Abstract
Vaccine trials with infants enrolled between 6 and 10 weeks of age (young infants) and 6 and 8 months of age (older infants) provided an opportunity to evaluate immunoglobulin G (IgG) isotype distribution and avidity maturation as indicators of antibody function and immunologic memory. Following vaccination with a strain-specific outer membrane vaccine, MeNZB, pre- and postvaccination sera were used to determine IgG isotype responses and avidity indices (AI) in subsets of vaccinated subjects. Measurements of IgG isotypes involved 100 infants from each trial. AI were measured in 50 infants from the young infant trial who received a fourth vaccine dose and in 40 older infants from whom serum was collected 7 months after the primary vaccination course. IgG1 and IgG3 dominated the responses to the vaccine. A modest linear correlation (P < 0.001) occurred between serum bactericidal antibody (SBAb) titers and the total IgG or the IgG1 antibody units in older infants. The young infants showed a modest linear correlation between SBAb and total IgG (P = 0.005) and a weak linear correlation between SBAb and IgG1 (P = 0.003). Increased avidity with age was demonstrated in both groups. The AI in the young infants increased from 51.5% (95% confidence interval [CI], 47.7 to 54.7) postvaccination to 68.7% (95% CI, 65.5 to 71.9%) following the fourth dose of vaccine (P < 0.001). The mean avidity of the older infants increased significantly (P = 0.00012) from 42.4% (95% CI, 39.1 to 45.3%) postvaccination to 50.4% (95% CI, 47.2 to 53.6%) 4 months later. A fourth dose of MeNZB is now being given to young infants at 10 months of age.
A strain-specific outer membrane vesicle vaccine formulation (MeNZB) containing 25 μg of protein antigens and alum as an adjuvant was developed in response to an epidemic of meningococcal disease caused by group B meningococci with the P1.7-2,4 PorA protein (8, 14). Blind observer, randomized controlled trials were conducted with young and older infants, toddlers, children, and adults to evaluate safety, reactogenicity, and immunogenicity of the vaccine (13, 14). The New Zealand clinical trials of the vaccine MeNZB were conducted using representative age group cross sections of the population in an area where rates of meningococcal disease were highest. Vaccinees in the older infant trial received 25 μg of MeNZB/dose and controls received Menjugate (14). Both vaccines were given as a three-dose schedule, with 6 weeks between doses. For measurement of antibody persistence, a subset (n = 40) of the older infants had a serum sample taken 7 months after the third vaccine dose. In the young infant trial, subjects received 25 μg of MeNZB/dose, concurrent with the vaccines in the childhood routine immunization schedule. Thus, the first two doses of MeNZB were given 6 weeks apart, and the third dose was given 8 weeks later at a minimum age of 5 months. Controls received only the routine childhood vaccines. A subset of 50 infants was given a fourth dose of vaccine at about 10 months of age.
The primary immunogenicity outcome measure for all trials was the induction of a serum bactericidal antibody (SBAb) response against Neisseria meningitidis serogroup B strain NZ98/254 (vaccine strain) using a validated serum bactericidal assay (7, 14). The requirement to achieve a titer of ≥1:8 for a seroresponse was based on the finding that serum antibody titer results below 1:4 lacked reproducibility and had a high coefficient of variation (7). Titrations of sera started at 1:2, and all sera with <50% killing at a 1:4 dilution were assigned a titer of 1:2, requiring the postvaccination serum to reach a minimum titer of 1:8 to achieve a fourfold rise (seroresponse) in SBAb (7). The secondary outcome measurement was a rise in total immunoglobulin G (IgG) antibodies measured using vesicles from the vaccine strain for antibody capture in an enzyme-linked immunosorbent assay (ELISA). The vaccine trials demonstrated that >70% of older infants, toddlers, and schoolchildren attained a fourfold SBAb rise in titer from their baseline and more than 90% achieved a SBAb titer of ≥4 against the vaccine strain NZ98/254 (14). A lower SBAb seroresponse rate (titer of ≥8) postvaccination of 53% (95% confidence interval [CI], 46 to 59%) and a rate with a titer of ≥4 of 76% (95% CI, 70 to 81%) were achieved with the young infants. However, within 6 weeks of a fourth dose of vaccine administered at 10 to 11 months of age, the SBAb antibody levels had increased and 69% (95% CI, 53 to 82%) of young infants had titers of ≥1:8. Demonstration of vaccine safety and acceptable seroresponse rates led to licensure of MeNZB in July 2004 for delivery to all those under 20 years of age in New Zealand (13, 14). A fourth dose of vaccine administered to a subset of 40 young infant trial subjects showed a safety profile similar to that of the primary vaccination schedule (unpublished data).
