Abstract
Recently, two different prohormone-processing enzymes, prohormone convertase 1 (PC1) and carboxypeptidase E, have been implicated in enhancing the storage of peptide hormones in endocrine secretory granules. It is important to know the extent to which such molecules may act as “sorting receptors” to allow the selective trafficking of cargo proteins from the trans-Golgi network into forming granules, versus acting as enzymes that may indirectly facilitate intraluminal storage of processed hormones within maturing granules. GH4C1 cells primarily store prolactin in granules; they lack PC1 and are defective for intragranular storage of transfected proinsulin. However, proinsulin readily enters the immature granules of these cells. Interestingly, GH4C1 clones that stably express modest levels of PC1 store more proinsulin-derived protein in granules. Even in the presence of PC1, a sizable portion of the proinsulin that enters granules goes unprocessed, and this portion largely escapes granule storage. Indeed, all of the increased granule storage can be accounted for by the modest portion converted to insulin. These results are not unique to GH4C1 cells; similar results are obtained upon PC1 expression in PC12 cells as well as in AtT20 cells (in which PC1 is expressed endogenously at higher levels). An in vitro assay of protein solubility indicates a difference in the biophysical behavior of proinsulin and insulin in the PC1 transfectants. We conclude that processing to insulin, facilitated by the catalytic activities of granule proteolytic enzymes, assists in the targeting (storage) of the hormone.
INTRODUCTION
Many specialized secretory cells maintain an abundant population of storage granules that undergo stimulus-dependent exocytosis. Granules are enriched in a concentrated subset of luminal proteins derived from the cell's biosynthetic apparatus, and potential mechanisms of sorting secretory granule proteins is an area of considerable interest (Thiele and Huttner, 1998b). It is believed that the design of regulated secretory proteins has evolved for their efficient targeting to granules. Conceivably, conserved structural features might allow for interaction with a conserved trans-Golgi network (TGN)-based sorting receptor (Chung et al., 1989) directing protein entry into immature granules (IGs), which is the first compartment in the regulated secretory pathway to acquire competence for stimulus-dependent release (Arvan et al., 1991; Tooze et al., 1991; Carnell and Moore, 1994). In addition, assembly of insoluble complexes, a common feature among regulated secretory proteins (Giannattasio et al., 1975; Reggio and Dagorn, 1978; Michael et al., 1987; Chanat and Huttner, 1991), leads to granule core formation within the intraluminal environment (Verbsky and Turkewitz, 1998) that may facilitate the ultimate targeting of regulated secretory proteins (Kuliawat and Arvan, 1992). Thus, after oligomerization in the early secretory pathway (Huang and Arvan, 1995; Thiele and Huttner, 1998a), the development of a higher-order quaternary structure within the distal secretory pathway may play a significant role in the intragranular storage of regulated secretory proteins (Thiele et al., 1997; Arvan and Castle, 1998).
In mature pancreatic β-secretory granules, insulin is found in a multimeric, highly condensed state (Michael et al., 1987). In contrast, the proinsulin precursor cannot assemble beyond soluble hexamers (Frank and Veros, 1968, 1970; Blundell et al., 1972; Grant et al., 1972; Steiner, 1973; Weiss et al., 1990). The inability of proinsulin to multimerize does not prevent its entry into forming β-granules, because other soluble proteins also enter this compartment (Kuliawat et al., 1997). However, in β-cells, additional quaternary structural maturation takes place upon proinsulin conversion to insulin (Kuliawat and Arvan, 1994), a process that is highly dependent on the activities of prohormone convertases PC1 and PC2 (Docherty et al., 1989; Rhodes et al., 1992; Smeekens et al., 1992; Sizonenko et al., 1993; Furuta et al., 1998) as well as carboxypeptidase E (CPE) (Naggert et al., 1995; Fricker et al., 1996; Irminger et al., 1997; Varlamov et al., 1997) within the IG compartment (Huang and Arvan, 1994). (PC1, the term used throughout this text, is also known as PC3 [Smeekens et al., 1991].)
Recently, there has been great interest in the idea that one or more of these prohormone-processing enzymes, which exhibit high expression in neuroendocrine cells (Seidah et al., 1994), could perform double duty by functioning in the TGN as sorting receptors for peptide hormone precursors. PC1, for example, has the potential to recognize its processing site (Rholam et al., 1986) before it has become fully enzymatically activated (Jutras et al., 1997), and recent evidence has suggested that the presence of the dibasic recognition/cleavage site is important for the ultimate granule targeting of prorenin, a potential PC1 substrate (Brechler et al., 1996). A second putative TGN-based sorting receptor has been suggested by Loh and colleagues to be the membrane-associated form of CPE (Cool and Loh, 1998). Because the majority of prohormone substrates tend to be proteolyzed within IGs of the regulated secretory pathway, a crucial aspect of this hypothesis is that CPE functions in the TGN independently of its catalytic activity (Tam et al., 1993; Cool and Loh, 1994, 1998; Cool et al., 1995, 1997; Shen and Loh, 1997). The homozygous Cpefat/Cpefat mutation has been proposed to result in a loss of sorting activity for proinsulin (Normant and Loh, 1998), leading to hyperproinsulinemia (Naggert et al., 1995) as well as defective trafficking and diminished intragranular storage of other peptide hormones in neuroendocrine cells (Shen and Loh, 1997). However, no defect in proinsulin entry into forming granules of Cpefat/Cpefat β-cells has been reported (Irminger et al., 1997; Varlamov et al., 1997), although there is a morphologically apparent defect in condensation within the β-granule core (Naggert et al., 1995). In pancreatic islets of mice with homozygous disruption of the PC2 gene, there is also no defect in proinsulin entry into forming β-granules and no effect on newly synthesized proinsulin secreted within the first 60 min after synthesis; however, morphologically apparent inhibition of granule core condensation is again detectable (Furuta et al., 1998). Finally, in transgenic mice with wild-type proinsulin-processing enzymes but expressing a mutant insulin (HisB10Asp) defective for condensation, this morphological phenotype in the β-granules has been shown to correlate with hypersecretion of pulse-labeled proinsulin and insulin under unstimulated conditions at chase times beyond 60 min (Carroll et al., 1988). This is consistent with enhanced constitutive-like secretion (Kuliawat and Arvan, 1992) that is enriched in secretory proteins exhibiting defective storage in maturing granules (Castle et al., 1997).
Clearly, a consensus has not yet been reached (Thiele et al., 1997; Loh, 1998) on the extent to which prohormone-processing enzymes can function as noncatalytic sorting receptors for entry into forming granules versus functioning as catalysts of prohormone conversion to mature hormones, thereby facilitating hormone storage within maturing granules. We have attempted to examine this issue in a cell line–based test system. It has long been known that upon introduction of a human proinsulin cDNA into AtT20 cells that express endogenous PC1 (Seidah et al., 1991), insulin becomes stored in mature granules (Moore et al., 1983). In contrast, expression of the same protein in GH4C1 cells lacking PC1 results in both a lack of conversion to insulin and a lack of efficient prohormone storage in mature secretory granules (which store endogenous prolactin) (Reaves et al., 1990). By stably expressing PC1 in GH4C1 cells, we are now able to pose two closely related questions: Does the presence of PC1 change the fractional storage efficiency for newly synthesized proinsulin? And does it change the fractional storage efficiency for newly synthesized insulin? The data presented here indicate that with or without PC1, newly synthesized proinsulin enters IGs in GH4C1 cells. PC1 does not enhance proinsulin storage in granules, but PC1-mediated excision of C-peptide from proinsulin dramatically improves the targeting efficiency of the processed peptide hormone in the regulated secretory pathway. These data are consistent with the idea that the catalytic activities of granule-processing enzymes may facilitate secretory protein sorting by retention within maturing granules.
MATERIALS AND METHODS
Antibodies and Other Materials
HRP-conjugated anti-rabbit serum (Bio-Rad Laboratories, Richmond, CA) was used as a secondary antibody to be used with ECL detection (Amersham, Arlington Heights, IL). Guinea pig anti-insulin antibody was from Linco (St. Louis, MO). Antibodies to rodent prolactin were obtained from Dr. A.F. Parlow (National Hormone and Pituitary Program, Bethesda, MD) and Dr. F. Reinhardt (Genzyme, Cambridge, MA). Aliquots of antisera against PC1 (and PC2) were generously provided by Drs. E. Eipper and R. Mains (Johns Hopkins University, Baltimore, MD), I. Lindberg (Louisiana State University, New Orleans, LA), and L. Devi (New York University School of Medicine, New York, NY). Texas Red–conjugated goat anti-rabbit antibody was from Southern Biotechnology Associates (Birmingham, AL). Thyrotropin-releasing hormone (TRH), protease inhibitors, saponin, zinc sulfate, insulin, EGF, estradiol, Dulbecco's modified Eagle's medium (DME), and protein A– and protein G–agarose were from Sigma Chemical (St. Louis, MO); G418, calf serum, and antibiotic solution were from GIBCO (Long Island City, NY); [35S]methionine/cysteine (Expre35S35S) was from New England Nuclear (New Bedford, MA); Zysorbin–protein A was from Zymed Laboratories (San Francisco, CA).
Cell Lines and Plasmids
The GH4C1 parental cell line and the PC12 cell line expressing PC1 were the generous gifts of Drs. P. Dannies (Yale University, New Haven, CT) and I. Lindberg (Louisiana State University), respectively. AtT20 cells were obtained from Dr. R. Mains (Johns Hopkins University). In all cases, GH4C1 cells were grown in DME plus 15% horse serum in the presence of 1 nM estradiol, 300 nM insulin, and 10 nM EGF for 7 d before experiments to maximally augment the granule storage pool (Scammell et al., 1986). GH4C1 cells were transfected with the mouse PC1 or PC2 cDNA in pR/cytomegalovirus (kindly provided by Dr. N. Seidah, University of Montreal, Canada) and selected with 0.5 mg/ml G418; after screening, clones were maintained in the presence of 0.2 mg/ml G418. PC12-PC1 cells were cultured in high-glucose DME plus 10% horse serum and 5% FBS, penicillin/streptomycin, and 0.2 mg/ml G418. The replication-deficient adenovirus vectors driving the expression of β-galactosidase or human proinsulin were the kind gifts of Drs. C. Newgard (University of Texas Southwestern, Dallas, TX) and S. Clarke (Beta-gene, Dallas, TX).
Indirect Immunofluorescence of GH4C1 Cells
Cells grown on collagen-coated coverslips were treated with 1 nM estradiol, 300 nM insulin, and 10 nM EGF for 7 d and with 5 mM sodium butyrate overnight before fixation with 3% formaldehyde in PBS, 50 μM CaCl2, 50 μM MgCl2, pH 7.4, at room temperature for 20 min. Cells were then quenched with 50 mM NH4Cl in PBS for 15 min, washed three times with PBS containing 0.2% fish skin gelatin, permeabilized in acetone at −20°C for 2 min, and blocked with 20% normal goat serum in PBS for 30 min. The cells were then incubated with polyclonal anti-PC1 antibody (1:800) for 1.5 h. After further washing, the cells were finally incubated for 1 h with Texas Red–conjugated secondary antibody (1:200). After more washing, the coverslips were mounted in ProLong Antifade mounting medium (Molecular Probes, Eugene, OR). Images were collected with the use of the SPOT digital camera (Diagnostic Instruments, Sterling Heights, MI) on a Zeiss (Thornwood, NY) Axioplan microscope.
