Abstract
Recent studies have demonstrated that fumarate addition and carboxylation are two possible mechanisms of anaerobic alkane degradation. In the present study, we surveyed metabolites formed during growth on hexadecane by the sulfate-reducing isolates AK-01 and Hxd3 and by a mixed sulfate-reducing consortium. The cultures were incubated with either protonated or fully deuterated hexadecane; the sulfate-reducing consortium was also incubated with [1,2-13C2]hexadecane. All cultures were extracted, silylated, and analyzed by gas chromatography-mass spectrometry. We detected a suite of metabolites that support a fumarate addition mechanism for hexadecane degradation by AK-01, including methylpentadecylsuccinic acid, 4-methyloctadecanoic acid, 4-methyloctadec-2,3-enoic acid, 2-methylhexadecanoic acid, and tetradecanoic acid. By using d34-hexadecane, mass spectral evidence strongly supporting a carbon skeleton rearrangement of the first intermediate, methylpentadecylsuccinic acid, was demonstrated for AK-01. Evidence indicating hexadecane carboxylation was not found in AK-01 extracts but was observed in Hxd3 extracts. In the mixed sulfate-reducing culture, however, metabolites consistent with both fumarate addition and carboxylation mechanisms of hexadecane degradation were detected, which demonstrates that multiple alkane degradation pathways can occur simultaneously within distinct anaerobic communities. Collectively, these findings underscore that fumarate addition and carboxylation are important alkane degradation mechanisms that may be widespread among phylogenetically and/or physiologically distinct microorganisms.
The contamination of marine and estuarine environments with crude oil or distillate fractions has become the focus of increasing regulation and public concern due to the toxicity of petroleum compounds (5). The remediation of petroleum-contaminated sites is often hindered by the recalcitrance of oil components. Hydrocarbons constitute the largest fraction of any crude oil (38). Aliphatic hydrocarbons, such as alkanes, are among the least reactive compounds because they contain apolar sigma bonds. Due to their chemical inertness, alkanes usually require activation by UV light, high temperatures, or catalysts to participate in chemical reactions (22). An important environmental fate process, however, for alkane transformation in natural environments is biodegradation by microorganisms.
Aerobic biodegradation of n-alkanes is mediated by different bacteria, yeasts, and filamentous fungi (24, 31, 41), and the mechanisms are well described. Under oxic conditions, oxygen is required both for respiration and as a reactant in the enzymatic activation of hydrocarbons. Monooxygenase or dioxygenase enzymes carry out terminal, subterminal, or diterminal oxidation of alkanes (34, 35, 41, 51). Terminal oxidation, resulting in an alkanol, is the most common metabolic pathway. The primary alcohol is further oxidized by dehydrogenases to aldehydes and then to fatty acids, which are metabolized through the β-oxidation pathway (13, 51, 52). The key role that oxygen plays in aerobic alkane transformation led to the belief, for many years, that anaerobic microorganisms could not utilize these compounds as growth substrates.
Research conducted within the last decade has shown that activation of hydrocarbons occurs under anoxic conditions by novel biochemical processes (for reviews, see references 12, 16, 17, 25, and 49). Thus far, at least two mechanisms appear to be important and widespread among several bacterial genera capable of anaerobic hydrocarbon utilization. Carboxylation of naphthalene and phenanthrene has been observed in sulfidogenic consortia (56, 57). Biodegradation of toluene, 2-methylnaphthalene, ethylbenzene, and o-, m- and p-xylenes under denitrifying, sulfate-reducing, iron-reducing, and/or methanogenic conditions can occur by a mechanism commonly referred to as fumarate addition (1, 4, 6, 7, 9, 18, 26, 27, 30). This mechanism has been well characterized for the biotransformation of toluene by denitrifiers wherein the methyl group of toluene is added to the double bond of fumarate to form benzylsuccinate (7, 9, 11, 21). This activation step is catalyzed by the glycyl radical enzyme, benzylsuccinate synthase (10, 18, 28, 32), and is stereospecific, yielding optically pure (R)-(+)-benzylsuccinate (8, 33). Such mechanistic studies ultimately led to the discovery of similar addition reactions that facilitate the biodegradation of linear alkanes.
In the case of n-alkanes, the subterminal carbon can be added to the double bond of fumarate to produce methyl-branched alkylsuccinates. This has been shown for sulfate-reducing consortia (20, 29), a denitrifying isolate (40, 55), and a sulfate-reducing isolate (19). Fumarate addition to cyclic alkanes has also recently been demonstrated (42, 54). The mechanism of n-alkane activation appears to be similar to glycyl radical processes wherein the abstracted hydrogen from the parent compound is retained (19, 29, 40).
To date, the only other mechanism elucidated for n-alkane activation is that of strain Hxd3, a sulfate reducer closely related to the genus Desulfococcus (2, 3). Strain Hxd3 utilizes C12-C20 alkanes and carboxylates hexadecane at the C-3 carbon, with subsequent elimination of the terminal and subterminal carbons (45). As a result, Hxd3 produces C-odd and C-even fatty acids when grown on C-even and C-odd alkanes, respectively (45). This mechanism of n-alkane degradation appears to be unique among strains studied thus far.
In contrast to strain Hxd3, studies have shown that sulfate-reducing strains AK-01, Pnd3, and Desulfatibacillum aliphaticivorans strain CV2803 produce C-even and C-odd fatty acids when grown on C-even and C-odd n-alkanes, respectively (3, 19, 47). Strains CV2803 and AK-01 also produce 2-, 4-, and 6-methyl branched fatty acids (19, 46). So and Young (46) first proposed that anaerobic activation of hexadecane by AK-01 occurred via carbon addition to the subterminal carbon. Metabolite analysis of cultures incubated with 13C-labeled bicarbonate showed that the carbon addition was not the result of a carboxylation mechanism (46).
Thus, one of the objectives of this study was to examine the initial step in the biodegradation of hexadecane by strain AK-01, a sulfate reducer isolated from the Arthur Kill, New York/New Jersey, waterway (47). Similar to strain CV2803, which has been shown to add fumarate to alkanes (19), strain AK-01 is closely related to the genus Desulfatibacillum in the delta subdivision of the Proteobacteria and utilizes C13-C18 alkanes, 1-alkenes (C15 and C16), and 1-alkanols (C15 and C16) as growth substrates (47). We hypothesized that AK-01 activates alkanes via fumarate addition. We compared metabolites produced during hexadecane biodegradation by strain AK-01 to those produced by a sulfate-reducing consortium and to strain Hxd3, which, due to its presumed carboxylation mechanism, served as a negative control for fumarate addition. As first reported by Callaghan et al. (15), we present evidence for fumarate addition to the subterminal carbon of hexadecane by strain AK-01 and for carbon skeleton rearrangement of the fumarate addition product. We also report evidence for both fumarate addition and carboxylation as mechanisms of alkane degradation by a mixed sulfate-reducing consortium. These findings, in addition to other studies, emphasize that both fumarate addition and carboxylation of alkanes are important degradation mechanisms in anoxic environments.