Following the initial dose of a vaccine, IgM usually precedes IgG antibody formation, although with subsequent vaccine doses, the IgG response is greater. The secondary response differs from the initial antigen priming in that the antibody binds more strongly, described as having a higher affinity (avidity) for the immunizing antigen (18). Variables determining the secondary response include the nature of the antigen and the time between primary and subsequent immunizations. To examine the characteristics of the immune responses to MeNZB in both the young and older infants, subsets of sera were used to determine IgG isotype distribution, antibody avidity postvaccination, the decay of antibodies following vaccination, and the impact of a fourth dose of vaccine on antibody levels.
MATERIALS AND METHODS
SBAb measurement.
A validated serum bactericidal assay was used in the vaccine trials to determine the serum bactericidal antibody responses to the vaccine strain NZ98/254 as previously described (7). Human serum complement, predetermined as suitable for strain NZ98/254, was used in the assay. Titers were reported as the reciprocal of the antibody dilution at the point where the serum antibody curve intersected the 50% kill/survival point (7). Results obtained for each serum during the vaccine trials were used in this study for correlation analyses with IgG isotypes and avidity results.
IgG isotype determination.
IgG isotype responses were measured in 100 vaccinees from the 260 young infants involved in the trial and in 100 vaccinees from the 233 older infants involved in the trial. Included in the 100-young-infant test group was the subset of 50 infants who had been given a fourth dose of vaccine 4 months after the primary vaccine schedule. Sera collected from these 50 infants prior to and 4 to 6 weeks following this fourth vaccination dose were also tested. The remaining 50 subjects were selected at random from those showing some IgG response to the vaccine outer membrane vesicle (OMV), but inclusion was based on there being sufficient serum available for the testing. With the older infant study, the 40 infants who had had a follow-up bleed 4 months after completion of the primary vaccination series were included. The remaining 60 infant sera were selected as described for the young infant trial.
NUNC-maxisorp (catalog no. 430341) 96-well microtiter plates were coated with 50 μl of the 4-μg/ml OMV preparation of strain NZ98/254 (vaccine strain) and held at 4°C for 48 h. PBS-Tw (phosphate-buffered saline [PBS], pH 7.4, containing 0.05% Tween 20) was used for all washing steps. Immediately prior to use, plates were washed and then blocked for 60 min at 37°C using 100 μl of PBS-Tw containing 1% skim milk powder (PBS-Tw-Sk)/well. Each serum sample was diluted (1:200) in PBS-Tw-Sk in duplicate, and 50 μl of each sample was added to the appropriate wells of a microtiter plate. Multiple serum samples for each individual were measured in the same assay. Strongly positive, weakly positive, and negative control serum samples obtained from adults were included on every test plate. Plates were incubated for 90 min at 37°C before being washed three times prior to the addition of 50 μl mouse anti-human IgG isotypes at dilutions shown by prior testing to give an optical density (OD) of 1.0 (Sigma I9388 clone HP6001 for IgG1, Sigma I9513 clone HP6002 for IgG2, Sigma I7260 clone HP6050 for IgG3, and Sigma I5885 clone GG5 for total IgG) to the appropriate wells. Following incubation (90 min, 37°C), the plates were washed, incubated with 50 μl goat anti-mouse IgG peroxidase conjugate (Sigma A0168) for 90 min at 37°C, and then washed again. TMB (3,3′,5,5′ tetramethylbenzidine) substrate (50 μl) was then added, and the reaction was stopped after 15 min with 50 μl of 2.5 M H2SO4 per well. Plates were read with a Labsystems Multiskan Ascent plate reader at 450 nm, with a reference wavelength of 540 nm. IgG indirect ELISA antibody unit values (ELISA units) were obtained by determining the mean OD value of the duplicate test samples tested at a 1:200 dilution. To adjust for interplate variation, a correction factor was applied to each result. This was calculated as the average OD for the positive control serum measured on all plates tested at the same time divided by the average OD for the positive control serum in that test plate. For acceptability of a test, the intra-assay variability of the control sera was required to be <5% and the interassay variability was required to be <15%. The standard deviation and coefficient of variation for reference sera in each set of assays were monitored using a Shewhart chart. Duplicate tests for each serum sample were required to have a variance of <0.5.