Expression of Human Proinsulin in GH4C1, PC12, and AtT20 Cells
For most experiments, a replication-deficient adenovirus (RDA) vector (see above) was used to express human proinsulin in heterologous regulated secretory cells. In preparation for these experiments, we initially exposed GH4C1 cells to an RDA vector encoding a fusion protein of β-galactosidase (containing a nuclear localization signal). After examination of fixed cells to screen for enzyme reaction product, ∼90% of cells were found to express the protein derived from the RDA vector. When immunofluorescence with an antibody to insulin, which is a somewhat less sensitive assay, was used, a diminished fraction (∼50%) of cells exhibited specific intracellular signal. RDAs were passed through HEK293 cells, in which they induce a lytic infection. Generally, virus preparation simply involved collecting the medium bathing infected cells, which was spun (3000 × g for 15 min) to remove intact cells and debris. The supernatant containing the viral vector was then exposed to GH4C1, PC12-PC1, or AtT20 cells. In some cases, a concentrated viral stock was prepared by adsorption to polyethylene glycol 8000 and elution in a small volume of 137 mM NaCl, 5 mM KCl, 1 mM MgCl2, 10 mM Tris, pH 7.4. The results were similar regardless of the method used for virus preparation. Cells were exposed to virus for 2 h and then returned overnight to normal growth medium. The next day, the cells were trypsinized and replated on fresh plastic. Experiments were performed on the third day after adenovirus infection. In a few experiments, a human proinsulin cDNA in pCDNA3 (Invitrogen, Carlsbad, CA) was introduced instead with the use of conventional transfection protocols into GH4C1 (Fugene 6, Boehringer Mannheim Biochemicals, Indianapolis, IN) or PC12 cells (calcium-phosphate method); control studies showed that neither transfection nor infection methods altered the outcome of our experiments.
Metabolic Labeling Protocols
For an approach to steady state, cells were labeled with ∼300 μCi of 35S-amino acids in complete growth medium for 2 d before experiments. For pulse-chase studies, cells were washed twice with met-free, cys-free DME before labeling for 30 min at 37°C in the same medium containing ∼300 μCi of 35S-amino acids. At the conclusion of the pulse, the cells were washed and chased in complete DME plus 5 mg/ml BSA. Routinely, cells were stimulated for 30 min with a secretagogue containing 50 mM KCl, 100 nM phorbol 12-myristate 13-acetate, and 1 μM Bay K8644 (except in the experiment shown in Figure 2B, in which 100 nM TRH was used). PC12-PC1 cells were treated similarly because K+ depolarization induces exocytosis in these cells (Carnell and Moore, 1994). AtT20 cells were stimulated for 30 min with 1 mM BaCl2 (Mains and Eipper, 1984). Cells were finally lysed in 100 mM NaCl, 10 mM EDTA, 25 mM Tris, pH 7.4, containing 1% Triton X-100, 0.2% deoxycholate, and 0.1% SDS. An anti-protease cocktail was added to the lysates to achieve final concentrations of aprotinin (1 mU/ml), leupeptin (1 μM), pepstatin (10 μM), EDTA (5 mM), diisopropylfluorophosphate (1 mM), E64 (1 μM), and iodoacetamide (1 mM). Both cells and media were spun briefly in a microfuge to remove debris before analysis. Immunoprecipitation with anti-insulin antibody was as described previously (Kuliawat and Arvan, 1992).
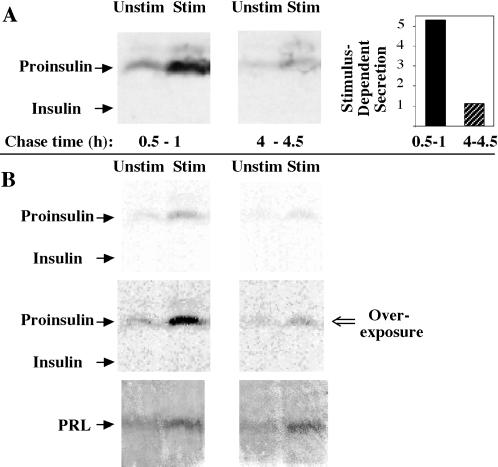
Figure 2.
Secretion of newly synthesized proinsulin upon expression in GH4C1 cells. (A) The cells were pulse labeled for 30 min with 35S-amino acids, and unstimulated (Unstim) and stimulated (Stim) media were collected during the indicated early and late chase intervals. The samples were immunoprecipitated with anti-insulin antibody and analyzed by Tricine–urea–SDS-PAGE and fluorography. The positions of proinsulin and insulin are indicated. Stimulus-dependent (stimulated minus unstimulated) secretion was calculated in arbitrary (phosphorimaging) units. The panel at right quantifies an approximately fivefold decrease in stimulus-dependent secretion of the labeled prohormone. (B) An independent experiment that used an identical protocol to that shown in A, except with 100 nM TRH as the secretagogue (Stim). The upper panels show a direct film scan, whereas the middle panels represent an intentional overexposure of the same scan by adjusting the contrast with the use of Adobe (Mountain View, CA) Photoshop to more readily compare with the direct film scan shown in A. In the lower panels, identical samples of media were analyzed by Western blotting for prolactin (PRL), demonstrating comparable total granule exocytosis at the two chase times.
SDS-PAGE, Fluorography, and Phosphorimaging
To detect the expression of prohormone convertases, cell lysates were normalized either to DNA content or to total protein before conventional SDS-PAGE and Western blotting. Anti-insulin immunoprecipitates were analyzed with 15% acrylamide SDS-PAGE plus urea with the use of a Tricine buffer system (Schagger and von Jagow, 1987). The gels were fixed initially in 20% trichloroacetic acid without alcohol, then in 12.5% trichloroacetic acid plus 50% methanol, and then were incubated briefly with water. Gels were then either dried before phosphorimaging or incubated with 1 M sodium salicylate for 20 min and dried before exposure to XAR film at −70°C. Phosphorimages or scanned x-ray films were analyzed with the use of the ImageQuant program (Molecular Dynamics, Sunnyvale, CA).
In Vitro Proinsulin and Insulin Solubility Assay
An in vitro assay of protein condensation state, patterned after the protocols of Chanat and Huttner (1991) and Schmidt and Moore (1994), was used. Briefly, 4 × 106 cells (either GH4C1 or PC12) plated on 15-cm dishes were used for each sample. Before lysis, the cells were rinsed twice with PBS plus 4 mM EGTA and once with homogenization buffer (HB) (0.25 M sucrose, 1 mM Mg acetate, 1 mM EDTA, 1.6 mM Na2SO4, 10 mM HEPES, pH 7.2, all at 4°C). The cells were collected by scraping into 1.8 ml of HB and pelleting in a microcentrifuge (13,000 × g) for 2 s. Cells were then lysed in 0.6 ml of HB plus a protease inhibitor cocktail (see above) by 8 passes through a 22-gauge needle followed by 10 passes in a cell cracker. For each sample, the nuclear/cell pellet was collected by sedimentation at 1500 × g for 4 min, rehomogenized as described above in an additional 0.6 ml of HB, and respun at 1500 × g for 4 min. The pooled postnuclear supernatant fraction was then spun sequentially, first at 6000 × g for 15 min and then at 25,000 × g for 20 min. The latter spin yielded a crude granule fraction pellet, which was then used for the in vitro assay of protein extractability. For this assay, the crude granule fraction pellet was resuspended in 0.2 ml of 10 mM CaCl2 plus 10 mM 2-(N-morpholino)ethanesulfonic acid, pH 6.0. Saponin was then added to achieve a final concentration of 0.5 mg/ml. The samples were incubated for 15 min on ice and then spun at 25,000 × g for 20 min. Both the pellet and the supernatant were then analyzed for proinsulin and insulin content by Western blotting. Unrelated other fractions generated from the fractionation protocol also were analyzed separately as a control for total proinsulin/insulin in each sample.
RESULTS
Proinsulin Processing and Insulin Storage in AtT20 Cells
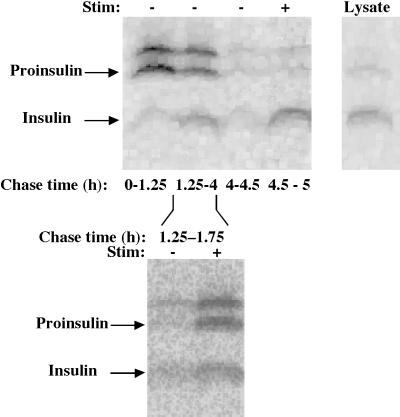
AtT20 cells express high levels of PC1 but very little PC2 (Bloomquist et al., 1991; Smeekens et al., 1992; Vindrola and Lindberg, 1992). Published work has highlighted the fact that after proinsulin expression in these cells, stimulation with secretagogues releases primarily insulin, rather than proinsulin, into the medium (Moore et al., 1983; Gross et al., 1989a,b; Ferber et al., 1991; Irminger et al., 1994). However, many studies have not focused specific attention on the size of the fraction of newly synthesized proinsulin that is actually converted to insulin and stored for release in a stimulus-dependent manner in AtT20 cells. To clarify this point, we expressed proinsulin in AtT20 cells with the use of a RDA vector. The cells were pulse labeled with 35S-amino acids for 30 min, and media from various early unstimulated (0–1.25 h, 1.25–4 h) and later unstimulated (4–4.5 h) plus stimulated (4.5–5 h) chase periods were collected to examine the extent to which labeled proinsulin-derived peptides were finally stored in mature AtT20 secretory granules. During 5 h of chase, >90% of the newly synthesized proinsulin was secreted in various forms, leaving <10% remaining intracellularly. Interestingly, in these cells, only a minor portion of the originally synthesized proinsulin was ever converted to insulin. In a series of four independent experiments with these cells, the maximum conversion to insulin was ∼33% and the minimum was ∼7%; in the experiment shown in Figure 1, ∼20% was converted to insulin. Irminger and colleagues (1994) have shown that in transfected AtT20 cells, by 60 min after pulse, constitutive proinsulin traffic has already occurred and intragranular processing is well under way; indeed, we also observed significant release of proinsulin and a probable des-31,32 (Irminger et al., 1994) conversion intermediate during the initial collection of medium up to 75 min of chase (Figure 1). Importantly, another major portion of newly synthesized proinsulin and conversion intermediate was secreted during the unstimulated period from 1.25 to 4 h of chase (Figure 1), a kinetic period that corresponds largely to events occurring within maturing AtT20 granules (Milgram et al., 1994; Castle et al., 1997). (Indeed, secretagogue addition beginning at 1.25 h elicited a strong stimulated release [Figure 1, lower panel], establishing that labeled proinsulin-derived peptides were in secretory granules during this period.) Finally, of the processed insulin, much was released during a terminal stimulation period (4.5–5 h; Figure 1), in good agreement with earlier reports (Quinn et al., 1991). In AtT20 cells, the final stimulus-dependent (stimulated minus unstimulated) secretion of insulin accounted for <10% of the originally synthesized proinsulin. These data establish that in AtT20 cells, the majority of newly synthesized proinsulin molecules are not actually converted to insulin and stored for stimulus-dependent release.
