Abstract
The gastrointestinal tract (GIT) of ruminants is the main reservoir of enterohemorrhagic Escherichia coli, which is responsible for food-borne infections in humans that can lead to severe kidney disease. Characterization of biotic and abiotic factors that influence the carriage of these pathogens by the ruminant would help in the development of ecological strategies to reduce their survival in the GIT and to decrease the risk of contamination of animal products. We found that growth of E. coli O157:H7 in rumen fluid was inhibited by the autochthonous microflora. Growth was also reduced when rumen fluid came from sheep fed a mixed diet composed of 50% wheat and 50% hay, as opposed to a 100% hay diet. In fecal suspensions, E. coli O157:H7 growth was not suppressed by the autochthonous flora. However, a probiotic strain of Lactobacillus acidophilus inhibited E. coli O157:H7 growth in fecal suspensions. The inhibitory effect was dose dependent. These lactic acid bacteria could be a relevant tool for controlling O157:H7 development in the terminal part of the ruminant GIT, which has been shown to be the main site of colonization by these pathogenic bacteria.
Enterohemorrhagic Escherichia coli (EHEC) is a food-borne pathogen that causes human diseases ranging from uncomplicated diarrhea to hemorrhagic colitis and life-threatening complications, such as the hemolytic-uremic syndrome. Cattle and other ruminants appear to be the main reservoir of EHEC strains. Human contamination occurs mainly through consumption of undercooked ground beef, water, or dairy products contaminated by bovine feces (2). It is clear that on-farm strategies that decrease the carriage of EHEC strains by ruminants would help to reduce the risk of food-borne disease in humans (51).
Experimental infections of ruminants have shown that the shedding of EHEC O157:H7 is a relatively transient event, with a mean duration of 14 days to 1 month (3, 7, 22, 46). Dietary factors can influence the resistance of cattle to transient E. coli O157:H7 colonization. However, studies investigating the effect of diet on the fecal shedding of E. coli O157:H7 are in some conflict due to considerable individual variability (see reference 9 for a review). In some studies, it appeared that hay-fed sheep or cattle shed E. coli O157:H7 longer than grain-fed animals (25, 33, 54). However, high-fiber diets have been reported to clear E. coli O157:H7 completely from the gastrointestinal tract (GIT), in contrast to low-fiber diets (32). Studies that have investigated the digestive location of EHEC in ruminants are also controversial. However, most studies have identified the lower GIT as the site of E. coli O157:H7 persistence and proliferation (34, 54). In experimentally inoculated calves, as well as in naturally infected animals, recent reports describe E. coli O157:H7 colonization at the terminal rectum in an area rich in lymphoid follicles (37, 38).
In vitro studies have shown that E. coli O157:H7 grows poorly in rumen fluid collected from recently fed cattle (within 4 h) and supplemented with glucose and Trypticase. In contrast, in rumen fluid collected 24 or 48 h after feeding and supplemented in the same way, growth of E. coli O157:H7 is rapid (43). However, only trace amounts of hexoses are actually available for E. coli in the rumen fluid (28). Therefore, these conditions were not representative of in vivo conditions. Boukhors et al. have shown in a more physiological model that E. coli O157:H7 persists in undiluted and unsupplemented rumen fluid under anaerobiosis up to 24 h without loss of viability but also without detectable growth (4). Growth of EHEC in fecal media and the influence of the ruminal and fecal autochthonous microflora on EHEC survival have never been investigated.
In an effort to develop methods of reducing or eliminating the carriage of E. coli O157:H7 in cattle on farms, probiotic yeast and bacteria have been evaluated for the capacity to reduce EHEC growth in vitro or EHEC shedding in cattle. Saccharomyces cerevisiae subsp. boulardii eradicated E. coli O157:H7 in clarified rumen fluid but did not show any inhibitory effect in continuous cultures containing the ruminal autochthonous flora (1). Some strains of Lactobacillus spp. have shown bactericidal activity against E. coli O157:H7 grown in complex medium, probably related to lactic acid production and a pH-reducing effect (40). More recently, Brashears et al. reported the isolation from cattle feces of lactic acid bacteria that significantly reduced E. coli O157 counts in sterilized and diluted rumen fluid (6). Several studies have shown that administration of probiotic bacteria as direct-fed microbials decreases fecal shedding of E. coli O157:H7 by ruminants (9, 52). In experimentally infected calves, administration of Streptococcus bovis and Lactobacillus gallinarum induced an increase in volatile fatty acids (VFAs) that correlated with a quick decrease in E. coli O157:H7 fecal shedding (41). In naturally infected finishing cattle, L. acidophilus and Propionibacterium freudenreichii decreased E. coli O157:H7 fecal shedding and contamination on hides without a detrimental effect on performance (5, 15, 57). In lambs experimentally infected with E. coli O157:H7, a mixture of L. acidophilus, Streptococcus faecium, Lactobacillus casei, Lactobacillus fermentum, and Lactobacillus plantarum significantly decreased the shedding of the pathogen and improved the average daily weight gain and gain-to-feed ratio (36). Taken together, studies addressing the use of probiotics as feed supplement indicate that direct feeding of microbials may be an efficient preharvest intervention strategy for reducing E. coli O157:H7 carriage in ruminants and consequently the risk of food-borne diseases in humans.
In this study, we used undiluted rumen fluid without supplementation with purified sugars to investigate the influence of diet and of the autochthonous microflora on the growth of E. coli O157:H7 in in vitro cultures mimicking in vivo conditions. We showed that anaerobiosis, a diet rich in grain, and the autochthonous microflora in rumen fluid exerted a strong inhibitory effect on E. coli O157:H7 growth. In contrast, E. coli O157:H7 growth was not restricted in fecal suspensions containing the autochthonous microflora. However, L. acidophilus BT-1386, a probiotic strain, was shown to be a potent inhibitor of E. coli O157:H7 growth in these fecal suspensions.
MATERIALS AND METHODS
Origin and preparation of digestive samples.
Two rumen-fistulated sheep were used for collection of ruminal and fecal samples. They were first fed with permanent grassland hay (H diet) for 4 weeks; then, after a transition period of 1 week, they received a mixed diet containing hay (50% on a dry-matter basis) and ground wheat (50%) for 2 weeks (HW diet). The diet (1 to 1.1 kg [dry matter]) was distributed twice a day.
