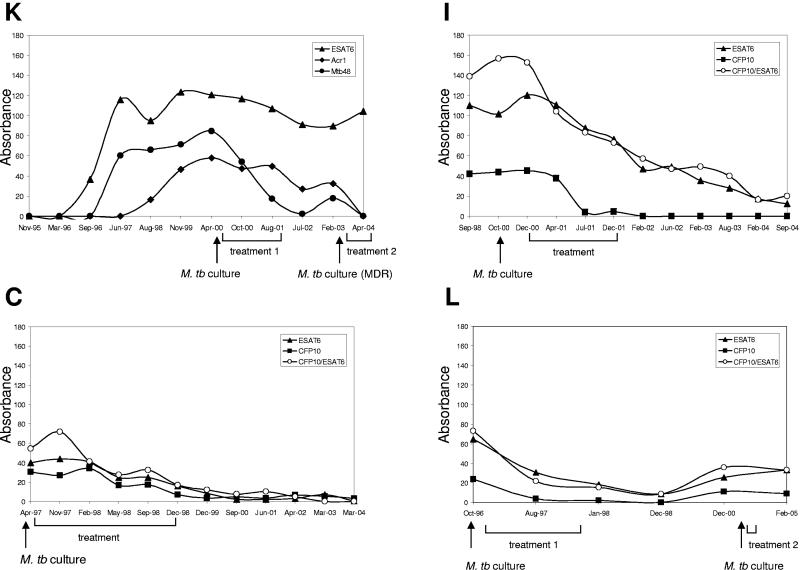
Effect of antimycobacterial therapy on antibody levels.
As mentioned above, humoral immune responses in all elephants with TB were robust and well established at the time of diagnosis. Antimycobacterial therapy was administered to four of the culture-positive elephants (L, C, K, and I). In most cases, the RT remained strongly reactive during and after treatment, showing little change over time (Fig. 3). In contrast, MAPIA demonstrated that the levels of antibody to certain antigens declined rather quickly down to baseline in response to treatment. Figures 1 and 4 show MAPIA strips and corresponding semiquantitative data obtained with selected antigens by scanning and densitometry. In elephant K, the antibody response to ESAT-6 (as well as a few other antigens) was not affected by therapy. In contrast, serum IgG reactivity to Acr1 and Mtb48, particularly, decreased to baseline levels during treatment. Interestingly, a short-lived spike of antibody response to Mtb48 in February 2003 appeared to coincide with a positive culture of M. tuberculosis from this elephant again, followed by its decline after treatment was reinitiated in March 2003. In elephants C and I, whose sera predominantly recognized ESAT-6 and CFP10, the antibody levels also decreased progressively and remained low or undetectable in the posttreatment period. In contrast, after a similar response to initial antimycobacterial therapy, elephant L showed a significant increase in antibody response in 2000, about 3 years after treatment and a year before another positive trunk wash culture. High levels of antibody lasted in this elephant until her death from TB in February 2005. Therefore, our findings demonstrate that MAPIA may have the potential for monitoring treatment and predicting relapses of TB in elephants.
FIG. 4.
Effect of antitubercular treatment on IgG antibody responses to selected antigens of M. tuberculosis in culture-positive elephants. MAPIA was performed as described in Materials and Methods. Each panel represents a set of serum samples collected over time from one elephant, as indicated (K, I, C, and L). Results are expressed in arbitrary units of optical density obtained from measuring intensities of MAPIA bands by scanning and densitometry using Scion Image (version Beta 4.0.2). The time of diagnosis by positive culture is shown by arrows, and treatment duration is indicated for each elephant.