MATERIALS AND METHODS
Inoculum growth conditions.
Strain AK-01 was grown under sulfate-limiting conditions (4 mM and 7 mM Na2SO4) in a mineral salts medium previously described (47). The vitamin stock solution (47) was modified to contain 50 mg of vitamin B12. Thirty milliliters of medium was dispensed into 150-ml serum bottles, crimp-sealed with butyl rubber stoppers, and autoclaved. After a cooling step, filter-sterilized stock solutions were added (47). Strain Hxd3 was also grown under sulfate-limiting conditions (5 mM Na2SO4) in a mineral salts medium (45). The vitamin and selenite-tungstate stock solutions (47) were the same as for AK-01 incubations, and the medium was prepared as it was for the AK-01 cultures. Replicate active (containing 10% inoculum, vol/vol) and sterilized cultures were amended with 15 μl of either H34-hexadecane or d34-hexadecane (approximately 51 μmol). Cultures were incubated horizontally in the dark at 30°C for approximately 8 months.
The hexadecane-degrading, sulfate-reducing consortium used for comparison with strains AK-01 and Hxd3 originated from hydrocarbon-impacted San Diego Bay sediments (14). For these studies, the consortium was incubated with a basal saltwater medium described by Widdel and Bak (53) except that resazurin was added (1 ml liter−1 of a 0.1% solution), and different vitamin and trace element solutions were included (50). For preparation, the medium at pH 7.2 was boiled and cooled under 20:80 CO2-N2, dispensed into 80-ml serum bottles, sealed with Teflon stoppers, and autoclaved. After cooling, the medium was amended with sterile solutions of bicarbonate, carbonate (53), and cysteine-sulfide (1 ml liter−1 of 2.5% solution). To duplicate bottles, 15 μl of either H34-hexadecane, d34-hexadecane, or [1,2-13C2]hexadecane was added (approximately 51 μmol). Cultures were incubated for approximately 11 months in the dark at 28°C.
Metabolic activity was monitored in the active cultures, relative to sterile controls, by measuring the loss of sulfate via ion chromatography as previously described (14, 47).
Metabolites of H34- and d34-hexadecane and [1,2-13C2]hexadecane.
At the end of the incubation period, all cultures were amended with 1 ml of 6 M NaOH and left for 30 min to hydrolyze coenzyme A (CoA) thioesters. The cultures were then acidified to a pH of 2 with 2 ml of 6 M HCl. The replicates were pooled (final volumes were between 80 and 125 ml) and extracted three times with 25 ml of ethyl acetate. Extracts were dried over anhydrous Na2SO4 and concentrated on a rotary evaporator to approximately 4 ml. This extract was further concentrated under a flow of N2 to 100 μl. Extracts were amended with 50 μl of N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA) and heated at 65°C for 10 min before analysis by gas chromatography-mass spectrometry (GC-MS) (29).
Derivatized samples were analyzed on an Agilent 6890N GC system equipped with a DB-5 column (30 m by 0.25-mm internal diameter; Palo Alto, CA). The oven temperature was held at 90°C for 2 min and increased at 4°C per minute to 270°C and held for 5 min. Helium was used as the carrier gas. Mass spectra of metabolites were obtained using an Agilent 5973 mass selective detector. The injection volume was 1 μl. Samples were analyzed in splitless mode. Data were acquired in scan mode from 50 to 600 mass units.
Chemical synthesis of n-hexadecylsuccinic acid.
The mass spectrum of the putative fumarate addition product, methylpentadecylsuccinic acid, was compared to that of a synthesized n-hexadecylsuccinic acid standard. The standard was prepared by base hydrolysis of n-hexadecylsuccinic anhydride as described by Kropp et al. (29) and derivatized with BSTFA prior to GC-MS analysis.
Chemicals.
n-Hexadecane (≥99%), nonadecanoic acid (99%), and tetradecanoic acid (99%) were purchased from Sigma Chemical (St. Louis, MO). 2-Methylhexadecanoic acid (≥95%) and n-hexadecylsuccinic anhydride were acquired from TCI America (Portland, OR). [1,2-13C2]hexadecane (99 atom %) was purchased from Isotec Inc. (Miamisburg, OH). d34-Hexadecane (98.4 atom %) was from CDN Isotopes (Pointe-Claire, PQ, Canada) and Aldrich Chemical Company (Milwaukee, WI). Ethyl acetate (high-performance liquid chromatography grade) was from Fisher Scientific (Pittsburgh, PA), whereas BSTFA was purchased from Sigma Chemical and Pierce (Rockford, IL).
RESULTS
AK-01 cultures incubated with either H34-hexadecane or d34-hexadecane.
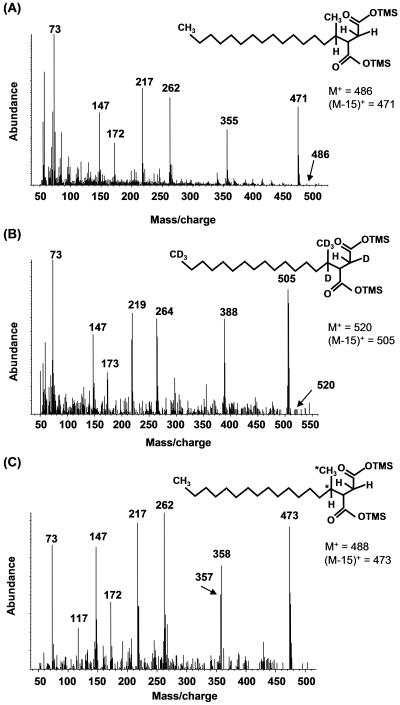
When AK-01 cultures were incubated with H34-hexadecane and subsequently extracted, derivatized, and analyzed by GC-MS, two metabolites eluted closely together but were distinguishable near the n-hexadecylsuccinic acid standard. The mass spectra of these compounds were identical, and the fragmentation features suggest these metabolites are diastereomers of methylpentadecylsuccinic acid (MPA), the presumed fumarate addition product. An example is shown in Fig. 1A. The derivatized metabolite has M+ and (M-15)+ ions at m/z 486 and m/z 471, respectively. The latter ion results from the loss of a methyl group from one of the trimethylsilyl (TMS) moieties, typical of TMS esters (39). The peak at m/z 355 (M-131)+ corresponds to simple cleavage of MPA between the C-2 and C-3 carbons on the succinyl moiety [loss of -CH2COO-Si(CH3)3]. MPA also underwent a McLafferty rearrangement (36) during GC-MS analyses to give a distinctive ion at m/z 262 (23, 29, 44). Further fragmentation of m/z 262 gives rise to the ion at m/z 172 (29), whereas the ion observed at m/z 217 is likely produced during the migration of a TMS group between carboxyl moieties (37). Other major ions included m/z 73, which is due to the TMS group in the BSTFA-derivatized metabolites, and m/z 147, which corresponds to a (CH3)2Si=OSi(CH3)3 ion, indicating that two functional groups have been silylated (39) (Table 1, column A). Protonated MPA was not found in the sterilized AK-01 controls.