Rumen samples were collected from the midventral sac before the morning feeding with both H and HW diets (three different samples were obtained per diet); with the HW diet, additional samples (n = 3) were collected 3 h after feeding. These samples were processed either under strictly anaerobic conditions or without any particular attention paid regarding anaerobiosis. To maintain anaerobiosis, rumen digesta were collected in O2-free, CO2-saturated sterile flasks. Traces of O2 had previously been removed from the CO2 used in all procedures by passage over hot copper filings in a furnace, as described by Hungate (27). Samples were immediately brought to the laboratory, where they were strained through four layers of cheesecloth and pooled in equal proportions (final volume, 300 ml) in sterile flasks under a 100% CO2 atmosphere. Half of the samples were centrifuged (twice for 20 min, 15,000 × g) in order to remove the autochthonous microflora (without flora [WOF]), whereas the other half still contained the flora (with flora [WF]). The efficacy of the centrifugation step was checked by inoculating an aliquot of the clarified sample obtained after centrifugation into a complex agar medium (35) classically used for enumeration of total anaerobic bacteria. The “aerobic” rumen samples were processed following the same filtration-centrifugation steps, except that no precaution was taken regarding anaerobiosis.
Fecal samples were obtained manually from the rectum of the animals fed the H diet at 5 to 6 h after the morning feeding. Ten to 12 grams of fresh feces per animal was collected and immediately suspended in a 0.9% NaCl sterile solution (saline) in order to obtain a final concentration of 0.1 g · ml−1. This fecal suspension was homogenized for 5 min with a Polytron (PT 2000; Kinematica GmbH, Switzerland) at maximum speed to ensure appropriate, even mixing of bacteria throughout each sample. Half of these samples were then centrifuged in screw-cap tubes (twice for 20 min, 15,000 × g) to remove the fecal flora (WOF), whereas the remaining portion was not centrifuged and still contained the flora (WF). Validation of the centrifugation step was done as described above for rumen contents. Half of the samples were processed by the same procedure as for the rumen samples to maintain strictly anaerobic conditions: the fecal material was collected in vials previously filled with CO2, the saline solution was prepared in order to maintain a low redox potential (the solution was boiled for 15 min, cooled under a 100% CO2 atmosphere for 20 min, and then autoclaved at 120°C for 20 min), and centrifugation was done in screw-cap tubes filled with CO2.
Bacterial strain characteristics.
The E. coli O157:H7 strain Sakaï, lacking the stx1 and stx2 genes, was kindly provided by Sasakawa (Department of Bacteriology, University of Tokyo, Tokyo, Japan). A spontaneous nalidixic acid-resistant derivative (Nalr) of this strain was isolated on Luria-Bertani (LB) agar plates containing 40 μg · ml−1 of nalidixic acid and was used throughout this study. L. acidophilus BT-1386 was provided by Lallemand Animal Nutrition (Milwaukee, Wis.). This strain is marketed as Micro-Cell LA (LA), which contains no less than 1 × 1010 CFU g−1 of L. acidophilus BT-1386.
In vitro incubations.
An E. coli O157:H7 (Sakaï Δstx1 stx2 Nalr) overnight aerobic culture in LB broth was diluted around 1,000-fold with O2-free, CO2-saturated sterile saline solution in the “anaerobic” digestive samples, and with sterile saline solution in the “aerobic” digestive samples, to reach approximately 5 × 105 CFU ml−1. Incubations were performed in triplicate in 5 ml of digestive contents for 24 h at 39°C, with gentle shaking, in screw-cap tubes fitted with butyl rubber stoppers (Bellco Glass, Vineland, N.J.). In order to simulate feed intake, 25 mg of ground feed (H or HW) was added to tubes containing ruminal samples, as previously described (16, 30). Cellulolytic bacteria were enumerated in ruminal samples before and after incubation to determine whether strictly anaerobic conditions had been respected during the experimental procedure. They were enumerated in the liquid medium of Halliwell and Bryant (20), in which filter paper cellulose was the energy source. After 2 weeks of incubation at 39°C, most probable numbers of cellulolytic bacteria were determined with McGrady tables (10). O157:H7 counts were enumerated following serial dilutions and plating on LB agar plates supplemented with nalidixic acid (40 μg · ml−1) before and after 24 h of incubation.
When needed, L. acidophilus BT-1386 was added to fecal suspensions to achieve different concentrations, which were first calculated from the concentration that was guaranteed in the commercial product. The true concentration of LA added to each fecal sample was checked by colony counting on lactobacillus enumeration medium from DeMan-Rogosa-Sharpe (MRS) (reference no. 69964; Fluka). In addition, lactic acid bacteria (LAB) were enumerated in fecal samples (WOF and WF) in which LA had been inoculated, or not, after 24 h of incubation at 39°C by direct counting on MRS agar plates.
pH measurement and analyses of simple sugars and fermentation products.
The pH of ruminal and fecal samples was recorded before and after 24 h of incubation. Acetate and d,l-lactate concentrations in ruminal and fecal samples, respectively, were determined by enzymatic methods (R-Biopharm, France). Hexose and pentose concentrations were determined in ruminal samples by the method of Dishe (13).
Statistical analysis.
Student's t test was used to assess significant differences in E. coli O157:H7 growth in fecal suspension with or without autochthonous flora and with or without L. acidophilus. One-way analysis of variance (SAS, version 8) was used to assess the effect of diet on E. coli O157:H7 growth in rumen contents. A linear model (GLM; SAS, version 8) was used to test the effects of diet, autochthonous flora, and oxygen and the interactions between them on E. coli O157:H7 growth.
RESULTS
In vitro growth of E. coli O157:H7 in rumen contents.
To ensure that the centrifugation steps efficiently removed the autochthonous flora from the WOF samples, total levels of anaerobic bacteria were investigated at the beginning of incubation and found to be undetectable, whereas in WF samples they reached an average of 6 × 109 cells · ml−1. To ensure that anaerobiosis was maintained during handling and preparation of the samples, the cellulolytic bacterial community, which includes some of the most oxygen-sensitive organisms in the rumen (17), was enumerated just before incubation. The population levels ranged between 8.9 × 106 and 2.8 × 107 cells · ml−1 and were found to be close to those generally recovered from fresh rumen contents (17).