DISCUSSION
Elephant TB has recently emerged as a serious infectious disease with zoonotic potential. Although it has been reported that about 3% of captive elephants in the United States are infected with M. tuberculosis (22, 27), recent estimates are up to 6% (S. K. Mikota, unpublished data). Since the estimation is based on the results of trunk wash culture and this method has inadequate sensitivity, the true number of elephants harboring TB is likely higher. In humans, TB diagnosis is based on evaluation of clinical symptoms and laboratory methods, such as chest X-ray, smear microscopy, culture, or PCR probes (7, 29). In cattle, the intradermal skin test remains the primary screening tool in most countries, while the in vitro whole-blood gamma interferon Bovigam assay (Prionics AG, Schlieren, Switzerland) is generally used as an ancillary test (28). None of these methods provides reliable TB diagnosis in elephants. Trunk wash culture, the only officially recommended diagnostic test in the United States, has poor sensitivity. It is labor-intensive, time-consuming, and costly. This test requires three trunk wash samples collected on different days within 1 week. Both elephant and caretaker must be appropriately trained to obtain a trunk wash sample of acceptable quality. To further complicate a timely diagnosis, most elephants with active TB have no clinical signs of disease. In fact, four of the five culture-positive elephants described in the present study were asymptomatic at the time of shedding. Therefore, early diagnosis of TB in elephants can be very elusive.
The present study is the first attempt to define immunodominant antigens of M. tuberculosis/M. bovis in elephant TB. We found that ESAT-6 is strongly recognized by serum IgG in all culture-positive elephants. Less frequently reactive antigens are CFP10, MPB83, Mtb48, Acr1, and the 38-kDa protein. Polyepitope fusions containing one or two of these protein sequences performed especially well in MAPIA, consistent with our previous reports on human, cattle, and deer TB serology (10, 18, 33). The predominant recognition of ESAT-6 is also found in nonhuman primates infected with M. tuberculosis (4). However, in other host species, including humans, badgers, cattle, white-tailed deer, and reindeer, this antigen is not the most important antigenic target for serum antibodies (9, 15, 16, 18, 33, 34). Thus, it appears to be critical to determine the immunodominant antigens for each new species to be evaluated for TB serodiagnosis.
Humoral immunity in TB is characterized by individual variation in antigen recognition (15, 17). Both pathogen- and host-related factors are believed to determine this heterogeneity. It has been suggested that differential expression of stage-specific M. tuberculosis genes can lead to shifting profiles of the predominant immune recognition of corresponding proteins in the course of disease (5, 16). Latent TB infection in humans is reportedly associated with up-regulation of the gene encoding the 16-kDa heat shock protein known as α-crystallin (Acr1, Rv2031c), whereas other antigens may be predominantly recognized by T cells and/or antibodies in the acute phase of disease (5, 6). The present study, however, did not provide any evidence for significant changes in antibody profiles during elephant infection with M. tuberculosis, except in response to therapy.
Although antitubercular therapy in animals is not common, treatment is a preferred choice for culture-positive elephants in the United States. Guidelines for the Control of Tuberculosis in Elephants (25) defines general principles and protocols of treatment. Drug dosages and regimens are continuously optimized (19, 20, 35). Insufficient or improper treatment of TB in humans is the major cause of emerging MDR strains of M. tuberculosis (30). In elephant K, the initial isolate was susceptible to all first-line drugs but converted to an MDR strain when found again 1 year after a 20-month treatment involving multiple interruptions mostly due to drug intolerance. Further, in elephant I, three different isolates of M. tuberculosis were found, one of which was resistant to RIF but not to INH. This rare pattern of drug resistance is found mostly in TB patients with AIDS who receive highly active antiretroviral therapy in combination with antitubercular treatment including RIF (G. Simpson, personal communication).
The effects of antitubercular therapy on immune responses of human TB patients have been described (2). In most cases, antibody levels in seropositive patients declined within several months after start of treatment (3, 11). Monitoring of treatment is particularly important in situations where drug doses are not fully optimized and regimens are not well established. A recent elephant TB serology study showed that the antibody levels measured by enzyme-linked immunosorbent assay (ELISA) using M. bovis culture filtrate changed during treatment (12). In agreement with that observation, the MAPIA data presented here demonstrate that IgG to particular proteins of M. tuberculosis can be a sensitive marker for monitoring anti-TB treatment. Some antigens were more informative than others for predicting treatment outcomes, and they were not the same in all treated elephants. Therefore, the kinetics of antibody responses should be analyzed on a case-by-case basis for each antigen used in MAPIA.