FIG. 1.
Mass spectra of the putative silylated metabolite methylpentadecylsuccinic acid from AK-01 cultures incubated with protonated hexadecane (A), AK-01 cultures incubated with d34-hexadecane (B), and the sulfate-reducing consortium incubated with [1,2-13C2]hexadecane (C). Chemical structures represented by the mass spectra are shown in insets. *, 13C-labeled carbon atoms.
TABLE 1.
Mass spectral data of metabolites identified in extracts of strain AK-01 and a sulfate-reducing consortium grown on protonated or labeled hexadecane that support the mechanism of fumarate addition
| Metabolite | Key ions detected under the specified conditionsa
|
||
|---|---|---|---|
| (A) Protonated conditions for strain AK-01 and a sulfate-reducing consortium | (B) Deuterated conditions for strain AK-01 and a sulfate-reducing consortium | (C) 13C-labeled conditions for a sulfate-reducing consortium | |
| Methylpentadecylsuccinic | 486 (M+), 471, 355, 262, 217, | 520 (M+), 505, 388, 264, 218, | 488 (M+), 473, 357, 358, 262, |
| acid | 172, 147, 73 | 219, 173, 147, 73 | 217, 172, 147, 73 |
| 4-Methyloctadecanoic acid | 370 (M+), 355, 313, 145, 132, | 404 (M+), 389, 340, 147, 133, | 372 (M+), 357, 315, 145, 132, |
| 129, 117, 73 | 130, 118, 73 | 129, 117, 73 | |
| 4-Methyloctadec-2,3-enoic | ND | 401, 402 (M+), 386, 387, 339, | ND |
| acid | 147, 132, 129, 117, 73 | ||
| 2-Methylhexadecanoic acid | 342 (M+), 327, 159, 146, 130, | ND | ND |
| 117, 73 | |||
| Tetradecanoic acid | 300 (M+), 285, 145, 132, 129, | 327 (M+), 312, 149, 135, 132, | 300 (M+), 285, 145, 132, 129, |
| 117, 73 | 119, 73 | 117, 73 | |
ND, not detected.
Under deuterated conditions, fumarate addition to d34-hexadecane also produced two metabolites that eluted closely together with identical mass spectra (Fig. 1B and Table 1, column B). Deuterated MPA has M+ and (M-15)+ ions at m/z 520 and m/z 505, respectively, accounting for all 34 deuterium atoms present in the deuterated parent substrate (20, 29, 40). A McLafferty rearrangement produced an ion at m/z 264, which fragments further to m/z 173 (29). The ion at m/z 388 (M+-132) corresponds to simple cleavage between the methylene and methine carbons on the succinic acid portion of the molecule (29) and is also consistent with the presence of one deuterium located at the C-3 carbon of the succinyl moiety. The ions at m/z 218 and m/z 219 presumably formed via long-range migration of a TMS moiety among carboxylic acid groups (37). The ion at m/z 147 could arise from another reaction forming distonic ions with loss of -CH3D to yield m/z 130 (see Fig. 3B). Deuterated MPA was not found in sterile cultures.
FIG. 3.
Formation of the McLafferty rearrangement ion (A) and a distonic ion (B) for protonated 4-methyloctadecanoic acid.
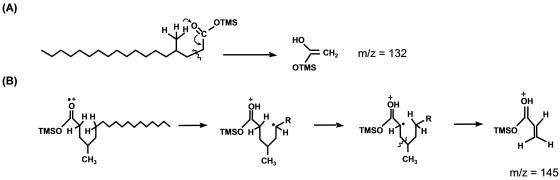
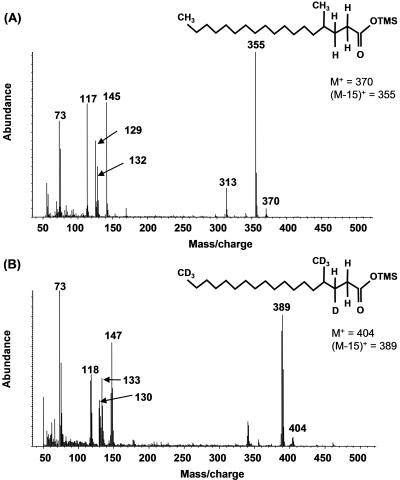
In addition to MPA, several other metabolites were identified during growth of isolate AK-01 with either H34- or d34-hexadecane (Table 1, columns A and B). A metabolite identified as 4-methyloctadecanoic acid was detected, which would result from the loss of CO2 from MPA. Under protonated conditions, this silylated metabolite has a mass (M+) of 370 and an (M-15)+ fragment at m/z 355 (Fig. 2A). The ion at m/z 313 represents an (M-57)+ fragment (loss of -CH2-CH2-CH2CH3), a radical site-driven alkyl group migration to a carbonyl oxygen commonly observed for methyl-branched carboxylic acids (37). The ion at m/z 145 is thought to be the result of a rearrangement in which distonic ions are formed (Fig. 3B) (48). Loss of -CH4 from m/z 145 yields a fragment ion at m/z 129 via a proton transfer reaction. The McLafferty rearrangement ion is at m/z 132 (Fig. 3A) and loss of a methyl radical species from this ion yielded a prominent ion at m/z 117 (37). Although an authentic standard of 4-methyloctadecanoic acid is not commercially available, the above key fragments are the same as in the mass spectra of other commercially available methyl-branched fatty acid standards that were also derivatized and analyzed (data not shown). Further, the mass spectrum of this putative metabolite was compared to that of an authentic nonadecanoic standard which has the same mass. The retention time of this standard was almost 1 min longer than that of the putative metabolite, and it lacked a prominent signal at m/z 313 (spectrum not shown), confirming that the metabolite cannot be nonadecanoic acid. 4-Methyloctadecanoic acid was not found in the AK-01 sterile controls containing protonated hexadecane. More importantly, this metabolite was also identified under deuterated conditions in active cultures (Fig. 2B and Table 1, column B) and not in deuterated sterile controls, indicating that it is indeed a metabolite formed during growth of the bacterium on hexadecane. β-Oxidation of 4-methyloctadecanoic acid would yield 4-methyloctadec-2,3-enoic acid. This metabolite was not identified under protonated conditions. However, under deuterated conditions, β-oxidation of deuterated 4-methyloctadecanoic acid could result in either the loss of a deuterium atom or hydrogen atom. Loss of a deuterium atom would produce M+ and (M-15)+ ions at m/z 401 and m/z 386, respectively. Loss of a hydrogen atom would yield m/z 402 and m/z 387. Fragment ions thus detected at m/z 386, 387, 401, 402, 339, 147, 129, 132, 117, and 73 suggested the formation of deuterated 4-methyloctadec-2,3-enoic acid, but no authentic standard was available for verification (Table 1, column B). This putative intermediate was also not found in sterile controls.