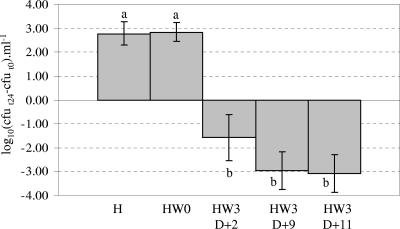
Significant growth of E. coli O157:H7 occurred after 24 h of anaerobic incubation in rumen fluid obtained from sheep fed the H diet and in rumen fluid obtained before feeding from sheep fed the HW diet (HW0) in WOF samples (Fig. 1). Before incubation, the mean pH of these samples was above 6.7 and acetate concentrations were rather low (below 44 mM); after incubation for 24 h, acetate concentrations were not modified (Table 1). When E. coli O157:H7 was inoculated into rumen contents obtained 3 h after HW feeding (HW3), high bacterial mortality was observed (Fig. 1). At the beginning of the incubation period, the mean pH of HW3 rumen fluid was lower and the acetate concentration was higher than the respective values obtained from H or HW0 ruminal samples (Table 1). After 24 h of incubation, the acetate concentration remained comparable to that measured at the beginning of the incubation period. The decline in E. coli O157:H7 counts was progressive and was greatest at the end of the HW feeding period (Fig. 1).
FIG. 1.
Effect of the nature of the diet on growth of E. coli O157:H7 in rumen fluid depleted of autochthonous microflora, under strictly anaerobic conditions. The y axis shows the difference between counts of the EHEC strain after 24 h of incubation (t24) in rumen fluid samples and counts of this strain at the beginning of incubation (t0). Growth is considered to occur if the difference is positive, and mortality is observed if the difference is negative. During the HW diet period, rumen fluid samples were obtained at 2 days (D+2), 9 days (D+9), and 11 days (D+11) after the diet shift. Means and standard deviations of triplicate values are shown; means with different superscripts are significantly different (P < 0.0001).
TABLE 1.
pH and acetate concentration in rumen samples before and after incubation with E. coli O157:H7
| Dieta | Mean acetate concn ± SDb (mM) and pH from indicated sample
|
|||||||
|---|---|---|---|---|---|---|---|---|
| WOF
|
WF
|
|||||||
| Before incubation
|
After a 24-h incubation
|
Before incubation
|
After a 24-h incubation
|
|||||
| Acetate | pH | Acetate | pH | Acetate | pH | Acetate | pH | |
| H | 38.1 ± 2 | 7.1 | 40.3 ± 1.1 | 6.8 | 38.1 ± 2 | 7.1 | 55.3 ± 0.4 | 6.5 |
| HW0 | 43.3 ± 7 | 6.9 | 45.7 ± 7.8 | 5.6 | 43.3 ± 7 | 6.9 | 81.4 ± 6.1 | 5.7 |
| HW3 | 65.7 ± 0.9 | 5.9 | 66.0 ± 1.4 | 5.1 | 65.7 ± 0.9 | 5.9 | 103.0 ± 0.5 | 4.8 |
Rumen samples were obtained from sheep fed hay or from sheep fed hay plus wheat before the morning feeding or 3 h after the morning feeding.
From three independent cultures.
Boukhors et al. (4) did not observe any growth of E. coli O157:H7 in a rumen fluid with characteristics close to those of HW0, without supplementation with ground feed. Therefore, to determine whether ground feed could be used for growth by E. coli O157:H7 in the absence of the autochthonous flora, sugar concentrations were measured. Hexose plus pentose concentration in rumen samples taken from sheep fed hay was 0.150 ± 0.016 mg · ml−1. After addition of 25 mg of ground hay and incubation for 24 h at 39°C, this concentration rose to 0.426 ± 0.027 mg · ml−1, due to the release of sugars from the feed. E. coli O157:H7 apparently utilized these soluble sugars released from ground hay, as there was a dramatic decrease in the hexose-plus-pentose concentration, to 0.030 ± 0.033 mg · ml−1, after 24 h of E. coli O157:H7 growth.
In H and HW0 samples (WOF), in which E. coli O157:H7 growth was observed under anaerobiosis, growth was significantly higher in the presence of oxygen than under strict anaerobiosis (Table 2). In HW3 samples, in which E. coli O157:H7 mortality was observed under anaerobiosis, the presence of oxygen did not restore growth but the bacteria did survive without mortality (data not shown).
TABLE 2.
Effects of the presence of the autochthonous microflora and of oxygen on E. coli O157:H7 growth in rumen fluid obtained from sheep fed hay or hay plus wheat
| Dieta | No. of EHEC cells (log10 · ml−1) at 24 h of incubation − no. of EHEC cells at 0 h of incubation for indicated sampleb
|
|||
|---|---|---|---|---|
| Anaerobiosis
|
Aerobiosis
|
|||
| WOF | WF | WOF | WF | |
| H | 1.74 ± 0.41 | −(0.40 ± 0.43) | 3.65 ± 0.16 | 3.01 ± 0.02 |
| HW0 | 2.16 ± 0.11 | −(1.50 ± 0.56) | 4.00 ± 0.10 | 1.21 ± 0.29 |
| HW0 without substrate | ND | −(0.30 ± 0.1) | ND | ND |
Rumen samples were obtained from sheep fed hay or from sheep fed hay plus wheat before the morning feeding. For each diet, the effect of the presence of oxygen was significant (P < 0.0001), the effect of the presence of autochthonous flora was significant (P < 0.0001), and the effect of the interaction between oxygen and the flora was significant (P < 0.01).
Values are means ± standard deviations from three independent cultures. Growth is considered to have occurred if the difference is positive, and mortality was observed if the difference is negative. ND, not done.