As point-of-care diagnostics have demonstrated certain advantages, novel assay formats for infectious diseases developed in recent years become increasingly attractive. Therefore, the RT for detection of TB in elephants (ElephantTB STAT-PAK kit) was recently designed by Chembio Diagnostic Systems, Inc., and was used in the present study to evaluate its potential as a first-line TB screening tool. The RT is a disposable device that employs lateral-flow technology and a unique cocktail of recombinant antigens of M. tuberculosis and/or M. bovis to detect specific antibodies of three immunoglobulin classes, IgG, IgM, and IgA. The RT requires only 30 μl of serum, plasma, or whole-blood sample and provides results within 20 min. The RT is stable at room temperature for up to 18 months and requires no laboratory environment or skilled personnel, as it is easy to perform and read. If necessary, this assay can be supplemented by use of an optical reading instrument that would overcome the issue of subjectivity of visual evaluation and allow a higher degree of test standardization, generating quantitative results, establishing numerical cutoffs, and archiving data. In the present study, the RT showed the potential for early antibody detection in African and Asian elephants infected with M. tuberculosis or M. bovis. In one infected animal, the RT revealed seroconversion 6 months before MAPIA did, presumably due to IgM detection. Thus, the RT may be the most suitable screening tool to improve elephant TB diagnosis and surveillance.
In addition to the five TB cases, we tested 95 sera from ten culture-negative elephants (1 to 22 samples from each animal) collected between October 1986 and January 2006. No antibody was detected in any sample. Importantly, four of the ten elephants were confirmed negative controls, as they either died or were euthanized for various reasons and no evidence of TB was found at necropsy. In one of these elephants, Mycobacterium intracellulare was isolated from the lungs. Although further evaluation of the immunoassays will require testing greater numbers of elephants, the absence of false-positive results by MAPIA or the RT in the present study suggests high serodiagnostic specificity.
A multiantigen ELISA for elephant TB was described recently (12). In that study, three preparations of M. bovis (heat-inactivated culture filtrate, bovine purified protein derivative, and protein MPB70), two lipoarabinomannan antigens purified from virulent (Erdman) and avirulent (H37Ra) strains of M. tuberculosis, and avian purified protein derivative produced from M. avium were used. Among these antigens, the M. bovis culture filtrate showed the highest degree of serological discrimination between seven M. tuberculosis culture-positive and 40 negative elephants. The calculated sensitivity of the ELISA was 100%, and the specificity was 95%. The authors of that report recognized that “only animals that were actively shedding M. tuberculosis were used as confirmed positives, and the degree of seroreactivity in nonclinical, nonshedding, infected elephants is unknown”. In the present study, we tested multiple serum samples collected long before positive culture results were obtained. In two elephants (K and N), for which earlier serum samples were available, retrospective testing revealed evident seroconversions. Most importantly, the antibody responses could be detected in infected elephants several years prior to shedding stages, demonstrating that serological assays might prove useful for early detection of TB in elephants. Antibody-based tests are unlikely to replace culture methods, as isolation of the pathogen from infected animals will always be important to confirm the diagnosis, identify the strain, and collect drug susceptibility data (23). However, management and control of TB in elephants and other nondomestic species would greatly benefit from early and rapid serodiagnosis. The cost of delayed diagnosis of TB in elephants may be extremely high. Elephants with undetected active TB may pose a serious threat of infection to other elephants, other animal species, and humans. Delayed diagnosis of TB in humans (>90 days) was found to increase rates of transmission to contacts (8) while placing the contacts at higher risk of infection. Therefore, earlier recognition of disease in elephants followed by immediate and appropriate interventions may prevent further spreading of M. tuberculosis.
Early stages of TB infection are associated with high expression of ESAT-6 (1), while the immune response to this antigen is known to correlate with progression and severity of disease (6, 32). In the present study, antibody to ESAT-6 was found in all culture-positive elephants by the time of diagnosis. Importantly, the response declined to baseline levels in those that appeared to be cured while remaining at high levels in those whose treatment turned out to be unsuccessful. The MAPIA predicted therapy failure in two elephants (L and K), as demonstrated by the sharp increase of antibody levels observed shortly before diseased animals started to shed organisms again. In human studies, higher rates of relapse after completion of therapy were found in TB patients who had prematurely suspended treatment (14). The other two treated elephants (C and I) produced MAPIA patterns predictive of cured TB without any relapse for several years after completion of therapy. One of these elephants was euthanized because of another disease and no evidence of TB was found at necropsy, whereas the other is alive and well at the time of this writing (over 4 years after treatment).