FIG. 2.
Mass spectra of silylated protonated 4-methyloctadecanoic acid (A) and deuterated 4-methyloctadecanoic acid (B) identified in AK-01 incubations.
Hydration of 4-methyloctadec-2,3-enoic acid, subsequent ketone formation, and loss of an acetyl moiety would produce 2-methylhexadecanoic acid (55). This latter compound was positively identified under protonated conditions by comparing it to the retention time and mass spectrum of an authentic standard (Table 1, column A). 2-Methylhexadecanoic acid was not found in sterile controls. Loss of a propionyl group from 2-methylhexadecanoic acid produces tetradecanoic acid, which was also identified under protonated conditions by comparing it to the retention time and mass spectrum of an authentic standard (Table 1, column A). Protonated tetradecanoic acid was detected in all protonated and deuterated samples, including the sterile controls. This was probably the result of the carryover of metabolites from the inoculum. The presence of deuterated tetradecanoic acid (Table 1, column B), however, in active cultures and not in the corresponding deuterated sterile controls strongly suggests that both protonated and deuterated tetradecanoic acid are metabolites formed during hexadecane degradation.
In addition to the identified metabolites which support a mechanism of hexadecane adding to the double bond of fumarate, no evidence suggesting carboxylation of hexadecane, such as deuterated pentadecanoic and tridecanoic acids (see below), was detected in strain AK-01 extracts.
Hxd3 incubated with H34- or d34-hexadecane.
So et al. (45) postulated that alkane degradation by strain Hxd3 involves carboxylation at the C-3 position with elimination of the two adjacent terminal carbons of the alkane, resulting in the production of pentadecanoic acid. The proposed carboxylated intermediate, 2-ethylpentadecanoic acid, was not identified in our study, nor was it identified by So et al. (45). However, using fully deuterated hexadecane as a substrate, we obtained data similar to that of So et al. (45) via the identification of deuterated pentadecanoic acid in Hxd3 extracts (data not shown). The mass spectrum was consistent with a pentadecanoic acid metabolite containing 27 deuterium atoms, wherein one hydrogen atom is located at the C-2 position and another hydrogen atom is located in an unknown position between C-3 and C-5 (fully deuterated pentadecanoic acid is expected to contain 29 deuterium atoms). So et al. (45) suggested that the replacement of two deuterium atoms with hydrogen atoms may be due to isotope exchange during the initial attack mechanism on the alkane. Further, we detected a metabolite whose mass spectrum is consistent with the structure of d26-10-methyl-pentadecanoic acid (data not shown). This metabolite is presumably formed by chain elongation via methylation of the identified d27-pentadecanoic acid, consistent with the findings of So et al. (45). In the Hxd3 extracts, we did not detect any metabolites attesting to a fumarate addition mechanism, including MPA, 4-methyloctadecanoic acid, or 2-methylhexadecanoic acid.
Sulfate-reducing consortium incubated with H34- or d34-hexadecane or [1,2-13C2]hexadecane.
When the sulfate-reducing consortium was incubated with protonated or deuterated hexadecane or [1,2-13C2] hexadecane, several metabolites were detected that were consistent with both fumarate addition and carboxylation mechanisms of alkane degradation.
With respect to fumarate addition under protonated conditions, MPA eluted as two peaks with identical mass spectra. 4-Methyloctadecanoic acid, 2-methylhexadecanoic acid and tetradecanoic acid were also identified. Fragmentation patterns were identical to those found in AK-01 incubations (Table 1, column A). Under deuterated conditions, MPA eluted as two peaks with identical mass spectra. Deuterated 4-methyloctadecanoic acid, 4-methyloctadec-2,3-enoic acid, and tetradecanoic acid were also identified (Table 1, column B) and not detected in controls. When the consortium was incubated with [1,2-13C2]hexadecane, the metabolites detected were 2 atomic mass units higher than the protonated metabolites until cleavage to tetradecanoic acid (Table 1, column C). The 2 atomic mass units account for the terminal and subterminal 13C atoms. The fumarate addition product eluted as two peaks with identical mass spectra (Fig. 1C). The M+ and (M-15)+ ions occurred at 488 and 473, respectively. The ion at m/z 358 was due to simple cleavage between the C-2 and C-3 carbons of the succinyl moiety (29). The fragment ions at m/z 262, 217, 172, 147, and 73 were produced similarly to those described for degradation of protonated hexadecane by AK-01. A further metabolite, [1,2-13C2]4-methyloctadecanoic acid, was also tentatively identified (Table 1, column C).
β-Oxidation, subsequent ketone formation, and loss of an acetyl moiety would produce [1,2-13C2]2-methylhexadecanoic acid. Although this metabolite was not detected, loss of a propionyl group from labeled 2-methylhexadecanoic acid would result in the elimination of the terminal and subterminal 13C-labeled carbon atoms, forming protonated tetradecanoic acid. This metabolite was identified and had a mass spectrum identical to that found in samples of AK-01 incubated with protonated hexadecane (Table 1, columns A and C).