E. coli O157:H7 counts were higher in the absence (WOF) than in the presence (WF) of the ruminal flora, showing a strong inhibitory effect of the autochthonous microflora on E. coli O157:H7 (Table 2). It is noteworthy that the acetate concentration increased during incubation in the presence of the microflora due to its fermentative activity (Table 1). To determine whether other factors besides the increase in VFA concentration and decrease in pH value could account for the inhibitory effect of the flora, the same experiment was repeated without the addition of substrate to the HW0 incubation. Under these conditions, no microflora-mediated fermentation occurred and the pH value and acetate concentration remained quite constant during the incubation. However, in this experiment in which the microflora was maintained, we observed a mortality rate similar to that observed in H samples containing substrate (Table 2), indicating that the microflora induced E. coli O157:H7 mortality by a pH- and VFA-independent mechanism. In agreement with this conclusion, a previous study from our laboratory showed that E. coli O157:H7 persisted for up to 24 h in rumen fluids with similar pH values and acetate concentrations, but without the microflora, without any loss of viability (4). It is noteworthy than in the absence of substrate, the mortality rate was lower than in substrate-containing HW0 (Table 2), suggesting that acidic conditions due to the fermentative activities of the microflora accounted in large part for the mortality rate observed in the presence of substrate.
Statistical analyses showed an interaction between the presence of the microflora and the presence of oxygen, indicating that the inhibition of E. coli O157:H7 growth by the rumen microflora increased under anaerobiosis.
Growth of E. coli O157:H7 in fecal contents.
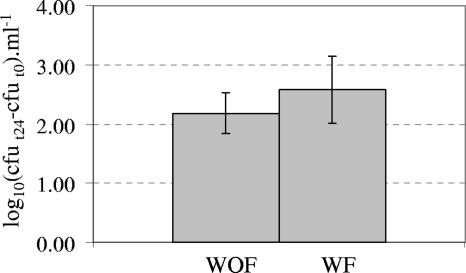
Although we used diluted feces to prepare fecal suspensions for bacterial cultures, total bacterial counts in the suspensions increased from 6.3 × 107 cells · ml−1 at the beginning of the incubation to 1.4 × 108 cells · ml−1 after 7 h and finally to 1 × 109 cells · ml−1 after 24 h. In WOF samples, no bacterial cells were recovered by culture methods at the beginning of the incubation periods. When the E. coli O157:H7 strain was incubated in fecal suspensions obtained from sheep fed the H diet, significant growth was observed within 24 h. Growth was slightly but not significantly favored in the presence of oxygen (data not shown). The fecal flora did not show any inhibitory effect on E. coli O157:H7 growth (Fig. 2).
FIG. 2.
Growth of E. coli O157:H7 in fecal suspensions in the absence or presence of autochthonous flora, under strictly anaerobic conditions. The y axis shows the difference between counts of the EHEC strain after 24 h of incubation (t24) and counts of this strain at the beginning of incubation (t0). Means and standard deviations of counts from triplicate incubations are shown.
Effect of L. acidophilus BT-1386 (LA) on growth of E. coli O157:H7 in fecal suspensions.
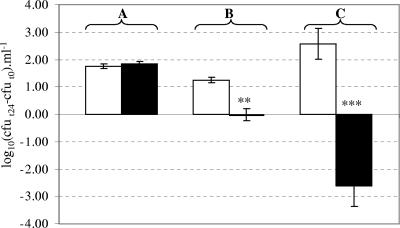
When LA was added to the fecal suspensions containing the autochthonous flora, a dose-dependent effect on E. coli O157:H7 was observed. Whereas no effect was observed with the lowest concentration of LA, E. coli O157:H7 numbers decreased as the concentration of LA increased (Fig. 3); this was particularly marked with the highest concentration of LA (8.5 × 107 CFU ml−1). At the same time, we observed a decrease in pH of the fecal suspensions and an increase in d,l-lactate concentration and in total LAB counts as the concentration of LA increased (Table 3). In the WOF samples, which were supplemented only with 8.5 × 107 CFU ml−1 of LA, a similar decrease in E. coli O157:H7 levels was observed (data not shown) and the pH of the fecal suspensions after incubation was also very low (3.84). Nevertheless, the d,l-lactate concentration was significantly lower (40.33 ± 7.06 mM) than in the WF samples (P < 0.001).
FIG. 3.
Effects of different concentrations of L. acidophilus BT-1386 (LA) counts on growth of the EHEC strain in fecal suspensions (WF), under strictly anaerobic conditions. The y axis shows the difference between counts of the EHEC strain after 24 h of incubation (t24) and counts of this strain at the beginning of incubation (t0). Means and standard deviations of counts from triplicate incubations are shown. (A) White bar, EHEC alone; black bar, EHEC plus 3.4 × 105 CFU ml−1 of LA. (B) White bar, EHEC alone; black bar, EHEC plus 3.2 × 106 CFU ml−1 of LA; **, effect of LA is significant (P < 0.001). (C) White bar, EHEC alone; black bar, EHEC plus 8.5 × 107 CFU ml−1 of LA; ***, effect of LA is significant (P < 0.0001).
TABLE 3.
Lactate concentration, pH, and total LAB concentration after 24 h of incubation in WF fecal suspensions inoculated with E. coli O157:H7 and supplemented or not with L. acidophilus BT-1386 (LA)
| Parameter | Mean ± SDa
|
|||
|---|---|---|---|---|
| Without LA | With LA at indicated concn (CFU · ml−1)
|
|||
| 3.4 × 105 | 3.2 × 106 | 8.5 × 107 | ||
| Lactate concn (mM) | 2.08 ± 0.79 | 3.36 ± 1.86 | 7.02 ± 0.85 | 53.01 ± 4.29 |
| pH | 6.3 | 6.2 | 5.7 | 3.9 |
| Total LAB concn (log10 CFU · ml−1) | 5.43 ± 0.15 | 6.16 ± 0.16 | 6.20 ± 0.35 | 7.39 ± 0.09 |
The effects of three different concentrations of LA were compared, and measurements were performed three times.