Figure 1.
Secretion of newly synthesized proinsulin upon expression in AtT20 cells. The cells were pulse labeled for 30 min with 35S-amino acids. During the indicated chase intervals (upper panels), sequential unstimulated (−) or stimulated (+) media and lysed cells were collected. In the lower panel, secretion into the medium beginning at 1.25 h of chase is shown, indicating that labeled proinsulin-derived peptides have already reached the stimulus-dependent secretory pathway by 1.25 h of chase. All samples were immunoprecipitated with anti-insulin antibody and analyzed by Tricine–urea–SDS-PAGE and fluorography. The positions of proinsulin and insulin are indicated; the band immediately above proinsulin probably represents the des-31,32 proinsulin conversion intermediate (Irminger et al., 1994). Results of a representative experiment (of four) are shown.
GH4C1 Cells Expressing PC1 Acquire the Ability to Convert a Fraction of Proinsulin to Insulin
GH4C1 cells lack PC1 and express little or no PC2 (Seidah et al., 1994; Rouille et al., 1995), and compared with AtT20 cells, heterologously expressed proinsulin-derived peptides exhibit defective storage in GH4C1 secretory granules (Reaves et al., 1990). To reaffirm this point, transfected GH4C1 cells expressing proinsulin from a RDA vector were pulse labeled with 35S-amino acids for 30 min, and at either an early (0.5–1 h) or late (4–4.5 h) chase interval, medium was collected from cells that were unstimulated or stimulated for 30 min. When considered as fold stimulation, labeled proinsulin secretion was augmented during both early (3.7-fold) and late (2.2-fold) chase periods; however, stimulus-dependent (stimulated minus unstimulated) secretion of proinsulin declined dramatically as the chase progressed (Figure 2A, quantified at right). During the early chase period, enhanced release of newly synthesized proinsulin was also observed after TRH stimulation (Figure 2B). The decline in stimulated proinsulin release did not reflect a loss of overall granule exocytosis, as assayed by prolactin release (Figure 2B, lower panels). Similar results were also obtained with KCl as the sole stimulus (our unpublished results). These data make clear that the reportedly abnormal handling of proinsulin in GH4C1 cells (Reaves et al., 1990) is not accounted for by a defect of newly synthesized proinsulin entry into IGs. Rather, a proinsulin storage defect in these cells is detected only after secretory granule maturation.
We then stably introduced the cDNA encoding PC1, driven by the cytomegalovirus promoter, in GH4C1 cells. Clones were initially selected for G418 resistance and then further screened by Western blotting (endogenous levels were below detection). In PC1 transfectants, the enzyme migrated as a mixture of precursor (∼83 kDa) and mature (∼66 kDa) forms (Figure 3), consistent with probable enzyme activation (Jutras et al., 1997). Most biochemical studies were performed with two independently selected clones (1-5 and 1-67) that were among our best PC1 expressors, although their levels fell far short of that observed in AtT20 cells (Figure 3). Immunofluorescence revealed that recombinant PC1 expression was detectable in all cells within a clone, with positive localization to the secretory pathway (Figure 4). Unlike in parental GH4C1 cells, some degree of conversion of proinsulin to insulin could be detected in metabolically labeled GH4C1 cells expressing PC1 (see below).
Figure 3.
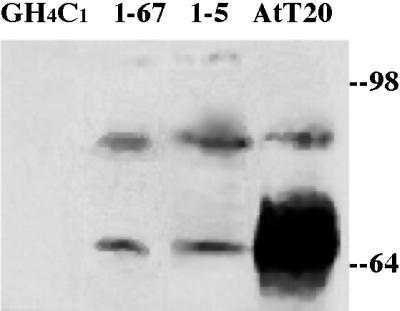
Western blot screening of GH4C1 clones transfected to express PC1. Eighty micrograms of cell lysate protein was loaded in each lane. Note that endogenous levels of PC1 in GH4C1 cells (left lane) were below detection. Clones 1-67 and 1-5 are the clones primarily used for studies in this paper; both clones show dramatically less immunoreactive PC1 (on a per cell protein basis) than do AtT20 cells.
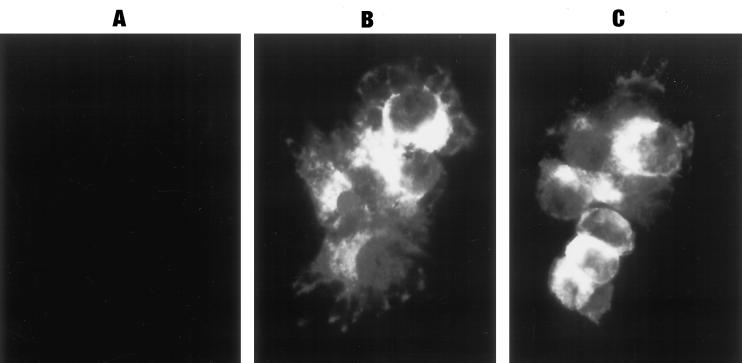
Figure 4.
Indirect immunofluorescent staining for PC1 in parental GH4C1 (A) and PC1-expressing GH4C1 cells (clone 1-5; B and C). Comparable numbers of cells and identical exposures are shown in the two fields. A similar specific immunofluorescent staining pattern was also seen for PC1 in the 1-67 clone.
Prolactin Storage and Secretion in GH4C1 Cells Is Not Affected by Expression of PC1 or Proinsulin
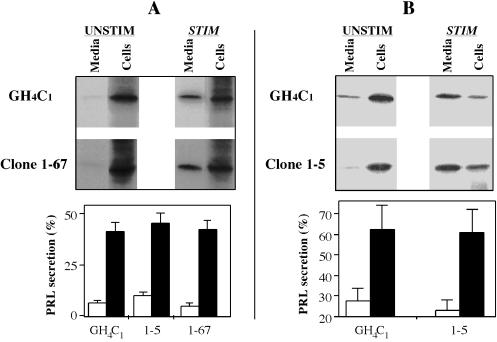
To confirm that the expression of PC1 and/or proinsulin did not fundamentally alter secretory granule formation or exocytosis in GH4C1 cells, we used a radiolabeling approach to examine the regulated exocytosis of prolactin, which normally undergoes no intracellular endoproteolysis after signal peptide removal. After overnight labeling to approach steady state, cells expressing proinsulin from the RDA vector were incubated for 30 min under unstimulated or secretagogue-containing conditions (Figure 5A, upper panel). Quantitation (Figure 5A, lower panel) established that PC1-expressing clones exhibited comparable stimulus-dependent secretion to parental GH4C1 cells. When a 3-h unstimulated chase was included before the 30-min test period to further enrich the cells for slow-turnover pools of labeled prolactin, a slightly higher fraction of the remaining labeled prolactin could be exported to the medium upon stimulation (Figure 5B). The data indicate that prolactin secretory behavior in GH4C1 cells is independent of the presence or absence of PC1 and proinsulin and that an analysis of stimulus-dependent secretion yields a meaningful estimate of the secretory granule storage pool.
Figure 5.
Storage of prolactin by GH4C1 cell clones expressing PC1. Cells were metabolically labeled to approach steady state, as described in the text. The cells were then either tested directly for secretion under unstimulated (UNSTIM) or stimulated (STIM) conditions (A) or tested after 3 initial unstimulated hours to further enrich for slow-turnover pools of secretory protein (B). As a consequence, in B, a majority of the remaining intracellular prolactin was exported to the media upon secretagogue exposure. From quantitative scanning of the media and cell lysate bands from each gel (the sum in each case was considered 100%), the percentages recovered in the media under unstimulated (open bars) and stimulated (closed bars) conditions are shown (bar graphs).
Time Course and Extent of Proinsulin Processing in the IGs of GH4C1 Cells Expressing PC1
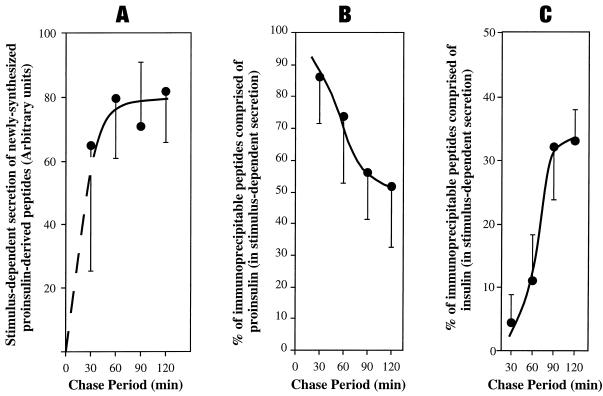
Excision of C-peptide from proinsulin has been reported to be an IG-specific event (Orci et al., 1985, 1986, 1987b; Rhodes and Halban, 1987; Rhodes et al., 1987; Steiner et al., 1987; Huang and Arvan, 1994). With this in mind, we used stimulus-dependent secretion to examine the time course of proinsulin arrival in the IGs of PC1-expressing GH4C1 cells and subsequent endoproteolysis therein. After a 30-min pulse-labeling period, the trend was for delivery of newly made proinsulin-derived peptides to appear in the regulated secretory pathway within the first 30 min of chase and to achieve a maximum by 60 min with a plateau thereafter, although error bars were unusually high for the 30-min measurement (Figure 6A). When proinsulin endoproteolysis was analyzed with the use of Tricine–urea–SDS-PAGE in conjunction with phosphorimaging, slightly less than half of the proinsulin in the IGs of GH4C1 cells transfected with PC1 was proteolytically cleaved by 2 h of chase (Figure 6B). Although the PC1-expressing GH4C1 clones did not approach the conversion efficiency of real β-cells or vaccinia-based expression systems (Kaufmann et al., 1997), a significant fraction (approximately one-third) of granule peptides were specifically recovered as insulin (Figure 6C). When release into constitutive and constitutive-like secretion was also taken into account (see below), it was clear that of each 100 molecules of newly made proinsulin, only ∼10 molecules were ultimately processed to insulin in the granules of these cells. This modest overall processing efficiency appears to reflect a combination of the level of PC1 expression (Figure 3) and the early unstimulated secretion of unprocessed precursor (such as that in AtT20 cells; Figure 1). Nevertheless, the kinetics of labeled prohormone delivery to the regulated secretory pathway (Figure 6A) and the occurrence of a maximum rate of prohormone conversion to insulin during the 60- to 90-min chase period (Figure 6, B and C) are comparable to those reported in pancreatic β-cells (Rhodes and Halban, 1987), suggesting that labeled proinsulin arrived maximally in IGs within 60 min after pulse labeling. Notably, there was no loss of recovery of proinsulin-derived peptides during these pulse-chase experiments. Importantly, the incomplete processing of proinsulin creates a potentially ideal system in which to examine the ability of PC1 to facilitate the storage of proinsulin versus insulin within secretory granules of the same cells.