AI determination.
For avidity index (AI) testing, sera available from the subset of 50 young infants and 40 older infants, as described for IgG isotype testing, were used. Measurement of antibody avidity involves the use of chaotropic agents, such as ammonium sulfate, sodium thiocyanate, or urea, that cause dissociation of antibody-antigen complexes that have low avidity, leaving intact those complexes with high avidity (10). The optimal assay conditions for measuring the AI were initially determined using different concentrations of sodium thiocyanate (NaSCN), ranging from 0 to 3 M with a range of sera. A 1.5 M solution gave good discrimination. This concentration was used in the assays reported. For testing, NUNC maxisorp plates were coated with 50 μl of a 4-μg/ml OMV preparation and blocked as for IgG testing. Starting at a dilution of 1:100, duplicate samples of each serum were diluted in serial twofold dilutions in PBS-Tw-Sk in each of two microtiter plates. Each plate also contained duplicate 1:200 dilutions of strongly positive, weakly positive, and negative control serum samples. Following incubation (90 min, 37°C), plates were washed (three times) with PBS-Tw. To one set of duplicated plates, 50 μl of a 1.5 M NaSCN solution in PBS was added to every well. PBS alone was added to the wells of the other plate. All plates were held at room temperature (15 min) before being washed with PBS-Tw (three times) and incubated (60 min, 37°C) with 50 μl rabbit anti-human IgG conjugated with horseradish peroxidase conjugate. The plates were washed again, and 50 μl of TMB substrate was added. The reaction was allowed to proceed for 15 min before being stopped by the addition of 50 μl of 2.5 M H2SO4 to each well. The plates were read with a Labsystems Multiskan Ascent plate reader at 450 nm, with a reference wavelength of 540 nm. Total IgG antibody unit values were obtained by determining the dilutions that yielded 50% of the maximal optical density for the same serum sample without NaSCN. Samples with antibody unit values beyond the assay's detection limit were retested starting at a higher dilution. The AI was calculated as the percentage of antibodies in a serum sample that remained bound after treatment with sodium thiocyanate and was calculated as follows: AI = (unit value with NaSCN)/(unit value without NaSCN) × 100 (20). Interplate variation with sera analyzed in triplicate in independent assays was monitored using control sera.
Statistical analyses.
Mean IgG isotype unit values were compared using nonparametric rather than parametric analyses, since IgG values are not normally distributed. Mean avidity percentages were compared using repeated measures of analysis of variance. Spearman's rho (ρ) correlation analysis was conducted to assess linear associations among SBAb, AI, and ELISA unit values. Correlations were classified as weak, modest, or strong if the coefficients were from 0 to 0.32, 0.33 to 0.65, or 0.66 to 0.1, respectively. An alpha level of 0.05 was set as the threshold for statistical significance. All analyses were performed using SPSS, version 12.5.
RESULTS
Serum bactericidal antibody results for the subsets used in this study.
All 100 young infants tested received MeNZB. The serum bactericidal antibody seroresponse rate for these young infants was 55% (55/100; 95% CI, 45% to 65%) (Table 1). At day 256, when the fourth vaccine dose was administered, the percentage of infants with a titer of ≥8 had reduced to 14% (7/50; 95% CI, 6% to 27%) but increased to 67% (31/46; 95% CI, 52% to 80%) following the fourth vaccine dose. The geometric mean titers (GMT) pre- and postvaccination and 7 months following the third dose are given in Table 1.
TABLE 1.
Serum bacterial antibody seroresponse rates for young infant subset
| Expt time point (day) | % of samples with titer of ≥1:8 (no. of samples/total no. of samples) (95% CI) | SBAb GMT (95% CI) |
|---|---|---|
| Prevaccination (1) | 5 (5/100) (2-11) | 1.26 (1.11-1.44) |
| Postvaccination (151) | 55 (55/100) (45-65) | 10 (7.57-14) |
| Pre-4th dose (256) | 14 (7/50) (6-27) | 2.23 (1.63-3.06) |
| Post-4th dose (298) | 67 (31/46) (52-80) | 21 (12-37) |
Of the 100 subjects selected in the older infant group, 91% (95% CI, 83% to 96%) were seroresponders postvaccination. Seven months after the third dose, only 5 (12.5%; 95% CI, 4 to 27%) of the 40 subjects tested who had received MeNZB vaccine had a titer of ≥8 (Table 2).