DISCUSSION
Although much research has been devoted to highlighting the role of the ruminant digestive tract as a reservoir of EHEC (21) and the impact of the nature of the diet on shedding of naturally occurring or experimentally inoculated O157:H7 strains by cattle (11, 25, 32, 33), very few reports have really focused on the physiology of these bacteria in ruminant digestive tract contents. Yet it is important to understand better how gut environmental conditions are likely to influence growth or survival of these potentially pathogenic bacteria. Most previous studies were done in sterile laboratory media or in diluted sterile rumen fluid supplemented with purified sugars or Trypticase (12, 43, 47, 56). In this report, we describe original in vitro models for growth of EHEC strains in digestive contents directly harvested from ruminants. The rumen fluid is used undiluted and unsupplemented with sugars, and our culture conditions at 39°C under anaerobiosis allow growth of the autochthonous flora. Although fecal samples must be diluted to obtain liquid media relevant for bacterial growth, the autochthonous flora reach physiological levels within 24 h. One limitation of such models is the absence of the cell wall, which could provide adhesion sites for O157:H7 (especially in the colon) and which produces oxygen, which could favor O157:H7 growth, or other, unidentified host factors having potential positive or negative effects on O157:H7 growth or survival. Also, it is not possible to supplement the rumen fluid with an amount of substrate reflecting the actual ratio of feed to total ruminal contents. In vivo, VFAs produced by bacterial fermentation of feed are rapidly absorbed through the rumen wall and do not accumulate, avoiding a dramatic decrease in pH, deleterious for animal health. In batch systems, removal of VFAs is not possible; thus, the amount of feed added to the ruminal fluid must be limited to avoid a too-high VFA concentration and low pH values. Nonetheless, these straightforward models are currently the most suitable and convenient in vitro models, and they best mimic the physiological conditions encountered by EHEC in the liquid phase of the rumen or in the fecal content. Using these models, we show for the first time that the ruminal microflora, but not the fecal flora, exerts a barrier effect on E. coli O157:H7. Furthermore, we demonstrate an inhibitory effect of an L. acidophilus strain in fecal cultures. This finding leads the way to potential on-farm intervention strategies.
Our results show that the composition of the ruminant diet influences E. coli O157:H7 growth in the rumen. Boukhors et al. provided evidence that O157:H7 strains were able to survive but not to multiply under anaerobiosis in sterilized rumen fluid harvested from sheep fed hay or hay plus corn, but the medium was not supplemented with any carbon source (4). In our study, we added finely ground feed, which released soluble sugars into the incubation medium to better reproduce the conditions EHEC would encounter in vivo. Under these conditions, E. coli O157:H7 growth and sugar consumption were observed when the rumen fluid came from sheep fed hay. However, bacterial mortality occurred in rumen samples harvested from sheep 3 h after being fed a mixed diet composed of hay and cereals. Mortality was likely due to the high VFA concentration in conjunction with mildly acidic pH in these samples. Other authors have also pointed out that diets rich in rapidly fermentable carbohydrates are less favorable to E. coli O157:H7 growth than are forage-based diets (25, 33, 54), mostly because the high fermentative activity of the microbial ecosystem in the presence of readily fermentable carbohydrates generates high concentrations of VFAs combined with a low pH (42). High organic acid concentrations, at low pH, greatly inhibit E. coli strains in vitro (43, 56); these acids are mainly undissociated under acidic conditions, and only this form is able to penetrate the bacterial cell (29, 41). A concern with acidogenic diets could be the induction of acid resistance mechanisms in EHEC, which could facilitate the transmission of these strains to humans. However, in vivo studies with experimentally infected cattle showed that E. coli O157:H7 from hay-fed or grain-fed animals is similarly acid resistant in all GIT locations (19).
In ruminal samples harvested before feeding from sheep fed the same hay-plus-cereal diet, pH values were higher than in the samples harvested 3 h after feeding, and E. coli O157:H7 growth, rather than mortality, was observed. These data indicate that low pH and a high VFA concentration, whose production depends on the dynamics of fermentation in the rumen, are only transitory and that the inhibitory effect of such a diet is not permanent.
An important question to be investigated is whether the autochthonous microflora is a key factor influencing E. coli O157:H7 growth. We show that in raw rumen fluid, growth of E. coli O157:H7 is limited or even suppressed due to the presence of the resident microflora, which exerts a strong barrier effect. These data agree with previous observations made with continuous cultures in 50% diluted rumen fluid (12). Taken together, our results indicate that this barrier effect is due mostly to the observed increase in acetate concentration (acetate represents 65 to 75% of the total VFA mixture) and decrease in pH, resulting from the fermentative activity of the microflora during incubation, but also to other direct or indirect effects not yet identified. We also observed that strictly anaerobic conditions limited E. coli O157:H7 growth, the introduction of oxygen enabling the bacteria to proliferate again. Oxygen enters the rumen during feed and water intake (39). Rumination and saliva production might therefore be favorable to the emergence of O157:H7 strains in the rumen. In situ measurements showed that a low concentration of O2 (3 μM) is present in the liquid phase for at least 18 h during the day in animals fed twice daily (23). The levels are higher in the headspace gases (48). Epithelial cells of the rumen wall could also provide oxygen. Strictly aerobic Pseudomonas aeruginosa strains attach to rumen epithelial cells in vitro, and it has been hypothesized that bacterial association with the rumen wall could provide a means for obtaining oxygen in vivo (14). Localization of commensal E. coli strains in the rumen has not been investigated in detail, but the presence of adherent enterobacteria on the rumen wall has been documented (45). Aerobic environments thus exist in the rumen and may be the ecological niches where O157:H7 strains proliferate. However, the inhibitory effect of the autochthonous microflora on E. coli O157:H7 growth, even in aerobiosis and under favorable acidic conditions, suggests that the ruminal compartment is probably neither the main reservoir of O157:H7 strains nor the source of fecal shedding of the organism. This finding is in agreement with in vivo studies in which O157:H7 was detected at necropsy in the most distal regions of the GIT of most experimentally infected cattle but was not consistently cultured from rumen contents (19, 38, 54).
Fecal suspensions constituted a more favorable medium than rumen fluid for growth of E. coli O157:H7. This is probably due in part to the limited fermentative activity of the fecal microflora, resulting in neutral pH and a low VFA concentration, but also to the poor barrier effect of this microflora against E. coli O157:H7, as demonstrated here. Although diluted feces were used to prepare fecal suspensions suitable for bacterial cultures, total bacterial counts were representative of the bacterial concentration in the distal colon and in the rectum of sheep, which has been found to be 2 × 109 cells · ml−1 (53). The total bacterial count is lower in the large intestine than in the rumen fluid (24), in which the bacterial population is considered to range between 1010 and 1011 cells · ml−1 (17). However, differences in composition of the respective bacterial communities might also be of importance in explaining the differences in levels of E. coli O157:H7 growth. According to these results, a number of reports suggest that the hindgut, and particularly the lower GIT, may be the site of E. coli O157:H7 persistence (8, 18, 19, 34, 54). In addition, recent findings of Naylor et al. (38) and Low et al. (37) provide evidence that the mucosal epithelium in the bovine terminal rectum is the primary site of E. coli O157:H7 colonization.