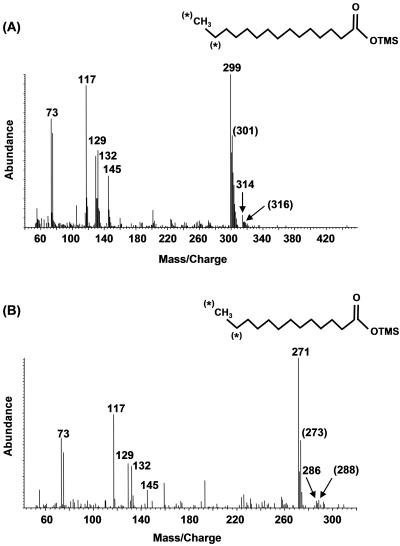
Two additional metabolites were identified, suggesting that carboxylation of hexadecane also occurred in the mixed sulfate-reducing consortium, analogous to the reported mechanism carried out by Hxd3 (44). Both pentadecanoic and tridecanoic acid were identified under protonated and 1,2-13C2-labeled conditions (data not shown). Under 1,2-13C2-labeled conditions, elimination of the two terminal carbons from a theoretical [13C2]2-ethylpentadecanoic acid metabolite would produce pentadecanoic acid having an M+ ion at either m/z 316 or m/z 314, depending on whether the carboxylation occurred at the labeled or unlabeled end of the molecule. Two metabolites coeluted with M+ ions at m/z 316 and 314 with corresponding (M-15)+ ions at m/z 301 and 299 (Fig. 4A). β-Oxidation of these metabolites would produce both labeled and unlabeled tridecanoic acids, which were also identified. The M+ ions occurred at m/z 288 and 286 with corresponding (M-15)+ ions at m/z 273 and 271 (Fig. 4B). These labeled fatty acids were not detected in controls.
FIG. 4.
Mass spectra of silylated protonated and 1,2-13C2-labeled pentadecanoic acid (A) and protonated and 12,13-13C2-labeled tridecanoic acid (B) produced by the sulfate-reducing consortium incubated with [1,2-13C2]hexadecane. *, 13C atoms that have not been eliminated during degradation. The m/z values shown in parentheses correspond to the m/z values for those labeled metabolites.
DISCUSSION
In this study, we present evidence supporting a mechanism of alkane addition to the double bond of fumarate by both the pure culture strain AK-01 and a sulfate-reducing consortium based on mass spectral analysis of protonated, deuterated, and/or 13C-labeled metabolites in active cultures versus sterile controls. We also identified metabolites consistent with carboxylation at C-3 in the sulfate-reducing consortium incubated with protonated hexadecane and [1,2-13C2]hexadecane. Evidence supporting carboxylation of hexadecane was also observed in Hxd3 cultures.
In AK-01 cultures grown on protonated or fully deuterated hexadecane, two peaks with identical mass spectra were resolved and tentatively identified as MPA. Two such peaks were also observed when the sulfate-reducing consortium was grown on protonated, deuterated, or 1,2-13C2-labeled hexadecane. The protonated, TMS-derivatized n-hexadecylsuccinic acid standard, however, eluted as one peak, with a retention time almost 1 min longer than that of the TMS-derivatized culture metabolites. These observations demonstrate that the succinyl moiety in the metabolites is not attached to the terminal carbon atom in the hexadecyl moiety but is, rather, in a subterminal position yielding chromatographically distinct stereoisomers (diastereomers). These findings are consistent with those previously reported (20, 29, 40).
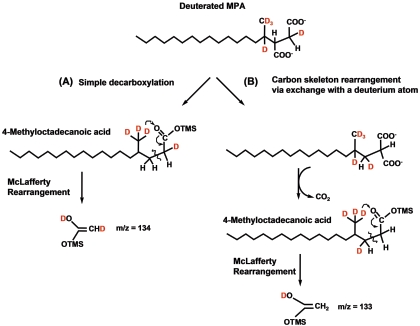
Wilkes et al. (55) proposed a pathway wherein fumarate addition to the subterminal carbon of hexane is followed by a speculated carbon skeleton rearrangement prior to β-oxidation. The results of our present study strongly support a carbon skeleton rearrangement whereby 4-methyloctadecanoic acid is a key metabolite (Fig. 2). This product could result from direct decarboxylation of MPA or decarboxylation after C-skeleton rearrangement of MPA. This study and others (20, 29, 40) have shown that all deuterium atoms are retained in the fumarate addition metabolite. For MPA, the retained deuterium atom is attached to the methylene atom (C-2 position) on the succinyl moiety, based on the observation of a fragment ion at m/z 388 (Fig. 1B). If simple decarboxylation of d34-MPA occurred, the deuterium atom would remain in the same position to yield 4-methyloctadecanoic acid. McLafferty rearrangement of this molecule (as a TMS derivative) in the MS would yield a fragment at m/z 134 (Fig. 5A). Instead, a McLafferty fragment at m/z 133 was observed (Fig. 2B), suggesting that the process involved exchange of the deuterium atom at C-3 with the carboxyl group at C-2 on the succinyl moiety (Fig. 5B). The observation of the McLafferty rearrangement signal at m/z 133 is consistent with the formation of 4-methyloctadecanoic acid following a C-skeleton rearrangement mechanism in a manner analogous to that observed for methylmalonyl-CoA transformation to succinyl-CoA (55).
FIG. 5.
McLafferty ions that result from decarboxylation of deuterated MPA that has not undergone carbon skeleton rearrangement (m/z 134) (A) and carbon skeleton rearrangement of MPA followed by decarboxylation (m/z 133) (B). The latter ion was experimentally observed in the mass spectrum of d34-4-methyloctadecanoic acid (Fig. 2B).
