Abstract
We investigated immunoglobulin G (IgG) subclass antibody responses to Plasmodium falciparum merozoite surface protein 1 (MSP-1) and MSP-2 in 112 malaria-exposed subjects in Brazil. IgG3 polarization was primarily epitope driven, being little affected by cumulative or current exposure to malaria and not affected by a subject's age and Fcγ receptor IIA genotype.
The polarization of antibody responses towards immunoglobulin G1 (IgG1) and IgG3 subclasses, which bind to Fcγ receptors (FcγR) on the surface of monocytes, macrophages, and neutrophils, is believed to play a key role in immunity to blood-stage Plasmodium falciparum infection (2). Cytophilic antibodies mediate parasite-killing responses such as opsonization and phagocytosis of extracellular parasites or parasitized red blood cells (8, 14) and antibody-dependent cellular inhibition of intracellular parasites (2). Antibody-mediated protection is further affected by polymorphism in FcγRIIA, one of the three receptors for human IgG (3). The replacement of arginine (R) with histidine (H) at position 131 defines a FcγRIIA allotype with increased avidity for IgG2 and IgG3; since only FcγRIIA-H131 interacts efficiently with IgG2, this polymorphism may determine whether parasite-specific IgG2 cooperates with effector cells (1). In addition, human monocytes bearing the FcγRIIA-H131 allotype are more efficient in the phagocytosis of IgG3-opsonized parasitized red blood cells (29).
The factors driving IgG subclass switching in P. falciparum-exposed subjects remain poorly characterized (13, 30). Polymorphic domains of two major P. falciparum vaccine candidate antigens, merozoite surface protein 1 (MSP-1) and MSP-2 (10), elicit atypical, IgG3-polarized antibody responses (5, 7, 9, 17, 23, 25, 27, 30), while dimorphic (7) and conserved (5, 30) domains of MSP-1 elicit comparable levels of IgG1 and IgG3 antibodies. Cumulative exposure to malaria (30), a subject's age (28, 30), and FcγR allotype (21) were also shown to affect the IgG subclass distribution of antibodies to MSP-1 and MSP-2 in African populations, but comparable data are not available for other areas of endemicity. Here we investigated patterns and determinants of IgG subclass responses to MSP-1 and MSP-2 in malaria-exposed subjects in Brazil.
We studied 112 adults (78.6% males), aged 18 to 52 (mean, 33.4) years, living in an opencast gold-mining area (Garimpo Satélite) in Mato Grosso, northwestern Brazil. Subjects were mostly migrants living in areas where malaria is endemic for 18.6 years on average (range, 2 to 50 years). This area is characterized by year-round transmission of both P. falciparum and Plasmodium vivax, with an increase in incidence at the end of the rainy season (March to April); the main malaria vector is Anopheles darlingi. The inhabitants of Apiacás experienced, throughout the 1990s, an average of one symptomatic malaria attack each year, and half of the diagnosed malaria infections were due to P. falciparum (11, 26). At the time of the field survey, 17% of the population (n = 527) had malaria parasites, as detected by thick-smear microscopy (11). P. falciparum was detected by microscopy or species-specific, PCR-based amplification of the 18S rRNA gene (20) in 57 plasma donors (50.9%) studied here; the remaining 55 subjects were free of malaria parasites. Subjects presenting with an acute febrile disease and P. falciparum infection (median load, 3,500 parasites per μl of blood; range, <10 to 71,000 parasites/μl) and individuals with P. falciparum infection (median load, 1,400 parasites per μl of blood; range, <10 to 13,275 parasites/μl) but without malaria symptoms at the time of blood collection were analyzed. These subjects were clinically reexamined ≥72 h after the initial parasite detection, and all remained infected (as detected by thick-smear microscopy and PCR) but free of any symptoms. We found no association of frequencies or levels of antibodies with clinical status (data not shown).