TABLE 2.
Serum bacterial antibody seroresponse rates for older infant subset
| Expt time point (day) | % of samples with titer of ≥1:8 (no. of samples/total no. of samples) (95% CI) | SBAb GMT (95% CI) |
|---|---|---|
| Prevaccination (1) | 1 (0/100) (0-4) | 1.02 (0.99-1.05) |
| Postvaccination (113) | 91 (91/100) (84-96) | 24 (20-29) |
| 7 mo postvaccination | 13 (5/40) (4-27) | 2.36 (1.61-3.46) |
IgG isotype distribution.
The percent coefficient of variation for reproducibility of results over all tests performed was determined to be 10.86 for the weakly positive control and 8.54 for the strongly positive control. For repeated IgG subtype determinations on the same sample, the average coefficient of variation was 13.9%.
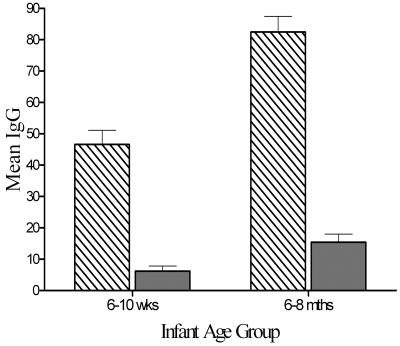
Following the primary three-dose vaccination series, the mean total IgG activity of 138.3 U, as measured at a dilution of 1:200, was significantly (P < 0.0001) higher in the older infant group than in the young infant group (117.9 U). At the same time point, the IgG isotype distribution for both groups (n = 100/group) was similar, with IgG1 unit levels being the highest, followed by much lower levels of IgG3 (Fig. 1). IgG2 levels were mostly undetected, and IgG4 levels were not measured.
FIG. 1.
Isotype distribution for IgG1 and IgG3 in the two infant age groups: 6 to 10 weeks (n = 100) and 6 to 8 months (n = 100). IgG1, bars with diagonal lines; IgG3, shaded bars. Error bars represent standard errors of the means.
Avidity indices.
Only sera with detectable anti-OMV IgG levels postvaccination were used. Avidity indices were calculated for 50 young infants who had a postvaccination bleed and were given a fourth dose of the vaccine 7 months following the primary three-dose series. The mean AI following the three-dose vaccination for the 50 young infants was 51.5% (95% CI, 47.7 to 54.7%). Prior to the fourth dose of vaccine, the AI had increased to 54.0% (95% CI, 50.3 to 57.6%), and 4 weeks after the fourth vaccination, around 11 months of age, the mean AI had increased significantly to 68.7% (95% CI, 65.5 to 71.9%; P < 0.001) (Fig. 2). For the 40 older infants tested, the mean AI postvaccination was 42.4% (95% CI, 39.1 to 45.3). By the visit at 4 to 6 months postvaccination, at around 15 months of age, without a fourth vaccination, the AI had increased significantly (P = 0.00012) to 50.4% (95% CI, 47.2 to 53.6).
FIG. 2.
Comparison of the mean AI (solid line) and the mean total IgG (dashed line) measured in young infants (n = 50). The primary three-dose vaccination series started at age 6 to 10 weeks. For the post series, serum was collected after the primary vaccination series (post-3). Prebooster serum was collected prior to delivery of the fourth dose (pre-4). Postbooster serum was collected at 4 weeks postbooster (post-4).
Correlation analyses.
Spearman's correlation analysis of 40 subjects in the young infant group and the 50 older infants was performed to compare SBAb, the AI, and total IgG ELISA units for those subjects who had shown an increase in SBAb (≥4) following the primary vaccination series. Results for 10 young infant subjects who did not have SBAb titers of ≥4 after the primary vaccination series were not included. Following the primary vaccination series for the young infants, there was a modest linear correlation between SBAb and AI (ρ = 0.33; P = 0.04), but after the fourth vaccine dose, the correlation between SBAb and AI ceased to be statistically significant (ρ = 0.19; P = 0.26). With the older infants, there was a modest linear correlation between SBAb and AI (ρ = 0.447; P = 0.004) but no significant correlation between ELISA levels and AI (ρ = 0.234; P = 0.152).