Figure 6.
Arrival in IGs and intragranule processing of labeled proinsulin in GH4C1 cells stably expressing PC1. Multiple identical wells of cells were pulse labeled for 30 min with 35S-amino acids. For the chase periods shown, the wells were divided into parallel secretagogue-stimulated and unstimulated conditions. Media were collected and analyzed by immunoprecipitation with anti-insulin antibody followed by SDS-PAGE and phosphorimaging. The stimulus-dependent secretion (stimulated minus unstimulated) was used as a specific measure of contents derived from secretory granules. (A) The sum of all labeled proinsulin-derived peptides released in the stimulus-dependent secretion. (B) Proinsulin as a fraction of granule contents. (C) Insulin as a fraction of granule contents. Means (n = 3) and SDs are shown.
Storage of Proinsulin and Insulin in the Secretory Granules of GH4C1 Cells
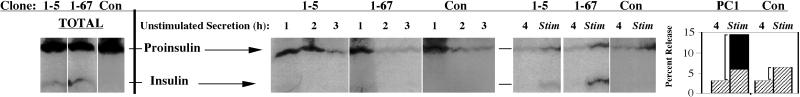
To test whether PC1-expressing clones of GH4C1 cells showed any improvement in storage capacity for the transfected prohormone, a protocol similar to that described for Figure 5B was used. Cells were labeled to approach steady state, and at the immediate termination of the labeling, data from 3 individual hours of unstimulated secretion were initially collected (Figure 7, lanes marked 1, 2, and 3). After the 3-h period (which enriches the cells for slow-turnover pools of labeled secretory proteins), data from two additional secretion periods were collected, one in the absence and one in the presence of secretagogue. As expected, all GH4C1-derived cells exhibited stimulated secretion of proinsulin, whereas only PC1 expressors exhibited detectable stimulated secretion of insulin (Figure 7). However, when the stimulus-dependent secretion was traced back to the total intracellular hormone pools of these peptides (Figure 7, far left panels), it became apparent that there were major sequelae for the clones that could make insulin. Specifically, although the stimulus-releasable proinsulin pool as a fraction of total cellular proinsulin was extremely small, the stimulus-releasable insulin pool as a fraction of total cellular insulin was very large. Indeed, total stimulus-dependent release of proinsulin-derived peptides increased approximately threefold in the PC1 expressors, and this increase represented precisely the extent to which insulin was made (Figure 7, bar graph at right).
Figure 7.
Relative storage of proinsulin and insulin in secretory granules of GH4C1 cells labeled to approach steady state. Different clones that either manufacture insulin (1-5 and 1-67) or do not manufacture insulin (Con) are shown. Cells were labeled as in Figure 5. Media collected during 3 initial unstimulated hours (middle panels) are shown as lanes marked 1, 2, and 3 for each clone. (As observed previously [Figure 5B], the 3-h period allows unstored peptides to drain through the intracellular transport pathway into the medium; this also enriches the cells for slow-turnover pools of labeled secretory protein.) Additional unstimulated and stimulated media at the end of the experiment are shown in each case in lanes 4 and Stim (panels at right). The total contents of labeled proinsulin and insulin produced by each clone were analyzed by immunoprecipitation in the gels shown at the far left. Quantitative analyses of the data for the proinsulin converters (PC1-expressing clones) and the nonconverter (Con) are shown in the bar graph at the far right: secretion of proinsulin is shown in the hatched bars, and secretion of insulin is shown in the closed bar. Conservation of cysteines and methionines between rat proinsulins and rat insulins allows the radioactivity in these bars to be directly additive. The stimulus-dependent secretion (stimulated minus unstimulated) is represented by square brackets in the bar graph. Note that all of the increase in stimulus-dependent release from the PC1-expressing clones is in the form of insulin.
Proinsulin Versus Insulin: Retention in Maturing Storage Granules
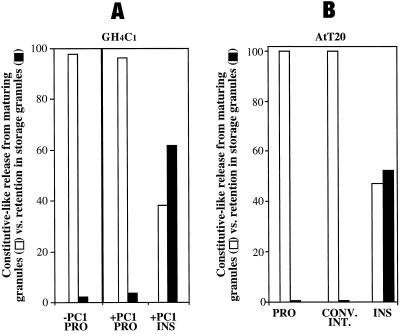
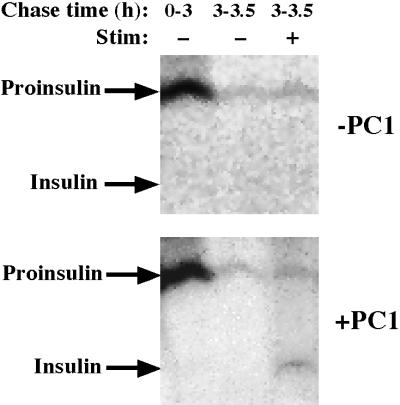
We then set out to directly compare the storage efficiency of proinsulin and insulin after entry into IGs of PC1-expressing GH4C1 cells versus control GH4C1 cells, with the use of a pulse-chase format. In all clones, the identical fraction of labeled proinsulin escaped into the unstimulated medium during the first 75 min of chase, which is enriched in constitutive secretion derived from the TGN (Kuliawat and Arvan, 1992; Irminger et al., 1994; Milgram et al., 1994). For this reason, we now directed our attention to comparisons beginning after 75 min of chase had already elapsed, thereby focusing on pathways derived primarily from maturing secretory granules. Thus, the unstimulated secretion of newly synthesized proinsulin and insulin during 75–210 min of chase, which encompasses the peak of constitutive-like secretion, was compared with the granule retention of these same proteins as assessed during a final period of stimulus-dependent secretion. As shown in Figure 8A, in the PC1 expressors, a much larger portion of proinsulin exited the cells in the constitutive-like secretion period (open bars) than was stored for release from mature granules (closed bar in the middle set of bars, representing an average of ∼3.5% from two independent clones). Importantly, this portion closely matched the degree of labeled proinsulin stored for release in mature granules of parental GH4C1 cells lacking PC1 (∼2.1%, n = 3, left set of bars). (Conceivably, the high level of unstimulated release of proinsulin despite the expression of PC1 might reflect heterogeneous, low-level, or abnormal packaging of recombinant PC1, leading to large amounts of proinsulin with no opportunity to interact with PC1 in the secretory pathway. Such a possibility seems unlikely, however, because AtT20 cells [which strongly express endogenous PC1] also showed a high level of unstimulated release of proinsulin [and conversion intermediate] [Figure 8B].)
Figure 8.
(A) Relative storage of proinsulin (PRO) and insulin (INS) in maturing secretory granules of PC1 expressors (+PC1) and parental control GH4C1 cells (−PC1). (B) Relative storage of proinsulin (PRO), conversion intermediate (CONV. INT.), and insulin (INS) in AtT20 cells. In all cases, the cells were pulse labeled for 30 min with 35S-amino acids, and 75 min of chase was allowed to pass to provide sufficient time for proinsulin arrival in the IG compartment (see Figure 6). Then, media were collected during an unstimulated period from 75 to 210 min of chase in GH4C1 cells (A) or from 75 to 240 min of chase in AtT20 cells (B), a time that includes constitutive-like secretion (open bars). Finally, proteins stored in mature granules were identified by stimulus-dependent secretion (closed bars). For the quantitation shown, release during the constitutive-like secretion period plus the stimulus-dependent secretion period were used to approximate all granule-derived secretion pathways and were assigned a value of 100%. (A) Two independent clones of GH4C1 cells (1-5 and 1-67) were internally controlled (i.e., 30 min of unstimulated and 30 min of stimulated release were obtained sequentially from the same sample) in three experiments for measurement of stimulus-dependent secretion; the mean values are shown, and SDs on these measurements were ≤5%. Identical results were obtained in three additional experiments that were externally controlled (i.e., 30 min of unstimulated and 30 min of stimulated release were obtained from two parallel sets of cells). (B) Data derived from a representative experiment.
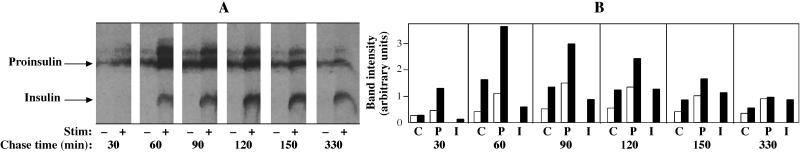
In contrast, there was a dramatic difference for newly synthesized insulin in the GH4C1 cells plus PC1 (Figure 8A, right set of bars). In this case, it was clear that the predominant fraction (∼62%) was stored in granules. This value is clearly an underestimate, because not all secretory granules undergo exocytosis during stimulation, but the stimulus-dependent secretion is nevertheless proportional to the size of the granule pool [Tatham et al., 1991; Hide et al., 1993].) When the labeled insulin retained in GH4C1 granules was compared with proinsulin retained, it appeared that IG insulin was stored ≥17.5-fold better than IG proinsulin. In an attempt to explain this major increase in insulin storage over proinsulin, stimulus-dependent secretion from pulse-labeled PC1-expressing GH4C1 cells was monitored every 30 min throughout the chase. As shown in Figure 9, there was marked stimulated proinsulin secretion at 30–60, 60–90, and 90–120 min of chase. However, as the chase progressed, stimulus-dependent secretion of labeled proinsulin waned (Figure 9). In contrast, labeled insulin behaved as an efficient granule storage marker at all chase times. Quantitative scanning data from the fluorograph for paired versions (unstimulated versus stimulated) of all labeled peptide species are shown in the bar graphs in Figure 9B. Together with the results shown in Figure 2, these data suggest that in spite of proinsulin entry into IGs of GH4C1 cells, proinsulin is poorly stored thereafter, rendering unlikely a significant noncatalytic role for PC1 either as a sorting receptor or as a helper protein for proinsulin storage within the regulated secretory pathway. In contrast, labeled insulin is formed only inefficiently in IGs of PC1-expressing GH4C1 cells, yet it is well stored thereafter. Such findings seem consistent with the strong homotypic polymerization properties of insulin that are not shared by proinsulin (Steiner, 1973).
Figure 9.
Entry and storage of proinsulin in secretory granules of GH4C1 cells. (A) Multiple identical wells of cells were pulse labeled with 35S-amino acids as in Figure 6. At different chase intervals, a 30-min secretion period (ending with the time shown below the gels) was collected under unstimulated (−) or stimulated (+) conditions. The media were immunoprecipitated and analyzed by Tricine–urea–SDS-PAGE and fluorography; the positions of insulin and proinsulin are marked, and a conversion intermediate migrates above proinsulin in unstimulated and stimulated media. (B) The bar graphs show quantitation of the data as arbitrary units with paired bars of unstimulated versus stimulated secretion as a function of chase time for each of the three species of labeled peptide in the fluorograph (C, conversion intermediate; P, proinsulin; I, insulin). At early chase times, proinsulin entry into the stimulus-dependent secretory pathway is clear, although its utility as a granule marker wanes as a function of chase time. In contrast, insulin behaves as an ideal granule marker from the moment it first appears.