Recombinant antigens for enzyme-linked immunosorbent assays (ELISA) included three versions (MAD20, 3D7, and RO33) of polymorphic block 2 of MSP-1 (4), one version (Wellcome) of the conserved C-terminal end of MSP-1 (MSP-119) (19), and six versions (25, AM89, FUP/CP, 3D7, S20, and FC27) of polymorphic blocks 2 and 3 of MSP-2 (31, 32) (Fig. 1A). Except for MSP-119 (expressed in Saccharomyces cerevisiae), antigens were expressed in Escherichia coli fused to Schistosoma japonicum glutathione S-transferase (GST). Antibodies were measured essentially as described previously (25, 31); results for MSP-1-derived antigens were partially reported elsewhere (25). Microplates (Nunc MaxiSorp, Roskilde, Denmark) were coated with 0.1 μg/well of recombinant antigens or GST alone, and test samples were assayed in duplicate (50 μl/well, 1:100 dilution). All mouse monoclonal antibodies used to detect human IgG subclasses, clone HP-6012 for IgG1, HP-6014 for IgG2, HP-6010 for IgG3, and HP-6025 for IgG4 (Sigma, St. Louis, MO), had previously been evaluated for use in ELISA (15). Monoclonal antibody binding was detected with peroxidase-conjugated rabbit anti-mouse Ig (Sigma) followed by o-phenylenediamine and hydrogen peroxide. Reactivity indices (RIs) were calculated as the ratio between the absorbance (measured at 490 nm) of each test sample and a cutoff value for each antigen and IgG subclass (range, 0.1 to 0.3) corresponding to average absorbance for samples from 40 malaria-naïve blood donors from Belo Horizonte (southeastern Brazil) plus 3 standard deviations. Corrected readings (absorbance for recombinant antigen minus absorbance for GST alone) were used to calculate RIs for GST-fused antigens. Positive samples had RIs of >1. Plasma donors were evaluated for FcγRIIA polymorphism (16); frequencies of H131 and R131 alleles were 0.69 and 0.31, respectively.
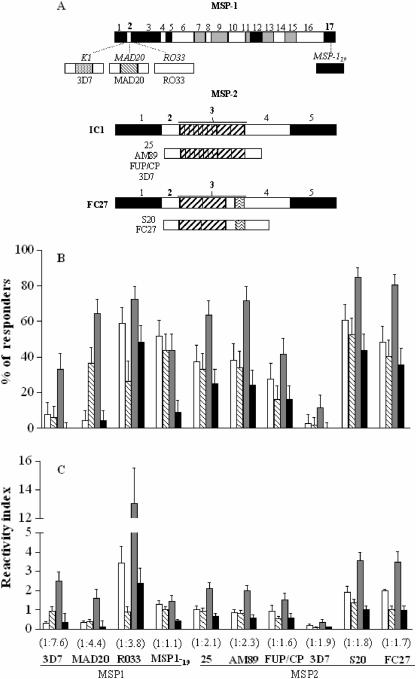
FIG. 1.
(A) Schematic representation of the recombinant antigens used for serological analysis. Variable, semiconserved, and conserved domains of P. falciparum MSP-1 and MSP-2 (10) are shown as white, gray, and black boxes; repetitive domains are indicated by the striped patterns. MSP-1 block 2 antigens represent the three major variants (MAD20, K1, and RO33) of this domain found in local parasites (proportions, 38, 42, and 23%, respectively [25]). MSP-2 antigens represent the central region (blocks 2 and 3) of two major allelic families: four IC1-type variants and two FC27-type variants. Three MSP-2 antigens (25, AM89, and S20) represent “local” variants commonly found in Amazonian isolates of P. falciparum (24, 32), while three “foreign” variants (FUP/CP, 3D7, and FC27) are commonly found on other continents but less frequently in Brazil. (B) Proportions (%) of malaria-exposed adult subjects living in northwestern Brazil with naturally acquired antibodies of each IgG subclass, detected by ELISA, to recombinant antigens derived from P. falciparum MSP-1 and MSP-2. IgG1, IgG2, IgG3, and IgG4 subclass antibodies are represented by white, hatched, gray, and black boxes, respectively. Error bars indicate the upper limit of exact 95% confidence intervals of proportions. For all antigens but MSP-119, the proportion of subjects with detectable IgG3 antibodies significantly exceeds that of subjects with IgG1 antibodies (by the MacNemar test, P was 0.24 for MSP-119 and P was <0.01 for all other antigens). (C) Mean levels of naturally acquired antibodies of each IgG subclass, measured as ELISA reactivity indices, to recombinant antigens derived from P. falciparum MSP-1 and MSP-2 among malaria-exposed adult subjects living in northwestern Brazil. Error bars indicate the upper limit of 95% confidence intervals of means. Note that levels of antibodies to RO33 (a highly conserved MSP-1 block 2 variant) are substantially higher than those to any other antigen tested. The IgG1/IgG3 ratio for each antigen is given in parentheses. For all antigens except MSP-119, levels of IgG3 antibodies significantly exceed those of IgG1 antibodies (by the Wilcoxon test, P was 0.30 for MSP-119 and P was <0.005 for all other antigens).