Considering that this terminal part of the gut rather than the rumen is the main site of O157:H7 persistence in ruminants, in-farm management designed to decrease fecal shedding of the pathogen should limit colonization at this site. We show that L. acidophilus BT-1386, which has been shown to enhance performance in feedlot cattle probably through its effect on the microbial balance in the gut (26, 55), inhibits growth of E. coli O157:H7 in fecal suspensions, likely through lactic acid production. Interestingly, the concentration of lactic acid was lower in WOF samples than in WF samples, suggesting a possible interaction between L. acidophilus BT-1386 and the autochthonous lactic acid-producing microflora, leading to an overall increase in lactic acid production in the fecal suspension. The addition to the diet of such a strain of L. acidophilus, able to produce high amounts of lactic acid in the digestive environment, would therefore be of great interest in limiting fecal shedding of E. coli O157:H7. Other modes of action of this L. acidophilus strain could also be involved in vivo: numerous reports provide evidence that some LAB are able to outcompete pathogenic strains for adhesion sites on the epithelium digestive tract (31, 44, 49, 50). However, the pH values achieved in in vitro incubations were quite low, especially with the highest dose of probiotics. Such values achieved in vivo could compromise the use of L. acidophilus as an effective prophylactic agent. Nonetheless, in contrast to in vitro batch systems, fermentation acids are efficiently removed in vivo by intestinal absorption. In vivo trials are required to determine the impact of L. acidophilus on colonic pH values and its effectiveness on O157:H7 shedding and, given the dose-dependent effect observed in vitro, to determine the exact L. acidophilus concentration required in the diet to achieve an efficient probiotic colonic concentration.
Acknowledgments
We thank Sasakawa (Department of Bacteriology, University of Tokyo, Tokyo, Japan) for the generous gift of the mutant E. coli O157:H7 strain Sakaï, lacking the stx1 and stx2 genes. We are very grateful to Daniel Thomas and his team at the Experimental Unit of the INRA Unit of Research on Herbivores for taking care of the fistulated sheep. We also thank Sébastien Masséglia (Lallemand) for skilled technical assistance, Jean-François Martin (Unit of Metabolic Diseases, INRA) for professional advice on statistical analyses, Jean-Pierre Girardeau (Unit of Microbiology, INRA) for helpful discussions, and Gordon Donaldson (Lallemand) for English language revision of the manuscript and helpful comments.
REFERENCES
- 1.Bach, S. J., T. A. McAllister, D. M. Veira, V. P. J. Gannon, and R. A. Holley. 2003. Effects of a Saccharomyces cerevisiae feed supplement on Escherichia coli O157:H7 in ruminal fluid in vitro. Anim. Feed Sci. Technol. 104:179-189. [Google Scholar]
- 2.Besser, R. E., P. M. Griffin, and L. Slutsker. 1999. Escherichia coli O157:H7 gastroenteritis and the hemolytic uremic syndrome: an emerging infectious disease. Annu. Rev. Med. 50:355-367. [DOI] [PubMed] [Google Scholar]
- 3.Besser, T. E., D. D. Hancock, L. C. Pritchett, E. M. McRae, D. H. Rice, and P. I. Tarr. 1997. Duration of detection of fecal excretion of Escherichia coli O157:H7 in cattle. J. Infect. Dis. 175:726-729. [DOI] [PubMed] [Google Scholar]
- 4.Boukhors, K., N. Pradel, J. P. Girardeau, V. Livrelli, A. M. Ou Said, M. Contrepois, and C. Martin. 2002. Effect of diet on Shiga toxin-producing Escherichia coli (STEC) growth and survival in rumen and abomasum fluids. Vet. Res. 33:405-412. [DOI] [PubMed] [Google Scholar]
- 5.Brashears, M. M., M. L. Galyean, G. H. Loneragan, J. E. Mann, and K. Killinger-Mann. 2003. Prevalence of Escherichia coli O157:H7 and performance by beef feedlot cattle given Lactobacillus direct-fed microbials. J. Food Prot. 66:748-754. [DOI] [PubMed] [Google Scholar]
- 6.Brashears, M. M., D. Jaroni, and J. Trimble. 2003. Isolation, selection, and characterization of lactic acid bacteria for a competitive exclusion product to reduce shedding of Escherichia coli O157:H7 in cattle. J. Food Prot. 66:355-363. [DOI] [PubMed] [Google Scholar]
- 7.Brown, C. A., B. G. Harmon, T. Zhao, and M. P. Doyle. 1997. Experimental Escherichia coli O157:H7 carriage in calves. Appl. Environ. Microbiol. 63:27-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buchko, S. J., R. A. Holley, W. O. Olson, V. P. Gannon, and D. M. Veira. 2000. The effect of different grain diets on fecal shedding of Escherichia coli O157:H7 by steers. J. Food Prot. 63:1467-1474. [DOI] [PubMed] [Google Scholar]
- 9.Callaway, T. R., R. C. Anderson, T. S. Edrington, K. J. Genovese, R. B. Harvey, T. L. Poole, and D. J. Nisbet. 2004. Recent pre-harvest supplementation strategies to reduce carriage and shedding of zoonotic enteric bacterial pathogens in food animals. Anim. Health Res. Rev. 5:35-47. [DOI] [PubMed] [Google Scholar]
- 10.Clarke, K., and N. J. P. Owens. 1983. A simple and versatile microcomputer program for the determination of “most probable number.” J. Microbiol. Methods 1:133-137. [Google Scholar]
- 11.Cray, W. C., Jr., T. A. Casey, B. T. Bosworth, and M. A. Rasmussen. 1998. Effect of dietary stress on fecal shedding of Escherichia coli O157:H7 in calves. Appl. Environ. Microbiol. 64:1975-1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Vaux, A., M. Morrison, and R. W. Hutkins. 2002. Displacement of Escherichia coli O157:H7 from rumen medium containing prebiotic sugars. Appl. Environ. Microbiol. 68:519-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dishe, Z. 1963. Sugars in polysaccharides, p. 313-358. In D. Glick (ed.), Methods in biochemical analysis, vol. II. Academic Press, New York, N.Y. [Google Scholar]
- 14.Duncan, S. H., C. J. Doherty, J. R. W. Govan, S. Neogrady, P. Galfi, and C. S. Stewart. 1999. Characteristics of sheep-rumen isolates of Pseudomonas aeruginosa inhibitory to the growth of Escherichia coli O157. FEMS Microbiol. Lett. 180:305-310. [DOI] [PubMed] [Google Scholar]
- 15.Elam, N. A., J. F. Gleghorn, J. D. Rivera, M. L. Galyean, P. J. Defoor, M. M. Brashears, and S. M. Younts-Dahl. 2003. Effects of live cultures of Lactobacillus acidophilus (strains NP45 and NP51) and Propionibacterium freudenreichii on performance, carcass, and intestinal characteristics, and Escherichia coli strain O157 shedding of finishing beef steers. J. Anim. Sci. 81:2686-2698. [DOI] [PubMed] [Google Scholar]
- 16.Fonty, G., M. Chavarot, Y. Mille, F. Michallon, and F. Chaucheyras-Durand. 1999. Establishment of hydrogenotrophic bacteria in the rumen of gnotobiotically-reared lambs. Microecol. Ther. 28:105-114. [Google Scholar]
- 17.Fonty, G., J. P. Jouany, E. Forano, and P. Gouet. 1995. L'écosystème microbien du réticulo-rumen, p. 299-347. In R. Jarrige, Y. Ruckebusch, C. Demarquilly, M. H. Farce, and M. Journet (ed.), Nutrition des ruminants domestiques. INRA, Paris, France.