Oddly, heterologous expression of secretory proteins with strong homotypic polymerization properties has in several instances been shown to lead to segregation into distinct dense-core organelles (i.e., other than endogenous granules) that do not undergo regulated exocytosis (Wagner et al., 1991; Voorberg et al., 1993; Castle et al., 1995). This raises the question of whether the efficient intragranular storage of insulin after PC1 expression in GH4C1 cells might somehow be a fortuitous result reflecting unanticipated heterotypic interactions, such as with endogenous prolactin that is manufactured in much higher quantities. Therefore, we screened PC12 cells for insulin storage in granules. Importantly, PC12 cells not expressing prohormone convertases were unable to synthesize insulin, whereas PC12 cells stably expressing PC1 converted a modest portion to insulin with efficiency and kinetics almost identical to those in GH4C1 cell clones (our unpublished results). After RDA-mediated expression of human proinsulin in PC12 cells with or without stable PC1 expression (see MATERIALS AND METHODS), newly synthesized proinsulin was released in large quantity into the early unstimulated chase medium, and only a very modest stimulus-dependent secretion of labeled proinsulin could be elicited during a subsequent secretagogue exposure (Figure 10). However, pulse-labeled insulin was hardly lost in early unstimulated secretion from PC12-PC1 cells; rather, it was released in response to a subsequent 30-min secretagogue stimulation (Figure 10). These data strongly support the idea that, regardless of the poor proinsulin storage (Reaves et al., 1990), insulin storage is enhanced by the specialized intragranule environment of neuroendocrine cells (i.e., GH4C1, PC12, AtT20, and β-cells), in spite of the fact that many of the major endogenous granule components vary significantly between these cell types.
Figure 10.
Storage of newly synthesized proinsulin and insulin in the secretory granules of control PC12 cells (−PC1) or PC12 cells stably expressing PC1 (+PC1). The cells were pulse labeled and chased for the times indicated under unstimulated (−) or stimulated (+) conditions, as described in MATERIALS AND METHODS. Despite the fact that conversion to insulin is quite inefficient in these cells (as is the case in GH4C1 cells), little or no insulin is lost during early unstimulated secretion, whereas relatively little proinsulin remains in the secretory granule storage pool after 3 h of chase.
An In Vitro Extractability Assay Supports Biophysical Differences between Proinsulin and Insulin in Transfected Cell Culture Models
In pancreatic β-cells in which insulin is the predominantly expressed protein (Hutton, 1989), homotypic polymerization follows proinsulin-to-insulin conversion (Kuliawat and Arvan, 1994). To determine if this change in biophysical properties might be relevant in the context of the transfected protein expressed at much lower levels in a foreign cell type, we followed the methods described by Chanat and Huttner (1991) and Schmidt and Moore (1994) for an in vitro assay to compare proinsulin and insulin solubility (extractability) from a saponin-permeabilized organelle fraction in an “aggregative milieu” (see MATERIALS AND METHODS). As shown in Figure 11, from GH4C1 as well as PC12-PC1 cells, most insulin was insoluble, whereas a major fraction of proinsulin was extracted into the supernatant. As expected (Giannattasio et al., 1975), prolactin (lower panel) exhibited behavior similar to that of insulin. These data support the idea that proinsulin and insulin exhibit differences in biophysical properties even when expressed in heterologous cell types that are primarily dedicated to the synthesis of unrelated secretory granule contents.
Figure 11.
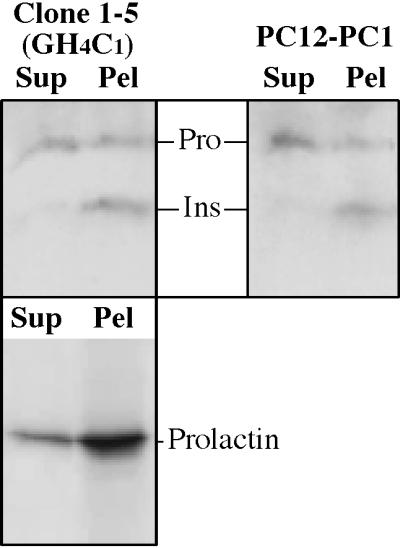
An in vitro extractability assay for proinsulin and insulin in PC1-expressing GH4C1 cells (clone 1-5) and PC12 cells. Crude granule fractions from these cells were permeabilized with 0.5 mg/ml saponin and extracted at pH 6.0 in the presence of 10 mM CaCl2 before sedimentation at 500,000 × g × min (see MATERIALS AND METHODS). The supernatant and pellet fractions (Sup and Pel, respectively) were analyzed by Western blotting with anti-insulin antibody. The positions of proinsulin (Pro) and insulin (Ins) are indicated. As a control, prolactin, the endogenous granule content protein, was analyzed in PC1-expressing GH4C1 cells (lower panel).
DISCUSSION
Classically, it has been reported that “sorting to granules” of endoproteolytically generated peptide hormones is typically more efficient than that of their precursors, regardless of whether these precursors are expressed endogenously (Gumbiner and Kelly, 1981; Tooze et al., 1987; Sossin et al., 1990) or after transfection (Stoller and Shields, 1988; Chu et al., 1990; Jung et al., 1993). In most if not all of these classic studies, no special mechanistic significance had been attributed to the efficient storage of fully processed hormones. Rather, this was felt to reflect the fact that most processed hormones are generated after the proproteins are contained within granules (Orci et al., 1985, 1987b; Stoeckel et al., 1985; Steiner et al., 1987; Tooze et al., 1987; Benjannet et al., 1992; Zhou et al., 1993; Huang and Arvan, 1994; Schmidt and Moore, 1995; Fernandez et al., 1997; Jutras et al., 1997; Tanaka et al., 1997; Urbe et al., 1997), whereas a failure to capture prohormones into forming granules would be coupled to constitutive secretion of these precursors (Bauerfeind et al., 1994). However, the more recent sorting-by-retention model of granule-based sorting (Arvan and Castle, 1998; Dumermuth and Moore, 1998; Thiele and Huttner, 1998b; Tooze, 1998) leaves open the possibility that some prohormones (as well as partially processed prohormones and other luminal proteins) may be removed from maturing secretory granules, with important consequences for the efficiency of regulated secretory protein targeting.
In pancreatic β-cells, processing to insulin has been shown to facilitate retention within maturing secretory granules (Huang and Arvan, 1994; Kuliawat and Arvan, 1994), and this is dependent on carboxypeptidase activity (Naggert et al., 1995; Irminger et al., 1997; Varlamov et al., 1997) and endopeptidase activities (Smeekens et al., 1991, 1992; Halban, 1994; Furuta et al., 1998), particularly PC1. Because PC1-mediated cleavage of human proinsulin at the usual R31R32 site (the “PC1 site”) facilitates cleavage of the second, K64R65 site (Docherty et al., 1989; Rhodes et al., 1992), PC1-mediated cleavage takes on special significance (Smeekens et al., 1992; Sizonenko et al., 1993). In GH4C1 cells, it has been shown that recombinant PC1 selectively processes its substrates (such as human prorenin) within secretory granules (Benjannet et al., 1992; Jutras et al., 1997).
In PC1-expressing GH4C1 cells, proinsulin arrives within 60 min of chase in secretory granules (Figure 6A), wherein conversion to insulin takes place (Figure 6C), with a maximum rate that occurs in the same period as that of authentic islet β-cells (Rhodes and Halban, 1987). However, compared with that in islet β-cells (Steiner et al., 1986), insulin formation in the granules of PC1-expressing GH4C1 cells is inefficient, providing a unique opportunity to compare the relative abilities of uncleaved proinsulin and insulin to be stored within the same cells. Such an analysis yields no evidence to support an effect of PC1 expression on the storage of proinsulin in secretory granules, rendering unlikely a significant noncatalytic action of PC1 in the sorting of this peptide hormone.
Remarkably, upon expression of PC1, GH4C1 cells (labeled to approach steady state) increase their intragranular storage of the transfected hormone in direct relation to the amount of insulin produced (Figure 7). However, prohormone convertase expression is clearly not required for secretory granule biogenesis (Figure 5), nor does it seem to be required for proinsulin entry into the IG compartment (Figure 2) (Irminger et al., 1997; Varlamov et al., 1997; Furuta et al., 1998). Indeed, in the PC1-expressing GH4C1 cells, there is major initial entry of proinsulin into the secretory granules, although proinsulin storage is not increased over the levels seen in GH4C1 parental cells (Figures 7 and 8). Instead, proinsulin behaves as an increasingly poor granule marker as a function of chase time, whereas insulin behaves as a nearly perfect secretory granule marker (Figure 9).
These findings may seem surprising because the expression of secretory proteins with strong homotypic condensation properties has not infrequently been shown to lead to their failure to be targeted to granules in foreign cell types, resulting instead in accumulation in completely novel dense-core organelles that exclude granule markers (Wagner et al., 1991; Colomer et al., 1994; Castle et al., 1995). However, PC1 expression in GH4C1 cells (Figures 8 and 9), PC12 cells (Figure 10), and AtT20 cells (Figures 1 and 8), i.e., cell types that store quite different mixtures of endocrine secretory proteins, leads in each case to a fraction of the heterologously expressed proinsulin being converted to insulin, which is stored with high efficiency in secretory granules, although the unconverted prohormone is not stored efficiently (Moore et al., 1983). Early studies led to the conclusion that the insulin-sorting determinant for granules resides in the mature peptide hormone (Powell et al., 1988); however, only recently has this finding begun to be explained by a growing number of studies that point to significant constitutive-like secretory traffic even in cultured cells (Milgram et al., 1994; Castle et al., 1997; Ciccotosto et al., 1999), which causes major selective loss of unprocessed prohormone and processing intermediates from maturing granules (Dumermuth and Moore, 1998). Storage in granules that is more efficient for fully processed hormones than for unprocessed prohormones is unlikely to be explained by sorting mechanisms that operate before the processing events have taken place.
The evidence suggests that for proinsulin, augmented sorting by PC1 primarily involves the catalytically mediated conversion to processed forms within maturing secretory granules, which tends to favor hormone retention (Arvan and Castle, 1998). Thus, we conclude that the inability to endoproteolytically generate insulin can explain the previously described dilemma of why parental GH4C1 cells are unable to efficiently store proinsulin in secretory granules (Reaves et al., 1990). Perhaps this behavior will help to explain granule storage of processed products from other peptide hormone precursors as well. Nevertheless, it must be acknowledged that the current findings are not inconsistent with the possibility that expression of CPE, in addition to its obvious enzymatic activity (Irminger et al., 1997; Varlamov et al., 1997), could also contribute via noncatalytic cocondensation within maturing secretory granules (Rindler, 1998) to secretory protein sorting (Cool et al., 1997; Shen and Loh, 1997; Cool and Loh, 1998; Normant and Loh, 1998). This is equally true for the enhanced hormone storage mediated by the expression of chromogranins (Natori and Huttner, 1996).