In summary, the present work improves our understanding of the immunobiology of elephant TB. We report here that elephants diagnosed with M. tuberculosis produce robust antibody responses to multiple antigens long before positive cultures can be detected from trunk washes. These findings open the possibility of serological application for earlier detection of TB in elephants. We identified the key antigens of M. tuberculosis recognized by elephant IgG antibodies. We demonstrated the potential for the RT as an efficient TB screening tool and for MAPIA as a useful approach for treatment monitoring. Further, MAPIA utilizing antigens that are highly specific for the M. tuberculosis complex can constitute a confirmatory test, in addition to the RT, to avoid false-positive results due to potential cross-reactivity with non-TB mycobacteria. The use of such immunoassays will make it possible to identify infected elephants with high accuracy and start treatment at the earliest stages of disease, long before shedding, potentially leading to improved cure rates, decreased likelihood for development of MDR strains, and reduced transmission of infection.
Acknowledgments
We are grateful to Mark Chambers for providing native MPB83 protein and to Raymond Houghton for providing recombinant Mtb8 and Mtb48 proteins.
This work was supported by Busch Gardens Tampa Bay and Chembio Diagnostic Systems, Inc.
REFERENCES
- 1.Andersen, P., M. E. Munk, J. M. Pollock, and T. M. Doherty. 2000. Specific immune-based diagnosis of tuberculosis. Lancet 356:1099-1104. [DOI] [PubMed] [Google Scholar]
- 2.Bothamley, G. H. 1995. Serological diagnosis of tuberculosis. Eur. Respir. J. 8(Suppl.):676s-688s. [PubMed] [Google Scholar]
- 3.Bothamley, G. H., R. Rudd, F. Festenstein, and J. Ivanyi. 1992. Clinical value of the measurement of Mycobacterium tuberculosis specific antibody in pulmonary tuberculosis. Thorax 47:270-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brusasca, P. N., R. L. Peters, S. L. Motzel, H. J. Klein, and M. L. Gennaro. 2003. Antigen recognition by serum antibodies in non-human primates experimentally infected with Mycobacterium tuberculosis. Comp. Med. 53:165-172. [PubMed] [Google Scholar]
- 5.Davidow, A., G. V. Kanaujia, L. Shi, J. Kaviar, X. Guo, N. Sung, G. Kaplan, D. Menzies, and M. L. Gennaro. 2005. Antibody profiles characteristic of Mycobacterium tuberculosis infection state. Infect. Immun. 73:6846-6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Demissie, A., E. M. Leyten, M. Abebe, L. Wassie, A. Aseffa, G. Abate, H. Fletcher, P. Owiafe, P. C. Hill, R. Brookes, G. Rook, A. Zumla, S. M. Arend, M. Klein, T. H. Ottenhoff, P. Andersen, and T. M. Doherty. 2006. Recognition of stage-specific mycobacterial antigens differentiates between acute and latent infections with Mycobacterium tuberculosis. Clin. Vaccine Immunol. 13:179-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garg, S. K., R. P. Tiwari, D. Tiwari, R. Singh, D. Malhotra, V. K. Ramnani, G. B. Prasad, R. Chandra, M. Fraziano, V. Colizzi, and P. S. Bisen. 2003. Diagnosis of tuberculosis: available technologies, limitations, and possibilities. J. Clin. Lab. Anal. 17:155-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Golub, J. E., S. Bur, W. A. Cronin, S. Gange, N. Baruch, G. W. Comstock, and R. E. Chaisson. 2006. Delayed tuberculosis diagnosis and tuberculosis transmission. Int. J. Tuberc. Lung Dis. 10:24-30. [PubMed] [Google Scholar]
- 9.Greenwald, R., J. Esfandiari, S. Lesellier, R. Houghton, J. Pollock, C. Aagaard, P. Andersen, R. G. Hewinson, M. Chambers, and K. Lyashchenko. 2003. Improved serodetection of Mycobacterium bovis infection in badgers (Meles meles) using multiantigen test formats. Diagn. Microbiol. Infect. Dis. 46:197-203. [DOI] [PubMed] [Google Scholar]