- 18.Grauke, L. J., I. T. Kudva, J. W. Yoon, C. W. Hunt, C. J. Williams, and C. J. Hovde. 2002. Gastrointestinal tract location of Escherichia coli O157:H7 in ruminants. Appl. Environ. Microbiol. 68:2269-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grauke, L. J., S. A. Wynia, H. Q. Sheng, J. W. Yoon, C. J. Williams, C. W. Hunt, and C. J. Hovde. 2003. Acid resistance of Escherichia coli O157:H7 from the gastrointestinal tract of cattle fed hay or grain. Vet. Microbiol. 95:211-225. [DOI] [PubMed] [Google Scholar]
- 20.Halliwell, G., and M. P. Bryant. 1963. The cellulolytic activity of pure strains of bacteria from the rumen of cattle. J. Gen. Microbiol. 32:441-448. [DOI] [PubMed] [Google Scholar]
- 21.Hancock, D. D., T. E. Besser, D. H. Rice, E. D. Ebel, D. E. Herriott, and L. V. Carpenter. 1998. Multiple sources of Escherichia coli O157 in feedlots and dairy farms in the northwestern USA. Prev. Vet. Med. 35:11-19. [DOI] [PubMed] [Google Scholar]
- 22.Harmon, B. G., C. A. Brown, S. Tkalcic, P. O. Mueller, A. Parks, A. V. Jain, T. Zhao, and M. P. Doyle. 1999. Fecal shedding and rumen growth of Escherichia coli O157:H7 in fasted calves. J. Food Prot. 62:574-579. [DOI] [PubMed] [Google Scholar]
- 23.Hillman, K., D. Lloyd, and A. G. Williams. 1985. Use of a portable quadrupole mass spectrometer for the measurement of dissolved gas concentrations in ovine rumen liquor in situ. Curr. Microbiol. 12:335-340. [Google Scholar]
- 24.Hoover, W. H. 1978. Digestion and absorption in the hindgut of ruminants. J. Anim. Sci. 46:1789-1799. [DOI] [PubMed] [Google Scholar]
- 25.Hovde, C. J., P. R. Austin, K. A. Cloud, C. J. Williams, and C. W. Hunt. 1999. Effect of cattle diet on Escherichia coli O157:H7 acid resistance. Appl. Environ. Microbiol. 65:3233-3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huck, G. L., K. K. Kreikemeier, and G. A. Ducharme. 1999. Effect of feeding Lactobacillus acidophilus BG2FO4 (MicroCell) and Propionibacterium freudenreichii P-63 (MicroCell PB) on growth performance of finishing heifers. J. Anim. Sci. 77(Suppl. 1):264. [Google Scholar]
- 27.Hungate, R. E. 1950. The anaerobic mesophilic cellulolytic bacteria. Bacteriol. Rev. 14:1-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hungate, R. E. 1975. The rumen microbial ecosystem. Annu. Rev. Ecol. Syst. 6:39-66. [Google Scholar]
- 29.Jordan, S. L., J. Glover, L. Malcolm, F. M. Thomson-Carter, I. R. Booth, and S. F. Park. 1999. Augmentation of killing of Escherichia coli O157 by combinations of lactate, ethanol, and low-pH conditions. Appl. Environ. Microbiol. 65:1308-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jouany, J. P., and B. Lassalas. 1999. Influence de la présence des protozoaires sur le métabolisme de l'écosystème ruminal. Rencontre des microbiologistes de l'INRA, 7 to 9 avril 1999. Dourdan, France.
- 31.Krehbiel, C. R., S. R. Rust, G. Zhang, and S. E. Gilliland. 2003. Bacterial direct-fed microbials in ruminant diets: performance response and mode of action. J. Anim. Sci. 81(Suppl. 2):E120-E132. [Google Scholar]
- 32.Kudva, I. T., P. G. Hatfield, and C. J. Hovde. 1995. Effect of diet on the shedding of Escherichia coli O157:H7 in a sheep model. Appl. Environ. Microbiol. 61:1363-1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kudva, I. T., C. W. Hunt, C. J. Williams, U. M. Nance, and C. J. Hovde. 1997. Evaluation of dietary influences on Escherichia coli O157:H7 shedding by sheep. Appl. Environ. Microbiol. 63:3878-3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laven, R. A., A. Ashmore, and C. S. Stewart. 2003. Escherichia coli in the rumen and colon of slaughter cattle, with particular reference to E. coli O157. Vet. J. 165:78-83. [DOI] [PubMed] [Google Scholar]
- 35.Leedle, J. A., and R. B. Hespell. 1980. Differential carbohydrate media and anaerobic replica plating techniques in delineating carbohydrate-utilizing subgroups in rumen bacterial populations. Appl. Environ. Microbiol. 39:709-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lema, M., L. Williams, and D. R. Rao. 2001. Reduction of fecal shedding of enterohemorrhagic Escherichia coli O157:H7 in lambs by feeding microbial feed supplement. Small Rumin. Res. 39:31-39. [DOI] [PubMed] [Google Scholar]
- 37.Low, J. C., I. J. McKendrick, C. McKechnie, D. Fenlon, S. W. Naylor, C. Currie, D. G. Smith, L. Allison, and D. L. Gally. 2005. Rectal carriage of enterohemorrhagic Escherichia coli O157 in slaughtered cattle. Appl. Environ. Microbiol. 71:93-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Naylor, S. W., J. C. Low, T. E. Besser, A. Mahajan, G. J. Gunn, M. C. Pearce, I. J. McKendrick, D. G. Smith, and D. L. Gally. 2003. Lymphoid follicle-dense mucosa at the terminal rectum is the principal site of colonization of enterohemorrhagic Escherichia coli O157:H7 in the bovine host. Infect. Immun. 71:1505-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Newbold, C. J. 1995. Microbial feed additives for ruminants, p. 259-278. In R. J. Wallace and A. Chesson (ed.), Biotechnology in animal feeds and animal feeding. VCH, Weinheim, Germany.