For these same infants, there was no significant correlation between the total IgG unit levels and AI (ρ = 0.04; P = 0.79), and this lack of correlation remained after the fourth dose. The young infants did show a modest linear correlation between SBAb and total IgG units (ρ = 0.44; P = 0.005) and a weak correlation between SBAb and IgG1 responses (ρ = 0.315; P = 0.003), whereas the older infants showed a modest correlation between SBAb and IgG unit levels (ρ = 0.612; P < 0.001) and between SBAb and IgG1 levels (ρ = 0.609; P < 0.001).
DISCUSSION
A serologic correlate for protection following immunization with group B meningococcal OMV vaccines has not been defined. In the New Zealand trials, seroresponders were defined as those achieving a fourfold rise in serum bactericidal antibody titer postvaccination; a titer of ≥1:8 from a baseline of <1:4 was required (7). SBAb specific for the P1.4 VR2 epitope on the PorA protein of the epidemic strain dominated the immune responses to MeNZB in each of the age group trials (9). In the vaccine trials, the seroresponse rate (53%) following vaccination with MeNZB was lower for the young infants, but following a fourth dose, the rate was similar to that achieved by the older infants after three doses of vaccine. The seroresponse rate (76%) achieved by the older infants was comparable to that achieved by the toddlers and schoolchildren (14). A ≥1:4 SBAb level postvaccination occurred in 92% of toddlers. Elevation of total IgG levels in the OMV ELISA occurred in 98% of both young infants and older infants after the primary vaccination schedule. The results presented in this study represent subpopulations of the two infant trials and therefore show some differences with respect to results reported using the total trial population.
T cells play an important role in the regulation of the immune response, including stimulation of B cells for antibody production. T cells are necessary for the establishment of immunological memory (avidity) and for activation of complement-mediated killing.
Of the four IgG isotypes, designated IgG1, IgG2, IgG3, and IgG4, occurring in human serum, IgG1 and IgG3 have been shown to be the most effective for complement binding and activation of complement-mediated killing of meningococci (1-3, 5, 11, 19). IgG2 is only effective at high epitope density, and IgG4 has not been shown to activate complement. In both infant groups, IgG1 was the predominant class of IgG measured following vaccination with MeNZB. The weak linear correlation (r = 0.315; P = 0.003) between postvaccination SBAb and IgG1 levels in the young infant group and a modest linear correlation in the older infant group (r = 0.609; P < 0.001) are consistent with a role of IgG1 in facilitating complement-mediated lysis of meningococci (11). Naess et al. (12) showed a similar correlation between IgG1 subclass antibody levels and SBAb levels (r = 0.62; P < 0.0001) in adults, although at a more significant level. While de Kleijn et al. (3) showed only a weak correlation between bactericidal levels and the levels of total IgG antibodies or isotype-specific levels (r = 0.2 to 0.64; P < 0.01) in toddlers immunized with the RIVM hexavalent vaccine, Vermont et al. (20) reported a strong correlation between SBAb and IgG1 levels (r = 0.83; P < 0.0001) in a study involving toddlers immunized with a P1.7-2,4 monovalent recombinant OMV vaccine (MonoMen). However, in a study of children (average age, 6.3 years) who were convalescing from meningococcal disease with B:4:P1.7-2,4, Vermont et al. (21) reported that although IgG1, followed by IgG3, dominated the IgG convalescent-phase serum response, serum bactericidal antibodies were not detected against the genetically modified strain of H44/76 expressing P1.7-2,4 PorA. The explanation for this is unclear. In another study, significantly increased levels of IgG1 and IgG3 (P < 0.001) were observed in patients 6 weeks after acute meningococcal disease (19). In that study, Sjursen and coworkers (19) reported that levels of IgG1 and IgG3 were significantly lower (P = 0.03 and 0.04, respectively) in patients admitted within 24 h of disease onset than in those admitted later. The highest levels were demonstrated 2 weeks after acute illness. These same workers also showed that following vaccination with a strain-specific OMV vaccine, the same antibody subclass pattern was induced. Only levels of total IgG and IgG1 increased significantly (P < 0.001) in the first 12 weeks postvaccination, and an increased level of IgG3 was not observed until 6 weeks after the second vaccine dose was given (P = 0.01). The levels of IgG2 and IgG4 were not shown to change with vaccination (19).