- 10.Houghton, R. L., M. J. Lodes, D. C. Dillon, L. D. Reynolds, C. H. Day, P. D. McNeill, R. C. Hendrickson, Y. A. W. Skeiky, D. P. Sampaio, R. Badaro, K. P. Lyashchenko, and S. G. Reed. 2002. Use of multiepitope polyproteins in serodiagnosis of active tuberculosis. Clin. Diagn. Lab. Immunol. 9:883-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imaz, M. S., and E. Zerbini. 2000. Antibody response to culture filtrate antigens of Mycobacterium tuberculosis during and after treatment of tuberculosis patients. Int. J. Tuberc. Lung Dis. 4:562-569. [PubMed] [Google Scholar]
- 12.Larsen, R. S., M. D. Salman, S. K. Mikota, R. Isaza, R. J. Montali, and J. Triantis. 2000. Evaluation of a multiple-antigen enzyme-linked immunosorbent assay for detection of Mycobacterium tuberculosis infection in captive elephants. J. Zoo Wildl. Med. 31:291-302. [DOI] [PubMed] [Google Scholar]
- 13.Lewerin, S. S., S. L. Olsson, K. Eld, B. Roken, S. Ghebremichael, T. Koivula, G. Kallenius, and G. Bolske. 2005. Outbreak of Mycobacterium tuberculosis infection among captive Asian elephants in a Swedish zoo. Vet. Rec. 156:171-175. [DOI] [PubMed] [Google Scholar]
- 14.Lopez-Cortes, L. F., A. Marin-Niebla, L. E. Lopez-Cortes, I. Villanego, M. Rodriguez-Diez, and R. Pascual-Carrasco. 2005. Influence of treatment and immunological recovery on tuberculosis relapses in HIV-infected patients. Int. J. Tuberc. Lung Dis. 9:1385-1390. [PubMed] [Google Scholar]
- 15.Lyashchenko, K., R. Colangeli, M. Houde, H. A. Jahdali, D. Menzies, and M. L. Gennaro. 1998. Heterogenous antibody responses in tuberculosis. Infect. Immun. 66:3936-3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lyashchenko, K. P., J. M. Pollock, R. Colangeli, and M. L. Gennaro. 1998. Diversity of antigen recognition by serum antibodies in experimental bovine tuberculosis. Infect. Immun. 66:5344-5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lyashchenko, K. P., M. Singh, R. Colangeli, and M. L. Gennaro. 2000. A multi-antigen print immunoassay for the serological diagnosis of infectious diseases. J. Immunol. Methods 242:91-100. [DOI] [PubMed] [Google Scholar]
- 18.Lyashchenko, K., A. O. Whelan, R. Greenwald, J. M. Pollock, P. Andersen, R. G. Hewinson, and H. M. Vordermeier. 2004. Association of tuberculin-boosted antibody responses with pathology and cell-mediated immunity in cattle vaccinated with Mycobacterium bovis BCG and infected with M. bovis. Infect. Immun. 72:2462-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maslow, J. N., S. K. Mikota, M. Zhu, R. Isaza, L. R. Peddie, F. Dunker, J. Peddie, H. Riddle, and C. A. Peloquin. 2005a. Population pharmacokinetics of isoniazid in the treatment of Mycobacterium tuberculosis among Asian and African elephants (Elephas maximus and Loxodonta africana). J. Vet. Pharmacol. Ther. 28:21-27. [DOI] [PubMed] [Google Scholar]
- 20.Maslow, J. N., S. K. Mikota, M. Zhu, H. Riddle, and C. A. Peloquin. 2005b. Pharmacokinetics of ethambutol (EMB) in elephants. J. Vet. Pharmacol. Ther. 28:321-323. [DOI] [PubMed] [Google Scholar]
- 21.Michalak, K., C. Austin, S. Diesel, M. J. Bacon, P. Zimmerman, and J. N. Maslow. 1998. Mycobacterium tuberculosis infection as a zoonotic disease: transmission between humans and elephants. Emerg. Infect. Dis. 4:283-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mikota, S. K., R. S. Larsen, and R. J. Montali. 2000. Tuberculosis in elephants in North America. Zoo Biol. 19:393-403. [Google Scholar]
- 23.Mikota, S. K., L. Peddie, J. Peddie, R. Isaza, F. Dunker, G. West, W. Lindsay, R. S. Larsen, M. D. Salman, D. Chatterjee, J. Payeur, D. Whipple, C. Thoen, D. S. Davis, C. Sedgwick, R. J. Montali, M. Ziccardi, and J. Maslow. 2001. Epidemiology and diagnosis Mycobacterium tuberculosis in captive Asian elephants (Elephas maximus). J. Zoo Wildl. Med. 32:1-16. [DOI] [PubMed] [Google Scholar]
- 24.Montali, R. J., S. K. Mikota, and L. I. Cheng. 2001. Mycobacterium tuberculosis in zoo and wildlife species. Rev. Sci. Tech. Off. Int. Epizoot. 20:291-303. [DOI] [PubMed] [Google Scholar]
- 25.National Tuberculosis Working Group for Zoo & Wildlife Species. 2003. Guidelines for the control of tuberculosis in elephants. Washington, D.C. United States Department of Agriculture, Animal and Plant Health Inspection Service, Washington, D.C. [Online.] www.aphis.usda.gov/ac/Elephant/TB2003.pdf.