- 40.Ogawa, M., K. Shimizu, K. Nomoto, R. Tanaka, T. Hamabata, S. Yamasaki, T. Takeda, and Y. Takeda. 2001. Inhibition of in vitro growth of Shiga toxin-producing Escherichia coli O157:H7 by probiotic Lactobacillus strains due to production of lactic acid. Int. J. Food Microbiol. 68:135-140. [DOI] [PubMed] [Google Scholar]
- 41.Ohya, T., T. Marubashi, and H. Ito. 2000. Significance of fecal volatile fatty acids in shedding of Escherichia coli O157 from calves: experimental infection and preliminary use of a probiotic product. J. Vet. Med. Sci. 62:1151-1155. [DOI] [PubMed] [Google Scholar]
- 42.Owens, F. N., D. S. Secrist, W. J. Hill, and D. R. Gill. 1998. Acidosis in cattle: a review. J. Anim. Sci. 76:275-286. [DOI] [PubMed] [Google Scholar]
- 43.Rasmussen, M. A., W. C. Cray, Jr., T. A. Casey, and S. C. Whipp. 1993. Rumen contents as a reservoir of enterohemorrhagic Escherichia coli. FEMS Microbiol. Lett. 114:79-84. [DOI] [PubMed] [Google Scholar]
- 44.Resta-Lenert, S., and K. E. Barrett. 2003. Live probiotics protect intestinal epithelial cells from the effects of infection with enteroinvasive Escherichia coli (EIEC). Gut 52:988-997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rieu, F., G. Fonty, and P. Gouet. 1989. Colony counts and characterization of bacteria adherent to the rumen wall and desquamated epithelial cells in conventional young lambs. Can. J. Microbiol. 35:698-705. [DOI] [PubMed] [Google Scholar]
- 46.Sanderson, M. W., T. E. Besser, J. M. Gay, C. C. Gay, and D. D. Hancock. 1999. Fecal Escherichia coli O157:H7 shedding patterns of orally inoculated calves. Vet. Microbiol. 69:199-205. [DOI] [PubMed] [Google Scholar]
- 47.Scott, K. P., and H. J. Flint. 1995. Transfer of plasmids between strains of Escherichia coli under rumen conditions. J. Appl. Bacteriol. 78:189-193. [DOI] [PubMed] [Google Scholar]
- 48.Scott, R. I., N. Yarlett, K. Hillman, T. N. Williams, A. G. Williams, and D. Lloyd. 1983. The presence of oxygen in rumen liquor and its effect on methanogenesis. J. Appl. Bacteriol. 55:143-149. [Google Scholar]
- 49.Servin, A. L. 2004. Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiol. Rev. 28:405-440. [DOI] [PubMed] [Google Scholar]
- 50.Servin, A. L., and M. H. Coconnier. 2003. Adhesion of probiotic strains to the intestinal mucosa and interaction with pathogens. Best Pract. Res. Clin. Gastroenterol. 17:741-754. [DOI] [PubMed] [Google Scholar]
- 51.Stevens, M. P., P. M. van Diemen, F. Dziva, P. W. Jones, and T. S. Wallis. 2002. Options for the control of enterohaemorrhagic Escherichia coli in ruminants. Microbiology 148:3767-3778. [DOI] [PubMed] [Google Scholar]
- 52.Tkalcic, S., T. Zhao, B. G. Harmon, M. P. Doyle, C. A. Brown, and P. Zhao. 2003. Fecal shedding of enterohemorrhagic Escherichia coli in weaned calves following treatment with probiotic Escherichia coli. J. Food Prot. 66:1184-1189. [DOI] [PubMed] [Google Scholar]
- 53.Ulyatt, M. J., D. W. Dellow, C. S. W. Reed, and T. Bauchop. 1975. Structure and function of the large intestine of ruminants, p. 119-131. In I. W. Mc Donald and A. I. C. Warner (ed.), Proceedings of the IV International Symposium on Ruminant Physiology. Armidale, New South Wales, Australia.
- 54.Van Baale, M. J., J. M. Sargeant, D. P. Gnad, B. M. DeBey, K. F. Lechtenberg, and T. G. Nagaraja. 2004. Effect of forage or grain diets with or without monensin on ruminal persistence and fecal Escherichia coli O157:H7 in cattle. Appl. Environ. Microbiol. 70:5336-5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ware, D. R., P. L. Read, and E. T. Manfredi. 1988. Lactation performance of two large dairy herds fed Lactobacillus acidophilus strain BT138 in a switchback experiment. J. Dairy Sci. 71(Suppl. 1):219. [Google Scholar]
- 56.Wolin, M. J. 1969. Volatile fatty acids and the inhibition of Escherichia coli growth by rumen fluid. Appl. Microbiol. 17:83-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Younts-Dahl, S. M., M. L. Galyean, G. H. Loneragan, N. A. Elam, and M. M. Brashears. 2004. Dietary supplementation with Lactobacillus- and Propionibacterium-based direct-fed microbials and prevalence of Escherichia coli O157 in beef feedlot cattle and on hides at harvest. J. Food Prot. 67:889-893. [DOI] [PubMed] [Google Scholar]