High-avidity antibodies have been shown to be superior to low-avidity antibodies for bactericidal activity, and their expression is age dependent (16). Low avidity has been reported in infants following infection with group B meningococci (15, 21). When comparing the characteristics of immune responses induced by meningococcal infection in young children with those detected in children immunized with monovalent P1.7-2,4 OMV vaccine, Vermont et al. (21) showed that the geometric mean AI in convalescent-phase sera (57%) was lower than the geometric mean AI of 73% induced by the monovalent vaccine (21). Those workers concluded that OMV vaccine may induce a better immune response than invasive meningococcal disease in young children. In an alternative study, Vermont et al. (20) reported that the RIVM monovalent OMV vaccine (MonoMen against the PorA P1.7-2,4) induced avidity maturation in toddlers and that there was a modest correlation between the serum bactericidal antibody level and the avidity index.
Our study showed that, in young infants, the AI increased a little from 51.5% postvaccination to 54.0% during the next 5 months without further vaccine doses (Fig. 2) but then increased significantly to 68.7% (P < 0.001) after a fourth dose at around 10 months of age, consistent with maturation of the antibody response (6, 17). Although higher-avidity antibodies have been shown to be more active than lower-avidity antibodies in eliciting complement-mediated bacteriolysis of meningococci, immunologic memory alone is insufficient to protect against development of disease. In the absence of circulating specific antibody, the memory response may take 4 to 7 days after exposure for an adequate antibody response to be mounted (unpublished data). Taking into account that the highest rates of disease occur in very young children (4) and that during the New Zealand trials the lowest seroconversion rates following vaccination with MeNZB occurred in young infants, the New Zealand Ministry of Health has scheduled a fourth dose of vaccine to be given to infants at 10 months of age.
Acknowledgments
Funding for this study was provided under our contract with the Ministry of Health for investigation of immune responses to the vaccine MeNZB.
We acknowledge the involvement of all members of the Vaccine Trials Teams, led by Diana Lennon, UniServices, Auckland University, who delivered the vaccine during the trials and collected the serum samples. We particularly thank Anne Glennie, Lisa McCallum, and Paul Blatchford, ESR staff who were involved with the meningococcal disease vaccine program, although not specifically with this study.
REFERENCES
- 1.Aase, A., E. A. Hoiby, and T. E. Michaelsen. 1998. Opsonophagocytic and bactericidal activity mediated by purified IgG subclass antibodies after vaccination with the Norwegian group B meningococcal vaccine. Scand. J. Immunol. 47:388-396. [DOI] [PubMed] [Google Scholar]
- 2.Aase, A., G. Bjune, E. A. Hoiby, E. Rosenqvist, A. K. Pedersen, and T. E. Michaelsen. 1995. Comparison among opsonic activity, antimeningococcal immunoglobulin G response, and serum bactericidal activity against meningococci in sera from vaccinees after immunization with a serogroup B outer membrane vesicle vaccine. Infect. Immun. 63:3531-3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Kleijn, E., L. van Eijndhoven, C. Vermont, B. Kuipers, H. van Dijken, H. Rumke, R. de Groot, L. van Alphen, and G. van der Dobbelsteen. 2001. Serum bactericidal activity and isotype distribution of antibodies in toddlers and schoolchildren after vaccination with RIVM hexavalent PorA vesicle vaccine. Vaccine 20:352-358. [DOI] [PubMed] [Google Scholar]
- 4.Dyet, K., A. Devoy, R. McDowell, and D. Martin. 2005. New Zealand's epidemic of meningococcal disease described using molecular analysis: implications for vaccine delivery. Vaccine 23:2228-2230. [DOI] [PubMed] [Google Scholar]
- 5.Garred, P., T. E. Michaelsen, and A. Aase. 1989. The IgG subclass pattern of complement activation depends on epitope density and antibody and complement concentration. Scand. J. Immunol. 30:379-382. [DOI] [PubMed] [Google Scholar]