The findings presented in this report may also help to explain an earlier conclusion that multimeric assembly is probably not important for regulated secretory protein sorting, because rat proinsulin transfected into AtT20 cells was found to be largely extractable into the soluble phase after permeabilization under aggregative ionic conditions (Schmidt and Moore, 1994). Indeed, we have confirmed these findings with transfected human proinsulin, but we have also expanded the analysis to include the essential point that, in contrast, insulin expressed in these cells is poorly extractable under identical conditions (similar to what is observed for endogenous prolactin; Figure 11). Of course, in vitro ionic exposure can only approximate the conditions found within intact granules. Also, because constitutive-like secretion appears to be a dynamic process that is limited to the granule maturation period, it is not a simple matter to quantitatively relate the degree of protein extractability in vitro to the degree of constitutive-like secretion under in vivo conditions (Figure 8). However, the data in Figure 11 support a correlation between luminal protein condensation and storage in secretory granules.
Together, the present findings provide a rationale for the observed major differences in the handling of unprocessed versus fully processed peptide hormones after their arrival in secretory granules. Allowing secretory proteins such as proinsulin to change their biophysical state (via endoproteolysis) after entry into secretory granules may be one way to ensure proper targeting and efficient capture of these molecules in the regulated secretory pathway. However, condensation of different regulated secretory proteins may begin at different stages along the secretory pathway, i.e., within granules (Kuliawat and Arvan, 1994), within the TGN (Chanat and Huttner, 1991), or perhaps even more proximally (Rambourg et al., 1988). How widely applicable prohormone endoproteolysis is as a general mechanism for enhancing the efficiency of intragranular peptide hormone storage in cells other than β-cells (Sossin et al., 1990; Jung et al., 1993) remains to be determined. Indeed, although insulin is condensed into insoluble granule cores (Michael et al., 1987), so are certain other polypeptide hormones that do not require any endoproteolytic cleavage (Giannattasio et al., 1975), suggesting that other storage features, most notably a specialized ionic milieu designed to match the granule type (Howell et al., 1978; Arvan and Castle, 1986; Orci et al., 1986, 1987a; Chanat and Huttner, 1991; Kuliawat and Arvan, 1994; Sun et al., 1996), also come into play. Obviously, the earlier that condensation occurs, the more questions are raised about how the condensed proteins can advance along the pathway and end up stored in granules. One recent view (Glick and Malholtra, 1998) is that this process could represent progressive sorting that takes place during cisternal maturation of the Golgi complex (Bonfanti et al., 1998). If cisternal maturation proves to be true, then granule protein sorting via interactions with the luminal side of a membrane domain destined to become a granule (Glombik et al., 1999) and granule protein sorting via the removal of poorly stored constituents (this report) are just two aspects of a single, continuous maturation process.
ACKNOWLEDGMENTS
We are indebted to Drs. C. Newgard and Sam Clarke for providing and instructing us on the use of RDA expression vectors. We thank Dr. P. Dannies for providing the GH4C1 strain and instructions for handling these cells. We are grateful to Dr. N. Seidah and to Dr. D. Steiner (University of Chicago, Chicago, IL) for providing plasmids encoding PC1 and PC2 and to Drs. E. Eipper, R. Mains, I. Lindberg, and L. Devi for providing aliquots of antisera against PC1 and PC2. We acknowledge the assistance of Drs. A.F. Parlow and F. Reinhardt for immunoprecipitating antibodies to rodent prolactin. We also acknowledge the assistance of the Analytical Imaging Facility at the Albert Einstein College of Medicine. This work was supported by National Institutes of Health grant DK48280 to P.A.
REFERENCES
- Arvan P, Castle D. Sorting and storage during secretory granule biogenesis: looking backward and looking forward. Biochem J. 1998;332:593–610. doi: 10.1042/bj3320593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvan P, Castle JD. Isolated secretion granules from parotid glands of chronically stimulated rats possess an alkaline internal pH and inward-directed H+ pump activity. J Cell Biol. 1986;103:1257–1267. doi: 10.1083/jcb.103.4.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvan P, Kuliawat R, Prabakaran D, Zavacki AM, Elahi D, Wang S, Pilkey D. Protein discharge from immature secretory granules displays both regulated and constitutive characteristics. J Biol Chem. 1991;266:14171–14174. [PubMed] [Google Scholar]
- Bauerfeind R, Ohashi M, Huttner WB. Biogenesis of secretory granules and synaptic vesicles. Ann NY Acad Sci. 1994;733:233–244. doi: 10.1111/j.1749-6632.1994.tb17273.x. [DOI] [PubMed] [Google Scholar]
- Benjannet S, Reudelhuber T, Mercure C, Rondeau N, Chretien M, Seidah NG. Proprotein conversion is determined by a multiplicity of factors including convertase processing, substrate specificity, and intracellular environment: cell type-specific processing of human prorenin by the convertase PC1. J Biol Chem. 1992;267:11417–11423. [PubMed] [Google Scholar]
- Bloomquist BT, Eipper BA, Mains RE. Prohormone-converting enzymes: regulation and evaluation of function using antisense RNA. Mol Endocrinol. 1991;5:2014–2024. doi: 10.1210/mend-5-12-2014. [DOI] [PubMed] [Google Scholar]
- Blundell TL, Cutfield JF, Cutfield SM, Dodson EJ, Dodson GG, Hodgkin DC, Mercola DA. Three-dimensional atomic structure of insulin and its relationship to activity. Diabetes. 1972;21(suppl 2):492–505. doi: 10.2337/diab.21.2.s492. [DOI] [PubMed] [Google Scholar]
- Bonfanti L, Mironov AAJ, Martinez-Menarguez JA, Martella O, Fusella A, Baldassarre M, Buccione R, Geuze HJ, Mironov AA, Luini A. Procollagen traverses the Golgi stack without leaving the lumen of cisternae: evidence for cisternal maturation. Cell. 1998;95:993–1003. doi: 10.1016/s0092-8674(00)81723-7. [DOI] [PubMed] [Google Scholar]
- Brechler V, Chu WN, Baxter JD, Thibault G, Reudelhuber TL. A protease processing site is essential for prorenin sorting to the regulated secretory pathway. J Biol Chem. 1996;271:20636–20640. doi: 10.1074/jbc.271.34.20636. [DOI] [PubMed] [Google Scholar]
- Carnell L, Moore H-PH. Transport via the regulated secretory pathway in semi-intact PC12 cells: role of intra-cisternal calcium and pH in the transport and sorting of secretogranin II. J Cell Biol. 1994;127:693–705. doi: 10.1083/jcb.127.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll RJ, Hammer RE, Chan SJ, Swift HH, Rubenstein AH, Steiner DF. A mutant human proinsulin is secreted from islets of Langerhans in increased amounts via an unregulated pathway. Proc Natl Acad Sci USA. 1988;85:8943–8947. doi: 10.1073/pnas.85.23.8943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle AM, Huang AY, Castle JD. Passive sorting in maturing granules of AtT-20 cells: the entry and exit of salivary amylase and proline-rich protein. J Cell Biol. 1997;138:45–54. doi: 10.1083/jcb.138.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle AM, Schwarzbauer JE, Wright RL, Castle JD. Differential targeting of recombinant fibronectins in AtT-20 cells based on their efficiency of aggregation. J Cell Sci. 1995;108:3827–3837. doi: 10.1242/jcs.108.12.3827. [DOI] [PubMed] [Google Scholar]
- Chanat E, Huttner WB. Milieu-induced, selective aggregation of regulated secretory proteins in the trans-Golgi network. J Cell Biol. 1991;115:1505–1520. doi: 10.1083/jcb.115.6.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu WN, Baxter JD, Reudelhuber TL. A targeting sequence for dense secretory granules resides in the active renin protein moiety of human preprorenin. Mol Endocrinol. 1990;4:1905–1913. doi: 10.1210/mend-4-12-1905. [DOI] [PubMed] [Google Scholar]
- Chung KN, Walter P, Aponte GW, Moore HPH. Molecular sorting in the secretory pathway. Science. 1989;243:192–197. doi: 10.1126/science.2911732. [DOI] [PubMed] [Google Scholar]
- Ciccotosto GD, Schiller MR, Eipper BA, Mains RE. Induction of integral membrane PAM expression in AtT-20 cells alters the storage and trafficking of POMC and PC1. J Cell Biol. 1999;144:459–471. doi: 10.1083/jcb.144.3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colomer V, Lal K, Hoops TC, Rindler MJ. Exocrine granule specific packaging signals are present in the polypeptide moiety of the pancreatic granule membrane protein GP2 and in amylase: implications for protein targeting to secretory granules. EMBO J. 1994;13:3711–3719. doi: 10.1002/j.1460-2075.1994.tb06680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cool DR, Fenger M, Snell CR, Loh YP. Identification of the sorting signal motif within pro-opiomelanocortin for the regulated secretory pathway. J Biol Chem. 1995;270:8723–8729. doi: 10.1074/jbc.270.15.8723. [DOI] [PubMed] [Google Scholar]
- Cool DR, Loh YP. Identification of a sorting signal for the regulated secretory pathway at the N-terminus of pro-opiomelanocortin. Biochimie. 1994;76:265–270. doi: 10.1016/0300-9084(94)90156-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cool DR, Loh YP. Carboxypeptidase E is a sorting receptor for prohormones: binding and kinetic studies. Mol Cell Endocrinol. 1998;139:7–13. doi: 10.1016/s0303-7207(98)00081-1. [DOI] [PubMed] [Google Scholar]
- Cool DR, Normant E, Shen F-s, Chen H-C, Pannel L, Zhang Y, Loh YP. Carboxypeptidase E is a regulated secretory pathway sorting receptor: genetic obliteration leads to endocrine disorders in Cpefat mice. Cell. 1997;88:73–83. doi: 10.1016/s0092-8674(00)81860-7. [DOI] [PubMed] [Google Scholar]
- Docherty K, Rhodes CJ, Taylor NA, Shennan KI, Hutton JC. Proinsulin endopeptidase substrate specificities defined by site-directed mutagenesis of proinsulin. J Biol Chem. 1989;264:18335–18339. [PubMed] [Google Scholar]
- Dumermuth E, Moore HP. Analysis of constitutive and constitutive-like secretion in semi-intact pituitary cells. Methods. 1998;16:188–197. doi: 10.1006/meth.1998.0666. [DOI] [PubMed] [Google Scholar]