As expected, antibodies to all antigens except MSP-119 were IgG3 biased, but in contrast to African populations (30), our subjects had antibodies of other IgG subclasses to most antigens detected (Fig. 1B and C). To test whether IgG3 polarization was correlated with cumulative exposure to malaria (measured as the time in years that each individual lived in an area where malaria is endemic), we calculated IgG1/IgG3 RI ratios for each antigen and compared IgG3 versus IgG1 RIs across terciles of cumulative exposure (2 to 10 years, 11 to 20 years, and 22 to 50 years). Levels of IgG3 antibodies to eight antigens significantly exceeded those of IgG1 in all terciles (P < 0.05, Wilcoxon's test), with similar IgG1/IgG3 ratios (ranges, 1:1.2 to 1:2.8 for MSP-2 and 1:3.5 to 1:8.9 for MSP-1 block 2) across exposure strata (data not shown). The exceptions were MSP-119 (no significant excess of IgG3 in any exposure stratum [IgG1/IgG3 ratios between 1:1.1 and 1:1.3]) and 3D7-type MSP-2 (IgG3 excess in only the second and third strata [IgG1/IgG3 ratios, 1:1.9 and 1:4.0]). Accordingly, the 3D7-type MSP-2 variant, relatively rare in local parasites (24, 32), was poorly recognized in this (Fig. 1B and C) and other Amazonian populations (18, 32).
This first comparison of IgG subclass responses in the same population to two P. falciparum vaccine candidate antigens known to elicit IgG3 polarization showed that antibodies to the block 2 domain of MSP-1 are much more biased towards IgG3 than those to MSP-2 (Fig. 1B and C). Since antibodies to the nonrepetitive RO33 variant were also IgG3 biased, the short repeat sequences in MSP-1 are not required for inducing IgG3 polarization. The IgG3/IgG1 ratio for RO33 is the lowest among block 2 antigens (Fig. 1C) but still higher than the corresponding ratio found for MSP-2-derived antigens. The fact that RO33 was recognized in more subjects than other block 2 antigens (MAD20 and K1) is not surprising, since RO33-type variants are highly conserved (no polymorphism found in Brazil) but MAD20-type and K1-type variants display extensive within-family diversity (25). These results implicate MSP-1 block 2, a major target for vaccine development (6, 22), as a useful model to investigate mechanisms underlying IgG subclass polarization in malaria-exposed populations.
We next used multiple linear regression models to investigate the independent contribution of a subject's age, FcγRIIA alleles, and cumulative exposure to malaria to IgG3 polarization. Because IgG3 antibodies are short-lived (9), the presence of current P. falciparum infection was also included as a covariate. We found little or no independent association of these parameters with levels of antibodies of any IgG subclass to most antigens tested (Table 1). Age correlated positively with levels of IgG1 (but not IgG3) antibodies to two MSP-2 variants, leading to a decreased IgG3 bias. However, since only adults were studied, age-related changes in IgG subclass antibody distribution could not be fully evaluated. Cumulative or current exposure to malaria parasites affected levels of IgG1 or IgG3 for only 3 of 10 antigens tested (Table 1). No significant association was found between FcγRIIA polymorphism and levels of any IgG subclass antibody for any antigen, in contrast to data recently reported for African children for IgG2 (21).
TABLE 1.
Factors associated with levels of naturally acquired IgG subclass antibodies to P. falciparum merozoite surface antigens, in multiple linear regression models, among malaria-exposed adult subjects from northwestern Brazil (n = 112)a
| Parameter | Slope (B) | 95% Confidence interval of B | P |
|---|---|---|---|
| Age (yr) associated with: | |||
| Increased levels of IgG1 to 25 (MSP-2) | 0.0281 | 0.002; 0.054 | 0.035 |
| Increased levels of IgG1 to FC27 (MSP-2) | 0.0984 | 0.031; 0.166 | 0.005 |
| Length of exposure to malaria (yr) associated with: | |||
| Increased levels of IgG3 to MAD20 (MSP-1) | 0.0360 | 0.010; 0.070 | 0.022 |
| Current Plasmodium falciparum infection (1 = yes) associated with: | |||
| Increased levels of IgG3 to MAD20 (MSP-1) | 1.122 | 0.222; 2.022 | 0.015 |
| Decreased levels of IgG1 to S20 (MSP-2) | −0.821 | −1.409; −0.233 | 0.0007 |
| Increased levels of IgG3 to FUP/CP (MSP-2) | 1.068 | 0.375; 1.761 | 0.003 |
The independent variables used in multiple linear regression analysis of reactivity indices of each IgG subclass antibody to each antigen were as follows: age (in years; continuous variable), length of exposure to malaria (in years of residence in an area where malaria is endemic), presence of current P. falciparum infection (1 = yes), and presence of the FcγRIIA allele (1 = yes). FcγRIIA polymorphism was entered in separate models as genotype data (H131/H131, H131/R131, and R131/R131) with quite similar results. Only variables associated with P values of <0.05 are shown. Analyses were made with version 13.0 of the SPSS software (SPSS Inc., Chicago, IL).