- 6.Longworth, L., R. Borrow, D. Goldblatt, P. Balmer, M. Dawson, N. Andrews, E. Miller, and K. Cartwright. 2002. Avidity maturation following vaccination with a meningococcal recombinant hexavalent PorA OMV vaccine in UK infants. Vaccine 20:2592-2596. [DOI] [PubMed] [Google Scholar]
- 7.Martin, D., L. McCallum, A. Glennie, N. Ruijne, P. Blatchford, J. O'Hallahan, and P. Oster. 2005. Validation of the serum bactericidal assay for measurement of functional antibodies against group B meningococci associated with vaccine trials. Vaccine 23:2218-2221. [DOI] [PubMed] [Google Scholar]
- 8.Martin, D. R., S. J. Walker, M. G. Baker, and D. R. Lennon. 1998. New Zealand epidemic of meningococcal disease identified by a strain with phenotype B:4:P1.4. J. Infect. Dis. 177:497-500. [DOI] [PubMed] [Google Scholar]
- 9.Martin, D. R., N. Ruijne, L. McCallum, J. O'Hallahan, and P. Oster. 2006. The VR2 epitope on the PorA P1.7-2,4 protein is the major target for the immune response elicited by the strain-specific group B meningococcal vaccine MeNZB. Clin. Vaccine Immunol. 13:486-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCloskey, N., M. W. Turner, and D. Goldblatt. 1997. Correlation between the avidity of mouse-human IgG subclass monoclonal antibodies measured by solid-phase elution ELISA and biospecific intraction analysis (BIA). J. Immunol. Methods 205:67-72. [DOI] [PubMed] [Google Scholar]
- 11.Michaelsen, T. E., P. Garred, and A. Aase. 1991. Human IgG subclass pattern of inducing complement-mediated cytolysis depends on antigen concentration and to a lesser extent on epitope patchiness, antibody affinity and complement concentration. Eur. J. Immunol. 2:11-16. [DOI] [PubMed] [Google Scholar]
- 12.Naess, L. M., T. Aarvak, A. Aase, F. Oftung, E. A. Hoiby, R. Sandin, and T. E. Michaelsen. 1999. Human IgG subclass responses in relation to serum bactericidal and opsonic activities after immunization with three doses of the Norwegian serogroup B meningococcal outer membrane vesicle vaccine. Vaccine 17:754-764. [DOI] [PubMed] [Google Scholar]
- 13.O'Hallahan, J., D. Lennon, P. Oster, R. Lane, S. Reid, K. Mulholland, J. Stewart, L. Penney, T. Percival, and D. Martin. 2005. From secondary prevention to primary prevention: a unique strategy that gives hope to a country ravaged by meningococcal disease. Vaccine 23:2197-2201. [DOI] [PubMed] [Google Scholar]
- 14.Oster, P., D. Lennon, J. O'Hallahan, K. Mulholland, S. Reid, and D. Martin. 2005. MeNZB: a safe and highly immunogenic tailor-made vaccine against the New Zealand Neisseria meningitidis serogroup B disease epidemic strain. Vaccine 23:2191-2196. [DOI] [PubMed] [Google Scholar]
- 15.Pollard, A. J., and M. Levin. 2000. Production of low-avidity antibody by infants after infection with serogroup B meningococci. Lancet 356:2065-2066. [DOI] [PubMed] [Google Scholar]
- 16.Pollard, A. J., R. Galassini, E. M. van der Voort, R. Booy, P. Langford, S. Nadel, C. Ison, J. S. Kroll, H. Poolman, and M. Levin. 1999. Humoral immune responses to Neisseria meningitidis in children. Infect. Immun. 67:2441-2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richmond, P., R. Borrow, D. Goldblatt, J. Findlow, S. Martin, R. Morris, K. Cartwright, and E. Miller. 2001. Ability of 3 different meningococcal C conjugate vaccines to induce immunologic memory after a single dose in UK toddlers. J. Infect. Dis. 183:160-163. [DOI] [PubMed] [Google Scholar]
- 18.Sell, S. 1987. Basic immunology: immune mechanisms in health and disease, p. 217-218. Elsevier Science Publishing Co., Amsterdam, The Netherlands.
- 19.Sjursen, H., E. Wedege, E. Rosenqvist, A. Naess, A. Halstensen, and R. Matre. 1990. IgG subclass antibodies to serogroup B meningococcal outer membrane antigens following infection and vaccination. APMIS 98:1061-1069. [DOI] [PubMed] [Google Scholar]
- 20.Vermont, C. L., H. H. van Dijken, C. J. P. van Limpt, R. de Groot, L. van Alphen, and G. P. J. M. van del Dobbelsteen. 2002. Antibody avidity and immunoglobulin G isotype distribution following immunization with a monovalent meningococcal B outer membrane vesicle vaccine. Infect. Immun. 70:584-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vermont, C. L., H. H. van Dijken, R. de Groot, and G. P. J. M. van den Dobbelstein. 2005. Porin A-specific antibody avidity in patients who are convalescing from meningococcal B disease. Pediatr. Res. 58:1-4. [DOI] [PubMed] [Google Scholar]