- Ferber S, Gross DJ, Villa-Komaroff L, Danehy F, Vollenweider F, Meyer K, Loekaen MR, Kahn CR, Halban PA. Heterogeneity of expression and secretion of native and mutant [AspB10]insulin in AtT20 cells. Mol Endocrinol. 1991;5:319–326. doi: 10.1210/mend-5-3-319. [DOI] [PubMed] [Google Scholar]
- Fernandez CJ, Haugwitz M, Eaton B, Moore H-PH. Distinct molecular events during secretory granule biogenesis revealed by sensitivities to brefeldin A. Mol Biol Cell. 1997;8:2171–2185. doi: 10.1091/mbc.8.11.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank BH, Veros AJ. Physical studies on proinsulin: association behavior and conformation in solution. Biochem Biophys Res Commun. 1968;32:155–160. doi: 10.1016/0006-291x(68)90362-8. [DOI] [PubMed] [Google Scholar]
- Frank BH, Veros AJ. Interaction of zinc with proinsulin. Biochem Biophys Res Commun. 1970;38:284–289. doi: 10.1016/0006-291x(70)90710-2. [DOI] [PubMed] [Google Scholar]
- Fricker LD, Berman YL, Leiter EH, Devi LA. Carboxypeptidase E activity is deficient in mice with the fat mutation: effect on peptide processing. J Biol Chem. 1996;271:30619–30624. doi: 10.1074/jbc.271.48.30619. [DOI] [PubMed] [Google Scholar]
- Furuta M, Carroll R, Martin S, Swift HH, Ravazzola M, Orci L, Steiner DF. Incomplete processing of proinsulin to insulin accompanied by elevation of des-31,32 proinsulin intermediates in islets of mice lacking active PC2. J Biol Chem. 1998;273:3431–3437. doi: 10.1074/jbc.273.6.3431. [DOI] [PubMed] [Google Scholar]
- Giannattasio G, Zanini A, Meldolesi J. J. Cell Biol. 64, 246–251. 1975. Molecular organization of rat prolactin granules. I. In vitro stability of intact and “membraneless” granules. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick BS, Malholtra V. The curious state of the Golgi apparatus. Cell. 1998;95:883–889. doi: 10.1016/s0092-8674(00)81713-4. [DOI] [PubMed] [Google Scholar]
- Glombik MM, Kromer A, Salm T, Huttner WB, Gerdes H-H. The disulfide-bonded loop of chromogranin B mediates membrane binding and directs sorting from the trans-Golgi network to secretory granules. EMBO J. 1999;18:1059–1070. doi: 10.1093/emboj/18.4.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant PT, Coombs TL, Frank BH. Differences in the nature of the interaction of insulin and proinsulin with zinc. Biochem J. 1972;126:433–440. doi: 10.1042/bj1260433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross DJ, Halban PA, Kahn CR, Weir GC, Villa-Komaroff L. Partial diversion of a mutant proinsulin (B10 aspartic acid) from the regulated to the constitutive secretory pathway in AtT20 cells. Proc Natl Acad Sci USA. 1989a;86:4107–4111. doi: 10.1073/pnas.86.11.4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross DJ, Villa-Komaroff L, Kahn CR, Weir GC, Halban PA. Deletion of a highly conserved tetrapeptide sequence of the proinsulin connecting peptide (C-peptide) inhibits proinsulin to insulin conversion. J Biol Chem. 1989b;264:21486–21490. [PubMed] [Google Scholar]
- Gumbiner B, Kelly RB. Secretory granules of an anterior pituitary cell line, AtT-20, contain only mature forms of corticotropin and beta-lipotropin. Proc Natl Acad Sci USA. 1981;78:318–322. doi: 10.1073/pnas.78.1.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halban PA. Proinsulin processing in the regulated and the constitutive secretory pathway. Diabetologia. 1994;37:S65–S72. doi: 10.1007/BF00400828. [DOI] [PubMed] [Google Scholar]
- Hide I, Bennett JP, Pizzey A, Boonen G, Bar-Sagi D, Gomperts B, Tatham PER. Degranulation of individual mast cells in response to Ca2+ and guanine nucleotides: an all-or-none event. J Cell Biol. 1993;123:585–593. doi: 10.1083/jcb.123.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell SL, Tyhurst M, Duvefelt H, Andersson A, Hellerstrom C. Role of zinc and calcium in the formation and storage of insulin in the pancreatic b-cell. Cell Tissue Res. 1978;188:107–118. doi: 10.1007/BF00220518. [DOI] [PubMed] [Google Scholar]
- Huang XF, Arvan P. Formation of the insulin-containing secretory granule core occurs within immature b-granules. J Biol Chem. 1994;269:20838–20844. [PubMed] [Google Scholar]
- Huang XF, Arvan P. Intracellular transport of proinsulin in pancreatic b-cells: structural maturation probed by disulfide accessibility. J Biol Chem. 1995;270:20417–20423. doi: 10.1074/jbc.270.35.20417. [DOI] [PubMed] [Google Scholar]
- Hutton JC. The insulin secretory granule. Diabetologia. 1989;32:271–281. doi: 10.1007/BF00265542. [DOI] [PubMed] [Google Scholar]
- Irminger J-C, Verchere CB, Meyer K, Halban PA. Proinsulin targeting to the regulated pathway is not impaired in carboxypeptidase E-deficient Cpefat/Cpefat mice. J Biol Chem. 1997;272:27532–27534. doi: 10.1074/jbc.272.44.27532. [DOI] [PubMed] [Google Scholar]
- Irminger JC, Vollenweider FM, Neerman-Arbez M, Halban PA. Human proinsulin conversion in the regulated and the constitutive pathways of transfected AtT20 cells. J Biol Chem. 1994;269:1756–1762. [PubMed] [Google Scholar]
- Jung LJ, Kreiner T, Scheller RH. Expression of mutant ELH prohormones in AtT-20 cells: the relationship between prohormone processing and sorting. J Cell Biol. 1993;121:11–21. doi: 10.1083/jcb.121.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutras I, Seidah NG, Reudelhuber TL, Brechler V. Two activation states of the prohormone convertase PC1 in the secretory pathway. J Biol Chem. 1997;272:15184–15188. doi: 10.1074/jbc.272.24.15184. [DOI] [PubMed] [Google Scholar]
- Kaufmann JE, Irminger JC, Mungall J, Halban PA. Proinsulin conversion in GH3 cells after coexpression of human proinsulin with the endoproteases PC2 and/or PC3. Diabetes. 1997;46:978–982. doi: 10.2337/diab.46.6.978. [DOI] [PubMed] [Google Scholar]
- Kuliawat R, Arvan P. Protein targeting via the “constitutive-like” secretory pathway in isolated pancreatic islets: passive sorting in the immature granule compartment. J Cell Biol. 1992;118:521–529. doi: 10.1083/jcb.118.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuliawat R, Arvan P. Distinct molecular mechanisms for protein sorting within immature secretory granules of pancreatic β-cells. J Cell Biol. 1994;126:77–86. doi: 10.1083/jcb.126.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuliawat R, Klumperman J, Ludwig T, Arvan P. Differential sorting of lysosomal enzymes out of the regulated secretory pathway in pancreatic β-cells. J Cell Biol. 1997;137:595–608. doi: 10.1083/jcb.137.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh YP. Protein secretion: puzzling receptors. Curr Biol. 1998;8:R41. doi: 10.1016/s0960-9822(98)70029-6. [DOI] [PubMed] [Google Scholar]
- Mains RE, Eipper BA. Secretion and regulation of two biosynthetic enzyme activities, peptidyl-glycine alpha-amidating monooxygenase and a carboxypeptidase, by mouse pituitary corticotropic tumor cells. Endocrinology. 1984;115:1683–1690. doi: 10.1210/endo-115-5-1683. [DOI] [PubMed] [Google Scholar]
- Michael J, Carroll R, Swift HH, Steiner DF. Studies on the molecular organization of rat insulin secretory granules. J Biol Chem. 1987;262:16531–16535. [PubMed] [Google Scholar]
- Milgram SL, Eipper BA, Mains RE. Differential trafficking of soluble and integral membrane secretory granule-associated proteins. J Cell Biol. 1994;124:33–41. doi: 10.1083/jcb.124.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore HPH, Walker MD, Lee F, Kelly RB. Expressing a human proinsulin cDNA in a mouse ACTH-secreting cell: intracellular storage, proteolytic processing, and secretion on stimulation. Cell. 1983;35:531–538. doi: 10.1016/0092-8674(83)90187-3. [DOI] [PubMed] [Google Scholar]
- Naggert JK, Fricker LD, Varlamov O, Nishina PM, Rouille Y, Steiner DF, Carroll RJ, Paigen BJ, Leiter EH. Hyperproinsulinaemia in obese fat/fat mice associated with carboxypeptidase E mutation which reduces enzyme activity. Nat Genet. 1995;10:135–142. doi: 10.1038/ng0695-135. [DOI] [PubMed] [Google Scholar]
- Natori S, Huttner WB. Chromogranin B (secretogranin I) promotes sorting to the regulated secretory pathway of processing intermediates derived from a peptide hormone precursor. Proc Natl Acad Sci USA. 1996;93:4431–4436. doi: 10.1073/pnas.93.9.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normant E, Loh YP. Depletion of carboxypeptidase E, a regulated secretory pathway sorting receptor, causes misrouting and constitutive secretion of proinsulin and proenkephalin, but not chromogranin A. Endocrinology. 1998;139:2137–2145. doi: 10.1210/endo.139.4.5951. [DOI] [PubMed] [Google Scholar]
- Orci L, Ravazzola M, Amherdt M, Madsen O, Perrelet A, Vassalli J-D, Anderson RGW. Conversion of proinsulin to insulin occurs coordinately with acidification of maturing secretory vesicles. J Cell Biol. 1986;103:2273–2281. doi: 10.1083/jcb.103.6.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L, Ravazzola M, Amherdt M, Madsen O, Vassalli JD. Direct identification of prohormone conversion site in insulin-secreting cells. Cell. 1985;42:671–681. doi: 10.1016/0092-8674(85)90124-2. [DOI] [PubMed] [Google Scholar]
- Orci L, Ravazzola M, Anderson RGW. The condensing vacuole of exocrine cells is more acidic than the mature secretory vesicle. Nature. 1987a;326:77–79. doi: 10.1038/326077a0. [DOI] [PubMed] [Google Scholar]
- Orci L, Ravazzola M, Storch MJ, Anderson RGW, Vassalli JD, Perrelet A. Proteolytic maturation of insulin is a post-Golgi event which occurs in acidifying clathrin-coated secretory vesicles. Cell. 1987b;49:865–868. doi: 10.1016/0092-8674(87)90624-6. [DOI] [PubMed] [Google Scholar]
- Powell SK, Orci L, Craik CS, Moore HPH. Efficient targeting to storage granules of human proinsulins with altered propeptide domain. J Cell Biol. 1988;106:1843–1851. doi: 10.1083/jcb.106.6.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn D, Orci L, Ravazzola M, Moore H-PH. Intracellular transport and sorting of mutant human proinsulins that fail to form hexamers. J Cell Biol. 1991;113:987–996. doi: 10.1083/jcb.113.5.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambourg A, Clermont Y, Hermo L. Formation of secretion granules in the Golgi apparatus of pancreatic acinar cells of the rat. Am J Anat. 1988;183:187–199. doi: 10.1002/aja.1001830302. [DOI] [PubMed] [Google Scholar]