We conclude that the IgG subclass distribution of naturally acquired antibodies to P. falciparum merozoite surface proteins in adults exposed to low to moderate levels of malaria transmission is primarily epitope driven. IgG3 polarization was more evident for polymorphic domains of MSP-1 than those of MSP-2, being little affected by cumulative or current exposure to malaria and not affected by the subject's age and FcγRIIA genotype. These findings have clear implications for the rational design and evaluation of antimalarial vaccines that induce antibody-mediated protection (12).
Acknowledgments
We thank David R. Cavanagh (University of Edinburg, Scotland) and David C. Kaslow (Vical Incorporated, San Diego, CA) for kindly providing MSP-1-derived recombinant antigens.
This work was supported by grants from the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). K.K.G.S. and M.U.F. are recipients of scholarships from CAPES and CNPq, respectively; M.U.F. is currently a visiting scholar at Daniel L. Hartl's laboratory at Harvard University, Cambridge, MA.
REFERENCES
- 1.Aucan, C., Y. Traore, F. Tall, B. Nacro, T. Traore-Leroux, F. Fumoux, and P. Rihet. 2000. High immunoglobulin G2 (IgG2) and low IgG4 levels are associated with human resistance to Plasmodium falciparum malaria. Infect. Immun. 68:1252-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boucharou-Tayoun, H., P. Attanath, A. Sabchareon, T. Chongcuphajaisiddi, and P. Druilhe. 1990. Antibodies that protect humans against Plasmodium falciparum blood stages do not on their own inhibit parasite growth and invasion in vitro, but act in cooperation with monocytes. J. Exp. Med. 172:1633-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braga, E. M., K. K. G. Scopel, N. T. Komatsu, M. da Silva-Nunes, and M. U. Ferreira. 2005. Polymorphism of the Fcγ receptor IIA and malaria morbity. J. Mol. Genet. Med. 1:5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cavanagh, D. R., and J. S. McBride. 1997. Antigenicity of recombinant proteins derived from Plasmodium falciparum merozoite surface protein 1. Mol. Biochem. Parasitol. 85:197-211. [DOI] [PubMed] [Google Scholar]
- 5.Cavanagh, D. R., C. Dobaño, I. M. Elhassan, K. Marsh, A. Elhassan, L. Hviid, E. A. T. G. Kahalil, T. G. Theander, D. Arnot, and J. S. McBride. 2001. Differential patterns of human immunoglobulin G subclass responses to distinct regions of a single protein, the merozoite surface protein 1 of Plasmodium falciparum. Infect. Immun. 69:1027-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conway, D. J., D. R. Cavanagh, K. Tanabe, C. Roper, Z. S. Mikes, N. Sakihama, K. A. Bojang, A. M. Oduola, P. G. Kremsner, D. E. Arnot, B. M. Greenwood, and J. S. McBride. 2000. A principal target of human immunity to malaria identified by molecular population genetic and immunological analyses. Nat. Med. 6:689-692. [DOI] [PubMed] [Google Scholar]
- 7.Da Silveira, L. A., M. L. Dorta, E. A. Kimura, A. M. Katzin, F. Kawamoto, K. Tanabe, and M. U. Ferreira. 1999. Allelic diversity and antibody recognition of Plasmodium falciparum merozoite surface protein 1 during hypoendemic malaria transmission in the Brazilian Amazon region. Infect. Immun. 67:5906-5916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrante, A., L. Kumaratilake, C. M. Rzepczyk, and J. M. Dayer. 1990. Killing of Plasmodium falciparum by cytokine activated effector cells (neutrophilis and macrophages). Immunol. Lett. 25:179-187. [DOI] [PubMed] [Google Scholar]
- 9.Ferrante, A., and C. M. Rzepczyk. 1997. Atypical IgG subclass antibody responses to Plasmodium falciparum asexual blood stage antigens. Parasitol. Today 13:145-148. [DOI] [PubMed] [Google Scholar]
- 10.Ferreira, M. U., M. da Silva-Nunes, and G. Wunderlich. 2004. Antigenic diversity and immune evasion by malaria parasites. Clin. Diagn. Lab. Immunol. 11:987-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fontes, C. J. F. 2001. Malária assintomática em áreas de garimpo no Brasil: estudos de fatores de risco. Ph.D. thesis. Universidade Federal de Minas Gerais, Belo Horizonte.