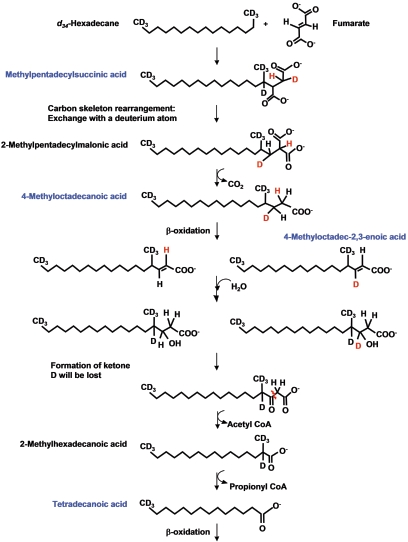
Based on these observations, a pathway for carbon skeleton rearrangement and subsequent degradation of d34-hexadecane by the sulfate reducer AK-01 is proposed which is analogous to that for hexane degradation under denitrifying conditions by strain HxN1 (55) (Fig. 6). The pathway is also likely carried out by some members of the sulfate-reducing consortium since many of the same metabolites were detected (Table 1). Although not shown, metabolites in the proposed degradation scheme presumably exist intracellularly as CoA thioesters following such activation of MPA prior to further degradation (55). After fumarate addition, C-skeleton rearrangement, and decarboxylation to 4-methyloctadecanoic acid, d34-hexadecane is presumably further degraded by a series of β-oxidation reactions beginning with a dehydrogenation reaction. The formation of the double bond could feasibly result in the loss of the deuterium atom or a hydrogen atom (Fig. 6). Our observation of peaks containing M+ and (M-15)+ fragments at m/z 401 and 386 as well as at m/z 402 and 387 suggests the presence of both d33- and d34-4-methyloctadec-2,3-enoic acid. The positive identification of d33-2-methylhexadecanoic acid and d27-tetradecanoic acid in d34-hexadecane-amended AK-01 supernatants also attests to further degradation of d34-4-methyloctadecanoic acid by two rounds of β-oxidation (Fig. 6).
FIG. 6.
Proposed mechanism of deuterated hexadecane degradation by strain AK-01. Putative deuterated metabolites are highlighted in blue.
As noted in Wilkes et al. (55), the final identified metabolite, tetradecanoic acid, would be further degraded via β-oxidation, ultimately leading to acetyl CoA. The propionic acid formed during β-oxidation of 2-methylhexadecanoic acid can be carboxylated and undergo a C-skeleton rearrangement, whereby fumarate is regenerated. Davidova et al. (20) offered evidence for such fumarate regeneration during the decomposition of isotopically heavy n-hexane by a sulfate-reducing culture. Alternatively, propionic acid could be further degraded to acetate that could enter the tricarboxylic acid cycle. Mineralization of hexadecane to 14CO2 by strain AK-01 was previously shown in 1-14C-labeled experiments by So and Young (47).
To date, most of the studied anaerobic alkane utilizers activate these hydrocarbons via a fumarate addition reaction (19, 20, 29, 40). In contrast, the sulfate-reducing strain Hxd3 is thought to utilize alkanes via carboxylation (45). In the present study, the detection of deuterated pentadecanoic and 10-methyl-pentadecanoic acids in Hxd3 cultures amended with fully deuterated hexadecane strongly suggests the elimination of the two terminal carbons following a C-3 carboxylation, supporting the findings of So et al. (45). Further, the identification of 14,15-13C2-labeled pentadecanoic acid and its presumed β-oxidation product 12,13-13C2-labeled tridecanoic acid in the sulfate-reducing consortium culture amended with [1,2-13C2]hexadecane is also consistent with a carboxylation mechanism. To our knowledge, this is the only additional report of a carboxylation mechanism involved in alkane degradation under anoxic conditions. The observation that metabolites consistent with both fumarate addition and carboxylation reactions were detected during hexadecane decay in the mixed consortium attests to the notion that multiple pathways for alkane degradation can occur simultaneously in mixed anaerobic ecosystems.
At the present time, the enzymes responsible for anaerobic alkane biodegradation reactions and the genes encoding them have not been defined. The activation of hydrocarbons under anoxic conditions requires novel biochemical reactions to overcome the energy barrier necessary to break hydrocarbon bonds. Glycyl radical enzymes, such as benzylsuccinate synthase, play a critical role in anaerobic metabolism by circumventing this energy barrier (43). Rabus et al. (40) used electron paramagnetic resonance spectroscopy to find evidence of a glycyl radical enzyme system in the denitrifying strain HxN1. Further investigation showed that strain HxN1 contains a small hexane-induced protein with an N-terminal sequence similar to the small subunit (BssC) of benzylsuccinate synthase (Behrends et al., unpublished data as reported in reference 40). Additionally, the retention of the abstracted deuterium atom from the parent molecule, as demonstrated in this study and others (19, 20, 29, 40), is consistent with a glycyl radical enzyme mechanism (7, 9). These findings, in addition to the current study, suggest that alkane-degrading organisms may share similar enzyme systems with each other and other hydrocarbon degraders.
Acknowledgments
This research was supported in part by grant NSFCHE-0221978 (L.Y.Y.) and in part by a fellowship to A.V.C. from the U.S. Department of Education GAANN, grant P200A010808.
We thank Sinéad Ní Chadhain and Boris Wawrik for editorial comment, Craig Phelps for technical assistance and editorial help, and Myron Dietz for help with graphics.
REFERENCES
- 1.Achong, G. R., A. M. Rodriguez, and A. M. Spormann. 2001. Benzylsuccinate synthase of Azoarcus sp. strain T: cloning, sequencing, transcriptional organization, and its role in anaerobic toluene and m-xylene mineralization. J. Bacteriol. 183:6763-6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aeckersberg, F., F. Bak, and F. Widdel. 1991. Anaerobic oxidation of saturated hydrocarbons to CO2 by a new type of sulfate-reducing bacterium. Arch. Microbiol. 156:5-14. [Google Scholar]
- 3.Aeckersberg, F., F. A. Rainey, and F. Widdel. 1998. Growth, natural relationships, cellular fatty acids and metabolic adaptation of sulfate-reducing bacteria that utilize long-chain alkanes under anoxic conditions. Arch. Microbiol. 170:361-369. [DOI] [PubMed] [Google Scholar]
- 4.Annweiler, E., A. Materna, M. Safinowski, A. Kappler, H. H. Richnow, W. Michaelis, and R. U. Meckenstock. 2000. Anaerobic degradation of 2-methylnaphthalene by a sulfate-reducing enrichment culture. Appl. Environ. Microbiol. 66:5329-5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baars, B. 2002. The wreckage of the oil tanker “Erica”—human health risk assessment of beach cleaning, sunbathing and swimming. Toxicol. Lett. 128:55-68. [DOI] [PubMed] [Google Scholar]
- 6.Beller, H. R., and E. A. Edwards. 2000. Anaerobic toluene activation by benzylsuccinate synthase in a highly enriched methanogenic culture. Appl. Environ. Microbiol. 66:5503-5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beller, H. R., and A. M. Spormann. 1997. Anaerobic activation of toluene and o-xylene by addition to fumarate in denitrifying strain T. J. Bacteriol. 179:670-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beller, H. R., and A. M. Spormann. 1998. Analysis of the novel benzylsuccinate synthase reaction for anaerobic toluene activation based on structural studies of the product. J. Bacteriol. 180:5454-5457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beller, H. R., and A. M. Spormann. 1997. Benzylsuccinate formation as a means of anaerobic toluene activation by sulfate-reducing strain PRTOL1. Appl. Environ. Microbiol. 63:3729-3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beller, H. R., and A. M. Spormann. 1999. Substrate range of benzylsuccinate synthase from Azoarcus sp. strain T. FEMS Microbiol. Lett. 178:147-153. [DOI] [PubMed] [Google Scholar]
- 11.Biegert, T., G. Fuchs, and J. Heider. 1996. Evidence that anaerobic oxidation of toluene in the denitrifying bacterium Thauera aromatica is initiated by formation of benzylsuccinate from toluene and fumarate. Eur. J. Biochem. 238:661-668. [DOI] [PubMed] [Google Scholar]
- 12.Boll, M., G. Fuchs, and J. Heider. 2002. Anaerobic oxidation of aromatic compounds and hydrocarbons. Curr. Opin. Chem. Biol. 6:604-611. [DOI] [PubMed] [Google Scholar]
- 13.Britton, L. N. 1984. Microbial degradation of aliphatic hydrocarbons, p. 89-129. In D. T. Gibson (ed.), Microbial degradation of organic compounds. Marcel Dekker, New York, N.Y.