- Reaves BJ, VanItallie CM, Moore HPH, Dannies PS. Prolactin and insulin are targeted to the regulated pathway in GH4C1 cells, but their storage is differentially regulated. Mol Endocrinol. 1990;4:1017–1026. doi: 10.1210/mend-4-7-1017. [DOI] [PubMed] [Google Scholar]
- Reggio H, Dagorn JC. Ionic interactions between bovine chymotrypsinogen A and chondroitin sulfate A.B.C. J Cell Biol. 1978;78:951–957. doi: 10.1083/jcb.78.3.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes CJ, Halban PA. Newly synthesized proinsulin/insulin and stored insulin are released from pancreatic B cells predominantly via a regulated, rather than a constitutive, pathway. J Cell Biol. 1987;105:145–153. doi: 10.1083/jcb.105.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes CJ, Lincoln B, Shoelson SE. Preferential cleavage of des-31,32-proinsulin over intact proinsulin by the insulin secretory granule type II endopeptidase: implications of a favored route for prohormone processing. J Biol Chem. 1992;267:22719–22727. [PubMed] [Google Scholar]
- Rhodes CJ, Lucas CA, Mutkoski RL, Orci L, Halban PA. Stimulation by ATP of proinsulin to insulin conversion in isolated rat pancreatic islet secretory granules. J Biol Chem. 1987;262:10712–10717. [PubMed] [Google Scholar]
- Rholam M, Nicolas P, Cohen P. Precursors for peptide hormones share common secondary structures forming features at the proteolytic processing sites. FEBS Lett. 1986;207:1–6. doi: 10.1016/0014-5793(86)80002-3. [DOI] [PubMed] [Google Scholar]
- Rindler MJ. Carboxypeptidase E, a peripheral membrane protein implicated in the targeting of hormones to secretory granules, co-aggregates with granule content proteins at acidic pH. J Biol Chem. 1998;273:31180–31185. doi: 10.1074/jbc.273.47.31180. [DOI] [PubMed] [Google Scholar]
- Rouille Y, Martin S, Steiner DF. Differential processing of proglucagon by the subtilisin-like prohormone convertases PC2 and PC3 to generate either glucagon or glucagon-like peptide. J Biol Chem. 1995;270:26488–26496. doi: 10.1074/jbc.270.44.26488. [DOI] [PubMed] [Google Scholar]
- Scammell JG, Burrage TG, Dannies PS. Hormonal induction of secretory granules in a pituitary tumor cell line. Endocrinology. 1986;119:1543–1548. doi: 10.1210/endo-119-4-1543. [DOI] [PubMed] [Google Scholar]
- Schagger H, von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Schmidt WK, Moore H-PH. J. Biol. Chem. 269, 27115–27124. 1994. Synthesis and targeting of insulin-like growth factor-I to the hormone storage granules in an endocrine cell line. [PubMed] [Google Scholar]
- Schmidt WK, Moore HP. Ionic milieu controls the compartment-specific activation of pro-opiomelanocortin processing in AtT-20 cells. Mol Biol Cell. 1995;6:1271–1285. doi: 10.1091/mbc.6.10.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidah NG, Chretien M, Day R. The family of subtilisin/kexin like pro-protein and pro-hormone convertases: divergent or shared functions. Biochimie. 1994;76:197–209. doi: 10.1016/0300-9084(94)90147-3. [DOI] [PubMed] [Google Scholar]
- Seidah NG, Marcinkiewicz M, Benjannet S, Gaspar L, Beaubien G, Mattei MG, Lazure C, Mbikay M, Chretien M. Cloning and primary sequence of a mouse candidate prohormone convertase PC1 homologous to PC2, furin, and Kex2: distinct chromosomal localization and messenger RNA distribution in brain and pituitary compared to PC2. Mol Endocrinol. 1991;5:111–122. doi: 10.1210/mend-5-1-111. [DOI] [PubMed] [Google Scholar]
- Shen F-S, Loh YP. Intracellular misrouting and abnormal secretion of adrenocorticotropin and growth hormone in Cpefat mice associated with a carboxypeptidase E mutation. Proc Natl Acad Sci USA. 1997;94:5314–5319. doi: 10.1073/pnas.94.10.5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sizonenko S, Irminger J-C, Buhler L, Deng S, Morel P, Halban PA. Kinetics of proinsulin conversion in human islets. Diabetes. 1993;42:933–936. doi: 10.2337/diab.42.6.933. [DOI] [PubMed] [Google Scholar]
- Smeekens SP, Avruch AS, LaMendola J, Chan SJ, Steiner DF. Identification of a cDNA encoding a second putative prohormone convertase related to PC2 in AtT20 cells and islets of Langerhans. Proc Natl Acad Sci USA. 1991;88:340–344. doi: 10.1073/pnas.88.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeekens SP, et al. Proinsulin processing by the subtilisin-related proprotein convertases furin, PC2, and PC3. Proc Natl Acad Sci USA. 1992;89:8822–8826. doi: 10.1073/pnas.89.18.8822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sossin WS, Fisher JM, Scheller RH. Sorting within the regulated secretory pathway occurs in the trans-Golgi network. J Cell Biol. 1990;110:1–12. doi: 10.1083/jcb.110.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner DF. Cocrystallization of proinsulin and insulin. Nature. 1973;243:528–530. doi: 10.1038/243528a0. [DOI] [PubMed] [Google Scholar]
- Steiner DF, Chan SJ, Welsh JM, Nielsen D, Michael J, Tager HS, Rubenstein AH. Models of peptide biosynthesis: the molecular and cellular basis of insulin production. Clin Invest Med. 1986;9:328–336. [PubMed] [Google Scholar]
- Steiner DF, Michael J, Houghten R, Mathieu M, Gardner PR, Ravazzola M, Orci L. Use of a synthetic peptide antigen to generate antisera reactive with a proteolytic processing site in native human proinsulin: demonstration of cleavage within clathrin-coated (pro)secretory vesicles. Proc Natl Acad Sci USA. 1987;84:6184–6188. doi: 10.1073/pnas.84.17.6184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckel ME, Schimchowitsch S, Garaud JC, Schmitt G, Vaudry H, Klein MJ, Porte A. Immunocytochemical evidence for intragranular processing of pro-opiomelanocortin in the melanotropic cells of the rabbit. Cell Tissue Res. 1985;242:365–370. doi: 10.1007/BF00214549. [DOI] [PubMed] [Google Scholar]
- Stoller TJ, Shields D. Retrovirus-mediated expression of preprosomatostatin: posttranslational processing, intracellular storage, and secretion of GH3 pituitary cells. J Cell Biol. 1988;107:2087–2095. doi: 10.1083/jcb.107.6.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Li SP, Dannies PS, Lee JC. Properties of human prolactin (PRL) and H27A-PRL, a mutant that does not bind Zn++ Mol Endocrinol. 1996;10:265–271. doi: 10.1210/mend.10.3.8833655. [DOI] [PubMed] [Google Scholar]
- Tam WW, Andreasson KI, Loh YP. The amino-terminal sequence of pro-opiomelanocortin directs intracellular targeting to the regulated secretory pathway. Eur J Cell Biol. 1993;62:294–306. [PubMed] [Google Scholar]
- Tanaka S, Yora T, Nakayama K, Inoue K, Kurosumi K. Proteolytic processing of pro-opiomelanocortin occurs in acidifying secretory granules of AtT-20 cells. J Histochem Cytochem. 1997;45:425–436. doi: 10.1177/002215549704500310. [DOI] [PubMed] [Google Scholar]
- Tatham PER, Duchen MR, Millar J. Monitoring exocytosis from single mast cells by fast voltametry. Pfluegers Arch. 1991;419:409–414. doi: 10.1007/BF00371124. [DOI] [PubMed] [Google Scholar]
- Thiele C, Gerdes HH, Huttner WB. Protein secretion: puzzling receptors. Curr Biol. 1997;7:R496–R500. doi: 10.1016/s0960-9822(06)00247-8. [DOI] [PubMed] [Google Scholar]
- Thiele C, Huttner WB. The disulfide-bonded loop of chromogranins, which is essential for sorting to secretory granules, mediates homodimerization. J Biol Chem. 1998a;273:1223–1231. doi: 10.1074/jbc.273.2.1223. [DOI] [PubMed] [Google Scholar]
- Thiele C, Huttner WB. Protein and lipid sorting from the trans-Golgi network to secretory granules: recent developments. Semin Cell Dev Biol. 1998b;9:511–516. doi: 10.1006/scdb.1998.0259. [DOI] [PubMed] [Google Scholar]
- Tooze J, Hollinshead M, Frank R, Burke B. An antibody specific for an endoproteolytic cleavage site provides evidence that pro-opiomelanocortin is packaged into secretory granules in AtT20 cells before its cleavage. J Cell Biol. 1987;105:155–162. doi: 10.1083/jcb.105.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze SA. Biogenesis of secretory granules in the trans-Golgi network of neuroendocrine and endocrine cells. Biochim Biophys Acta. 1998;1404:231–244. doi: 10.1016/S0167-4889(98)00059-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze SA, Flatmark T, Tooze J, Huttner WB. Characterization of the immature secretory granule, an intermediate in granule biogenesis. J Cell Biol. 1991;115:1491–1504. doi: 10.1083/jcb.115.6.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbe S, Dittie AS, Tooze SA. pH-dependent processing of secretogranin II by the endopeptidase PC2 in isolated immature secretory granules. Biochem J. 1997;321:65–74. doi: 10.1042/bj3210065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlamov O, Fricker LD, Furukawa H, Steiner DF, Langley SH, Leiter EH. β-cell lines derived from transgenic Cpefat/Cpefat mice are defective in carboxypeptidase E and proinsulin processing. Endocrinology. 1997;138:4883–4892. doi: 10.1210/endo.138.11.5506. [DOI] [PubMed] [Google Scholar]
- Verbsky JW, Turkewitz AP. Proteolytic processing and Ca2+-binding activity of dense-core vesicle polypeptides in tetrahymena. Mol Biol Cell. 1998;9:497–511. doi: 10.1091/mbc.9.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vindrola O, Lindberg I. Biosynthesis of the prohormone convertase mPC1 in AtT-20 cells. Mol Endocrinol. 1992;6:1088–1094. doi: 10.1210/mend.6.7.1508222. [DOI] [PubMed] [Google Scholar]
- Voorberg J, Fontijn R, Calafat J, Janssen J, van Mourik JA, Pannekoek H. Biogenesis of von Willebrand factor-containing organelles in heterologous transfected CV-1 cells. EMBO J. 1993;12:749–758. doi: 10.1002/j.1460-2075.1993.tb05709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner DD, Saffaripour S, Bonfanti R, Sadler JE, Cramer EM, Chapman B, Mayadas TN. Induction of specific storage organelles by von Willebrand factor propolypeptide. Cell. 1991;64:403–413. doi: 10.1016/0092-8674(91)90648-i. [DOI] [PubMed] [Google Scholar]
- Weiss MA, Frank BH, Khait I, Pekar A, Heiney R, Shoelson SE, Neuringer LJ. NMR and photo-CIDNP studies of human proinsulin and prohormone processing intermediates with application to endopeptidase recognition. Biochemistry. 1990;29:8389–8401. doi: 10.1021/bi00488a028. [DOI] [PubMed] [Google Scholar]
- Zhou A, Bloomquist BT, Mains RE. The prohormone convertase PC1 and PC2 mediate distinct endoproteolytic cleavages in a strict temporal order during proopiomelanocortin biosynthetic processing. J Biol Chem. 1993;268:1763–1769. [PubMed] [Google Scholar]