- 12.Garraud, O., S. Mahanty, and R. Perraut. 2003. Malaria-specific antibody subclass in immune individuals: a key source of information for vaccine design. Trends Immunol. 24:30-34. [DOI] [PubMed] [Google Scholar]
- 13.Garraud, O., R. Perraut, A. Diouf, W. S. Nambei, A. Tall, A. Spiegel, S. Longracre, D. C. Kaslow, H. Jouin, D. Mattei, G. M. Engler, T. B. Nutman, E. M. Riley, and O. Mercereau-Puijalon. 2002. Regulation of antigen-specific immunoglobulin G subclasses in response to conserved and polymorphic Plasmodium falciparum antigens in an in vitro model. Infect. Immun. 70:2820-2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Groux, H., and J. Gysin. 1990. Opsonization as an effector mechanism in human protection against asexual blood stages of Plasmodium falciparum: functional role of IgG subclass. Res. Immunol. 141:529-542. [DOI] [PubMed] [Google Scholar]
- 15.Jefferis, R., C. B. Reimer, F. Skvaril, G. De Lange, N. R. Linge, J. Lowe, M. R. Walker, D. J. Phillips, C. H. Aloisio, T. W. Wells, J. P. Vaerman, C. G. Magnusson, H. Kubagawa, M. Cooper, F. Vertidal, B. Vandvik, J. J. Haaijman, O. Makela, A. Sarnesto, Z. Lando, J. Gergely, E. Rajnavolgyi, G. Laslo, J. Radl, and G. A. Molinaro. 1985. Evaluation of monoclonal antibodies having specificity for human IgG subclasses: results of an IUIS/WHO collaborative study. Immunol. Lett. 10:223-252. [DOI] [PubMed] [Google Scholar]
- 16.Jiang, X. M., G. Arepally, M. Poncz, and S. E. McKenzie. 1996. Rapid detection of the Fc gamma RIIA-H/R 131 ligand-binding polymorphism using an allele-specific restriction enzyme digestion (ASRED). J. Immunol. Methods 199:55-59. [DOI] [PubMed] [Google Scholar]
- 17.Jouin, H., C. Rogier, J. F. Trape, and O. Mercereau-Puijalon. 2001. Fixed, epitope-specific, cytophilic antibody response to the polymorphic block 2 domain of the Plasmodium falciparum merozoite surface antigen MSP-1 in humans living in a malaria-endemic area. Eur. J. Immunol. 31:539-550. [DOI] [PubMed] [Google Scholar]
- 18.Kanunfre, K. A., F. M. Leoratti, E. H. Hoffmann, R. R. Durlacher, A. W. Ferreira, S. L. Moraes-Avila, and M. U. Ferreira. 2003. Differential recognition of Plasmodium falciparum merozoite surface protein 2 variants by antibodies from malaria patients in Brazil. Clin. Diagn. Lab. Immunol. 10:973-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaslow, D. C., G. Hut, and S. Kumar. 1994. Expression and antigenicity of Plasmodium falciparum major merozoite surface protein (MSP1-19) variants secreted from Saccharomyces cerevisae. Mol. Biochem. Parasitol. 63:283-289. [DOI] [PubMed] [Google Scholar]
- 20.Kimura, M., O. Kaneko, Q. Liu, M. Zhou, F. Kawamoto, Y. Wataya, S. Otani, Y. Yamaguchi, and K. Tanabe. 1997. Identification of the four species of human malaria parasites by nested PCR that targets variant sequences in the small subunit rRNA gene. Parasitol. Int. 46:91-95. [Google Scholar]
- 21.Ntoumi, F., L. Flori, P. I. Mayengue, D. W. M. Maya, S. Issifou, P. Deleron, B. Lell, P. G. Kremsner, and P. Rihet. 2005. Influence of carriage of hemoglobin AS and the Fcγ receptor IIa-R131 allele on levels of immunoglobulin G2 antibodies to Plasmodium falciparum merozoite antigens in Gabonese children. J. Infect. Dis. 192:1975-1980. [DOI] [PubMed] [Google Scholar]