- 26.Oh, P., R. Granich, J. Scott, B. Sun, M. Joseph, C. Stringfield, S. Thisdell, J. Staley, D. Workman-Malcolm, L. Borenstein, E. Lehnkering, P. Ryan, J. Soukup, A. Nitta, and J. Flood. 2002. Human exposure following Mycobacterium tuberculosis infection of multiple animal species in a metropolitan zoo. Emerg. Infect. Dis. 8:1290-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Payeur, J. B., J. L. Jarnagin, J. G. Marquardt, and D. L. Whipple. 2002. Mycobacterial isolations in captive elephants in the United States. Ann. N. Y. Acad. Sci. 969:256-258. [DOI] [PubMed] [Google Scholar]
- 28.Pollock, J. M., M. D. Welsh, and J. McNair. 2005. Immune response in bovine tuberculosis: towards new strategies for the diagnosis and control disease. Vet. Immunol. Immunopathol. 18:37-43. [DOI] [PubMed] [Google Scholar]
- 29.Schluger, N. M. 2003. The diagnosis of tuberculosis: what's old, what's new. Semin. Respir. Infect. 18:241-248. [DOI] [PubMed] [Google Scholar]
- 30.Sharma, S. K., and A. Mohan. 2004. Multidrug-resistant tuberculosis. Indian J. Med. Res. 120:354-376. [PubMed] [Google Scholar]
- 31.Thoen, C. O., K. Mills, and M. P. Hopkins. 1980. Enzyme-linked protein A: an enzyme-linked immunosorbent assay reagent for detecting antibodies in tuberculous exotic animals. Am. J. Vet. Res. 41:833-835. [PubMed] [Google Scholar]
- 32.Vordermeier, H. M., M. A. Chambers, P. J. Cockle, A. O. Whelan, J. Simmons, and R. G. Hewinson. 2002. Correlation of ESAT-6-specific gamma interferon production with pathology in cattle following Mycobacterium bovis BCG vaccination against experimental bovine tuberculosis. Infect. Immun. 70:3026-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waters, W. R., M. V. Palmer, J. P. Bannantine, D. L. Whipple, R. Greenwald, J. Esfandiari, P. Andersen, J. McNair, J. M. Pollock, and K. P. Lyashchenko. 2004. Antigen recognition by serum antibodies in white tailed-deer (Odocoileus virginianus) experimentally infected with Mycobacterium bovis. Clin. Diagn. Lab. Immunol. 11:849-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Waters, W. R., M. V. Palmer, J. P. Bannantine, R. Greenwald, J. Esfandiari, P. Andersen, J. McNair, J. M. Pollock, and K. P. Lyashchenko. 2005. Antibody responses in reindeer (Rangifer tarandus) infected with Mycobacterium bovis. Clin. Diagn. Lab. Immunol. 12:727-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu, M., J. N. Maslow, S. K. Mikota, R. Isaza, F. Dunker, H. Riddle, and C. A. Peloquin. 2005. Population pharmacokinetics of pyrazinamide in elephants. J. Vet. Pharmacol. Ther. 28:403-409. [DOI] [PubMed] [Google Scholar]