- 14.Caldwell, M. E., R. M. Garrett, R. C. Prince, and J. M. Suflita. 1998. Anaerobic biodegradation of long-chain n-alkanes under sulfate-reducing conditions. Environ. Sci. Technol. 32:2191-2195. [Google Scholar]
- 15.Callaghan, A. V., L. M. Gieg, K. G. Kropp, J. M. Suflita, and L. Y. Young. 2003. Fumarate addition during hexadecane degradation by the sulfate-reducer AK-01, abstr. Q-038, p. 521. Abstr. 103rd Gen. Meet. Am. Soc. Microbiol. 2003. American Society of Microbiology, Washington, D.C.
- 16.Chakraborty, R., and J. D. Coates. 2004. Anaerobic degradation of monoaromatic hydrocarbons. Appl. Microbiol. Biotechnol. 64:437-446. [DOI] [PubMed] [Google Scholar]
- 17.Colberg, P. J. S., and L. Y. Young. 1995. Anaerobic degradation of nonhalogenated homocyclic aromatic compounds coupled with nitrate, iron and sulfate reduction, p. 301-324. In L. Y. Young and C. E. Cerniglia (ed.), Microbial transformation and degradation of toxic organic chemicals. Wiley-Liss Inc., New York, N.Y.
- 18.Coschigano, P. W., T. S. Wehrman, and L. Y. Young. 1998. Identification and analysis of genes involved in anaerobic toluene metabolism by strain T1: putative role of a glycine free radical. Appl. Environ. Microbiol. 64:1650-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cravo-Laureau, C., V. Grossi, D. Raphel, R. Matheron, and A. Hirschler-Réa. 2005. Anaerobic n-alkane metabolism by a sulfate-reducing bacterium, Desulfatibacillum aliphaticivorans strain CV2803. Appl. Environ. Microbiol. 71:3458-3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davidova, I. A., L. M. Gieg, M. Nanny, K. G. Kropp, and J. M. Suflita. 2005. Stable isotopic studies of n-alkane metabolism by a sulfate-reducing bacterial enrichment culture. Appl. Environ. Microbiol. 71:8174-8182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evans, P. J., W. Ling, B. Goldschmidt, E. R. Ritter, and L. Y. Young. 1992. Metabolites formed during anaerobic transformation of toluene and o-xylene and their proposed relationship to the initial steps of toluene mineralization. Appl. Environ. Microbiol. 58:496-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fessenden, R. J., and J. S. Fessenden. 1982. Organic Chemistry. PWS Publishers, Boston, Mass.
- 23.Gieg, L. M., and J. M. Suflita. 2002. Detection of anaerobic metabolites of saturated and aromatic hydrocarbons in petroleum-contaminated aquifers. Environ. Sci. Technol. 36:3755-3762. [DOI] [PubMed] [Google Scholar]
- 24.Harayama, S., H. Kishira, Y. Kassai, and K. Shutsubo. 1999. Petroleum biodegradation in marine environments. J. Mol. Microbiol. Biotechnol. 1:63-70. [PubMed] [Google Scholar]
- 25.Heider, J., A. M. Spormann, H. R. Beller, and F. Widdel. 1999. Anaerobic bacterial metabolism of hydrocarbons. FEMS Microbiol. Rev. 22:459-473. [Google Scholar]
- 26.Kane, S. R., H. R. Beller, T. C. Legler, and R. T. Anderson. 2002. Biochemical and genetic evidence of benzylsuccinate synthase in toluene-degrading, ferric iron-reducing Geobacter metallireducens. Biodegradation 13:149-154. [DOI] [PubMed] [Google Scholar]
- 27.Kniemeyer, O., T. Fischer, H. Wilkes, F. O. Glöckner, and F. Widdel. 2003. Anaerobic degradation of ethylbenzene by a new type of marine sulfate-reducing bacterium. Appl. Environ. Microbiol. 69:760-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krieger, C. J., W. Roseboom, S. P. Albracht, and A. M. Spormann. 2001. A stable organic free radical in anaerobic benzylsuccinate synthase of Azoarcus sp. strain T. J. Biol. Chem. 276:12924-12927. [DOI] [PubMed] [Google Scholar]
- 29.Kropp, K. G., I. A. Davidova, and J. M. Suflita. 2000. Anaerobic oxidation of n-dodecane by an addition reaction in a sulfate-reducing bacterial enrichment culture. Appl. Environ. Microbiol. 66:5393-5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kube, M., J. Heider, J. Amann, P. Hufnagel, S. Kuhner, A. Beck, R. Reinhardt, and R. Rabus. 2004. Genes involved in the anaerobic degradation of toluene in a denitrifying bacterium, strain EbN1. Arch. Microbiol. 181:182-194. [DOI] [PubMed] [Google Scholar]
- 31.Leahy, J. G., and R. R. Colwell. 1990. Microbial degradation of hydrocarbons in the environment. Microbiol. Rev. 54:305-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leuthner, B., C. Leutwein, H. Schulz, P. Horth, W. Haehnel, E. Schiltz, H. Schagger, and J. Heider. 1998. Biochemical and genetic characterization of benzylsuccinate synthase from Thauera aromatica: a new glycyl radical enzyme catalysing the first step in anaerobic toluene metabolism. Mol. Microbiol. 28:615-628. [DOI] [PubMed] [Google Scholar]
- 33.Leutwein, C., and J. Heider. 1999. Anaerobic toluene-catabolic pathway in denitrifying Thauera aromatica: activation and beta-oxidation of the first intermediate, (R)-(+)-benzylsuccinate. Microbiology 145:3265-3271. [DOI] [PubMed] [Google Scholar]
- 34.Maeng, J. H., Y. Sakai, T. Ishige, Y. Tani, and N. Kato. 1996. Diversity of dioxygenases that catalyze the first step of oxidation of long-chain n-alkanes in Acinetobacter sp. M-1. FEMS Microbiol. Lett. 141:177-182. [Google Scholar]
- 35.May, S. W., and A. G. Katoposis. 1990. Hydrocarbon monooxygenase system of Pseudomonas oleovorans. Methods Enzymol. 188:3-9. [DOI] [PubMed] [Google Scholar]
- 36.McLafferty, F. W. 1980. Interpretation of mass spectra, 3rd ed. University Science Books, Mill Valley, Calif.