- 22.Polley, S. D., K. K. Tetteh, D. R. Cavanagh, R. J. Pearce, J. M. Lloyd, K. A. Bojang, D. M. Okenu, B. M. Greenwood, J. S. McBride, and D. J. Conway. 2003. Repeat sequences in block 2 of Plasmodium falciparum merozoite surface protein 1 are targets of antibodies associated with protection from malaria. Infect. Immun. 71:1833-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rzepczyk, C., K. Hale, N. Woodroffe, A. Bobogare, P. Csurhes, A. Ishii, and A. Ferrante. 1997. Humoral immune responses of Solomon Islanders to the merozoite surface antigen 2 of Plasmodium falciparum show pronounced skewing towards antibodies of the immunoglobulin G3 subclass. Infect. Immun. 65:1098-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salleneve-Sales, S., M. F. Ferreira-da-Cruz, C. P. Faria, C. J. Cerruti, C. T. Daniel-Ribeiro, and M. G. Zalis. 2003. Plasmodium falciparum: limited genetic diversity of MSP-2 in isolates circulating in Brazilian endemic areas. Exp. Parasitol. 103:127-135. [DOI] [PubMed] [Google Scholar]
- 25.Scopel, K. K. G., C. J. F. Fontes, M. U. Ferreira, and E. M. Braga. 2005. Plasmodium falciparum: IgG subclass antibody response to merozoite surface protein-1 among Amazonian gold miners, in relation to infection status and disease expression. Exp. Parasitol. 109:124-134. [DOI] [PubMed] [Google Scholar]
- 26.Scopel, K. K. G., C. J. F. Fontes, A. C. Nunes, M. F. Horta, and E. M. Braga. 2004. Low sensitivity of nested PCR using Plasmodium DNA extracted from stained thick blood smears: an epidemiological retrospective study among subjects with low parasitemia in an endemic area of the Brazilian Amazon region. Malar. J. 3:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor, R. R., D. B. Smith, V. J. Robinson, J. S. McBride, and E. M. Riley. 1995. Human antibody response to Plasmodium falciparum merozoite surface protein 2 is serogroup specific and predominantly of the immunoglobulin G3 subclass. Infect. Immun. 63:4382-4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taylor, R. R., S. J. Allen, B. M. Greenwood, and E. M. Riley. 1998. IgG3 antibodies to Plasmodium falciparum merozoite surface protein 2 (MSP2): increasing prevalence with age and association with clinical immunity to malaria. Am. J. Trop. Med. Hyg. 58:406-413. [DOI] [PubMed] [Google Scholar]
- 29.Tebo, A. E., P. G. Kremsner, and J. F. Luty. 2002. Fcγ receptor-mediated phagocytosis of Plasmodium falciparum-infected erythrocytes in vitro. Exp. Parasitol. 130:300-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tongren, J. E., C. J. Drakeley, S. L. R. McDonald, H. G. Reyburn, A. Manjurano, W. M. M. Nkya, M. M. Lemnge, C. D. Gowda, J. E. Todd, P. H. Corran, and E. M. Riley. 2006. Target antigen, age, and duration of antigen exposure independently regulate immunoglobulin G subclass switching in malaria. Infect. Immun. 74:257-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tonhosolo, R., G. Wunderlich, and M. U. Ferreira. 2001. Differential antibody recognition of four allelic variants of the merozoite surface protein-2 (MSP-2) of Plasmodium falciparum. J. Eukaryot. Microbiol. 48:556-564. [DOI] [PubMed] [Google Scholar]
- 32.Tonon, A. P., E. H. E. Hoffmann, L. A. Da Silveira, A. G. Ribeiro, C. R. S. Gonçalves, P. E. M. Ribolla, G. Wunderlich, and M. U. Ferreira. 2004. Sequence diversity, antibody recognition and evolution of the malaria vaccine candidate antigen merozoite surface protein 2 (MSP2) of Plasmodium falciparum. Exp. Parasitol. 108:114-125. [DOI] [PubMed] [Google Scholar]