- 37.Murphy, R. C. 1993. Mass spectrometry of lipids, p. 71-130. In F. Snyder (ed.), Handbook of lipid research, vol. 7. Plenum Press, New York, N.Y. [Google Scholar]
- 38.Petrov, A. A. 1984. Petroleum hydrocarbons. Springer-Verlag, New York, N.Y.
- 39.Pierce, A. E. 1968. Silylation of organic compounds. Pierce Chemical Company, Rockford, Ill.
- 40.Rabus, R., H. Wilkes, A. Behrends, A. Armstroff, T. Fischer, A. J. Pierik, and F. Widdel. 2001. Anaerobic initial reaction of n-alkanes in a denitrifying bacterium: evidence for (1-methylpentyl)succinate as initial product and for involvement of an organic radical in n-hexane metabolism. J. Bacteriol. 183:1707-1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rehm, H. J., and I. Reiff. 1981. Mechanisms and occurrence of microbial oxidation of long-chain alkanes, p. 175-215. In A. Fiechter (ed.), Advances in biochemical engineering, vol. 19. Springer-Verlag, Berlin, Germany. [Google Scholar]
- 42.Rios-Hernandez, L. A., L. M. Gieg, and J. M. Suflita. 2003. Biodegradation of an alicyclic hydrocarbon by a sulfate-reducing enrichment from a gas condensate-contaminated aquifer. Appl. Environ. Microbiol. 69:434-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sawers, G. 1999. Biochemistry, physiology and molecular biology of glycyl radical enzymes. FEMS Microbiol. Rev. 22:543-551. [Google Scholar]
- 44.Silverstein, R. M., G. C. Bassler, and T. C. Morrill. 1991. Spectrometric identification of organic compounds. John Wiley & Sons, Inc., New York, N.Y.
- 45.So, C. M., C. D. Phelps, and L. Y. Young. 2003. Anaerobic transformation of alkanes to fatty acids by a sulfate-reducing bacterium, strain Hxd3. Appl. Environ. Microbiol. 69:3892-3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.So, C. M., and L. Y. Young. 1999. Initial reactions in anaerobic alkane degradation by a sulfate reducer, strain AK-01. Appl. Environ. Microbiol. 65:5532-5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.So, C. M., and L. Y. Young. 1999. Isolation and characterization of a sulfate-reducing bacterium that anaerobically degrades alkanes. Appl. Environ. Microbiol. 65:2969-2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spiteller, G., M. Spiteller-Friedmann, and R. Houriet. 1966. Elucidation of mass-spectroscopic fragmentation mechanisms by use of cold ion sources and low energy electrons. Aliphatic Esters Monatsh. Chem. 1:121-128. [Google Scholar]
- 49.Spormann, A. M., and F. Widdel. 2000. Metabolism of alkylbenzenes, alkanes, and other hydrocarbons in anaerobic bacteria. Biodegradation 11:85-105. [DOI] [PubMed] [Google Scholar]
- 50.Tanner, R. S. 2002. Cultivation of bacteria and fungi. In C. J. Hurst, R. L. Crawford, G. R. Knudson, M. J. NcInerney, and L. D. Stetzenbach (ed.), Manual of environmental microbiology, 2nd ed. ASM Press, Washington, D.C.
- 51.Van Beilen, J. B., M. G. Wubbults, and B. Witholt. 1994. Genetics of alkane oxidation by Pseudomonas oleovorans. Biodegradation 5:161-174. [DOI] [PubMed] [Google Scholar]
- 52.Watkinson, R. J., and P. Morgan. 1990. Physiology of aliphatic hydrocarbon-degrading microorganisms. Biodegradation 1:79-92. [DOI] [PubMed] [Google Scholar]
- 53.Widdel, F., and F. Bak. 1992. Gram-negative mesophilic sulfate-reducing bacteria, p. 3352-3378. In A. Balows, H. G. Truper, M. Dworkin, W. Harder, and K. H. Schleifer (ed.), The prokaryotes, vol. 4. Springer-Verlag, New York, N.Y. [Google Scholar]
- 54.Wilkes, H., S. Kuehner, C. Bolm, T. Fischer, A. Classen, F. Widdel, and R. Rabus. 2003. Formation of n-alkane- and cycloalkane-derived organic acid during anaerobic growth of a denitrifying bacterium with crude oil. Org. Geochem. 34:1313-1323. [Google Scholar]
- 55.Wilkes, H., R. Rabus, T. Fischer, A. Armstroff, A. Behrends, and F. Widdel. 2002. Anaerobic degradation of n-hexane in a denitrifying bacterium: further degradation of the initial intermediate (1-methylpentyl)succinate via C-skeleton rearrangement. Arch. Microbiol. 177:235-243. [DOI] [PubMed] [Google Scholar]
- 56.Zhang, X., E. R. Sullivan, and L. Y. Young. 2000. Evidence for aromatic ring reduction in the biodegradation pathway of carboxylated naphthalene by a sulfate reducing consortium. Biodegradation 11:117-124. [DOI] [PubMed] [Google Scholar]
- 57.Zhang, X., and L. Y. Young. 1997. Carboxylation as an initial reaction in the anaerobic metabolism of naphthalene and phenanthrene by sulfidogenic consortia. Appl. Environ. Microbiol. 63:4759-4764. [DOI] [PMC free article] [PubMed] [Google Scholar]